Abstract
Spontaneous haemorrhage in patients with haemophilia is generally considered to occur randomly and without a predictable temporal or seasonal pattern; however, there is a lack of evidence in the literature on the effects of weather, temperature and atmosphere on bleeding episodes. This post hoc analysis of a multicentre, open-label crossover study examined the influence of seasonality on bleeding frequency and patient-assessed pain in patients with moderately severe and severe (FIX C ≤ 2%) haemophilia B. Fifty patients were enrolled and treated on-demand for 16 weeks; 47 were subsequently randomized to one of two prophylactic regimens (nonacog alfa 100 IU kg−1 once weekly or 50 IU kg−1 twice weekly) for 16 weeks. Patients then underwent an 8-week washout period of on-demand therapy before being crossed over to the other prophylactic regimen for 16 weeks. Bleeding episodes during the on-demand treatment periods were analysed. To assess for temporal trends, data were graphed as scatter plots. The primary end point was the annualized bleeding rate (ABR). Additional measures included raw and median pain scores during every joint bleeding event (spontaneous or traumatic), with pain scored using the Brief Pain Inventory (0 = ‘no pain’ to 10 = ‘pain as bad as you can imagine’). The observed ABRs during the on-demand periods showed no distinguishable trend over time. Analysis of pain associated with joint bleeding episodes also did not demonstrate any discernible temporal trend. No apparent seasonal variation in bleeding pattern or patient-reported pain was observed in this analysis of patients with haemophilia B.
Keywords: factor IX, haemophilia B, pain, recombinant factor IX, seasonality
Introduction
Haemophilia is a rare, X-linked congenital bleeding disorder that occurs in about 1 in 10 000 newborns. Caused by a partial or total deficiency of coagulation factor IX (FIX), haemophilia B is less common than haemophilia A (deficiency of factor VIII), accounting for approximately 15–20% of the total haemophilia population 1. The severity of haemophilia B is typically classified according to the levels of coagulation factor activity in the blood. Severely affected patients have <1% of normal factor levels, patients with moderate disease, 1–5%, and patients with mild disease, 5–40% 2. Patients with severe disease constitute approximately 30% of the haemophilia B patient population 3.
Bleeding events associated with haemophilia are characterized by spontaneous or trauma-related haemorrhage into soft tissue, muscles and joints, with repeated bleeding leading to arthropathy and pain 1. Spontaneous haemorrhage is generally considered to occur randomly and without a predictable temporal or seasonal pattern; however, a few reports have examined the effects that weather, temperature and atmosphere might have on spontaneous bleeding. Based on these reports, there is a lack of consensus in the medical literature regarding consistent seasonal variation in bleeding risk in patients with haemophilia 4–10. In addition, regulatory agencies have expressed concern regarding the potential for seasonal influence on bleeding rates and have relayed requests for clinical trial analyses of on-demand treatment over a period of 1 year to appropriately assess the impact of seasonal variation on bleeding risk.
Replacement of deficient FIX with high purity plasma-derived or recombinant FIX concentrates used either as on-demand or prophylaxis therapy, is a mainstay of treatment for haemophilia B 1. In a multicentre, randomized, open-label crossover study, Valentino et al. evaluated the efficacy and safety associated with the use of nonacog alfa (BeneFIX®; Wyeth [Pfizer], Philadelphia, PA, USA), currently the only commercially available recombinant FIX, as a prophylaxis regimen compared with administration on an on-demand basis 11. The objective of this post hoc analysis was to assess the influence of seasonality on bleeding frequency and patient-assessed pain in these patients with haemophilia B receiving on-demand therapy.
Methods and patients
Study design
Data for this post hoc analysis were obtained from a multicentre, randomized, open-label, four-period crossover study that assessed the efficacy and safety of nonacog alfa as secondary prophylaxis in patients with moderately severe to severe haemophilia B. The design and primary results of this study have been reported previously 11. In brief, patients received on-demand treatment with nonacog alfa for 16 weeks, with dosing adjusted based on the investigator's judgment, according to body weight and bleeding severity (Period 1). Patients were then randomized to prophylaxis therapy with nonacog alfa 50 IU kg−1 twice weekly or 100 IU kg−1 once weekly for 16 weeks (Period 2; Fig.1). This was followed by an 8-week period during which patients received on-demand treatment only, with a dose and treatment plan similar to that of Period 1 (Period 3). Patients were then crossed over to receive prophylaxis with the alternative treatment regimen for 16 weeks (Period 4).
