Animal viruses have developed various strategies for infecting cells, and all begin with adsorption to cell surface receptors, penetration into the cytosol, uncoating or release of the viral genome, and targeting the genome and any required accessory proteins toward the correct cellular organelle or compartment for replication (26, 48, 63). Since genome delivery and release require the rearrangement of the viral structures, infection is normally a multistep process involving various viral and cellular components. Viruses that replicate in the nucleus must have mechanisms for transporting the genome and other components to the vicinity of the nuclear pore and into the nucleus (84). The endosomal pathways of the cell are used by many viruses; vesicles are transported by microtubule-dependent motors through the cytoplasm. Nucleus-replicating viruses may require cytoskeleton-driven transport of the capsid or of viral components mediated by either microtubules or actin or, in some cases, by both structures (39, 66). Nuclear transport of viral components involves signal-mediated interactions with the nuclear import machinery (84). While enveloped viruses enter the cell by glycoprotein-mediated fusion of the viral envelope with a cellular membrane, much less is known about the entry of most nonenveloped viruses, but capsid-dependent mechanisms for penetrating the cell membrane or lysing endosomes are likely to be involved.
PARVOVIRUSES
The parvoviruses are among the smallest animal DNA viruses. The family Parvoviridae contains two subfamilies: the Parvovirinae, which infect vertebrates and include the three genera Parvovirus, Erythrovirus, and Dependovirus, and the Densovirinae, which infect invertebrates (22, 41). The dependoviruses depend on a helper adenovirus or herpesvirus to supply functions needed for their replication. When added to cells alone, they still infect the host cell efficiently, but the genome undergoes an incomplete replication and may integrate into the host genome to establish latency (15, 56). Human adeno-associated viruses (AAVs) include several different serotypes, and the prototype strain (AAV2) infects many types of cells and tissues of various species, including humans, dogs, and mice (40, 41, 60). The autonomous parvoviruses, Parvovirus and Erythrovirus, do not need a helper virus but require cellular S-phase functions for their DNA replication. The autonomous parvoviruses infect only a restricted range of host and tissues, and densoviruses infect invertebrates (10, 19).
PARVOVIRUSES AND DISEASE
The human parvovirus B19 virus causes aplastic anemia in patients with sickle cell disease, childhood fifth disease (erythema infectiosum), arthropathy in adults, and rare fetal infections (11). Canine parvovirus (CPV) and feline panleukopenia virus (FPV) are closely related parvoviruses. CPV appeared in 1978 as a new virus infecting dogs and is the result of a mutation in a strain of FPV that allowed the virus to expand its host range to canines (46). The minute virus of mice (MVM) and other rodent parvoviruses cause a variety of diseases in neonatal and older animals, and MVM shows various tissue tropisms (12, 46). The AAVs are being developed as vectors for gene therapy, with alterations in the tissue tropism of the viruses being introduced to alter the target tissues for alternative therapies.
The 26-nm-diameter parvovirus icosahedral capsid packages a single-stranded DNA genome of around 5,000 bases (74, 87). The capsid is assembled from 60 copies of the structural proteins, which come in two or more different overlapping forms (17). VP1 and VP2 of MVM and CPV are translated from alternatively spliced messages, while VP3 is formed in full capsids by the cleavage of a peptide from the N terminus of VP2 exposed outside the capsid (72, 76, 82). VP1 contains the complete sequence of VP2 and a unique 143-residue N-terminal sequence necessary for viral infectivity but not for capsid formation (76, 78). The VP1-unique regions contain a phospholipase A2 (PLA2) motif which appears active when released from the capsid (91). AAV capsids are formed from VP1, VP2, and VP3, which are formed by alternative splicing and start codons (9). The atomic structures of the capsid surfaces of the mammalian viruses CPV, FPV, MVM, and AAV have a variety of features, and the host ranges of CPV, FPV, and MVM are controlled by sequences on the capsid surfaces (1-3, 14, 30, 75, 86). An insect parvovirus has a relatively smooth surface compared to that of the vertebrate parvoviruses (65).
CELL RECEPTORS
Both carbohydrate and protein receptors have been reported for many viruses (Table 1). AAV2 and AAV3 use heparan sulfate proteoglycan (HSPG) for low-affinity attachment to the cell surface and for infection of host cells (53, 71), although AAV2 mutants that do not bind HSPG are still infectious (43, 61). Other receptors associated with cell infection by AAV2 include αVβ5 integrin and basic fibroblast growth factor receptor 1 (51, 70), although Qiu and Brown reported that αVβ5 integrin is not involved (52). AAV4 and AAV5 bind sialic acids during the infection of cells, although the type of sialic acid bound differs: AAV4 binds to α2-3-linked sialic acids, and AAV5 binds to both α2-3- and α2-6-linked sialic acids (32, 80). AAV5 can also use platelet-derived growth factor receptor for cell binding and infection (47). AAVs appear to be quite promiscuous in their use of receptors. A variety of different cross-linking ligands were able to mediate the virus infection, and AAVs could be adapted to a variety of alternative receptors with mutations or insertions within the capsid protein (6, 23, 54, 62, 85, 89).
TABLE 1.
