Abstract
Purpose
Dentin hypersensitivity is a potential threat to oral health. Laser irradiation may provide reliable and reproducible treatment but remains controversial. The present study aimed to evaluate the effects of CO2 or erbium-doped yttrium aluminium garnet (Er:YAG) laser therapy, and to assess mineral content.
Methods
Eighteen human single-rooted teeth affected with advanced periodontitis were obtained. Buccal and lingual surfaces were planed to form 36 specimens. Ethylenediaminetetraacetic acid gel (24%) was applied to remove the smear layer and simulate hypersensitive teeth. The experimental groups were: group 1, control (no irradiation); group 2, CO2 laser (repetitive pulsed mode, 2 W, 2.7 J/cm2); and group 3, Er:YAG laser (slight contact mode, 40 mJ/pulse and 10 Hz). To evaluate dentinal tubule occlusion, six specimens per group (2-mm thickness) were prepared and observed using scanning electron microscopy (SEM) for calculation of the occlusion percentage. To evaluate the mineral content, six specimens per group (0.6-mm thickness) were used, and then the levels of Ca, K, Mg, Na, and P were measured by inductively coupled plasma-atomic emission spectrometry. In addition, the surface temperature of the specimens during laser irradiation was analyzed by a thermograph.
Results
The SEM photomicrographs indicated melted areas around exposed dentinal tubules and a significantly greater percentage of tubular occlusion in the CO2 and Er:YAG laser groups than the control, and in the Er:YAG group than the CO2 laser group. In addition, no significant differences were noted among the experimental groups for the mineral elements analyzed. The CO2 laser group showed an evident thermal effect compared to the Er:YAG group.
Conclusions
CO2 and Er:YAG laser are effective in treating dentin hypersensitivity and reducing its symptoms. However, the Er:YAG laser has a more significant effect; thus, it may constitute a useful conditioning item. Furthermore, neither CO2 nor Er:YAG lasers affected the compositional structure of the mineral content.
Graphical Abstract
Keywords: Dentin, Hypersensitivity, Laser, Minerals
INTRODUCTION
Tooth hypersensitivity is characterized by a specific type of pain that most commonly occurs when exposed cervical tooth surfaces (cementum/dentin) are subjected to different stimuli through different tooth wear processes [1]. It sometimes shows an exaggerated response to a sensory stimulus and causes chronic irritation that affects eating, drinking and even breathing [2]. Several types of stimuli may cause irritation: thermal (cold, warm), evaporative (air blast, air jet stimulator), osmotic (hypertonic solutions, sugars), mechanical-tactile (probing, scaling), electrical (pulp testers), or chemical stimuli (acids) [1]. The interactions between different tooth wear processes such as attrition (wear due to tooth friction), abrasion (wear between teeth and different materials), and erosion (dissolution of apatite structure by acids) have been thoroughly described and implicated in the development of dentin hypersensitivity (DHS) [3]. Other factors have also been reported to increase the risk for hypersensitive teeth such as: caries, abrasion due to brushing, dietary erosion, abnormal tooth positioning, a parafunctional habit, aging, gingival recession, chronic periodontal disease, and gingival and/or periodontal surgery as well as orthodontic, prosthetic, and restorative treatments [4,5].
The exact mechanism responsible for DHS is still unclear and its cause is not well understood; thus, there is no effective treatment. A convincing and widely accepted theory to explain this pain sensation is the so called "hydrodynamic" theory [6], which considers that the stimulus could cause a convective fluid flow in the dentinal tubules, and thus the permeability of the dentin would be related to the extent of the effect of the stimulus. However, the condition has been treated by various chemical agents, which have been claimed to reduce pain, mostly by aiming to occlude or block exposed dentinal tubules, such as desensitizing dentifrices, potassium oxalate and sodium fluoride [7,8]. However, Kerns et al. [9] have reported that the effects of tubule occlusion are relatively short-term, and Jacobsen and Bruce [10] also indicated that none of these treatments have produced consistently effective or long-lasting results.
Therefore, many investigators have been searching for other therapeutic agents or methods that are effective and relatively long-lasting. The effects of laser irradiation on dentin in vitro have been studied because it can close and seal the dentinal tubules and may possibly reduce DHS. In some reports, most of the therapies have failed to satisfy the patients; however, other authors have reported that laser irradiation may provide reliable and reproducible treatment [11]. Laser technology has gained popularity over recent years, with many proposed applications in dentistry and medicine. Laser irradiation of dental hard tissue causes morphological and chemical changes. The extent of these changes is affected by the absorption characteristics of the tissue, and hence, the changes are likely to be varied according to the type of laser and dental tissue [12].