Figure 1.
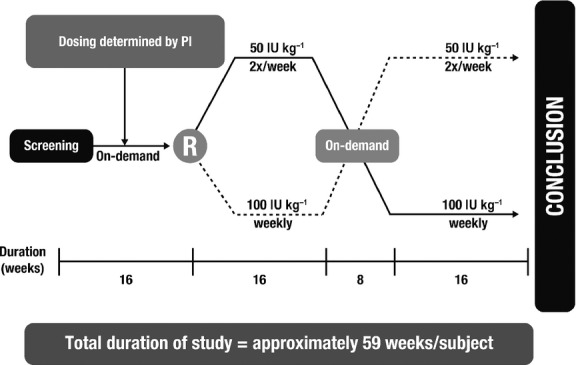
Study design. PI, principal investigator; R, randomization.
Patients
The study enrolled male patients aged 6–65 years with moderately severe to severe haemophilia B (FIX activity [FIX:C] ≤2%) who reported at least 12 bleeding episodes within the previous 12 months, at least 6 of which were joint bleeding events. Reasons for exclusion included current use of primary prophylaxis, human immunodeficiency virus infection with CD4 count <200/μL, hepatic or renal impairment, thrombocytopaenia, another bleeding disorder, major surgery or orthopaedic surgery within the previous 3 months or planned during the study period, past or current FIX inhibitor, known hypersensitivity to FIX or hamster proteins, or any other condition that could interfere with study participation or confound the interpretation of the results. Written informed consent was obtained from all patients prior to study participation.
Assessments
Data were obtained from study periods 1 and 3 when patients received on-demand treatment with nonacog alfa. The on-demand periods were selected for analysis to evaluate bleeding events without the influence of prophylactic regimens. Assessments included the annualized number of bleeding episodes, expressed as the annualized bleeding rate (ABR) and patient-reported pain 24 h after every joint bleeding event (spontaneous or traumatic), rated according to the Brief Pain Inventory (BPI) questionnaire (0 indicated ‘no pain’; 10 indicated ‘pain as bad as you can imagine’) 12.
Statistical analysis
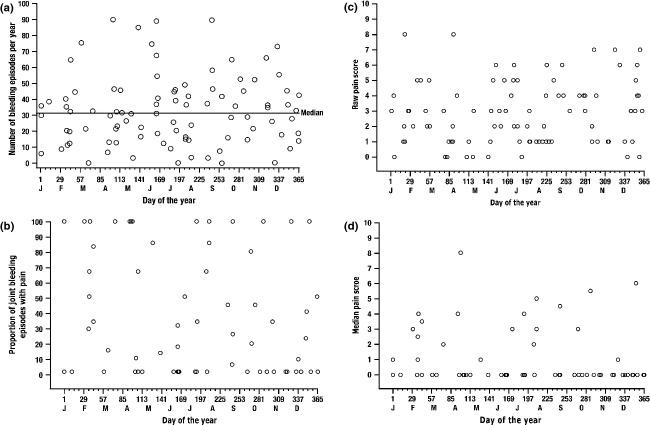
No sample size calculations were required for this post hoc analysis. The analyses of the outcomes were prepared as scatter plots. The following were plotted: (i) distribution of ABRs in each on-demand treatment period for each patient by day of the year (the time point for each patient's ABR being the midpoint of that patient's participation in the on-demand treatment period); (ii) the proportion of joint bleeding episodes during which a patient reported pain (answered ‘Yes’ to question 1 on the BPI: ‘Have you experienced significant pain today?’), with the value plotted at the midpoint of the patient's participation in that period; (iii) the raw pain scores for patients reporting pain from a joint bleeding episode, plotted on the day the episode occurred; and (iv) the median pain score recorded for each patient for all joint bleeding episodes (inputting a score of 0 for any joint bleeding episode for which the patient reported no significant pain), with the median pain score plotted at the midpoint of the patient's participation during that period.