Parvoviral receptors
| Virus | Receptor (reference) | Coreceptor (reference) |
|---|---|---|
| AAV2 | Heparan sulfate proteoglycan (71) | αVβ5 integrin (70) |
| Human fibroblast growth factor receptor (51) | ||
| AAV4 | α2-3-linked sialic acid (80) | |
| AAV5 | α2-3-linked sialic acid (34) | |
| α2-6-linked sialic acid (34) | ||
| Platelet-derived growth factor (47) | ||
| B19V | Erythrocyte P antigena (12) | |
| ADV | ADV binding protein | |
| CPV | Transferrin receptor (46) | |
| Sialic acid (5) | ||
| FPV | Transferrin receptor (46) |
Globoside.
The human parvovirus B19 virus replicates only in human erythroid progenitor cells, and cell binding and infection require the erythrocyte P antigen (globoside) (10). However, a number of cells which express globoside on their surfaces are not susceptible to infection, which may be due partly to an intracellular blocking of the transcription of viral messages in nonerythroid cells (13, 35). It is also likely that there is another protein-based receptor for the parvovirus B19 virus (83).
Both CPV and FPV bind the sialic acid on some host cells, and sialic acid binding appears to be specific for N-glycolyl neuraminic acid (NeuGC), which is found on the erythrocytes and cells of most cats, monkeys, and horses but not on the cells of most dogs. NeuGC binding is temperature and pH dependent and is controlled by residues adjacent to a depressed region (the dimple) on the virus capsid (5, 73). Mutations within a loop between VP2 residues at amino acid positions 359 to 377 or of the VP2 residue at amino acid position 323 make sialic acid binding dependent on low pH (a pH of <6.5) or stop sialic acid binding (5, 64, 73). However, sialic acid binding does not mediate infection of tissue-cultured cells, as the viruses can infect cells displaying only N-acetylneuraminic acid and non-sialic acid binding mutants infect the same range of cells as wild-type viruses. FPV capsids bind sialic acids on feline cells most efficiently under the nonphysiological conditions of pH below 6.5 and a temperature of 4°C. CPV can bind the sialic acid on feline cells at pH values of >7.0, at least partially, and that inhibits infection, as mutants which do not bind the sialic acid are selected by passage of CPV in cat cells (5). The transferrin receptor (TfR) is used by CPV and FPV for infecting cells, and differences between the feline and canine TfR specifically control the binding of viruses to cells (31, 45). The specific contact between the CPV or FPV capsids and the TfR appears to be important for successful cell infection, as some changes in the virus or the receptor may still allow capsid binding to occur but do not mediate infection (30). The host ranges of CPV and FPV were found to be controlled by their cell binding abilities. Both CPV and FPV capsids bound and entered feline cells, but only CPV bound to the canine cells. This specificity appears to be due to TfR binding by the capsids, as the feline TfR expressed on Chinese hamster ovary-derived TRVb cells allowed efficient CPV and FPV binding and infection, while the canine TfR bound CPV but not FPV capsids and allowed infection by CPV (31).
ENDOCYTOSIS, CYTOPLASMIC TRAFFICKING, AND NUCLEAR ENTRY
All parvoviruses require receptor-mediated endocytosis for cell infection. CPV, MVM, and AAV are taken up rapidly into cells, most likely by clathrin-mediated endocytosis, as CPV and AAV2 endocytosis was inhibited by overexpression of the Lys44Ala (K44A) dominant interfering mutant of dynamin-2 (7, 21, 34, 44). Both CPV and AAV infections can be inhibited by treatment of cells with lysosomotropic agents, including NH4Cl and bafilomycin A1 (7, 8, 27, 44), indicating that low endosomal pH is required for infection or for endosomal trafficking. Shortly after uptake, CPV capsids colocalize with transferrin in perinuclear endosomes (Fig. 1) (44, 69).
FIG. 1.
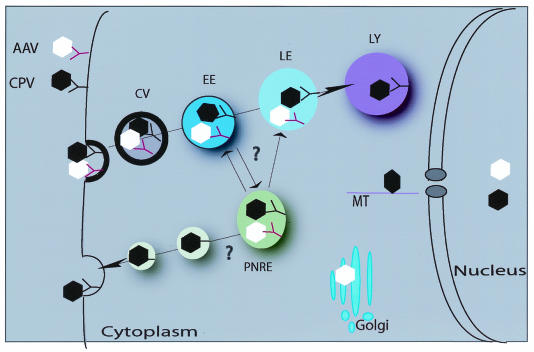
A schematic representation of the entry mechanisms utilized by parvoviruses within the host cell. Cell entry of autonomous parvovirus CPV and AAV is shown. After binding to their cell surface receptors, both viruses are internalized into clathrin-coated vesicles (CV), followed by transport to early (EE), late (LE), or perinuclear recycling endosomes (PNRE). Later in entry, in the case of AAV, capsids are found in Golgi compartments, whereas CPV can be found in lysosomes (LY). The site of the capsid escape from endocytic vesicles into the cytosol is still unclear. CPVs make use of microtubules (MT) during the traffic through the cytosol toward the nucleus. Viral capsids are able to enter the nucleus in intact form without apparent deformation.
