Abstract
RNA interference has been applied for the prevention of virus infections in mammalian cells but has not succeeded in eliminating infections from already infected cells. We now show that the transfection of JC virus-infected SVG-A human glial cells with small interfering RNAs that target late viral proteins, including agnoprotein and VP1, results in a marked inhibition both of viral protein expression and of virus production. RNA interference directed against JC virus genes may thus provide a basis for the development of new strategies to control infections with this polyomavirus.
JC virus (JCV) belongs to the polyomavirus family of double-stranded DNA viruses and causes progressive multifocal leukoencephalopathy (PML) in humans (23). PML is often observed in immunosuppressed individuals, such as those with AIDS or advanced malignancies. Although highly active antiretroviral therapy, which includes treatment with protease inhibitors, improves the survival rate of patients with AIDS-related PML (2, 7), current therapeutic approaches to PML are not satisfactory. Treatment with cytosine arabinoside (8) or cidofovir (15) has failed to prove efficacious in individuals with PML. Trials of topotecan, which inhibits DNA topoisomerase and blocks JCV replication in vitro (11), are currently under way in such individuals. RNA interference (RNAi) with small interfering RNAs (siRNAs) has recently become a widely used approach for repressing cellular or viral gene expression (5, 6, 10, 16). Although several studies have shown that virus infections can be prevented by a prior or concomitant administration of siRNAs, the elimination of established infections from cells or tissues by RNAi has not been demonstrated (1).
To attempt to inhibit JCV production in infected cells, we designed the following siRNAs (Dharmacon) to target three different JCV proteins (Fig. 1a): VP274 and VP691 for VP1, Ag122 and Ag147 for agnoprotein, and LT78 and LT134 for the large T antigen (T-Ag). The JCV early and late RNAs are generated by alternative splicing. The early RNAs encode T-Ag and the small t antigen (14), whereas the major late RNA encodes both agnoprotein and VP1 (21). We introduced the JCV-specific siRNAs into cells of the SVG-A (simian virus 40 [SV40]-transformed human fetal glial cells) line (13) that had been inoculated with JCV (Mad-1/SVEΔ strain; 1,024 hemagglutination activity units per 3 × 105 cells) 4 days previously. JCV late proteins, including VP1 and agnoprotein, were detected by an immunoblot analysis at 2 days postinfection (dpi) and were abundant at 4 dpi (Fig. 1b). At 4 and 6 dpi, each siRNA (120 pmol per 6 × 104 cells) was introduced individually into SVG-A cells by the use of Lipofectamine 2000 (Invitrogen) (Fig. 1c). About 80% of the SVG-A cells were successfully transfected with a fluorescein-conjugated Ag122 siRNA (data not shown). The abundance of JCV proteins in siRNA-transfected cells was examined 48 h after the second transfection by an immunoblot analysis with antibodies specific for agnoprotein (3, 18, 19), VP1 (12, 22), or SV40 T-Ag (Ab-2; Oncogene Research Products) (20). Cells transfected with Ag122, Ag147, or VP274 manifested a marked depletion of viral proteins compared with cells transfected with a control siRNA with a scrambled sequence which is not present in mammalian cells (Dharmacon) (Fig. 1d). Ag122 inhibited the expression of VP1 as well as that of agnoprotein in a dose-dependent manner, but it did not affect the abundance of T-Ag, lamin A/C, or actin (Fig. 1d and e). The antibodies to SV40 T-Ag did not allow for differentiation between JCV T-Ag and SV40 T-Ag in SV40-transformed cells, as these two proteins share >70% amino acid sequence identity (4). We therefore assessed the effects of LT78 and LT134 on JCV T-Ag expression by reverse transcription (RT) and PCR; the abundance of JCV T-Ag mRNA was not affected by the transfection of cells with either siRNA (data not shown).
FIG. 1.
