Abstract
The presence of the prion agent in skeletal muscle is thought to be due to the infection of nerve fibers located within the muscle. We report here that the pathological isoform of the prion protein, PrPSc, accumulates within skeletal muscle cells, in addition to axons, in the tongue of hamsters following intralingual and intracerebral inoculation of the HY strain of the transmissible mink encephalopathy agent. Localization of PrPSc to the neuromuscular junction suggests that this synapse is a site for prion agent spread between motor axon terminals and muscle cells. Following intracerebral inoculation, the majority of PrPSc in the tongue was found in the lamina propria, where it was associated with sensory nerve fibers in the core of the lingual papillae. PrPSc staining was also identified in the stratified squamous epithelium of the lingual mucosa. These findings indicate that prion infection of skeletal muscle cells and the epithelial layer in the tongue can be established following the spread of the prion agent from nerve terminals and/or axons that innervate the tongue. Our data suggest that ingestion of meat products containing prion-infected tongue could result in human exposure to the prion agent, while sloughing of prion-infected epithelial cells at the mucosal surface of the tongue could be a mechanism for prion agent shedding and subsequent prion transmission in animals.
In prion diseases, the prion agent is primarily found in the lymphoreticular and nervous systems (8, 23). In both natural and experimental prion infections, the prion agent is commonly amplified by replicating in the lymphoreticular system prior to entry into nerve cells and subsequent invasion of the brain (1, 18, 23). Spread within the nervous system can occur by transport along axons and between synaptically linked neurons. Experimental studies have demonstrated that the initial pattern of prion agent spread in the spinal cord and brain follows defined autonomic, sensory, and motor pathways (3, 4, 12, 13, 27).
Modest levels of prion agent replication in skeletal muscle have been reported in a few studies following intracerebral or extraneural inoculation of the prion agent. Prion infectivity in skeletal muscle was first demonstrated in mink with transmissible mink encephalopathy (TME); the amount of infectious agent in skeletal muscle was 10,000-fold less than the amount found in brain (26). Prion infectivity or accumulation of the abnormal form of the prion protein, PrPSc, in skeletal muscle has more recently been described in experimental prion infection of rodents and in sporadic Creutzfeldt-Jakob disease (CJD) of humans (14), but the cellular location of agent deposition and/or replication in muscle has not been identified (3, 6, 40). In natural prion diseases, PrPSc is found in the peripheral nervous system of scrapie-infected sheep and goats, and it has been proposed that it is the peripheral nerves which transverse skeletal muscle that are the source of prion infectivity in muscle (16, 17, 19, 20). Attempts to measure the prion agent in muscle of goats infected with the scrapie agent (18, 20) and cattle infected with the bovine spongiform encephalopathy (BSE) agent (10, 42) have not detected prion infectivity. However, a murine bioassay was used to measure prion infectivity in these studies, and this assay cannot detect below 104.1 lethal median doses (LD50) of the BSE agent (10, 42). Therefore, low levels of prion agent that could be present in muscle tissue of domestic livestock would not be found using this bioassay. Routine testing for PrPSc in brains from cattle in Europe and in lymph nodes from deer and elk in the United States has been implemented to reduce human exposure to BSE and chronic wasting disease by removing infected animals from the food supply. However, human consumption of skeletal muscle from a prion-infected animal is currently considered a low risk for contracting a prion disease.
In this study, we report that in hamsters infected with the TME agent, by either an intralingual or intracerebral route, PrPSc can accumulate in skeletal muscle cells of the tongue. Localization of PrPSc to the neuromuscular junction (NMJ) indicates that this synapse can be a site for prion agent replication and may provide a path by which the prion agent spreads between skeletal muscle cells and axon terminals. PrPSc was also found in the lingual papillae of the tongue, specifically in nerve fibers of the lamina propria and in the stratified squamous epithelium of the tongue surface. These findings have public health implications and suggest that (i) ingestion of prion-infected tongue could be a source of prion exposure for humans, since livestock tongue is not included in the specified risk material; and (ii) normal shedding of the outer layer of the tongue epithelium can release the prion agent into saliva, which may be a source for prion agent transmission in livestock and cervids.
MATERIALS AND METHODS
Animal inoculations.
All procedures involving animals were approved by the Creighton University Institutional Animal Care and Use Committee and were in compliance with the Guide for the Care and Use of Laboratory Animals (31). Hamsters were inoculated in the lingual muscles of the tongue or the cerebrum with 20 μl of a brain homogenate from a TME-infected hamster which contained 107.5 intracerebral LD50 per ml of the HY TME agent. Following inoculation with the HY TME agent, hamsters were observed three times per week for the onset of clinical symptoms. Hamsters were euthanized at either specific intervals postinfection or after the onset of disease. In the latter case, animals were culled at 75 to 85 days postinfection for the intracerebral inoculation group and at 95 to 105 days for the intratongue inoculation group. Tongue and brain were collected for PrPSc analysis by Western blotting and immunohistochemistry. At least two independent mock-infected and HY TME-infected tissue samples were used for each of these analyses, but three or more tissue samples were typically used in each study. Western blotting and immunohistochemistry results were similar among individual animals within each control and experimental group.