Results
Patient demographics
A total of 50 patients were enrolled in the study. Of these, 49 patients received on-demand treatment in period 1, 47 patients were randomized to the two prophylactic treatment sequences, and 39 patients received on-demand treatment during period 3. Patient demographics are summarized in Table1. The majority of patients were aged 18–50 years, and all but 1 were white.
Table 1.
Baseline patient demographics
| Demographic | 100 IU kg−1 QW then 50 IU kg−1 BW (n = 22) | 50 IU kg−1 BW then 100 BW then 100 IU kg−1 QW (n = 25) |
|---|---|---|
| Age, years | ||
| Mean (SD) | 31.7 (13.4) | 25 (14.4) |
| Median (min, max) | 28.0 (9.0, 57.0) | 24.0 (6.0, 64.0) |
| Age ranges, n (%) | ||
| 6–12, years | 1 (4.5) | 6 (24.0) |
| >12–18, years | 3 (13.6) | 1 (4.0) |
| >18–30, years | 8 (36.4) | 12 (48.0) |
| >30–50, years | 8 (36.4) | 5 (20.0) |
| >50, years | 2 (9.1) | 1 (4.0) |
| Gender, male, n (%) | 22 (100) | 25 (100) |
| Race, n (%) | ||
| White | 21 (96.5) | 25 (100) |
| Black | 1 (4.5) | 0 |
BW, twice weekly; QW, once weekly.
Bleeding patterns and patient-reported pain
To determine the appropriateness of combining the data from both on-demand treatment periods to have all months represented, the two on-demand ABRs were plotted against one another for each patient (Fig.2). Most patients had relatively similar ABR values for both on-demand periods; thus, the data were combined. The ABRs for patients in each on-demand treatment period with data available are shown in Fig.3a, with up to two values per patient. The observed ABRs showed no distinguishable trend over time.
Figure 2.
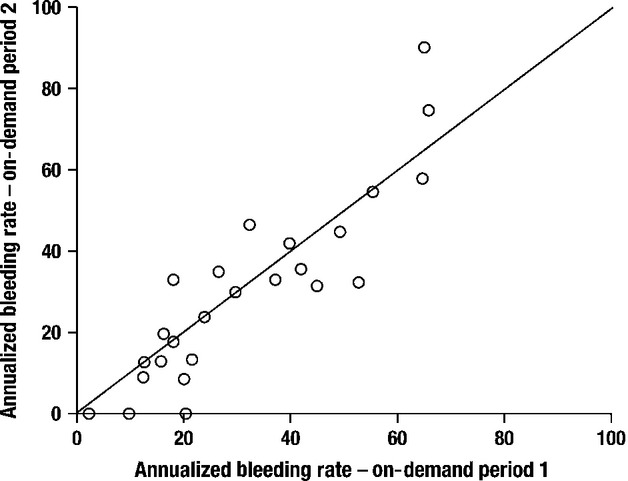
Distribution of annualized bleeding events for on-demand period 1 vs. on-demand period 2 for each patient.
Figure 3.
(a) Distribution of annualized bleeding events in each on-demand treatment period per patient, by day of the year. Data from both on-demand periods were pooled so that all months could be represented. Each circle represents up to two data values for an individual patient. The time point represents the midpoint of the patient's participation in that period. (b) Proportion of joint bleeding episodes associated with pain per patient in each on-demand treatment period, by day of the year. Each circle represents the proportion of joint bleeding episodes for which the patient answered ‘Yes’ to question 1 on the BPI (‘Have you experienced significant pain today?’), plotted at the midpoint of the patient's participation in that period. (c) Raw pain scores for patients reporting pain from a joint bleeding episode during either on-demand period, by day and year of occurrence, for each reported episode. Each circle represents a raw pain score, which is plotted on the day of occurrence. (d) Median pain score in each on-demand treatment period per patient, by day of the year. Each point represents the median pain score for each patient, plotted at the midpoint of the patient's participation in that period. A pain score of 0 was imputed for any joint bleeding episode for which a patient reported no significant pain.
The proportion of joint bleeding episodes during which patients reported pain is shown in Fig.3b. No evident pattern of pain over time was observed over the course of the study. Raw pain scores for patients who reported significant pain as the result of a joint bleeding episode are shown in Fig.3c and median pain scores for all joint bleeding episodes are shown in Fig.3d. No distinguishable temporal pattern was observed for either of these measures.
