Abstract
The anti-apoptotic proteins Bcl-XL and Bcl-2 are abundantly expressed in hematopoietic stem cells and/or progenitor cells. Furthermore, leukemic cells expressing these proteins are enriched in minimal residual disease cell populations. This prompted us to test the BH3-mimetic compound ABT-737 for its ability to eradicate putative leukemic stem cells. ABT-737 demonstrated potent cytotoxic effects in all patient samples tested. The efficacy of ABT-737 against AML blasts and the primitive CD34+/CD38− population was equal and independent of sensitivity to cytarabine/daunorubicin. These results, together with previously reported synergistic effects of ABT-737 with chemotherapeutics make BH3-mimetics promising candidates for future AML treatment regimens.
Keywords: Acute myeloid leukemia, Putative leukemic stem cells, CD34+/CD38−, ABT-737, BH3-mimetic
Highlights
-
•
ABT-737 potently kills AML blast cells.
-
•
ABT-737 has a cytotoxic effect against both bone marrow-derived AML blasts and putative Leukemic Stem cells (pLSCs).
-
•
ABT-737 has an equally high cytotoxic efficacy in disseminated AML putative Leukemic Stem cells (pLSCs).
1. Introduction
Acute myeloid leukemia (AML) is a biologically heterogeneous group of related diseases in urgent need of better therapeutic options. Despite sensitivity to initial treatment, 70–90% of elderly patients relapse within 5 years. There is accumulating evidence that a small population of primitive cells, called putative leukemic stem cells (pLSCs), resistant to treatment in bone marrow niche sites is responsible for disease relapse [1,2]. Anti-apoptotic Bcl-2 proteins have long been known to play a major role in the long-term survival of hematopoietic stem and progenitor cells [3]. Pluripotent hematopoietic stem cells identified as CD34+/lin−/CD38− abundantly express Bcl-XL, while progenitor cells (CD34+) produce high amounts of Bcl-2 [3]. Additionally, treatment with chemotherapeutics have been shown to induce anti-apoptotic Bcl-2 protein expression and minimal residual disease cells also express high levels of Bcl-2/Bcl-XL [4].
Despite this long-known feature of primitive hematopoietic cells and pLSCs, the potential of the recently developed Bcl-2/Bcl-XL/Bcl-W inhibitor, ABT-737 in eradicating AML putative leukemic stem cells has not been thoroughly examined. In this study, we show that ABT-737 is potent and equally effective in killing both AML blasts and CD34+/CD38− progenitors. The effect did not depend on the source of the AML cells (bone marrow or peripheral blood), or the resistance of the pLSCs to the mainstream chemotherapeutic drug combination of cytarabine (AraC) and daunorubicin (DnR).
2. Materials and methods
2.1. Patient samples
AML patient׳s bone marrow aspirates and peripheral blood samples were kindly provided by Department of Hematology, University Hospital Galway. Mononuclear cells (MNCs) were separated by standard ficoll separation protocol and then cultured ex vivo as described before [5].
2.2. Ethical approval
The project was approved by the Galway University Hospital ethics committee. Informed consent was obtained from patients before collection of material.
2.3. Cell lines and ex vivo culture system
The human stromal cell line HS-5 (expressing GFP) was cultured in DMEM supplemented with 10% FBS. Patient-derived MNCs were seeded on HS-5 cells of 70% confluency at a concentration of 3×105 cells/well for 24 h prior to treatment. Cells were treated with ABT-737 or clinically relevant dosages of cytarabine+daunorubicin combination for 24 h (in a molar concentration ratio of 3:1).
2.4. Flow cytometry
MNCs were incubated with anti-CD34 and anti-CD38 antibodies (BD Bioscience, San Diego, USA) in PBS/1% BSA (Sigma) following the manufacturer׳s instructions. After washing off unbound antibodies cells were stained with 2 μM of 7-aminoactinomycin D (7-AAD; Life Technologies) to label the dead cell population for 15 min at room temperature. Samples were analyzed on a BD Canto II flow cytometer by collecting 105 events. HS-5 cells were excluded by gating out GFP+/FSСhigh events. Statistical analysis and data visualization were performed using GraphPad Prism software (GraphPad Software inc., La Jolla, USA).