Energy from the long-wavelength, nonpenetration type CO2 and erbium-doped yttrium aluminium garnet (Er:YAG) lasers is absorbed by water, and is used for many applications such as surgical applications, root canal therapy, and treatment of DHS [13,14]. The first use of a laser for the treatment of DHS was reported by Matsumoto et al. [11] who used a neodymium-doped:yttrium aluminium garnet (Nd:YAG) laser. Laser therapy has also been recommended by others [15] to treat DHS and was reportedly found to be effective. The Er:YAG laser was approved by the U.S. Food and Drug Administration (in 1997) for application to dental hard tissue [16]. However, issues such as the effects of laser irradiation on DHS have received little attention. Therefore, our in vitro research has focused on the transmission of photochemical energy to dentin via two types of lasers (CO2 and Er:YAG) to attain an effective laser level below ablation threshold in a trial.
Dentin is a complex mineralized tissue composed of approximately 70% mineral, 20% organic component and 10% fluid by weight [17]. Ca, P and the calcium-to-phosphorus mineral ratio (Ca/P) present in hydroxyapatite crystals are the major inorganic components and indicate the basic composition of the dental hard tissue surfaces. It has been reported that some chemical agents cause alterations in the chemical structure of human dentin and change the Ca/P mineral ratio of the dentin surface [18]. Since both ions (Ca and P) are ultimately related to remineralization, most research efforts have been directed toward their deposition or enhancement of their concentration in the dental structure [19]. Alterations in the Ca/P mineral ratio can change the original ratio between organic and inorganic components which in turn changes the permeability and solubility characteristics of dentin [18]; Moreover, in addition to Ca and P, small amounts of Mg are always detectable in dentin, which is thought to influence the mineralization process, especially crystal growth [20].
The inductively coupled plasma-atomic emission spectrometry (ICP-AES) technique is one of the most attractive detection systems for determining the levels of trace elements in the dentin. With this technique, polishing is not necessary and dentin chips are sufficient for element detection [18]. ICP-AES uses a sample that is passed through argon in a ray fluorescence field. When the sample is introduced into the plasma, the atoms are excited and emit very stable light of varying wavelengths that permits the identification of the elements. This technique has become highly popular for element analysis [21]. However, limited studies have been performed on how lasers change the elemental composition. The purpose of the present study was to evaluate and compare the effects of using CO2 or Er:YAG laser therapy in the treatment of DHS, in an attempt to reach an effective laser level below the ablation threshold, and also to assess effect of using a laser on the mineral content.
MATERIALS AND METHODS
Patient inclusion and exclusion criteria
The patients fulfilled the following criteria:
The presence of an advanced type of periodontal disease
Complaint of gingival recession and hypersensitive dentin
No periodontal therapy during the past year
No other systemic diseases
No smoking
No pregnancy for female patients
Willing to participate and continue in the study
Preparation of experimental specimens
The present study contained 18 single-rooted human extracted teeth (free of caries and/or restoration) from 10 patients (5 males and 5 females) affected with advanced periodontitis. The teeth were wiped with gauze, cleaned with a fine soft brush and stored in distilled water at room temperature immediately after extraction. The teeth were then mounted on molds with acrylic resin. The crowns of the teeth were removed and cut with a slow-speed diamond saw sectioning machine under water cooling.
A thorough scaling and root planning of the buccal & lingual surfaces of all eighteen teeth was performed by Gracey periodontal curettes (Hu-Friedy, Chicago, IL, USA) to form 36 specimens. The specimens were prepared using ethylenediaminetetraacetic acid gel (24%) for two minutes to remove the smear layer and simulate hypersensitive teeth. The specimens were then washed with distilled water, dried, and autoclaved. Finally, they were stored in distilled water containing 0.2% thymol at 4℃ to inhibit microbial growth. Cuts were made perpendicular to the long axis of the tooth and parallel to the mesial and distal line angles, with an area of 4 mm2. Written informed consent forms were obtained from all participants before initiating the experiment.
To evaluate dentinal tubule occlusion, 18 specimens (6 per group of 3 experimental groups) of 2-mm thickness were obtained from one side of each tooth. These specimens were prepared and observed using scanning electron microscopy (SEM) to calculate the percentage of occluded tubules. On the other hand, to assess the mineral content, another 18 specimens (6 per group) of 0.6-mm thickness were obtained from the other opposing side of the same tooth. The levels of Ca, K, Mg, Na, and P were then measured by inductively coupled plasma-atomic emission spectrometry.
Experimental groups
The specimens were randomly and equally divided into 3 groups (12 specimens "from 6 teeth" per group), and subjected to one of the following treatments:
(1) Group 1: Control; a nonirradiated group, that is without laser irradiation
(2) Group 2: CO2 laser (Luxar LX-20 Dental Laser, Panasonic, Tokyo, Japan); in a repetitive pulsed mode, at 4-mm target distance and set at a power setting of 2 W, which is equivalent to an energy density of 2.7 J/cm2
(3) Group 3: Er:YAG laser (Erwin, Morita, Tokyo, Japan); in a slight contact mode, under continuous water irrigation, set at 40 mJ/pulse and 10 Hz
Laser irradiation
CO2 and Er:YAG laser irradiation were performed in groups 2 and 3 respectively, in parallel paths and in three different dimensions as follows: (1) horizontally: from coronal to apical, (2) vertically: from mesial to distal, and (3) obliquely: across these directions. This achieved an overlapping laser application technique to confirm complete lasing of the whole specimen's surface. Laser irradiation was maintained at room temperature (25℃). This laser irradiation condition was chosen on the basis of the data of a pilot study and the manufacturer's recommendation. On the other hand, the surface temperature of the specimens during laser irradiation was analyzed by a thermograph.