AUTONOMOUS PARVOVIRUSES
After endocytosis, penetration of CPV capsids into the cytosol is a slow process. Antibodies against the TfR cytoplasmic tail reduced virus infection when injected into cells 4 h after inoculation, showing that many infecting capsids remain associated with the TfR in endocytic compartments for several hours after uptake (44). Antibodies to the CPV capsid injected into the cytoplasm also prevented virus infection when injected 4 or more hours after inoculation of the virus, indicating that the capsids pass slowly out of the endosomal vesicle (78, 79). After uptake, capsids were detected in perinuclear compartments for several hours (44, 69). After fluorescence in situ hybridization (FISH), CPV DNA colocalized with the capsids in perinuclear compartments for at least 8 h (69). MVM capsids also require uptake through the endosomal system, where infection could be blocked by bafilomycin A1 or chloroquin for several hours after uptake from the cell surface (55). The mechanism of escape from endocytic vesicles into the cytosol is still unknown, although there does not appear to be a wholesale lysis of the endosomal vesicle, as there is little transport of alpha-sarcin into the cytoplasm (44, 68). A PLA2 homologous sequence is present in the VP1-unique region of most parvoviruses and has been shown by mutation of its predicted active site and by inhibitor studies to be active (Fig. 2) (19, 68, 91).
FIG. 2.
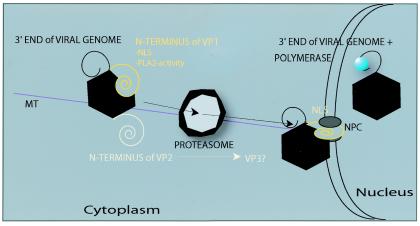
Intracellular trafficking and modifications of autonomous parvovirus capsids. Once inside the cell, capsids undergo modifications, exposing on their surfaces not only the N-terminal end of the VP1 capsid protein but also the N-terminal sequence of the VP2 capsid protein and 3′ end of the viral genome. In the cytosol, capsids are also affected by the activity of proteasomes, possibly causing the intracellular formation of the VP3 capsid protein. Later in entry, viral capsids are imported into the nucleus through the NPC with the help of the NLS. MT, microtubules.
The capsids are most likely released into the cytoplasm from a vesicle in a perinuclear location, and there is likely further processing in the cytoplasm and transport to the nucleus. MVM capsids are affected by the activity of the proteasome, since infection can be reduced by some proteasome inhibitors, including inhibitors of chymotrypsin-like activity (N-tosyl-l-phenylalanine chloromethyl ketone and aclarubin), but not by inhibitors of trypsin-like activity (55). It is not clear what mechanisms are involved in that activity, but a direct proteolytic activity or a role in controlling the ubiquitination of the capsid may be involved. Active transport mechanisms are also likely to be required for the particles or the viral DNA to reach the nuclear pore. The cytoplasm contains a lattice-like mesh of microtubules, actin microfilaments, and intermediate filaments which restrict the diffusion of macromolecular complexes (38, 59). CPV capsids injected into the cytoplasm rapidly became localized in a perinuclear location, and the capsids mostly entered the nucleus only 3 to 6 h later (79). Transport to the perinuclear region and nucleus were prevented by nocodazole treatment to depolymerize microtubules and by an antibody against the intermediate chain of the microtubule-based, minus-end-directed motor protein dynein (67, 69, 79). By electron microscopy, capsids were associated with tubulin and with apparent dynein structures in vitro. Viral capsids could be precipitated from infected cells along with intermediate chains of dynein. These results suggest that microtubules and dynein move CPV capsids within the cytoplasm, facilitating their transport to the nucleus and infection of cells (67).
Transport of the capsid and/or viral DNA into the nucleus is an important step in infection of cells with an intact nucleus and occurs principally through the nuclear pore complex (NPC). Although small macromolecules can diffuse freely through the pore, transport of larger molecules is specific, requiring ATP and soluble cytosolic factors, including Ran-1, and is mediated by nuclear localization sequences (NLSs) (25, 42). Parvovirus capsids appear to be able to pass through the NPC intact, and over a period of 2 or more h, microinjected CPV capsids enter the nucleus, where they are recognized by antibodies that bind only intact capsids (79). Modification of viral capsids may allow exposure of NLSs needed for nuclear targeting and transport of viral particles (Fig. 2). Only two basic sequences that might be classical NLSs are present in the VP2 capsid protein sequence of CPV, while the VP1-unique region contains several such sequences. A VP1 sequence (PAKRARRGYK) between residues at amino acid positions 4 to 13 functions for nuclear transport when conjugated to bovine serum albumin (77). That N-terminal unique sequence was detected on capsids between 1 and 4 h after injection of the capsids into cells, and it was also exposed on incoming infectious virions, since antibodies specific for the VP1-unique region blocked infection when injected into cells before inoculation of the virus (78). In addition, some specific changes in the VP1 N-terminal basic sequence reduced the relative infectivity of the capsids (78). MVM capsids have NLSs in both VP1 and VP2, with two NLSs mapped near the VP1-specific N terminus, while VP2 appears to make use of an internal basic sequence (KGKLTMRAKLR) in a conformation-dependent manner (36, 37). An intact VP1-unique region that could mediate efficient nuclear transport was also required for efficient infection of cells by MVM capsids (Fig. 2) (37).