Tissue preparation for PrPSc Western blotting.
PrPSc was enriched from tongue prior to Western blotting by tissue extraction in detergent and differential ultracentrifugation as previously described (3, 4). PrPSc-enriched samples were digested with proteinase K and resuspended in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample loading buffer. Five-percent brain homogenates were also digested with proteinase K and prepared for Western blotting as previously described (4). SDS-PAGE and Western blot analysis were performed using monoclonal antibody 3F4 hybridoma supernatant (22) (a gift of Victoria Lawson, NIH Rocky Mountain Labs, Hamilton, Mont.) as previously described. Quantification of PrPSc signal in Western blot assays was performed using ImageQuant software as previously described (4).
PrPSc immunohistochemistry, immunofluorescence, and confocal microscopy.
Immunostaining for PrPSc in tongue was performed as previously described (3). Briefly, animals were intracardially perfused with paraformaldehye-lysine-periodate fixative followed by postfixation in paraformaldehye-lysine-periodate for 5 h. Paraffin-embedded tissue sections (4 μm) were subjected to antigen retrieval by pretreatment with formic acid for 20 min. A minimum of 10 tissue sections per animal was examined for each antibody staining procedure. PrPSc was detected by incubation with monoclonal 3F4 hybridoma supernatant (1:600 dilution) and the ABC-horseradish peroxidase Elite staining method (Vector Laboratories, Burlingame, Calif.). For PrPSc staining in the double immunofluorescence procedure, the ABC-horseradish peroxidase incubation step in the ABC method was replaced by incubation with an Alexa Fluor 488 streptavidin conjugate (Molecular Probes, Portland, Oreg.) at a 1:400 dilution. PrPSc immunofluorescence staining was combined with rabbit polyclonal antibody staining for either desmin (1:50 dilution; DAKO Corp., Carpinteria, Calif.), synaptophysin (1:50 dilution; DAKO Corp.), S100 (1:800 dilution; DAKO Corp.), cytokeratin (1:800 dilution; DAKO Corp.), or calcitonin growth-related peptide (CGRP; 1:125 dilution; Peninsula Labs, Inc., San Carlos, Calif.). This latter group of primary antibodies was visualized by incubation with anti-rabbit antibody conjugated to Alexa Fluor 568 (Molecular Probes). The nuclear counterstain TO-PRO3 (Molecular Probes) was applied to some tissue sections at a concentration of 0.25 μM for 10 min. Tissue sections were mounted with Vectashield Antifade (Vector Laboratories). Images were visualized with epifluorescence using a Bio-Rad Radiance 2000 confocal system attached to a Nikon Eclipse 800 microscope.
RESULTS
Inoculation of the HY TME agent into the lingual muscles of the tongue and the cerebrum resulted in incubation periods of 83 ± 3 days and 62 ± 2 days, respectively, in Golden Syrian hamsters (mean ± standard error of the mean). At the onset of clinical symptoms, both groups had evidence of PrPSc deposition in the tongue (Fig. 1). Quantification of the PrPSc polypeptide bands using ImageQuant software indicated that the amount of PrPSc in the tongue was approximately fourfold higher following intratongue inoculation than that following intracerebral inoculation. However, the amount of PrPSc in the brain was approximately 1,000-fold higher than that found in comparable amounts of TME-infected tongue following either route of inoculation (Fig. 1). Immunohistochemistry revealed that PrPSc was located in three regions of the tongue, including the skeletal muscle (Fig. 2B), the lamina propria of the papillae (Fig. 2C), and the stratified squamous epithelium of the tongue mucosa (see below).
FIG. 1.
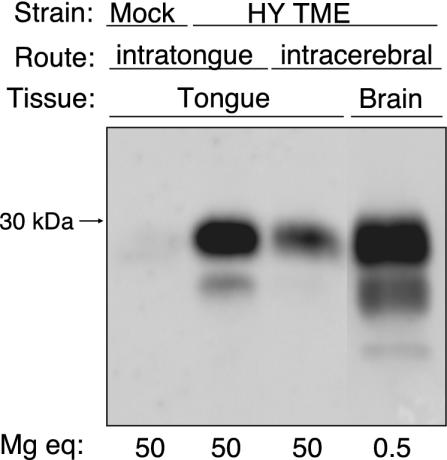
PrPSc deposition in tongue and brain following inoculation of the HY TME agent. Hamsters were inoculated with the TME agent or a normal brain homogenate (mock) by either the intratongue or intracerebral route. Tongue and brain tissues were collected from animals that were sacrificed after the onset of clinical disease as described in the text. PrPSc in brain homogenates or PrPSc-enriched preparations from tongue digested with proteinase K were prepared as described in Materials and Methods and analyzed by SDS-PAGE and PrP Western blotting. The amount of tissue analyzed in each lane is indicated in milligram tissue equivalents (mg eq), and the molecular masses of polypeptides in kilodaltons are indicated to the left of the panel.