Evaluation of dentinal tubule occlusion
The specimens were processed and prepared for scanning electron microscopic examination. The specimens were dehydrated in a graded series of ethanol solutions from 50% to 100%, and in 3-methyl butyl acetate (isoamyl acetate) using the critical-point drying method. The dried specimens were sputter-coated with a layer of titanium and observed using SEM (S-4500 SEM, Hitachi Ltd., Hitachinaka, Japan). To evaluate the extent of occlusion of the exposed dentinal tubules, the central area of each specimen was scanned in order to view tubules in a circular cross section. SEM photomicrographs were then taken in order to analyze the number of tubules that were occluded and also the number of tubules that were patent per unit area. To evaluate and to facilitate the manual counting process, grids were applied on the photomicrographs using Adobe Photoshop software. Tubules at the right and upper sides of the margins of the grids were counted and included.
Assessment of mineral content using the ICP-AES technique
The specimens were dehydrated at 70℃ in a desiccator until they reached a fixed weight recorded by electronic balance. Five milliliters of nitric acid (HNO3) and 2 mL of hydrogen peroxide (H2O2) were added to the specimens, and the specimens were burned and dissolved at 180℃ in a microwave. After calibration of the ICP-AES instrument (Vista AX, Varian, Australia), we took 2 mL of the solution, and the solutions were conveyed into a nebulizer with the help of a peristaltic pump. The specimens were aerosolized and carried in the form of an argon spray. The aerosols were heated by conduction and radiation to reach a high temperature of approximately 10,000℃, at which they were completely atomized, and energy was released. The light was transferred to a detector and every element was described according to its wavelength [22]. The present study performed repeated measurements of every element of each solution and the means were calculated in milligrams per liter (parts per million) using a computer. The levels of the five elements (Ca, K, Mg, Na, and P) in each specimen were measured using ICP-AES, and the mineral contents were calculated as percentage weights.
Statistical analysis
The statistical analyses were performed by calculating mean percentage weights of the five elements and mean percentage of tubule occlusion (mean and standard deviation) per each group. The sample size was determined using PASS sample size software (NCSS statistical software, Kaysville, UT, USA). A Kolmogorov-Smirnov test of normality was done. The results of the test revealed that all of the variables were parametric, so all variables were compared among the three experimental groups using analysis of variance (ANOVA) followed by Tukey test as a post hoc test, and between the two laser groups using an unpaired Student t-test. Statistical differences were considered significant at P-value of ≤0.05. All of the statistical analyses were performed using IBM SPSS Statistics ver. 21.0 (IBM Co., Armonk, NY, USA).
RESULTS
Evaluation of dentinal tubule occlusion ability
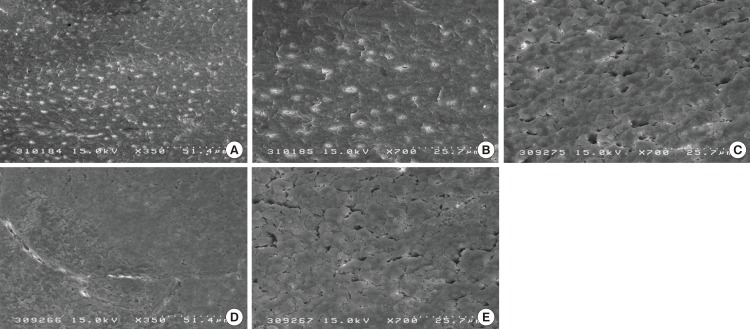
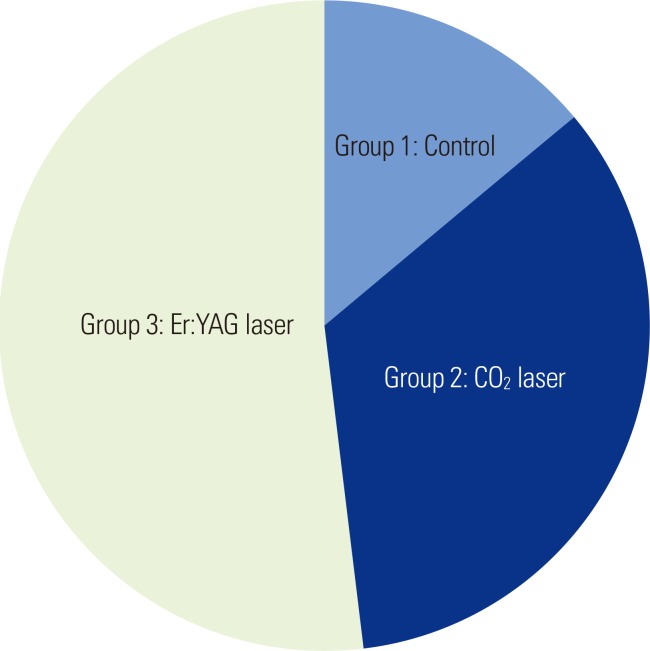
The occlusion ability of the dentinal tubules was measured by calculation of the percentage of the occluded tubules. The SEM photomicrographs indicated that there were melted areas around the exposed dentinal tubules, and a significantly greater percentage of the tubular occlusion in the groups treated with CO2 (Fig. 1D and E) and Er:YAG (Fig. 2) lasers than that in the control group (Fig. 1A-C). However, the Er:YAG laser group showed a significantly greater percentage of tubular occlusion than that found in the CO2 laser group (Table 1, Fig. 3).