3. Results and discussion
ABT-737 is a BH3-mimetic targeting Bcl-2, Bcl-XL and Bcl-W. The aim of the experiments was to test whether inhibition of Bcl-2 and Bcl-XL using ABT-737 can eradicate the CD34+/CD38− AML cell population. Bone marrow MNCs from patients (Table 1) were cultured on stromal-feeder layer for 24 h and then treated with dosages of ABT-737 of 30 nM and 300 nM. The co-culture system has been optimized for supporting the survival of AML cells ex vivo and to model features of the bone marrow microenvironment that drive drug-resistance [8]. The dose range was selected based on the in vitro effect of ABT-737 on secondary AML cell lines [8]. The resting cell viability of the patient samples was between 60% and 90% after 24 h of stromal-feeder co-culture. ABT-737 potently killed the AML cells in all samples with the 300 nM dose causing a 75% average loss in viability (Fig.1A). Similar results were obtained from a parallel study with ALL patient samples [data not shown].
Table 1.
List of AML patients׳ bone marrow and blood samples used.
| Patient number | Sample type (PB/BM) | AML type | Remarks |
|---|---|---|---|
| 1 | BM | Refractory cytopenia with excess of blasts II (15%), normal karyotype, FLT3 positive | Palliative care |
| 2 | BM, PB | Acute myeloid leukemia with multilineage dysplasia, complex karyotype, FLT3 negative | Treatment: clofarabine-refractory |
| 3 | BM | Acute myeloid leukemia normal karyotype, FLT3 positive | Treatment: daunorubicin and cytarabine – complete remission |
| 4 | BM, PB | Acute myeloid leukemia, not otherwise categorized, Acute myeloid leukemia without maturation, normal karyotype, FLT3 negative | Treatment: daunorubicin and cytarabine – refractory |
Note: samples were received upon diagnosis.
Fig. 1.
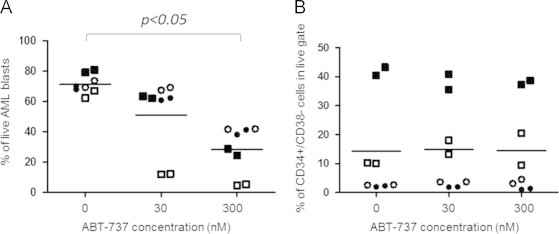
ABT-737 has a potent cytotoxic effect on bone marrow-derived AML pLSCs. Bone marrow-derived primary AML cells were cultured on a stromal-feeder layer for 24 h and then treated with 30 nM and 300 nM of ABT-737 for 24 h. AML cell viability was evaluated using7-AAD staining and flow cytometry. (A) The cytotoxic effect of ABT-737 on the overall blast population. The graph shows the percentage of live cells for each sample. (B) ABT-737 is equally efficient in eradicating AML pLSCs. The graph shows the percentage of the CD34+/CD38− cells within the surviving AML cell population in each sample. E Horizontal lines indicate mean values. Different shapes indicate data-points corresponding to particular patient samples (Patients cross-referencing with Table 1: Patient 1 (○), Patient 2 (■), Patient 3 (•), Patient 4 (□)).
To check the activity of ABT-737 against pLSCs, the proportion of CD34+/CD38− cells was evaluated within the surviving cell population. The relative percentage of pLSCs did not increase in response to ABT-737 treatment highlighting that the CD34+/CD38− pLSCs-containing population has the same sensitivity to ABT-737 as the more differentiated AML blasts. Resistance of pLSCs to ABT-737 would have resulted in the relative enrichment of the CD34+/CD38− pLSCs compartment (Fig. 1B). Of note, the two samples that carried Fms-like tyrosine kinase 3 internal tandem duplication (FLT3-ITD) mutation (patients 2 and 4) showed higher percentage of pLSCs within the sample, but these pLSCs were also sensitive to ABT-737. On the contrary, AraC+DnR combination treatment showed variable efficacy on the AML blasts, with enrichment of the CD34+/CD38− pLSCs population in most samples (Fig. 2A and B). Finally, the efficacy of ABT-737 on bone marrow-residing versus disseminated AML cells was compared using matched patient samples. The bone marrow-derived and peripheral blood-derived cells were cultured and treated as described above and overall cell death, and cell death within the CD34+/CD38− LIC compartment was determined. ABT-737 showed comparable efficacy on disseminated AML cells, including the circulating pLSCs population (Fig. 3A and B).