FIG. 2.
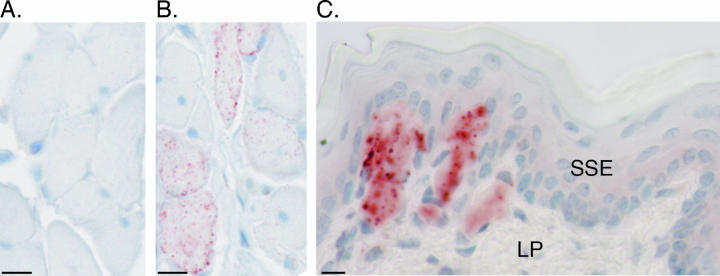
PrPSc deposition in tongue following inoculation of the HY TME agent. Hamsters were inoculated with a normal brain homogenate (A) or the TME agent (B and C) by either the intratongue (A and B) or intracerebral (C) route. Tongues were collected from animals that were sacrificed after the onset of clinical disease and prepared for immunohistochemistry as described in Materials and Methods. PrPSc immunohistochemistry illustrated PrPSc (red punctate staining) in skeletal muscle cells (B) and the lamina propria (C) but an absence of immunostaining in mock-infected tongue (A). The tissue was counterstained with hematoxylin. The lamina propria (LP) and stratified squamous epithelium (SSE) of the tongue are indicated. Bar, 10 μm.
Confocal microscopy was used to localize PrPSc to specific cell types in the tongue. Following intratongue inoculation of the HY TME agent, the majority of PrPSc staining was found within skeletal muscle cells. This was demonstrated in the tongues of clinically ill hamsters at greater than 95 days postinfection by colocalization of desmin staining (i.e., an intermediate filament protein in skeletal muscle cells) in PrPSc-positive cells (Fig. 3A to C). While PrPSc was widely distributed within skeletal muscle cells, it was often present in higher amounts at the periphery of the cell (Fig. 3A). At 1 week postinoculation of the tongue, PrPSc staining was found in less than three skeletal muscle cells per tissue section; the number of PrPSc-positive cells as well as the amount of PrPSc staining progressively increased in skeletal muscle cells during the course of subclinical TME infection (data not shown). At 95 days postinoculation, when hamsters were clinically affected, skeletal muscle cells throughout the tongue were PrPSc positive. It is worth noting that PrPSc-positive muscle cells were located between PrPSc-negative cells (Fig. 2B and 3A). These results demonstrate that the TME agent can accumulate within skeletal muscle cells and suggest that replication can also occur in these cells. At the clinical stages of TME, a lower amount of PrPSc staining was found in skeletal muscle cells of the tongue in hamsters that were intracerebrally inoculated compared to those inoculated into the tongue with the HY TME agent (data not shown). This finding was consistent with PrPSc Western blotting data of tongue from intracerebral- and intratongue-inoculated hamsters (Fig. 1). In mock-infected controls, specific binding of anti-PrP monoclonal 3F4 antibody was not observed (Fig. 2A).
FIG. 3.
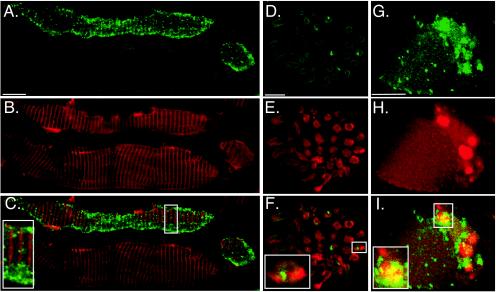
Confocal microscopy of PrPSc in skeletal muscle cells and axons. Hamsters were inoculated in the lingual muscles with the HY TME agent. At the onset of clinical symptoms, the tongue was prepared for immunofluorescence staining and confocal microscopy as described in Materials and Methods. Following immunofluorescence staining for identification of PrPSc with Alexa Fluor 488 (A, D, and G), tissue sections were also stained for either desmin (B), S100 (E), or synaptophysin (H) with goat anti-rabbit immunoglobulin conjugated to Alexa Fluor 568. To investigate the colocalization of PrPSc with desmin (C), S100 (F), and synaptophysin (I), green and red fluorescent images were merged. Bar, 10 μm. The insets at the lower left corner (C, F, and I) are two- to threefold enlargements of smaller boxes in each of the corresponding panels.
Previously, we demonstrated that PrPSc was associated with axons in nerve fascicles in the tongue (3). In the present report, PrPSc staining in nerve fascicles was demonstrated after 95 days postinfection following intratongue inoculation of HY TME, but it did not colocalize with S100 staining, which was used as a marker for Schwann cells (Fig. 3D to F). This finding suggested that the majority of PrPSc was located within the axons in this experimental model and was less likely to be within the Schwann cells that surround axons in the peripheral nervous system. Both large nerve fascicles that travel between skeletal muscle fascicles and small nerve fascicles located in the lamina propria were found to contain punctate PrPSc staining following intratongue inoculation.