Figure 1.
Scanning electron microscopy image of the root surfaces for the different experimental groups: (A-C) Group 1: control, clearly showed openings of the dentinal tubules with wide and funnel shaped orifices; (D and E) Group 2: the CO2 laser group, which generally showed a reduction in open dentinal tubules and reduced diameters when compared to the controls (×350 and ×700).
Figure 2.
Scanning electron microscopy image of the root surfaces of group 3, which was treated by erbium-doped yttrium aluminium garnet laser. Narrowing of the orifices and a reduction in open dentinal tubules (A and B) as well as melting of the tubule orifices (C and D) can be noted (×350 and ×700).
Table 1.
Comparison among the experimental groups, using an ANOVA test (Tukey post hoc test) for the difference in the degree of occlusion of a dentinal tubule.

ANOVA: analysis of variance, Group 1: control, Group 2: CO2 laser, Group 3: erbium-doped yttrium aluminium garnet laser, SD: standard deviation.
a)Statistically significant.
Figure 3.
Pie chart of the mean percentage of occluded tubules among the experimental groups. Er:YAG, erbium-doped yttrium aluminium garnet laser.
Assessment of mineral content
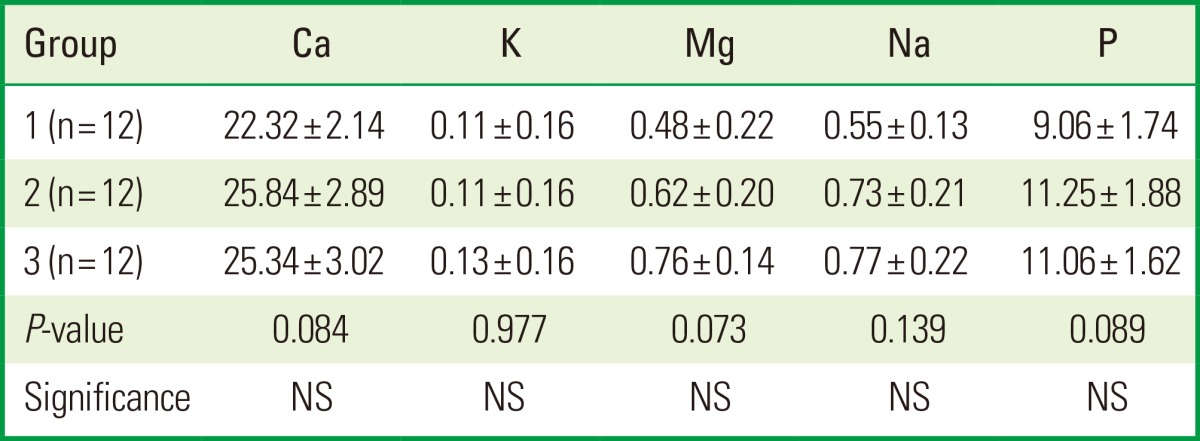
The mineral content was assessed by the mean percentage weights of the included elements using the ICP-AES technique. The present study results demonstrated that there were no significant differences detected among the experimental groups for the mineral content (Ca, K, Mg, Na, and P) (Table 2).
Table 2.
Comparison among the experimental groups, using an ANOVA test (Tukey post hoc test) for the weighted values (%) of the included elemental analysis.
Values are presented as mean±standard deviation.
ANOVA: analysis of variance, Group 1: control, Group 2: CO2 laser, Group 3: erbium-doped yttrium aluminium garnet laser, NS: not significant.
Temperature rise during laser irradiation
The effect of the temperature rise on the specimen's surface during laser irradiation was assessed using a thermograph and the intergroup comparison was performed by an unpaired Student t-test. It was demonstrated that the CO2 laser group showed a significantly greater thermal effect, with a mean temperature rise of 6.4℃, compared to that obtained in the Er:YAG laser group: 0.9℃ (Table 3).
Table 3.
Comparison among the laser groups, using Student t-test for the effect of the mean temperature rise on the specimen's surface during laser irradiation.