AAV CELL ENTRY AND INFECTION OR TRANSDUCTION PATHWAYS
The cell entry and infection pathways of AAV capsids appear to differ in several ways from those used by the autonomous parvoviruses (Fig. 1). AAV capsids enter cells through endosomal uptake, but a variety of different results have been reported. In a study which followed individual, labeled capsids into cells, the capsids appeared to follow different routes: some entered the cytoplasm quickly after endosomal uptake, while others remained within the endosomal system for longer periods (58). The capsids then appeared to traffic within the cell by three different pathways: microtubule-dependent transport within endosomes, free diffusion within the cytoplasm, and rapid transport in the cytoplasm in association with microtubular motors (58). In another study of AAV2 entry, capsids appeared to escape from early endosomes into the cytosol and then to become localized within the nucleus within 2 h (7). AAV2 capsids have also been reported to traffic through both early and late endosomes prior to nuclear translocation. The susceptibility of AAV2 infection to bafilomycin A1 treatment differed between human 293 cells and mouse 3T3 cells (20, 27). AAV capsids have also been seen to colocalize with transferrin within perinuclear vesicles for long periods (21). Cell binding by AAV2 led to activation of Rac1, most likely through binding and clustering of αVβ5 integrins. Infection was reduced by a dominant negative inhibitor of Rac1 and by inhibitors of phosphatidylinositol (PI) 3-kinase, indicating that signaling through a Rac1 and PI 3-kinase activation cascade might be involved (57). AAV5 capsids accumulate in the Golgi compartment after uptake (4). Within the cytoplasm, the AAV2 and AAV5 capsids are ubiquitinated and may be degraded by the proteasome; proteasome inhibitors increased the transduction efficiency of virions (20, 88).
The processes of nuclear transport and second-strand synthesis during AAV infection appear to vary among different studies; this variation may reflect differences in both cells and methods. The VP2 N terminus is important in the nuclear translocation of AAV2 proteins when they are expressed as virus-like particles, although whether the N-terminal sequences are also involved in controlling infection is not clear (29) A high proportion of the viral DNA can be recovered from a nuclear fraction as double-stranded forms. The VP1 of AAV2 contains a PLA2 activity which is required for cell infection, and when that sequence was mutated, the viruses showed a delayed infectivity (24). The mechanism of AAV trafficking to the nucleus is not known, although, as with the autonomous parvoviruses, viral particles may be transported into the nucleus through the nuclear pore (57). Moreover, uptake of AAV2 capsids into the purified nuclei was examined and reported not to require transport through the nuclear pore; however, the mechanism of that alternative pathway was not defined (28).
VIRAL DNA RELEASE FROM THE CAPSID AND INITIATION OF REPLICATION
Full capsids of MVM, CPV, and AAV2 have 20 to 30 nucleotides of the 5′ end of the viral genome exposed on the outside of the capsid, and the NS1 protein is covalently attached to the 5′ end (18, 49, 81). That DNA is thought to pass through a pore at the 5-fold axis of the capsid (86, 87). The 3′ end of the viral DNA can also be exposed outside the capsid after treatments which do not cause capsid disintegration, and the 3′ terminal hairpin can act as a template for the DNA polymerase in vitro (16, 78). This finding suggests that there is a mechanism whereby this DNA exposed within the nucleus can be used as a template for initiating DNA replication by the host cell DNA polymerase, whereby the DNA can be removed without disassembly of the stable capsid.
AAV particles can efficiently transduce nondividing cells, and in the absence of a helper herpesvirus or adenovirus their genomic DNA becomes integrated into the cellular DNA (33, 90). The release of AAV DNA has not been well characterized, but in some cells the A region adjacent to the terminal sequence (the D region) may be bound by a nonphosphorylated form of a cellular protein, SKFBP, which blocks infection (50).
CONCLUSIONS
The process of cell infection by parvoviruses shows many of the features seen for other viruses which replicate in the nucleus, but the small and stable parvovirus particles must undergo more subtle changes during the various steps involved. The relatively high particle-to-infectivity ratio suggests that the virus is inefficient at infection, but this might also be a reflection of the numerous steps that the virus has to accomplish during the trafficking from the cell surface to the nucleus. Our ability to detect small changes in the virus and to follow small numbers of particles into the cell should allow us to define more specifically the principles involved in intracellular trafficking events.
Acknowledgments
We thank John Parker and Matti Vuento for valuable discussions and helpful comments on the manuscript.
REFERENCES
- 1.Agbandje, M., R. McKenna, M. G. Rossmann, M. L. Strassheim, and C. R. Parrish. 1993. Structure determination of feline panleukopenia virus empty particles. Proteins 16:155-171. [DOI] [PubMed] [Google Scholar]