The spatial relationship between PrPSc and the NMJ was investigated following intratongue inoculation of the HY TME agent, since PrPSc was found in both skeletal muscle cells and axons in the tongue. An antibody to synpatophysin, a marker for synaptic vesicles in the axon terminal, was used for identification of NMJs in hamster tongue from clinically ill hamsters at 95 days postinfection. Sixty-four percent of synaptophysin-positive skeletal muscle cells were also PrPSc positive; colocalization of synaptophysin and PrPSc was found in 25% of these cells (Fig. 3G to I). In the example illustrated (Fig. 3G to I), PrPSc staining was localized to the NMJ, indicating that this is one potential site for PrPSc accumulation in skeletal muscle cells and/or axon terminals.
The majority of PrPSc staining in the lingual mucosa was located in the lamina propria following intracerebral inoculation of HY TME, especially within the connective tissue core of the lingual papillae (Fig. 2C). To investigate the spatial relationship between PrPSc and nerve fibers in the lamina propria, we examined PrPSc and CGRP staining in the tongue at 75 to 85 days postinfection following intracerebral inoculation of the HY TME agent. CGRP staining in the lamina propria was used to identify a subset of sensory nerves, since not all sensory fibers are CGRP positive. PrPSc immunostaining was identified in 35% of CGRP-positive papillae, but colocalization of CGRP and PrPSc was found in only 5% of CGRP-positive papillae (Fig. 4A to C). In 4% of the lingual papillae, PrPSc staining was also found in the stratified squamous epithelium of the tongue mucosa following intracerebral inoculation (Fig. 4D). In these examples, the PrPSc and cytokeratin staining were in close proximity in the papillary epithelium (Fig. 4D to F); cytokeratin immunohistochemistry was used as a marker for the stratified squamous epithelial cells of the tongue. However, there was no direct overlap of the two staining patterns (Fig. 4F, inset). A greater amount of PrPSc staining was found associated with tongue papillae following intracerebral inoculation compared to inoculation of the HY TME agent into the lingual muscles. No PrPSc-specific staining was observed in the tongue of mock-infected hamsters (Fig. 4G to I).
FIG. 4.
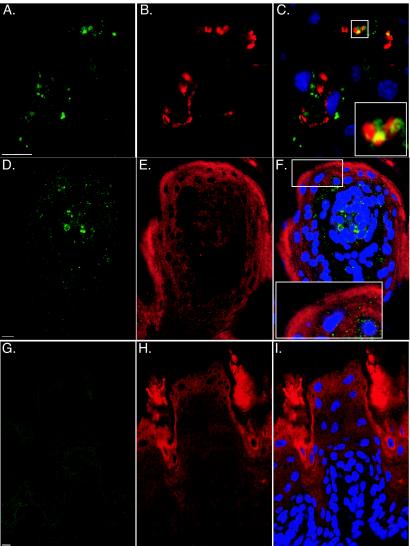
Confocal microscopy of PrPSc in sensory nerve fibers and the stratified squamous epithelium. Hamsters were inoculated in the cerebrum with the HY TME agent (A to F) or a normal brain homogenate (G to I) (mock infected). At the onset of clinical symptoms, the tongue was prepared for confocal microscopy as described in Materials and Methods. Following immunofluorescence staining for PrPSc with Alexa Fluor 488 (A, D, and G), tissue sections were stained for either CGRP (B) or cytokeratin (E and H) with goat anti-rabbit immunoglobulin conjugated to Alexa Fluor 568. The stain TO-PRO3 was used to visualize nuclei under blue fluorescence. To investigate the colocalization of PrPSc with CGRP (C) and cytokeratin (F and I), green and red fluorescent images were merged. Bar, 10 μm. The insets at the bottom (C and F) are two- to threefold enlargements of smaller boxes in each of the corresponding panels.
PrPSc staining in the tongue mucosa did not appear in cell groups that had the morphology of the lingual tonsils. However, we cannot exclude the possibility that PrPSc was associated with diffuse lymphoid tissue that is found in the tongue of some species.
DISCUSSION
In this study we report that the prion agent is located within skeletal muscle cells following inoculation of the HY TME agent into either the tongue or the cerebrum. While the TME agent was distributed throughout the individual muscle cells, PrPSc was also localized to the NMJ in 25% of synaptophysin-positive muscle cells. These findings do not distinguish whether the PrPSc staining was present in the presynaptic axon terminal, postsynaptic skeletal muscle, or in both locations in the NMJ. PrPSc was also identified in sensory axons of the lamina propria, mainly in the core of the lingual papillae, but also within the stratified squamous epithelium. This tissue distribution of the prion agent in skeletal muscle and oral mucosa has not been previously reported.