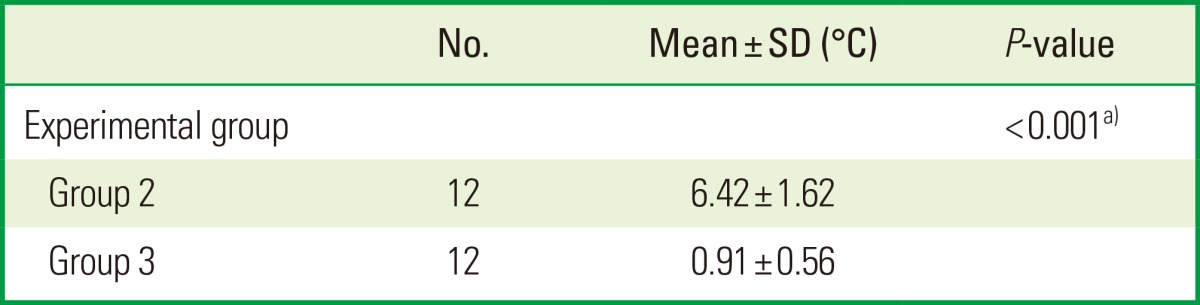
SD: standard deviation, Group 2: CO2 laser, Group 3: erbium-doped yttrium aluminium garnet laser.
a)Statistically significant.
DISCUSSION
In general, the results of the present study indicated that laser irradiation can be used to close exposed dentinal tubules. The Er:YAG laser showed a significantly more promising role with less severe thermal side effects when compared to the CO2 laser. Some previous reports [23,24] have indicated that laser irradiation has curative effects in patients with DHS, may have long-term effectiveness, does not produce notable adverse effects especially when used at low powers, and is a simple and a quick technique. These characteristics may make it an effective and attractive therapy for DHS.
It has been shown that sensitive teeth have much greater numbers of open tubules per unit area of exposed dentin, two times wider tubular diameters and about a hundred times greater fluid diffusion than nonsensitive teeth [25]. According to the hydrodynamic theory, the decrease of dentin fluid movements through evaporation of its superficial layers may directly lead to the reduction of DHS [26]. Therefore, one method of treatment is to block the exposed dentinal tubules, and the other is to reduce the excitability of sensory nerves. Blockage may include the reduction of the number of open tubules through their occlusion or even decreasing tubule diameters as a therapeutic goal for sensitive teeth and as a prerequisite to attain an effective degree of desensitization. In addition, hydroxyapatite crystals melt in the presence of a sufficient quantity of energy, such as the interaction of a laser and tooth surface that liberates a certain degree of heat, leading to closure of the dentinal tubules [27].
The CO2 laser irradiation of root surface specimens in the present study resulted in smaller tubule orifices, melted areas around the exposed dentinal tubules, and a significantly greater percentage of tubular occlusion than in the controls. Thus, the positive effects of the CO2 laser on dentinal hypersensitivity are due to the occlusion or narrowing of the dentinal tubules. In accordance, Bonin et al. [28] reported that using the CO2 laser at 1.0 W moderate energy density resulted mainly in sealing of the dentinal tubules, as well as a reduction of their permeability. Zhang et al. [13] reported that CO2 laser irradiation is useful for treatment of cervical dentinal hypersensitivity without thermal damage to the pulp. In an in vitro study, Moritz et al. [29] proposed that CO2 laser irradiation is associated with the occurrence of occlusion or narrowing of dentinal tubules. Thereafter, in another in vivo study, Moritz et al. [30] postulated that dentin may be fused during CO2 laser treatment of dental necks, which may lead to elimination of DHS.
The obtained positive results of Er:YAG laser in the present study may be explained by the expectation of its efficient use through its thermomechanical ablation and high absorption by water [31], which is suggested as approximately fifteen times greater than that of CO2 and 20,000× greater than Nd:YAG laser [32]. This high absorption in water may lead to evaporation of the dentinal fluid and the surface smear layer, since the dentin is dissolved and the water on the dentin surface is evaporated explosively by the laser energy [33], thus may possibly lead to blockade of the dentinal tubule orifices. The change of temperature on the dentin surface was checked during laser irradiation. In the present results, only CO2 laser irradiation has raised temperature for 6.4℃, but Er:YAG laser was not significantly changed (0.91℃). Er:YAG laser has been suggested to act as an efficient tool in removing the smear layer [34], and it could be postulated that the precipitation of insoluble salts in the exposed dentinal tubules are responsible for their obturation. In this context, it is important to refer that the principal mechanism of fluoride to relieve DHS is its chemical ability to reduce and block fluid movements in the dentinal tubules through formation of Ca-P precipitates as well as calcium fluoride and fluorapatite [35]. Petersson [1] in a recent review study has also suggested that most of the fluoride preparations in combination with dentinal fluid obstructive agents are beneficial to reduce DHS.