- 2.Agbandje-McKenna, M., A. L. Llamas-Saiz, F. Wang, P. Tattersall, and M. G. Rossmann. 1998. Functional implications of the structure of the murine parvovirus, minute virus of mice. Structure 6:1369-1381. [DOI] [PubMed] [Google Scholar]
- 3.Ball-Goodrich, L. J., and P. Tattersall. 1992. Two amino acid substitutions within the capsid are coordinately required for acquisition of fibrotropism by the lymphotropic strain of minute virus of mice. J. Virol. 66:3415-3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bantel-Schaal, U., B. Hub, and J. Kartenbeck. 2002. Endocytosis of adeno-associated virus type 5 leads to accumulation of virus particles in the Golgi compartment. J. Virol. 76:2340-2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbis, D. P., S.-F. Chang, and C. R. Parrish. 1992. Mutations adjacent to the dimple of canine parvovirus capsid structure affect sialic acid binding. Virology 191:301-308. [DOI] [PubMed] [Google Scholar]
- 6.Bartlett, J. S., J. Kleinschmidt, R. C. Boucher, and R. J. Samulski. 1999. Targeted adeno-associated virus vector transduction of nonpermissive cells mediated by a bispecific F(ab′γ)2 antibody. Nat. Biotechnol. 17:181-186. [DOI] [PubMed] [Google Scholar]
- 7.Bartlett, J. S., R. Wilcher, and R. J. Samulski. 2000. Infectious entry pathway of adeno-associated virus and adeno-associated virus vectors. J. Virol. 74:2777-2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Basak, S., and H. Turner. 1992. Infectious entry pathway for canine parvovirus. Virology 186:368-376. [DOI] [PubMed] [Google Scholar]
- 9.Becerra, S. P., F. Koczot, P. Fabisch, and J. A. Rose. 1988. Synthesis of adeno-associated virus structural proteins requires both alternative mRNA splicing and alternative initiations from a single transcript. J. Virol. 62:2745-2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown, K. E., S. M. Anderson, and N. S. Young. 1993. Erythrocyte P antigen: cellular receptor for B19 parvovirus. Science 262:114-117. [DOI] [PubMed] [Google Scholar]
- 11.Brown, K. E., N. S. Young, and J. M. Liu. 1994. Molecular, cellular and clinical aspects of parvovirus B19 infection. Crit. Rev. Oncol. Hematol. 16:1-31. [DOI] [PubMed] [Google Scholar]
- 12.Brownstein, D. G., A. L. Smith, R. O. Jacoby, E. A. Johnson, G. Hansen, and P. Tattersall. 1991. Pathogenesis of infection with a virulent allotropic variant of minute virus of mice and regulation by host genotype. Lab. Investig. 65:357-364. [PubMed] [Google Scholar]
- 13.Brunstein, J., and C. R. Astell. 1997. Analysis of the internal replication sequence indicates that there are three elements required for efficient replication of minute virus of mice minigenomes. J. Virol. 71:9087-9095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang, S. F., J. Y. Sgro, and C. R. Parrish. 1992. Multiple amino acids in the capsid structure of canine parvovirus coordinately determine the canine host range and specific antigenic and hemagglutination properties. J. Virol. 66:6858-6867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiorini, J. A., S. M. Wiener, L. Yang, R. H. Smith, B. Safer, N. P. Kilcoin, Y. Liu, E. Urcelay, and R. M. Kotin. 1996. The roles of AAV Rep proteins in gene expression and targeted integration. Curr. Top. Microbiol. Immunol. 218:25-33. [DOI] [PubMed] [Google Scholar]
- 16.Cotmore, S. F., A. M. D'Abramo, Jr., C. M. Ticknor, and P. Tattersall. 1999. Controlled conformational transitions in the MVM virion expose the VP1 N-terminus and viral genome without particle disassembly. Virology 254:169-181. [DOI] [PubMed] [Google Scholar]
- 17.Cotmore, S. F., and P. Tattersall. 1987. The autonomously replicating parvoviruses of vertebrates. Adv. Virus Res. 33:91-174. [DOI] [PubMed] [Google Scholar]
- 18.Cotmore, S. F., and P. Tattersall. 1988. The NS-1 polypeptide of minute virus of mice is covalently attached to the 5′ termini of duplex replicative form DNA and progeny single strands. J. Virol. 62:851-860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dorsch, S., G. Liebisch, B. Kaufmann, P. von Landenberg, J. H. Hoffmann, W. Drobnik, and S. Modrow. 2002. The VP1 unique region of parvovirus B19 and its constituent phospholipase A2-like activity. J. Virol. 76:2014-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Douar, A. M., K. Poulard, D. Stockholm, and O. Danos. 2001. Intracellular trafficking of adeno-associated virus vectors: routing to the late endosomal compartment and proteasome degradation. J. Virol. 75:1824-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duan, D., Q. Li, A. W. Kao, Y. Yue, J. E. Pessin, and J. F. Engelhardt. 1999. Dynamin is required for recombinant adeno-associated virus type 2 infection. J. Virol. 73:10371-10376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fauquet, C. M., and M. A. Mayo. 2001. The 7th ICTV report. Arch. Virol. 146:189-194. [DOI] [PubMed] [Google Scholar]
- 23.Girod, A., M. Ried, C. Wobus, H. Lahm, K. Leike, J. Kleinschmidt, G. Deleage, and M. Hallek. 1999. Genetic capsid modifications allow efficient re-targeting of adeno-associated virus type 2. Nat. Med. 5:1052-1056. [DOI] [PubMed] [Google Scholar]
- 24.Girod, A., C. E. Wobus, Z. Zadori, M. Ried, K. Leike, P. Tijssen, J. A. Kleinschmidt, and M. Hallek. 2002. The VP1 capsid protein of adeno-associated virus type 2 is carrying a phospholipase A2 domain required for virus infectivity. J. Gen. Virol. 83:973-978. [DOI] [PubMed] [Google Scholar]