Prior studies have demonstrated PrPSc accumulation in the tongue (3, 40) or prion infectivity in skeletal muscle (6, 26, 40), but PrPSc staining by immunomorphological methods has only been reported in nerve fibers that transverse skeletal muscle (3). In the present study, PrPSc deposition was found in nerve fibers and skeletal muscle cells of the tongue. The normal isoform of the prion protein, PrPC, has been demonstrated to spread within nerve fibers by axonal transport (5) and is located in synapses, including subsynaptic areas in both the presynaptic and postsynaptic cells of the NMJ (2, 15). Since PrPC is required for PrPSc formation (7), we propose that the NMJ is a site for PrPSc formation and/or PrPSc spread between skeletal muscle cells and axon terminals. In this case, PrPSc spread to, and accumulation in, skeletal muscle cells in the tongue following intracerebral inoculation could be due to TME agent replication in the brain and subsequent anterograde transport within the hypoglossal nerve, which provides motor innervation to the lingual muscles. PrPSc may spread across the NMJ and enter skeletal muscle cells, where additional TME agent replication can occur, since PrPC is also expressed in skeletal muscle cells (15, 29). This hypothesis is supported by studies that describe (i) the spread of the prion agent in both the anterograde and retrograde directions between synaptically linked nerve cell groups in the central nervous system (4, 12, 13, 27), (ii) the retrograde transport of the HY TME agent from the tongue to the brain in the hypoglossal nerve following intratongue inoculation (3), and (iii) prion infectivity in skeletal muscle of transgenic mice that express PrPC in myocytes (6). Although the NMJ is morphologically distinct from synapses in the central nervous system, our findings are consistent with this peripheral synapse serving as a pathway for intercellular spread of the prion agent between motor axon terminals and muscle cells.
PrPSc deposition in the lamina propria below the mucosal epithelium was associated with sensory nerve fibers; however, we cannot exclude the possibility that other types of cells or axons are infected with the TME agent, since only a portion of PrPSc staining colocalized with CGRP staining. Given that CGRP staining only labels a subset of sensory fibers, it is possible that other types of sensory fibers located in the lamina propria also contain PrPSc. Based on the location of PrPSc staining and morphology of the adjacent tissue, there was no evidence that PrPSc was present in the lingual tonsils, which are also a potential target for TME agent replication. However, from these studies we cannot exclude that the lingual tonsils are a site of TME agent replication. PrPSc staining in the lamina propria was often found in the connective tissue in the core of papillae; this area has a high concentration of sensory fibers that innervate mechanoreceptors and taste buds in the tongue (30, 35). PrPSc staining in the lamina propria and tongue epithelium was more pronounced following intracerebral inoculation compared to intralingual inoculation. This finding could be due to TME agent replication in the brain and subsequent transganglionic transport within the tongue-associated sensory cranial nerves. PrPSc that was localized to cytokeratin-positive cells could also be explained by TME infection of sensory nerve fibers that project into the epithelial cell layers of the tongue (11, 25, 32, 38). Alternatively, prion agent could spread to basal keratinocytes from sensory nerve endings and lead to PrPSc formation, since keratinocytes also express PrPC (33, 39). This proposed pathway for prion agent spread to the mucosa along sensory fibers is analogous to the previous reports of spread of the CJD agent from the brain to the cornea and olfactory mucosa in sporadic CJD (24, 45; P. Duffy, J. Wolf, G. Collins, A. G. DeVoe, B. Streeten, and D. Cowen, Letter, N. Engl. J. Med. 290:692-693, 1974). The low level or absence of the prion agent in the lymphoreticular system in sporadic CJD (14, 21) suggests that the optic and olfactory cranial nerves, respectively, transport the CJD agent from the brain to these mucosal or exposed surfaces. Ultrastructural studies will be necessary to further define the location of PrPSc in the lamina propria and sensory nervous system in the tongue and its spatial relationship to epithelial cells of the tongue.