Fig. 2.
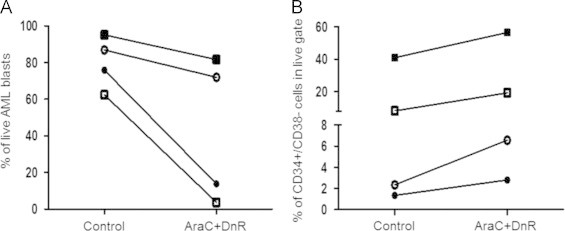
AML cells show variable sensitivity to the combination of cytarabine and daunorubicin. Bone marrow primary AML cells were cultured on a stromal-feeder layer and then were treated with 4.5 μM cytarabine (AraC)+1.35 μM daunorubicin (DnR) for 24 h. AML cell viability was evaluated using 7-AAD staining and flow cytometry. (A) The cytotoxic effect of AraC+DnR on the overall blast population. The graph shows the percentage of live cells for each sample. (B) Treatment with AraC+DnR leads to enrichment of the CD34+/CD38− cell population in the surviving fraction. The graph shows the percentage of the CD34+/CD38− cells within the surviving AML cell population in each sample. Different shapes indicate data-points corresponding to different patient samples. (Patients cross-referencing with Table 1: Patient 1 (○), Patient 2 (■), Patient 3 (●), Patient 4 (□)).
Fig. 3.
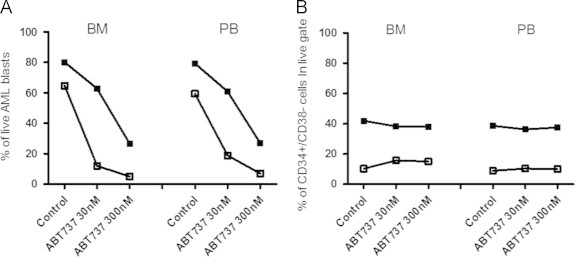
ABT-737 has an equally high cytotoxic efficacy in disseminated AML pLSCs. Bone marrow and peripheral blood-derived primary AML cells from 2 matching patients were cultured and treated and the percentage of surviving cells determined as described before. (A) Viability of bone marrow-derived and peripheral blood-derived overall AML population after exposure to ABT-737. (B) The percentage of CD34+/CD38− cells within the surviving population of leukemic blasts in both bone marrow and peripheral blood-derived matching sample sets showing equal efficacy of ABT-737 compound against AML blasts and pLSCs. The % of pLSCs within live AML cell population was evaluated as a number of CD34+/CD38−-7AAD− events. Different shapes indicate data-points corresponding to different patient samples. (Patients cross-referencing with Table 1: Patient 2 (■), Patient 4 (□)).
The current data, in line with previously published studies, clearly show that ABT-737 demonstrates efficacy against AML blasts comparable or superior to the activity of traditional drugs in current clinical use, such as AraC and [9]. Moreover, several studies have shown that combination of ABT-737 with chemotherapeutics, such as daunorubicin [6] and 5-Azacytidine [7] have a synergistic cytotoxic effect on AML blast cells. However, no studies have focused on examining the effect ABT-737 on the pLSCs subpopulation and the potential effect of the bone marrow microenvironment on ABT-737 resistance of pLSCs has not been sufficiently studied either. This is of key importance as the interaction with stromal bone marrow components can induce the expression of Bcl-2 and Bcl-XL in the AML cells. Our findings prove that the pLSC-containing CD34+/CD38− population can be efficiently targeted via inhibition of Bcl-2 and Bcl-XL even in the presence of bone marrow stroma which was able to provide resistance against AraC and DnR.