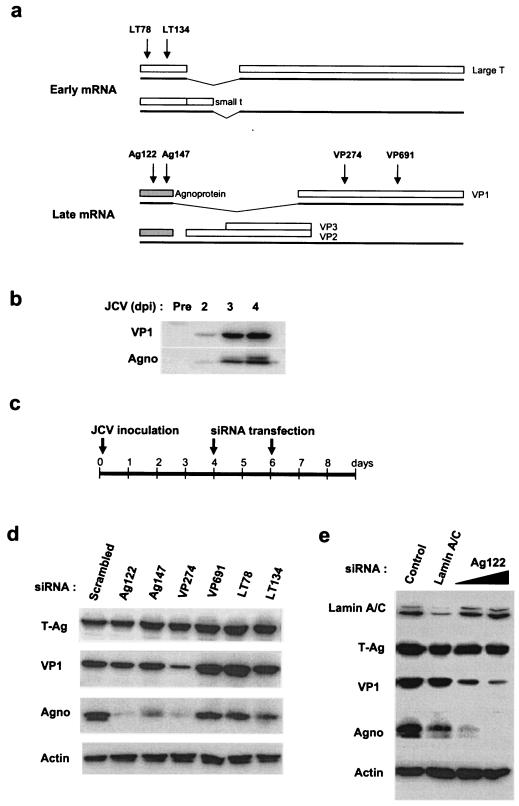
Effects of postinfection RNAi on the abundance of JCV proteins in JCV-infected SVG-A cells. (a) Schematic representation of major early and late mRNAs of JCV. Major early mRNAs encode the small t antigen and T-Ag, which are translated as splicing variants. Two major forms of late mRNA encode either agnoprotein and VP1 or agnoprotein, VP2, and VP3. The regions of the viral RNAs targeted by the siRNAs are indicated by arrows. (b) Immunoblot analysis of the abundance of VP1 and agnoprotein of JCV in SVG-A cells at the indicated times after infection with JCV. (c) Schedule for JCV infection and siRNA transfection in SVG-A cells. (d) Immunoblot analysis of the indicated proteins in JCV-infected cells subjected to transfection with the indicated siRNAs. (e) Immunoblot analysis of the indicated proteins in JCV-infected cells subjected to transfection with the Ag122 siRNA at 60 or 120 pmol/well or with a lamin A/C-specific siRNA. Control (infected) cells were subjected to mock transfection.
We also examined the effects of Ag122 and VP274 siRNAs by an indirect immunofluorescence analysis in JCV-infected cells. At 48 h posttransfection, methanol-fixed cells were stained with antibodies to VP1 or agnoprotein and then with Alexa Fluor 488-conjugated goat antibodies to rabbit immunoglobulin G (Molecular Probes). Cells positive for VP1 or agnoprotein were visualized with a laser-scanning confocal microscope (Olympus) and counted in six fields of view. The proportion of agnoprotein-positive cells was significantly reduced for cells transfected with Ag122, VP274, or both siRNAs compared with the value for cells transfected with the scrambled siRNA (Fig. 2a). Similarly, the percentage of VP1-positive cells was also reduced by transfection with Ag122, VP274, or both Ag122 and VP274. We confirmed the inhibition of the expression of agnoprotein and VP1 in cells transfected with Ag122, VP274, or both siRNAs by an immunoblot analysis (Fig. 2b). The extent of inhibition of viral protein expression achieved with the combination of Ag122 and VP274 did not differ significantly from that achieved with either siRNA alone. The observed inhibition of both agnoprotein and VP1 expression by either Ag122 or VP274 was likely due to the degradation of the polycistronic late RNA for both of these proteins induced by each siRNA.
FIG. 2.