In natural prion diseases, prion agent transport from the brain to the tongue could have implications for human food safety and animal prion transmission. In sheep with scrapie, cattle with BSE, and deer with chronic wasting disease, the brain stem regions containing the tongue-associated cranial nerve nuclei have been reported to be targets for prion infection. In one or more of these diseases, PrPSc has been found in the hypoglossal nucleus (9, 37, 43), the nucleus of the solitary tract (9, 34, 37, 43), and several of the trigeminal nuclei (9, 34, 37, 43, 44). The ability of the prion agent to establish infection in brain nuclei that contribute to, or synapse with, the four cranial nerves that innervate the tongue suggests that this could be a pathway for centrifugal prion agent spread. The tongue is one of the most densely innervated extraneural tissues in the human body, with a very high concentration of both motor and sensory axons (28, 30, 35, 36, 41). Anterograde prion transport within the hypoglossal nerve would be expected to result in prion infection of the lingual muscles, while transganglionic transport within the trigeminal, facial, and glossopharyngeal nerves could result in prion spread to the sensory fibers in the tongue. It is noteworthy that prion infectivity has been found in the trigeminal ganglia of sheep with scrapie (19) and cattle with BSE (42), indicating that centrifugal spread of these prion agents within the trigeminal nerve can occur and could result in spread to peripheral sites including the tongue. Since livestock and cervid tongues are not banned for human consumption, it is possible that humans could be exposed to animal prion diseases by ingestion of prion-infected tongue. Attempts to identify prion infectivity in bovine tongues have not been successful. However, these studies have used an infectivity bioassay that cannot detect below 104.1 LD50 of BSE prions, which may not detect low levels of the prion agent (10, 42). Our findings also have implications for intraspecies transmission of animal prion diseases. Establishment of prion infection in the stratified squamous epithelial cells of the tongue, as suggested by our findings, could result in sloughing of the prion agent into the saliva, since this tissue is undergoing continual cell turnover. In this case, animal behaviors resulting in the exchange of saliva between hosts may play a role in prion transmission.
Acknowledgments
This work was supported by grants from NIH NIAID (1RO1 AI055043-01), NIH NCRR (RR15635), and USDA/CREES/NRICGP (2002-35204-12584).
Special thanks are given to Maria Christensen and Emily Hansen for excellent technical assistance.
REFERENCES
- 1.Andreoletti, O., P. Berthon, D. Marc, P. Sarradin, J. Grosclaude, L. van Keulen, F. Schelcher, J. M. Elsen, and F. Lantier. 2000. Early accumulation of PrPSc in gut-associated lymphoid and nervous tissues of susceptible sheep from a Romanov flock with natural scrapie. J. Gen. Virol. 81:3115-3126. [DOI] [PubMed] [Google Scholar]
- 2.Askanas, V., M. Bilak, W. K. Engel, A. Leclerc, and F. Tome. 1993. Prion protein is strongly immunolocalized at the postsynaptic domain of human normal neuromuscular junctions. Neurosci. Lett. 159:111-114. [DOI] [PubMed] [Google Scholar]
- 3.Bartz, J. C., A. E. Kincaid, and R. A. Bessen. 2003. Rapid prion neuroinvasion following tongue infection. J. Virol. 77:583-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartz, J. C., A. E. Kincaid, and R. A. Bessen. 2002. Retrograde transport of transmissible mink encephalopathy within descending motor tracts. J. Virol. 76:5759-5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borchelt, D. R., V. E. Koliatsos, M. Guarnieri, C. A. Pardo, S. S. Sisodia, and D. L. Price. 1994. Rapid anterograde axonal transport of the cellular prion glycoprotein in the peripheral and central nervous systems. J. Biol. Chem. 269:14711-14714. [PubMed] [Google Scholar]
- 6.Bosque, P. J., C. Ryou, G. Telling, D. Peretz, G. Legname, S. J. DeArmond, and S. B. Prusiner. 2002. Prions in skeletal muscle. Proc. Natl. Acad. Sci. USA 99:3812-3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bueler, H., A. Aguzzi, A. Sailer, R. A. Greiner, P. Autenried, M. Aguet, and C. Weissmann. 1993. Mice devoid of PrP are resistant to scrapie. Cell 73:1339-1347. [DOI] [PubMed] [Google Scholar]
- 8.Eklund, C. M., R. C. Kennedy, and W. J. Hadlow. 1967. Pathogenesis of scrapie virus infection in the mouse. J. Infect. Dis. 117:15-22. [DOI] [PubMed] [Google Scholar]
- 9.Ersdal, C., M. J. Ulvund, S. L. Benestad, and M. A. Tranulis. 2003. Accumulation of pathogenic prion protein (PrPSc) in nervous and lymphoid tissues of sheep with subclinical scrapie. Vet. Pathol. 40:164-174. [DOI] [PubMed] [Google Scholar]
- 10.European Commission for Food Safety, Scientific Steering Commitee. 2002. Opinion on TSE infectivity distribution in ruminant tissues (state of knowledge, December 2001). European Commission Health and Consumer Protection Directorate-General, Brussels, Belgium.
- 11.Farbman, A. I., and J. P. Allgood. 1971. Innervation, sensory receptors and sensitivity of the oral mucosa, p. 250-273. In C. A. Squier and J. Meyer (ed.), Current concepts of the histology of the oral mucosa. Charles C. Thomas Publisher, New York, N.Y.