- 25.Gorlich, D., and U. Kutay. 1999. Transport between the cell nucleus and the cytoplasm. Annu. Rev. Cell. Dev. Biol. 15:607-660. [DOI] [PubMed] [Google Scholar]
- 26.Greber, U. F. 2002. Signalling in viral entry. Cell. Mol. Life Sci. 59:608-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hansen, J., K. Qing, and A. Srivastava. 2001. Adeno-associated virus type 2-mediated gene transfer: altered endocytic processing enhances transduction efficiency in murine fibroblasts. J. Virol. 75:4080-4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hansen, J., K. Qing, and A. Srivastava. 2001. Infection of purified nuclei by adeno-associated virus 2. Mol. Ther. 4:289-296. [DOI] [PubMed] [Google Scholar]
- 29.Hoque, M., K.-I. Ishizu, A. Matsumoto, S.-I. Han, F. Arisaka, M. Takayama, K. Suzuki, K. Kato, T. Kanda, H. Watanabe, and H. Handa. 1999. Nuclear transport of the major capsid protein is essential for adeno-associated virus capsid formation. J. Virol. 73:7912-7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hueffer, K., L. Govindasamy, M. Agbandje-McKenna, and C. R. Parrish. 2003. Combinations of two capsid regions controlling canine host range determine canine transferrin receptor binding by canine and feline parvoviruses. J. Virol. 77:10099-10105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hueffer, K., J. S. Parker, W. S. Weichert, R. E. Geisel, J. Y. Sgro, and C. R. Parrish. 2003. The natural host range shift and subsequent evolution of canine parvovirus resulted from virus-specific binding to the canine transferrin receptor. J. Virol. 77:1718-1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaludov, N., K. E. Brown, R. W. Walters, J. Zabner, and J. A. Chiorini. 2001. Adeno-associated virus serotype 4 (AAV4) and AAV5 both require sialic acid binding for hemagglutination and efficient transduction but differ in sialic acid linkage specificity. J. Virol. 75:6884-6893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kotin, R. M., R. M. Linden, and K. I. Berns. 1992. Characterization of a preferred site on human chromosome 19q for integration of adeno-associated virus DNA by non-homologous recombination. EMBO J. 11:5071-5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Linser, P., H. Bruning, and R. W. Armentrout. 1979. Uptake of minute virus of mice into cultured rodent cells. J. Virol. 31:537-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu, J. M., S. W. Green, T. Shimada, and N. S. Young. 1992. A block in full-length transcript maturation in cells nonpermissive for B19 parvovirus. J. Virol. 66:4686-4692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lombardo, E., J. C. Ramírez, M. Agbandje-McKenna, and J. M. Almendral. 2000. A beta-stranded motif drives capsid protein oligomers of the parvovirus minute virus of mice into the nucleus for viral assembly. J. Virol. 74:3804-3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lombardo, E., J. C. Ramirez, J. Garcia, and J. M. Almendral. 2002. Complementary roles of multiple nuclear targeting signals in the capsid proteins of the parvovirus minute virus of mice during assembly and onset of infection. J. Virol. 76:7049-7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luby-Phelps, K. 2000. Cytoarchitecture and physical properties of cytoplasm: volume, viscosity, diffusion, intracellular surface area. Int. Rev. Cytol. 192:189-221. [DOI] [PubMed] [Google Scholar]
- 39.Mabit, H., M. Y. Nakano, U. Prank, B. Saam, K. Dohner, B. Sodeik, and U. F. Greber. 2002. Intact microtubules support adenovirus and herpes simplex virus infections. J. Virol. 76:9962-9971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muzyczka, N. 1992. Use of adeno-associated virus as a general transduction vector for mammalian cells. Curr. Top. Microbiol. Immunol. 158:97-129. [DOI] [PubMed] [Google Scholar]
- 41.Muzyczka, N., and K. I. Berns. 2001. Parvoviridae: the viruses and their replication, p. 2327-2359. In D. M. Knipe, P. M. Howley, D. E. Griffen, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, vol. 2. Lippincott Williams and Wilkins, Philadelphia, Pa. [Google Scholar]
- 42.Nigg, E. A. 1997. Nucleocytoplasmic transport: signals, mechanisms and regulation. Nature (London) 386:779-787. [DOI] [PubMed] [Google Scholar]
- 43.Opie, S. R., K. H. Warrington, Jr., M. Agbandje-McKenna, S. Zolotukhin, and N. Muzyczka. 2003. Identification of amino acid residues in the capsid proteins of adeno-associated virus type 2 that contribute to heparan sulfate proteoglycan binding. J. Virol. 77:6995-7006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parker, J. S., and C. R. Parrish. 2000. Cellular uptake and infection by canine parvovirus involves rapid dynamin-regulated clathrin-mediated endocytosis, followed by slower intracellular trafficking. J. Virol. 74:1919-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parker, J. S. L., W. J. Murphy, D. Wang, S. J. O'Brien, and C. R. Parrish. 2001. Canine and feline parvoviruses can use human or feline transferrin receptors to bind, enter, and infect cells. J. Virol. 75:3896-3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parrish, C. R. 1990. Emergence, natural history, and variation of canine, mink, and feline parvoviruses. Adv. Virus Res. 38:403-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pasquale, G. D., B. L. Davidson, C. S. Stein, I. Martins, D. Scudiero, A. Monks, and J. A. Chiorini. 2003. Identification of PDGFR as a receptor for AAV-5 transduction. Nat. Med. 9:1306-1312. [DOI] [PubMed] [Google Scholar]