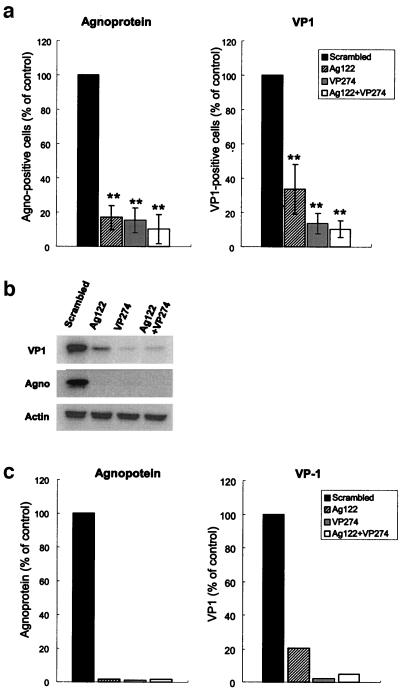
Indirect immunofluorescence analysis of the effects of RNAi on viral protein expression in JCV-infected SVG-A cells. (a) The proportion of cells that were positive for agnoprotein or VP1 was determined by an indirect immunofluorescence analysis 48 h after transfection with the indicated siRNA(s). The data are expressed as percentages of the proportion determined for JCV-infected cells transfected with the scrambled siRNA (control) and are means ± standard deviations (SD) of values from at least three independent experiments. **, P < 0.02 versus the value for cells transfected with the scrambled siRNA (Student's t test). (b) Immunoblot analysis of VP1, agnoprotein, and actin expression in JCV-infected cells transfected with the indicated siRNA(s). (c) The signals for agnoprotein and VP1 were quantified with an image analyzer and expressed as percentages of the value for cells transfected with the scrambled siRNA.
It is thought that siRNAs target mRNAs containing the same sequences and induce their cleavage. We therefore examined the effects of Ag122 and VP274 on the abundance of JCV mRNAs. Total RNAs were isolated from cells 12 or 24 h after transfection with a siRNA, treated with DNase I, and subjected to RT with a Superscript first-strand synthesis system (Invitrogen) followed by real-time quantitative PCR with a GeneAmp5700 instrument (Applied Biosystems). The amount of each viral mRNA was normalized to that of β-actin mRNA in the same sample. The abundance of agnoprotein and VP1 mRNAs was significantly reduced in JCV-infected cells transfected with Ag122, VP274, or both siRNAs (Fig. 3). The reduction in the amounts of viral mRNAs, however, was not as large as that in the amounts of the encoded proteins. We eliminated the possibility of contamination of the viral DNA in the RT-PCR samples by (i) performing a DNase I treatment prior to RT-PCR, without the reverse transcriptase, and (ii) performing an RNase A treatment prior to the reverse transcriptase reaction. For both treatments, the RT-PCR signal was lost, suggesting that there was no contamination of the viral DNA in the RT-PCR samples. Whereas siRNAs are known to be incorporated into an RNA-induced silencing complex and to direct RNA-induced silencing complex-mediated sequence-specific mRNA degradation (9), the detailed mechanism of this process remains unclear. One possible explanation for the difference in the magnitude of the effects of the JCV-specific siRNAs on the amounts of viral RNAs and proteins is that the target mRNA bound with a siRNA might be detected by RT-PCR before its degradation.
FIG. 3.
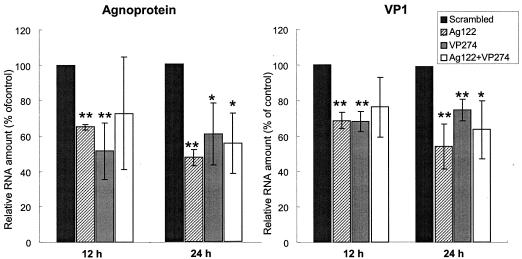
Depletion of viral RNAs by RNAi in JCV-infected SVG-A cells. Total RNAs isolated from JCV-infected cells 12 or 24 h after transfection with the indicated siRNAs were subjected to an RT-PCR analysis of agnoprotein and VP1 mRNAs. The data were normalized to the amount of β-actin mRNA and are expressed as percentages of the normalized value for JCV-infected cells transfected with the scrambled siRNA (control); they are means ± SD of values from at least three independent experiments. *, P < 0.05, and **, P < 0.02 versus the value for cells transfected with the scrambled siRNA.
To examine the effect of RNAi on JCV production, we measured the hemagglutination activity (17, 22) of JCV-infected SVG-A cells 36 h after siRNA transfection. The hemagglutination activities of cells transfected with Ag122, VP274, or both of these siRNAs were 6.7, 9.3, and 4.1%, respectively, of that for cells transfected with the scrambled siRNA (Fig. 4). Thus, siRNAs that target agnoprotein or VP1 greatly inhibited JCV production in infected cells.
FIG. 4.