- 12.Fraser, H. 1982. Neuronal spread of scrapie agent and targeting of lesions within the retino-tectal pathway. Nature 295:149-150. [DOI] [PubMed] [Google Scholar]
- 13.Fraser, H., and A. G. Dickinson. 1985. Targeting of scrapie lesions and spread of agent via the retino-tectal projection. Brain Res. 346:32-41. [DOI] [PubMed] [Google Scholar]
- 14.Glatzel, M., E. Abela, M. Maissen, and A. Aguzzi. 2003. Extraneural pathologic prion protein in sporadic Creutzfeldt-Jakob disease. N. Engl. J. Med. 349:1812-1820. [DOI] [PubMed] [Google Scholar]
- 15.Gohel, C., V. Grigoriev, F. Escaig-Haye, C. I. Lasmezas, J. P. Deslys, J. Langeveld, M. Akaaboune, D. Hantai, and J. G. Fournier. 1999. Ultrastructural localization of cellular prion protein (PrPc) at the neuromuscular junction. J. Neurosci. Res. 55:261-267. [DOI] [PubMed] [Google Scholar]
- 16.Groschup, M. H., M. Beekes, P. A. McBride, M. Hardt, J. A. Hainfellner, and H. Budka. 1999. Deposition of disease-associated prion protein involves the peripheral nervous system in experimental scrapie. Acta Neuropathol. (Berlin) 98:453-457. [DOI] [PubMed] [Google Scholar]
- 17.Groschup, M. H., F. Weiland, O. C. Straub, and E. Pfaff. 1996. Detection of scrapie agent in the peripheral nervous system of a diseased sheep. Neurobiol. Dis. 3:191-195. [DOI] [PubMed] [Google Scholar]
- 18.Hadlow, W. J., C. M. Eklund, R. C. Kennedy, T. A. Jackson, H. W. Whitford, and C. C. Boyle. 1974. Course of experimental scrapie virus infection in the goat. J. Infect. Dis. 129:559-567. [DOI] [PubMed] [Google Scholar]
- 19.Hadlow, W. J., R. C. Kennedy, and R. E. Race. 1982. Natural infection of Suffolk sheep with scrapie virus. J. Infect. Dis. 146:657-664. [DOI] [PubMed] [Google Scholar]
- 20.Hadlow, W. J., R. C. Kennedy, R. E. Race, and C. M. Eklund. 1980. Virologic and neurohistologic findings in dairy goats affected with natural scrapie. Vet. Pathol. 17:187-199. [DOI] [PubMed] [Google Scholar]
- 21.Hill, A. F., R. J. Butterworth, S. Joiner, G. Jackson, M. N. Rossor, D. J. Thomas, A. Frosh, N. Tolley, J. E. Bell, M. Spencer, A. King, S. Al-Sarraj, J. W. Ironside, P. L. Lantos, and J. Collinge. 1999. Investigation of variant Creutzfeldt-Jakob disease and other human prion diseases with tonsil biopsy samples. Lancet 353:183-189. [DOI] [PubMed] [Google Scholar]
- 22.Kascsak, R. J., R. Rubenstein, P. A. Merz, M. Tonna-DeMasi, R. Fersko, R. I. Carp, H. M. Wisniewski, and H. Diringer. 1987. Mouse polyclonal and monoclonal antibody to scrapie-associated fibril proteins. J. Virol. 61:3688-3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kimberlin, R. H., and C. A. Walker. 1979. Pathogenesis of mouse scrapie: dynamics of agent replication in spleen, spinal cord and brain after infection by different routes. J. Comp. Pathol. 89:551-562. [DOI] [PubMed] [Google Scholar]
- 24.Lang, C. J., J. G. Heckmann, and B. Neundorfer. 1998. Creutzfeldt-Jakob disease via dural and corneal transplants. J. Neurol. Sci. 160:128-139. [DOI] [PubMed] [Google Scholar]
- 25.Marlow, C. D., R. K. Winkelmann, and J. A. Gibilsco. 1965. General sensory innervation of the human tongue. Anat. Rec. 152:503-512. [Google Scholar]
- 26.Marsh, R. F., D. Burger, and R. P. Hanson. 1969. Transmissible mink encephalopathy: behavior of the disease agent in mink. Am. J. Vet. Res. 30:1637-1642. [PubMed] [Google Scholar]
- 27.McBride, P. A., W. J. Schulz-Schaeffer, M. Donaldson, M. Bruce, H. Diringer, H. A. Kretzschmar, and M. Beekes. 2001. Early spread of scrapie from the gastrointestinal tract to the central nervous system involves autonomic fibers of the splanchnic and vagus nerves. J. Virol. 75:9320-9327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McClung, J. R., and S. J. Goldberg. 2000. Functional anatomy of the hypoglossal innervated muscles of the rat tongue: a model for elongation and protrusion of the mammalian tongue. Anat. Rec. 260:378-386. [DOI] [PubMed] [Google Scholar]
- 29.Moudjou, M., Y. Frobert, J. Grassi, and C. La Bonnardiere. 2001. Cellular prion protein status in sheep: tissue-specific biochemical signatures. J. Gen. Virol. 82:2017-2024. [DOI] [PubMed] [Google Scholar]
- 30.Mu, L., and I. Sanders. 1999. Neuromuscular organization of the canine tongue. Anat. Rec. 256:412-424. [DOI] [PubMed] [Google Scholar]
- 31.National Research Council. 1996. Guide for the care and use of laboratory animals. National Academies Press, Washington, D.C.