- 48.Pelkmans, L., and A. Helenius. 2003. Insider information: what viruses tell us about endocytosis. Curr. Opin. Cell Biol. 15:414-422. [DOI] [PubMed] [Google Scholar]
- 49.Prasad, K. M., and J. P. Trempe. 1995. The adeno-associated virus Rep78 protein is covalently linked to viral DNA in a preformed virion. Virology 214:360-370. [DOI] [PubMed] [Google Scholar]
- 50.Qing, K., J. Hansen, K. A. Weigel-Kelley, M. Tan, S. Zhou, and A. Srivastava. 2001. Adeno-associated virus type 2-mediated gene transfer: role of cellular FKBP52 protein in transgene expression. J. Virol. 75:8968-8976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qing, K., C. Mah, J. Hansen, S. Zhou, V. Dwarki, and A. Srivastava. 1999. Human fibroblast growth factor receptor 1 is a co-receptor for infection by adeno-associated virus 2. Nat. Med. 5:71-77. [DOI] [PubMed] [Google Scholar]
- 52.Qiu, J., and K. E. Brown. 1999. Integrin alphaVbeta5 is not involved in adeno-associated virus type 2 (AAV2) infection. Virology 264:436-440. [DOI] [PubMed] [Google Scholar]
- 53.Qiu, J., A. Handa, M. Kirby, and K. E. Brown. 2000. The interaction of heparin sulfate and adeno-associated virus 2. Virology 269:137-147. [DOI] [PubMed] [Google Scholar]
- 54.Ried, M. U., A. Girod, K. Leike, H. Buning, and M. Hallek. 2002. Adeno-associated virus capsids displaying immunoglobulin-binding domains permit antibody-mediated vector retargeting to specific cell surface receptors. J. Virol. 76:4559-4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ros, C., C. J. Burckhardt, and C. Kempf. 2002. Cytoplasmic trafficking of minute virus of mice: low-pH requirement, routing to late endosomes, and proteasome interaction. J. Virol. 76:12634-12645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Samulski, R. J. 1993. Adeno-associated virus: integration at a specific chromosomal locus. Curr. Opin. Genet. Dev. 3:74-80. [DOI] [PubMed] [Google Scholar]
- 57.Sanlioglu, S., P. K. Benson, J. Yang, E. M. Atkinson, T. Reynolds, and J. F. Engelhardt. 2000. Endocytosis and nuclear trafficking of adeno-associated virus type 2 are controlled by Rac1 and phosphatidylinositol-3 kinase activation. J. Virol. 74:9184-9196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seisenberger, G., M. U. Ried, T. Endress, H. Buning, M. Hallek, and C. Brauchle. 2001. Real-time single-molecule imaging of the infection pathway of an adeno-associated virus. Science 294:1929-1932. [DOI] [PubMed] [Google Scholar]
- 59.Seksek, O., J. Biwersi, and A. S. Verkman. 1997. Translational diffusion of macromolecule-sized solutes in cytoplasm and nucleus. J. Cell Biol. 138:131-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shaughnessy, E., D. Lu, S. Chatterjee, and K. K. Wong. 1996. Parvoviral vectors for the gene therapy of cancer. Semin. Oncol. 23:159-171. [PubMed] [Google Scholar]
- 61.Shi, W., G. S. Arnold, and J. S. Bartlett. 2001. Insertional mutagenesis of the adeno-associated virus type 2 (aav2) capsid gene and generation of aav2 vectors targeted to alternative cell-surface receptors. Hum. Gene Ther. 12:1697-1711. [DOI] [PubMed] [Google Scholar]
- 62.Shi, W., and J. S. Bartlett. 2003. RGD inclusion in VP3 provides adeno-associated virus type 2 (AAV2)-based vectors with a heparan sulfate-independent cell entry mechanism. Mol. Ther. 7:515-525. [DOI] [PubMed] [Google Scholar]
- 63.Sieczkarski, S. B., and G. R. Whittaker. 2002. Dissecting virus entry via endocytosis. J. Gen. Virol. 83:1535-1545. [DOI] [PubMed] [Google Scholar]
- 64.Simpson, A. A., V. Chandrasekar, B. Hebert, G. M. Sullivan, M. G. Rossmann, and C. R. Parrish. 2000. Host range and variability of calcium binding by surface loops in the capsids of canine and feline parvoviruses. J. Mol. Biol. 300:597-610. [DOI] [PubMed] [Google Scholar]
- 65.Simpson, A. A., P. R. Chipman, T. S. Baker, P. Tijssen, and M. G. Rossmann. 1998. The structure of an insect parvovirus (Galleria mellonella densovirus) at 3.7 Å resolution. Structure 6:1355-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sodeik, B. 2000. Mechanisms of viral transport in the cytoplasm. Trends Microbiol. 8:465-472. [DOI] [PubMed] [Google Scholar]
- 67.Suikkanen, S., T. Aaltonen, M. Nevalainen, O. Valilehto, L. Lindholm, M. Vuento, and M. Vihinen-Ranta. 2003. Exploitation of microtubule cytoskeleton and dynein during parvoviral traffic toward the nucleus. J. Virol. 77:10270-10279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Suikkanen, S., M. Antila, A. Jaatinen, M. Vihinen-Ranta, and M. Vuento. 2003. Release of canine parvovirus from endocytic vesicles. Virology 316:267-280. [DOI] [PubMed] [Google Scholar]
- 69.Suikkanen, S., K. Saajarvi, J. Hirsimaki, O. Valilehto, H. Reunanen, M. Vihinen-Ranta, and M. Vuento. 2002. Role of recycling endosomes and lysosomes in dynein-dependent entry of canine parvovirus. J. Virol. 76:4401-4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Summerford, C., J. S. Bartlett, and R. J. Samulski. 1999. αVβ5 integrin: a co-receptor for adeno-associated virus type 2 infection. Nat. Med. 5:78-82. [DOI] [PubMed] [Google Scholar]