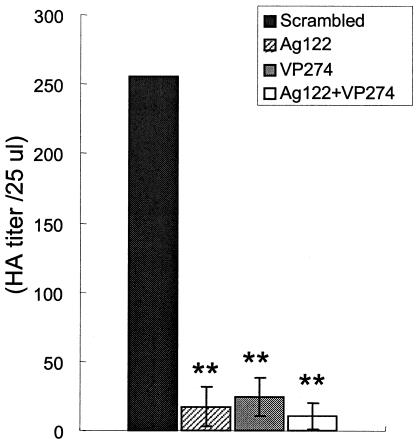
Inhibition of JCV production by RNAi in SVG-A cells. Extracts prepared from JCV-infected cells 36 h after transfection with the indicated siRNAs were assayed for hemagglutination activity (HA). The data are expressed as HA titers per 25 μl of cell extract and are means ± SD of values from at least three independent experiments. **, P < 0.02 versus the value for cells transfected with the scrambled siRNA.
In summary, we have achieved a marked inhibition of JCV production by RNAi in cells already infected with the virus. Our results may have important implications for the development of a new approach to the treatment of PML. The application of an RNAi-based antiviral strategy for PML will require an efficient and specific delivery of siRNAs to the central nervous system.
Acknowledgments
We thank Mayumi Sasada for technical assistance.
This study was supported in part by grants from the Ministry of Education, Science, Sports, and Culture and by grants from the Ministry of Health, Labour and Welfare, Japan.
REFERENCES
- 1.Andino, R. 2003. RNAi puts a lid on virus replication. Nat. Biotechnol. 21:629-630. [DOI] [PubMed] [Google Scholar]
- 2.Clifford, D. B. 1999. Opportunistic viral infections in the setting of human immunodeficiency virus. Semin. Neurol. 19:185-192. [DOI] [PubMed] [Google Scholar]
- 3.Endo, S., Y. Okada, Y. Orba, H. Nishihara, S. Tanaka, K. Nagashima, and H. Sawa. 2003. JC virus agnoprotein colocalizes with tubulin. J. Neurovirol. 9(Suppl. 1):10-14. [DOI] [PubMed] [Google Scholar]
- 4.Frisque, R. J., G. L. Bream, and M. T. Cannella. 1984. Human polyomavirus JC virus genome. J. Virol. 51:458-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ge, Q., M. T. McManus, T. Nguyen, C. H. Shen, P. A. Sharp, H. N. Eisen, and J. Chen. 2003. RNA interference of influenza virus production by directly targeting mRNA for degradation and indirectly inhibiting all viral RNA transcription. Proc. Natl. Acad. Sci. USA 100:2718-2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gitlin, L., S. Karelsky, and R. Andino. 2002. Short interfering RNA confers intracellular antiviral immunity in human cells. Nature 418:430-434. [DOI] [PubMed] [Google Scholar]
- 7.Giudici, B., B. Vaz, S. Bossolasco, S. Casari, A. M. Brambilla, W. Luke, A. Lazzarin, T. Weber, and P. Cinque. 2000. Highly active antiretroviral therapy and progressive multifocal leukoencephalopathy: effects on cerebrospinal fluid markers of JC virus replication and immune response. Clin. Infect. Dis. 30:95-99. [DOI] [PubMed] [Google Scholar]
- 8.Hall, C. D., U. Dafni, D. Simpson, D. Clifford, P. E. Wetherill, B. Cohen, J. McArthur, H. Hollander, C. Yainnoutsos, E. Major, L. Millar, and J. Timpone. 1998. Failure of cytarabine in progressive multifocal leukoencephalopathy associated with human immunodeficiency virus infection. AIDS Clinical Trials Group 243 Team. N. Engl. J. Med. 338:1345-1351. [DOI] [PubMed] [Google Scholar]
- 9.Hammond, S. M., E. Bernstein, D. Beach, and G. J. Hannon. 2000. An RNA-directed nuclease mediates post-transcriptional gene silencing in Drosophila cells. Nature 404:293-296. [DOI] [PubMed] [Google Scholar]