It has to be noted that in some AMLs, especially the ones that harbor a FLT3-ITD mutation, the LICs can express very high levels of another anti-apoptotic Bcl-2 family member, Mcl-1 that ABT-737 or the orally bioavailable ABT-737 derivative, ABT-263 cannot inhibit [10]. The FLT3-ITD positive samples studied here did not show resistance to ABT-737, but testing of a larger sample cohort would be necessary to confirm these findings. Eradication of high Mcl-1 expressing AML pLSCs is thus likely to require a combination of BH3-mimetics with complementary anti-apoptotic Bcl-2 protein binding profile, such as the combination of ABT-263 with Mcl-1-targeting BH3-mimetics, for example BIMS2A or MCL-1 SAHB (currently in pre-clinical evaluation). Other, non-BH3 motif-like drugs which are also able to target Mcl-1 are also being developed. One such example is the small molecule drug S1 that inhibits Mcl-1 by causing induction of the pro-apoptotic BH3-only Bcl-2 family member Noxa, the unique BH3-binding partner and inhibitor of Mcl-1.
The current progress in the development of BH3-mimetics and the emerging results of clinical trials corroborate the potential of strategies targeting anti-apoptotic Bcl-2 proteins to kill AML pLSCs and achieve a long-lasting cure.
Conflict of interest
All authors have no conflicts of interest to declare.
Acknowledgments
The project has been supported by grants from Science Foundation Ireland, Ireland (Starting Investigator Research Grant to ES, 09/SIRG/B1575, TIDA to ES 12/TIDA/B2388) and by the Millennium Fund of NUIG.
References
- 1.Valent P. Targeting of leukemia-initiating cells to develop curative drug therapies: straightforward but nontrivial concept. Curr Cancer Drug Targets. 2011;11:56–71. doi: 10.2174/156800911793743655. [DOI] [PubMed] [Google Scholar]
- 2.Doan P., Chute J. The vascular niche: home for normal and malignant hematopoietic stem cells. Leukemia. 2012;26(1):54–62. doi: 10.1038/leu.2011.236. [DOI] [PubMed] [Google Scholar]
- 3.Park J.R., Bernstein I.D., Hockenbery D.M. Primitive human hematopoietic precursors express Bcl-x but not Bcl-2. Blood. 1995;86(3):868–876. [PubMed] [Google Scholar]
- 4.Andreeff M., Jiang S., Zhang X., Konopleva M., Estrov Z., Snell V.E. Expression of Bcl-2-related genes in normal and AML progenitors: changes induced by chemotherapy and retinoic acid. Leukemia. 1999;13(11):1881–1892. doi: 10.1038/sj.leu.2401573. [DOI] [PubMed] [Google Scholar]
- 5.Szegezdi E., Reis C.R., van der Sloot A.M., Natoni A., O׳Reilly A., Reeve J. Targeting AML through DR4 with a novel variant of rhTRAIL. J Cell Mol Med. 2011;15(10):2216–2231. doi: 10.1111/j.1582-4934.2010.01211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dariushnejad H., Zarghami N., Rahmati M., Ghasemali S., Sadeghi Z., Davoodi Z. ABT-737, synergistically enhances daunorubicin-mediated apoptosis in acute myeloid leukemia cell lines. Adv Pharm Bull. 2014;4(2):185–189. doi: 10.5681/apb.2014.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bogenberger J.M., Kornblau S.M., Pierceall W.E., Lena R., Chow D., Shi C.X. BCL-2 family proteins as 5-azacytidine-sensitizing targets and determinants of response in myeloid malignancies. Leukemia. 2014;28(8):1657–1665. doi: 10.1038/leu.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruth C., Morrell et al. The BH3 mimetic, ABT-737, overcomes stromal-mediated pro-survival signals and synergizes with PHA-767491, a dual Cdc7/CDK9 inhibitor, in acute myeloid leukemia. 53rd ASH annual meeting abstract; 2010.
- 9.Konopleva M., Contractor R., Tsao T., Samudio I., Ruvolo P.P., Kitada S. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell. 2006;10(5):375–388. doi: 10.1016/j.ccr.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Goichi Yoshimoto, Miyamoto T., Jabbarzadeh-Tabrizi S., Iino T., Rocnik J.L., Kikushige Y. FLT3-ITD up-regulates MCL-1 to promote survival of stem cells in acute myeloid leukemia via FLT3-ITD–specific STAT5 activation. Blood. 2009;114(24):5034–5043. doi: 10.1182/blood-2008-12-196055. [DOI] [PMC free article] [PubMed] [Google Scholar]