- 32.Nishimoto, T., M. Akai, S. Inagaki, S. Shiosaka, Y. Shimizu, K. Yamamoto, E. Senba, M. Sakanaka, K. Takatsuki, Y. Hara, H. Takagi, T. Matsuzaki, Y. Kawai, and M. Tohyama. 1982. On the distribution and origins of substance P in the papillae of the rat tongue: an experimental and immunohistochemical study. J. Comp. Neurol. 207:85-92. [DOI] [PubMed] [Google Scholar]
- 33.Pammer, J., A. Suchy, M. Rendl, and E. Tschachler. 1999. Cellular prion protein expressed by bovine squamous epithelia of skin and upper gastrointestinal tract. Lancet 354:1702-1703. [DOI] [PubMed] [Google Scholar]
- 34.Ryder, S. J., Y. I. Spencer, P. J. Bellerby, and S. A. March. 2001. Immunohistochemical detection of PrP in the medulla oblongata of sheep: the spectrum of staining in normal and scrapie-affected sheep. Vet. Rec. 148:7-13. [DOI] [PubMed] [Google Scholar]
- 35.Sato, O., T. Maeda, S. Kobayashi, T. Iwanaga, and T. Fujita. 1988. Filiform papillae as a sensory apparatus in the tongue: an immunohistochemical study of nervous elements by use of neurofilament protein (NFP) and S-100 protein antibodies. Cell Tissue Res. 252:231-238. [DOI] [PubMed] [Google Scholar]
- 36.Sawczuk, A., and K. M. Mosier. 2001. Neural control of tongue movement with respect to respiration and swallowing. Crit. Rev. Oral Biol. Med. 12:18-37. [DOI] [PubMed] [Google Scholar]
- 37.Spraker, T. R., R. R. Zink, B. A. Cummings, M. A. Wild, M. W. Miller, and K. I. O'Rourke. 2002. Comparison of histological lesions and immunohistochemical staining of proteinase-resistant prion protein in a naturally occurring spongiform encephalopathy of free-ranging mule deer (Odocoileus hemionus) with those of chronic wasting disease of captive mule deer. Vet. Pathol 39:110-119. [DOI] [PubMed] [Google Scholar]
- 38.Suemune, S., T. Nishimori, M. Hosoi, Y. Suzuki, H. Tsuru, T. Kawata, K. Yamauchi, and N. Maeda. 1992. Trigeminal nerve endings of lingual mucosa and musculature of the rat. Brain Res. 586:162-165. [DOI] [PubMed] [Google Scholar]
- 39.Sugaya, M., K. Nakamura, T. Watanabe, A. Asahina, N. Yasaka, Y. Koyama, M. Kusubata, Y. Ushiki, K. Kimura, A. Morooka, S. Irie, T. Yokoyama, K. Inoue, S. Itohara, and K. Tamaki. 2002. Expression of cellular prion-related protein by murine Langerhans cells and keratinocytes. J. Dermatol. Sci. 28:126-134. [DOI] [PubMed] [Google Scholar]
- 40.Thomzig, A., C. Kratzel, G. Lenz, D. Kruger, and M. Beekes. 2003. Widespread PrPSc accumulation in muscles of hamsters orally infected with scrapie. EMBO Rep. 4:530-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weddel, G., J. A. Harpman, and D. G. Lambley. 1940. The innervation of the musculature of the tongue. J. Anat. 74:255-267. [PMC free article] [PubMed] [Google Scholar]
- 42.Wells, G. A., S. A. Hawkins, R. B. Green, A. R. Austin, I. Dexter, Y. I. Spencer, M. J. Chaplin, M. J. Stack, and M. Dawson. 1998. Preliminary observations on the pathogenesis of experimental bovine spongiform encephalopathy (BSE): an update. Vet. Rec. 142:103-106. [DOI] [PubMed] [Google Scholar]
- 43.Wells, G. A., and J. W. Wilesmith. 1995. The neuropathology and epidemiology of bovine spongiform encephalopathy. Brain Pathol. 5:91-103. [DOI] [PubMed] [Google Scholar]
- 44.Wells, G. A., J. W. Wilesmith, and I. S. McGill. 1991. Bovine spongiform encephalopathy: a neuropathological perspective. Brain Pathol. 1:69-78. [DOI] [PubMed] [Google Scholar]
- 45.Zanusso, G., S. Ferrari, F. Cardone, P. Zampieri, M. Gelati, M. Fiorini, A. Farinazzo, M. Gardiman, T. Cavallaro, M. Bentivoglio, P. G. Righetti, M. Pocchiari, N. Rizzuto, and S. Monaco. 2003. Detection of pathologic prion protein in the olfactory epithelium in sporadic Creutzfeldt-Jakob disease. N. Engl. J. Med. 348:711-719. [DOI] [PubMed] [Google Scholar]