Another explanation of the effects of the Er:YAG laser on dentinal hypersensitivity is perhaps due to sealing of dentinal tubule orifices, in consequence to destruction of the hydroxyapatite crystals on the dentin surface, especially when using laser with parameters below ablation threshold. In accordance, Aranha et al. [14] reported that Er:YAG laser irradiation at 60 mJ is useful for decreasing dentin permeability, highly effective in reducing the diameters of dentin tubules under specific conditions and also partially obliterates the tubules below ablation threshold. Dilber et al. [36] in a recent study reported also that Er:YAG laser when used with parameters of 100 mJ/pulse, 10 Hz, and defocus mode, was lower than the ablation threshold of dentin, and expected that this treatment would partially seal the tubule orifices and decrease the fluid movements. This mechanism involves modification of the dentin tubular structure by melting and fusing the hard tissue or smear layer and subsequently sealing the dentinal tubules. In addition, Schwarz et al. [26] used the Er:YAG laser in a controlled prospective clinical study for the treatment of hypersensitive dentin and suggested that their energy settings of 80 mJ/pulse, at 3 Hz (under water irrigation, in a defocused mode and a lasing time of 2 minutes per tooth) were lower than the ablation thresholds of dental hard tissues. However, the present study used the Er:YAG laser with energy settings of 40 mJ/pulse, and a repetition rate of 10 Hz (under water irrigation, in a slight contact mode and a lasing time of 47 seconds per specimen) in a trial to attain a laser level below the ablation threshold.
On the other hand, because the exact mechanism responsible for DHS is still unclear, it has been hypothesized [37] that free radicals are generated by He-Ne laser and Ga-Al-As laser irradiation with H2O2, NaClO, or cultured cells. We believe that upon using the non-penetration-type lasers (CO2 and Er:YAG), free radicals might be generated on the surface of dentin, the dentin structure is then destroyed and crumbled, and the dentinal tubules are finally obturated. However, the mechanism of action of the Er:YAG laser for the treatment of DHS remains unclear and further study is needed.
The mineral content of dentin can be measured using a SEM/energy dispersive spectrometer (EDS) (SEM with X-ray microanalysis), with amounts detectable at the parts per million (mg/L) level. In addition, the measurements should be repeated for a second element in the SEM/EDS technique [19]. In contrast, with the ICP-AES technique, the mineral content can be detected at the parts per billion (µg/L) level and multiple elements can be measured at the same time. In the present study, we used the ICP-AES technique for elemental analysis for the above mentioned reasons.
In this study, the compositional change of the dentin surfaces irradiated by Er:YAG and CO2 lasers was evaluated, and the mineral content of the groups were compared using the ICP-AES technique. The mean percentage weights of Ca, K, Mg, Na, and P showed no significant differences among the groups. In accordance, Lee et al. [38] demonstrated that Er:YAG laser irradiation with water spray does not significantly change the structure and composition of the dentin. Secilmis et al. [22] observed also that laser treatment at 2 W and 3 W did not significantly affect the mean percentage weight of any of the elements. However, Rohanizadeh et al. [12] evaluated the ultrastructural and compositional changes in dentin after irradiation with a short pulse laser (Q-switched Nd:YAG). The investigation was done by SEM and transmission electron microscopy, micro-electron diffraction, and electron microscope analysis of dispersive energy. The Ca/P mineral ratio was lower in the irradiated areas than that in the nonirradiated areas.
It can thus be concluded that the usage of the CO2 and Er:YAG laser at the determined power settings can treat DHS and reduce its symptoms significantly. However, the Er:YAG laser has a greater effect on tubular occlusion with less thermal change; thus it may act as a useful conditioning tool under such conditions. Furthermore, neither the CO2 nor Er:YAG laser has a significant effect on the compositional structure, specifically, the mineral content.