Discussion
The risk for bleeding in patients with haemophilia strongly correlates with endogenous coagulation factor levels; however, there is evidence that other factors may influence bleeding tendencies. For example, a small proportion (10–15%) of patients with severe haemophilia have bleeding patterns typically associated with mild disease (i.e. less spontaneous bleeding or less joint damage) despite having a coagulation factor activity of <1% 13. The presence of target joints and synovial hypertrophy, extent of haemophilic arthropathy, physical activity and age may also influence bleeding patterns in patients with haemophilia 14,15. Research has also included investigation into physiological issues such as seasonality and weather in haemophilia bleeding episodes 4–10. The findings from this post hoc analysis showed no seasonal or temporal pattern for bleeding episodes (spontaneous and traumatic) in patients with moderately severe to severe haemophilia B receiving on-demand treatment with nonacog alfa. In addition, the pattern of severe pain associated with joint bleeding events did not fit a seasonal or temporal pattern.
The findings from this analysis are generally consistent with data in the literature. In a recent study, Broderick, et al. evaluated the effect of physical activity on bleeding risk in paediatric patients with haemophilia A and B. The latter study included an assessment of bleeding frequency according to week of year of study participation, and no trend was observed 9. A case study by Benbasset of adult patients with haemophilia A found monthly peaks in bleeding in individual patients; however, the timing of the peaks varied widely between patients and did not show a seasonal preference for the group as a whole 8. Likewise, an observational study of adolescent boys with haemophilia A found no seasonal variation in bleeding episodes over a period of 2 academic years 4. A retrospective analysis of data from clinical trials of patients with haemophilia A, including both children and adults, found an age-dependent elevation of the risk for joint bleeds during the summer months. In patients aged 10–17 years and 18–65 years, 43% and 46%, respectively, experienced their bleeding events from June to August, compared with 21% for patients aged 1–6 years 10. The authors hypothesized that the pattern of increased bleeding was likely related to the increased physical activity levels in teenage and adult patients during this time. Only one study published in 1990 found a correlation between weather patterns/atmospheric pressure and bleeding risk in patients with haemophilia 5–7; however, it appears that this study was not followed up or repeated.
In this study, patients were prompted to complete a pain assessment whenever they reported a joint bleed. A recently published study found that scores for acute pain associated with a bleed event were higher (5.97/10) than scores for pain not associated with a bleed (4.22/10) 16.This is consistent with existing literature which suggests that although acute and chronic pain in patients with haemophilia are qualitatively similar, patients experience acute pain as being more intense 17. However, findings from a recent study evaluating 15 spontaneous, non-traumatic pain episodes (six ankles, seven knee, two muscles) in 11 haemophilic adults suggested that patients and physicians misclassified the pain aetiology for the majority of episodes 18. Of 10 episodes of patient-perceived joint bleeding, only three were confirmed by ultrasound imaging to actually be associated with bleeding. Furthermore, swelling, warmth and loss of range of motion were present in only half of the confirmed bleeding episodes, demonstrating that these findings alone are not clinically reliable assessments of pain associated with bleeding.
The limitations of this analysis include its post hoc nature. In addition, the relatively small number of patients may be a limiting factor; however, this is inherent in studies of rare diseases such as haemophilia. Finally, this analysis may have been limited by the collection of data from study periods other than those used for the primary assessments (e.g. from the washout period between the two prophylaxis periods).
More data are clearly needed to determine patient risk profiles to optimize therapy with FIX replacement agents. Based on the present analysis, the frequency of bleeding in patients with moderate to severe haemophilia B does not vary according to a seasonal or temporal pattern, and similarly, there is no seasonal or temporal pattern to the intensity of pain during joint bleeding episodes.
Acknowledgments
The authors thank the research support staff and data management personnel for their participation in this study. They also thank Bina J. Patel, PharmD, of Peloton Advantage for medical writing and editorial assistance, which was funded by Pfizer.
Author contribution
FS, PR and LS collected and assembled post hoc data. NV, MC, FS and LS analysed and interpreted the data. FS prepared the manuscript. All authors reviewed, revised and approved the manuscript.