- 10.Jacque, J. M., K. Triques, and M. Stevenson. 2002. Modulation of HIV-1 replication by RNA interference. Nature 418:435-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kerr, D. A., C. F. Chang, J. Gordon, M. A. Bjornsti, and K. Khalili. 1993. Inhibition of human neurotropic virus (JCV) DNA replication in glial cells by camptothecin. Virology 196:612-618. [DOI] [PubMed] [Google Scholar]
- 12.Komagome, R., H. Sawa, T. Suzuki, Y. Suzuki, S. Tanaka, W. J. Atwood, and K. Nagashima. 2002. Oligosaccharides as receptors for JC virus. J. Virol. 76:12992-13000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu, C. K., G. Wei, and W. J. Atwood. 1998. Infection of glial cells by the human polyomavirus JC is mediated by an N-linked glycoprotein containing terminal α(2-6)-linked sialic acids. J. Virol. 72:4643-4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lynch, K. J., and R. J. Frisque. 1991. Factors contributing to the restricted DNA replicating activity of JC virus. Virology 180:306-317. [DOI] [PubMed] [Google Scholar]
- 15.Marra, C. M., N. Rajicic, D. E. Barker, B. A. Cohen, D. Clifford, M. J. Donovan Post, A. Ruiz, B. C. Bowen, M. L. Huang, J. Queen-Baker, J. Andersen, S. Kelly, and S. Shriver. 2002. A pilot study of cidofovir for progressive multifocal leukoencephalopathy in AIDS. AIDS 16:1791-1797. [DOI] [PubMed] [Google Scholar]
- 16.Novina, C. D., M. F. Murray, D. M. Dykxhoorn, P. J. Beresford, J. Riess, S. K. Lee, R. G. Collman, J. Lieberman, P. Shankar, and P. A. Sharp. 2002. siRNA-directed inhibition of HIV-1 infection. Nat. Med. 8:681-686. [DOI] [PubMed] [Google Scholar]
- 17.Nukuzuma, S., Y. Yogo, J. Guo, C. Nukuzuma, S. Itoh, T. Shinohara, and K. Nagashima. 1995. Establishment and characterization of a carrier cell culture producing high titres of polyoma JC virus. J. Med. Virol. 47:370-377. [DOI] [PubMed] [Google Scholar]
- 18.Okada, Y., S. Endo, H. Takahashi, H. Sawa, T. Umemura, and K. Nagashima. 2001. Distribution and function of JCV agnoprotein. J. Neurovirol. 7:302-306. [DOI] [PubMed] [Google Scholar]
- 19.Okada, Y., H. Sawa, S. Endo, Y. Orba, T. Umemura, H. Nishihara, A. C. Stan, S. Tanaka, H. Takahashi, and K. Nagashima. 2002. Expression of JC virus agnoprotein in progressive multifocal leukoencephalopathy brain. Acta Neuropathol. (Berlin) 104:130-136. [DOI] [PubMed] [Google Scholar]
- 20.Safak, M., R. Barrucco, A. Darbinyan, Y. Okada, K. Nagashima, and K. Khalili. 2001. Interaction of JC virus agno protein with T antigen modulates transcription and replication of the viral genome in glial cells. J. Virol. 75:1476-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shishido-Hara, Y., Y. Hara, T. Larson, K. Yasui, K. Nagashima, and G. L. Stoner. 2000. Analysis of capsid formation of human polyomavirus JC (Tokyo-1 strain) by a eukaryotic expression system: splicing of late RNAs, translation and nuclear transport of major capsid protein VP1, and capsid assembly. J. Virol. 74:1840-1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suzuki, S., H. Sawa, R. Komagome, Y. Orba, M. Yamada, Y. Okada, Y. Ishida, H. Nishihara, S. Tanaka, and K. Nagashima. 2001. Broad distribution of the JC virus receptor contrasts with a marked cellular restriction of virus replication. Virology 286:100-112. [DOI] [PubMed] [Google Scholar]
- 23.Weber, T., and E. O. Major. 1997. Progressive multifocal leukoencephalopathy: molecular biology, pathogenesis and clinical impact. Intervirology 40:98-111. [DOI] [PubMed] [Google Scholar]