ACKNOWLEDGEMENTS
The author would like to thank all the kind contributors to the preparation and revision of this manuscript, especially Ms. Afaf El-Ewaidy.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Petersson LG. The role of fluoride in the preventive management of dentin hypersensitivity and root caries. Clin Oral Investig. 2013;17(Suppl 1):S63–S71. doi: 10.1007/s00784-012-0916-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tonguc MO, Ozat Y, Sert T, Sonmez Y, Kirzioglu FY. Tooth sensitivity in fluorotic teeth. Eur J Dent. 2011;5:273–280. [PMC free article] [PubMed] [Google Scholar]
- 3.Barbour ME, Rees GD. The role of erosion, abrasion and attrition in tooth wear. J Clin Dent. 2006;17:88–93. [PubMed] [Google Scholar]
- 4.Lee BS, Chang CW, Chen WP, Lan WH, Lin CP. In vitro study of dentin hypersensitivity treated by Nd:YAP laser and bioglass. Dent Mater. 2005;21:511–519. doi: 10.1016/j.dental.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Markowitz K, Pashley DH. Discovering new treatments for sensitive teeth: the long path from biology to therapy. J Oral Rehabil. 2008;35:300–315. doi: 10.1111/j.1365-2842.2007.01798.x. [DOI] [PubMed] [Google Scholar]
- 6.Brannstrom M, Astrom A. The hydrodynamics of the dentine; its possible relationship to dentinal pain. Int Dent J. 1972;22:219–227. [PubMed] [Google Scholar]
- 7.Arrais CA, Micheloni CD, Giannini M, Chan DC. Occluding effect of dentifrices on dentinal tubules. J Dent. 2003;31:577–584. doi: 10.1016/s0300-5712(03)00115-5. [DOI] [PubMed] [Google Scholar]
- 8.Pashley DH, Galloway SE. The effects of oxalate treatment on the smear layer of ground surfaces of human dentine. Arch Oral Biol. 1985;30:731–737. doi: 10.1016/0003-9969(85)90185-2. [DOI] [PubMed] [Google Scholar]
- 9.Kerns DG, Scheidt MJ, Pashley DH, Horner JA, Strong SL, Van Dyke TE. Dentinal tubule occlusion and root hypersensitivity. J Periodontol. 1991;62:421–428. doi: 10.1902/jop.1991.62.7.421. [DOI] [PubMed] [Google Scholar]
- 10.Jacobsen PL, Bruce G. Clinical dentin hypersensitivity: understanding the causes and prescribing a treatment. J Contemp Dent Pract. 2001;2:1–12. [PubMed] [Google Scholar]
- 11.Matsumoto K, Funai H, Shirasuka T, Wakabayashi H. Effects of Nd: YAG- laser in treatment of cervical hypersensitive dentine. Jpn J Conserv Dent. 1985;28:760–765. [Google Scholar]
- 12.Rohanizadeh R, LeGeros RZ, Fan D, Jean A, Daculsi G. Ultrastructural properties of laser-irradiated and heat-treated dentin. J Dent Res. 1999;78:1829–1835. doi: 10.1177/00220345990780121001. [DOI] [PubMed] [Google Scholar]
- 13.Zhang C, Matsumoto K, Kimura Y, Harashima T, Takeda FH, Zhou H. Effects of CO2 laser in treatment of cervical dentinal hypersensitivity. J Endod. 1998;24:595–597. doi: 10.1016/S0099-2399(98)80117-9. [DOI] [PubMed] [Google Scholar]
- 14.Aranha AC, Domingues FB, Franco VO, Gutknecht N, Eduardo Cde P. Effects of Er:YAG and Nd:YAG lasers on dentin permeability in root surfaces: a preliminary in vitro study. Photomed Laser Surg. 2005;23:504–508. doi: 10.1089/pho.2005.23.504. [DOI] [PubMed] [Google Scholar]
- 15.Kimura Y, Wilder-Smith P, Yonaga K, Matsumoto K. Treatment of dentine hypersensitivity by lasers: a review. J Clin Periodontol. 2000;27:715–721. doi: 10.1034/j.1600-051x.2000.027010715.x. [DOI] [PubMed] [Google Scholar]
- 16.Kantorowitz Z, Featherstone JD, Fried D. Caries prevention by CO2 laser treatment: dependency on the number of pulses used. J Am Dent Assoc. 1998;129:585–591. doi: 10.14219/jada.archive.1998.0276. [DOI] [PubMed] [Google Scholar]
- 17.Ten Cate AR. Composition formation and structure of dentin. In: Nanci A, Ten Cate AR, editors. Ten Cate's oral histology: development, structure, and function. 7th ed. St. Louis: Mosby Elsevier; 2008. pp. 166–168. [Google Scholar]
- 18.Ari H, Erdemir A. Effects of endodontic irrigation solutions on mineral content of root canal dentin using ICP-AES technique. J Endod. 2005;31:187–189. doi: 10.1097/01.don.0000137643.54109.81. [DOI] [PubMed] [Google Scholar]
- 19.Pulido MT, Wefel JS, Hernandez MM, Denehy GE, Guzman-Armstrong S, Chalmers JM, et al. The inhibitory effect of MI paste, fluoride and a combination of both on the progression of artificial caries-like lesions in enamel. Oper Dent. 2008;33:550–555. doi: 10.2341/07-136. [DOI] [PubMed] [Google Scholar]
- 20.Wiesmann HP, Tkotz T, Joos U, Zierold K, Stratmann U, Szuwart T, et al. Magnesium in newly formed dentin mineral of rat incisor. J Bone Miner Res. 1997;12:380–383. doi: 10.1359/jbmr.1997.12.3.380. [DOI] [PubMed] [Google Scholar]