- 71.Summerford, C., and R. J. Samulski. 1998. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J. Virol. 72:1438-1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tattersall, P., A. J. Shatkin, and D. C. Ward. 1977. Sequence homology between the structural polypeptides of minute virus of mice. J. Mol. Biol. 111:775-794. [DOI] [PubMed] [Google Scholar]
- 73.Tresnan, D. B., L. Southard, W. Weichert, J. Y. Sgro, and C. R. Parrish. 1995. Analysis of the cell and erythrocyte binding activities of the dimple and canyon regions of the canine parvovirus capsid. Virology 211:123-132. [DOI] [PubMed] [Google Scholar]
- 74.Tsao, J., M. S. Chapman, M. Agbandje, W. Keller, K. Smith, H. Wu, M. Luo, T. J. Smith, M. G. Rossmann, R. W. Compans, and C. R. Parrish. 1991. The three-dimensional structure of canine parvovirus and its functional implications. Science 251:1456-1464. [DOI] [PubMed] [Google Scholar]
- 75.Tsao, J., M. S. Chapman, H. Wu, M. Agbandje, W. Keller, and M. G. Rossmann. 1992. Structure determination of monoclinic canine parvovirus. Acta Crystallogr. Sect. B Struct. Sci. 48:75-88. [DOI] [PubMed] [Google Scholar]
- 76.Tullis, G. E., L. R. Burger, and D. J. Pintel. 1992. The trypsin-sensitive RVER domain in the capsid proteins of minute virus of mice is required for efficient cell binding and viral infection but not for proteolytic processing in vivo. Virology 191:846-857. [DOI] [PubMed] [Google Scholar]
- 77.Vihinen-Ranta, M., L. Kakkola, A. Kalela, P. Vilja, and M. Vuento. 1997. Characterization of a nuclear localization signal of canine parvovirus capsid proteins. Eur. J. Biochem. 250:389-394. [DOI] [PubMed] [Google Scholar]
- 78.Vihinen-Ranta, M., D. Wang, W. S. Weichert, and C. R. Parrish. 2002. The VP1 N-terminal sequence of canine parvovirus affects nuclear transport of capsids and efficient cell infection. J. Virol. 76:1884-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vihinen-Ranta, M., W. Yuan, and C. R. Parrish. 2000. Cytoplasmic trafficking of the canine parvovirus capsid and its role in infection and nuclear transport. J. Virol. 74:4853-4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Walters, R. W., S. Yi, S. Keshavjee, K. E. Brown, M. J. Welsh, J. A. Chiorini, and J. Zabner. 2001. Binding of adeno-associated virus type 5 to 2,3-linked sialic acid is required for gene transfer. J. Biol. Chem. 276:20610-20616. [DOI] [PubMed] [Google Scholar]
- 81.Wang, D., and C. R. Parrish. 1999. A heterogeneous nuclear ribonucleoprotein A/B-related protein binds to single-stranded DNA near the 5′end or within the genome of feline parvovirus and can modify virus replication. J. Virol. 73:7761-7768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Weichert, W. S., J. S. Parker, A. T. M. Wahid, S. F. Chang, E. Meier, and C. R. Parrish. 1998. Assaying for structural variation in the parvovirus capsid and its role in infection. Virology 250:106-117. [DOI] [PubMed] [Google Scholar]
- 83.Weigel-Kelley, K. A., M. C. Yoder, and A. Srivastava. 2001. Recombinant human parvovirus B19 vectors: erythrocyte P antigen is necessary but not sufficient for successful transduction of human hematopoietic cells. J. Virol. 75:4110-5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Whittaker, G. R., and A. Helenius. 1998. Nuclear import and export of viruses and virus genomes. Virology 246:1-23. [DOI] [PubMed] [Google Scholar]
- 85.Wu, P., W. Xiao, T. Conlon, J. Hughes, M. Agbandje-McKenna, T. Ferkol, T. Flotte, and N. Muzyczka. 2000. Mutational analysis of the adeno-associated virus type 2 (AAV2) capsid gene and construction of AAV2 vectors with altered tropism. J. Virol. 74:8635-8647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xie, Q., W. Bu, S. Bhatia, J. Hare, T. Somasundaram, A. Azzi, and M. S. Chapman. 2002. The atomic structure of adeno-associated virus (AAV-2), a vector for human gene therapy. Proc. Natl. Acad. Sci. USA 99:10405-10410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xie, Q., and M. S. Chapman. 1996. Canine parvovirus capsid structure, analyzed at 2.9 Å resolution. J. Mol. Biol. 264:497-520. [DOI] [PubMed] [Google Scholar]
- 88.Yan, Z., R. Zak, G. W. Luxton, T. C. Ritchie, U. Bantel-Schaal, and J. F. Engelhardt. 2002. Ubiquitination of both adeno-associated virus type 2 and 5 capsid proteins affects the transduction efficiency of recombinant vectors. J. Virol. 76:2043-2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang, Q., M. Mamounas, G. Yu, S. Kennedy, B. Leaker, J. Merson, F. Wong-Staal, M. Yu, and J. R. Barber. 1998. Development of novel cell surface CD34-targeted recombinant adenoassociated virus vectors for gene therapy. Hum. Gene Ther. 9:1929-1937. [DOI] [PubMed] [Google Scholar]
- 90.Young, S. M., Jr., W. Xiao, and R. J. Samulski. 2000. Site-specific targeting of DNA plasmids to chromosome 19 using AAV cis and trans sequences. Methods Mol. Biol. 133:111-126. [DOI] [PubMed] [Google Scholar]
- 91.Zadori, Z., J. Szelei, M.-C. Lacoste, P. Raymond, M. Allaire, I. R. Nabi, and P. Tijssen. 2001. A viral phospholipase A2 is required for parvovirus infectivity. Dev. Cell 1:291-302. [DOI] [PubMed] [Google Scholar]