- 21.Deutsch AS, Cohen BI, Musikant BL. Inductively coupled plasma-emission spectroscopy and atomic absorption for the use of elemental analysis of a root canal after lasing with a holmium:YAG laser. J Endod. 2003;29:404–406. doi: 10.1097/00004770-200306000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Secilmis A, Altintas S, Usumez A, Berk G. Evaluation of mineral content of dentin prepared by erbium, chromium:yttrium scandium gallium garnet laser. Lasers Med Sci. 2008;23:421–425. doi: 10.1007/s10103-007-0498-y. [DOI] [PubMed] [Google Scholar]
- 23.Khabbaz MG, Makropoulou MI, Serafetinides AA, Papadopoulos D, Papagiakoumou E. Q-switched versus free-running Er:YAG laser efficacy on the root canal walls of human teeth: a SEM study. J Endod. 2004;30:585–588. doi: 10.1097/01.don.0000121612.74269.0d. [DOI] [PubMed] [Google Scholar]
- 24.Hamachi T, Iwamoto Y, Hirofuji T, Kabashima H, Maeda K. Clinical evaluation of GaAlAs-semicondoctor laser in the treatment of cervical hypersensitivity dentin. Jpn J Conserv Dent. 1992;35:12–17. [Google Scholar]
- 25.Absi EG, Addy M, Adams D. Dentine hypersensitivity. A study of the patency of dentinal tubules in sensitive and non-sensitive cervical dentine. J Clin Periodontol. 1987;14:280–284. doi: 10.1111/j.1600-051x.1987.tb01533.x. [DOI] [PubMed] [Google Scholar]
- 26.Schwarz F, Arweiler N, Georg T, Reich E. Desensitizing effects of an Er:YAG laser on hypersensitive dentine. J Clin Periodontol. 2002;29:211–215. doi: 10.1034/j.1600-051x.2002.290305.x. [DOI] [PubMed] [Google Scholar]
- 27.Lan WH, Lee BS, Liu HC, Lin CP. Morphologic study of Nd:YAG laser usage in treatment of dentinal hypersensitivity. J Endod. 2004;30:131–134. doi: 10.1097/00004770-200403000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Bonin P, Boivin R, Poulard J. Dentinal permeability of the dog canine after exposure of a cervical cavity to the beam of a CO2 laser. J Endod. 1991;17:116–118. doi: 10.1016/S0099-2399(06)81741-3. [DOI] [PubMed] [Google Scholar]
- 29.Moritz A, Gutknecht N, Schoop U, Wernisch J, Lampert F, Sperr W. Effects of CO2 laser irradiation on treatment of hypersensitive dental necks: results of an in vitro study. J Clin Laser Med Surg. 1995;13:397–400. doi: 10.1089/clm.1998.16.211. [DOI] [PubMed] [Google Scholar]
- 30.Moritz A, Gutknecht N, Schoop U, Goharkhay K, Ebrahim D, Wernisch J, et al. The advantage of CO2-treated dental necks, in comparison with a standard method: results of an in vivo study. J Clin Laser Med Surg. 1996;14:27–32. doi: 10.1089/clm.1996.14.27. [DOI] [PubMed] [Google Scholar]
- 31.Aoki A, Ando Y, Watanabe H, Ishikawa I. In vitro studies on laser scaling of subgingival calculus with an erbium:YAG laser. J Periodontol. 1994;65:1097–1106. doi: 10.1902/jop.1994.65.12.1097. [DOI] [PubMed] [Google Scholar]
- 32.Walsh JT, Jr, Flotte TJ, Deutsch TF. Er:YAG laser ablation of tissue: effect of pulse duration and tissue type on thermal damage. Lasers Surg Med. 1989;9:314–326. doi: 10.1002/lsm.1900090403. [DOI] [PubMed] [Google Scholar]
- 33.Matsui S, Kozuka M, Takayama J, Ueda K, Nakamura H, Ito K, et al. Stimulatory Effects of CO(2) Laser, Er:YAG Laser and Ga-Al-As Laser on Exposed Dentinal Tubule Orifices. J Clin Biochem Nutr. 2008;42:138–143. doi: 10.3164/jcbn.2008020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takeda FH, Harashima T, Kimura Y, Matsumoto K. A comparative study of the removal of smear layer by three endodontic irrigants and two types of laser. Int Endod J. 1999;32:32–39. doi: 10.1046/j.1365-2591.1999.00182.x. [DOI] [PubMed] [Google Scholar]
- 35.Rösing CK, Fiorini T, Liberman DN, Cavagni J. Dentine hypersensitivity: analysis of self-care products. Braz Oral Res. 2009;23(Suppl 1):56–63. doi: 10.1590/s1806-83242009000500009. [DOI] [PubMed] [Google Scholar]
- 36.Dilber E, Malkoc MA, Ozturk AN, Ozturk F. Effect of various laser irradiations on the mineral content of dentin. Eur J Dent. 2013;7:74–80. [PMC free article] [PubMed] [Google Scholar]
- 37.Kashima-Tanaka M, Tsujimoto Y, Kawamoto K, Senda N, Ito K, Yamazaki M. Generation of free radicals and/or active oxygen by light or laser irradiation of hydrogen peroxide or sodium hypochlorite. J Endod. 2003;29:141–143. doi: 10.1097/00004770-200302000-00013. [DOI] [PubMed] [Google Scholar]
- 38.Lee BS, Lin CP, Hung YL, Lan WH. Structural changes of Er:YAG laser-irradiated human dentin. Photomed Laser Surg. 2004;22:330–334. doi: 10.1089/pho.2004.22.330. [DOI] [PubMed] [Google Scholar]