Disclosures
All authors are employees of Pfizer Inc. F. Shafer, L. Smith, P. Rendo and M. Carr also stockholders in Pfizer.
References
- 1.Srivastava A, Brewer AK, Mauser-Bunschoten EP, et al. Guidelines for the management of hemophilia. Haemophilia. 2013;19:e1–47. doi: 10.1111/j.1365-2516.2012.02909.x. [DOI] [PubMed] [Google Scholar]
- 2.White GC, Rosendaal F, Aledort LM, Lusher JM, Rothschild C, Ingerslev J. Definitions in hemophilia. Recommendation of the scientific subcommittee on factor VIII and factor IX of the scientific and standardization committee of the International Society on Thrombosis and Haemostasis. Thromb Haemost. 2001;85:560. [PubMed] [Google Scholar]
- 3.Zappa S, McDaniel M, Marandola J, Allen G. Treatment trends for haemophilia A and haemophilia B in the United States: results from the 2010 practice patterns survey. Haemophilia. 2012;18:e140–53. doi: 10.1111/j.1365-2516.2012.02770.x. [DOI] [PubMed] [Google Scholar]
- 4.Rainsford SG, Hall A. A three-year study of adolescent boys suffering from haemophilia and allied disorders. Br J Haematol. 1973;24:539–51. doi: 10.1111/j.1365-2141.1973.tb01680.x. [DOI] [PubMed] [Google Scholar]
- 5.Linde P, Syrbe G. [Meteorologic effects on hemorrhagic diathesis in hemophilia. 2. Weather effects on hemophilia with special reference to the atmospheric temperature-humidity complex] Z Gesamte Inn Med. 1990;45:659–62. [PubMed] [Google Scholar]
- 6.Linde P, Syrbe G. [Recommendations for a medical-meteorologic prognosis for hemophilia] Folia Haematol Int Mag Klin Morphol Blutforsch. 1990;117:631–4. [PubMed] [Google Scholar]
- 7.Katanic D, Gebauer E. [Bleeding in hemophilia as a function of meteorologic changes and the effect of tissue hormones] Med Pregl. 1990;43:218–20. [PubMed] [Google Scholar]
- 8.Benbassat J. Periodic haemorrhages in haemophilia A. Br Med J. 1971;3:771. doi: 10.1136/bmj.3.5777.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Broderick CR, Herbert RD, Latimer J, et al. Association between physical activity and risk of bleeding in children with hemophilia. JAMA. 2012;308:1452–9. doi: 10.1001/jama.2012.12727. [DOI] [PubMed] [Google Scholar]
- 10.Fischer K, Collins P, Bjorkman S, et al. Trends in bleeding patterns during prophylaxis for severe haemophilia: observations from a series of prospective clinical trials. Haemophilia. 2011;17:433–8. doi: 10.1111/j.1365-2516.2010.02450.x. [DOI] [PubMed] [Google Scholar]
- 11.Valentino LA, Plushch OP, Rusen L, et al. 2011. Kyoto, Japan A multicenter, randomized, open-label study to compare on-demand treatment with two prophylaxis regimens of recombinant coagulation factor IX in subjects with hemophilia B [poster]. Presented at: Biennial Congress of the International Society on Thrombosis and Haemostasis, July 23-28,
- 12.Daut RL, Cleeland CS, Flanery RC. Development of the Wisconsin Brief Pain Questionnaire to assess pain in cancer and other diseases. Pain. 1983;17:197–210. doi: 10.1016/0304-3959(83)90143-4. [DOI] [PubMed] [Google Scholar]
- 13.Jayandharan GR, Srivastava A, Srivastava A. Role of molecular genetics in hemophilia: from diagnosis to therapy. Semin Thromb Hemost. 2012;38:64–78. doi: 10.1055/s-0031-1300953. [DOI] [PubMed] [Google Scholar]
- 14.Collins PW. Personalized prophylaxis. Haemophilia. 2012;18(suppl 4):131–5. doi: 10.1111/j.1365-2516.2012.02838.x. [DOI] [PubMed] [Google Scholar]
- 15.Fischer K. Prophylaxis for adults with haemophilia: one size does not fit all. Blood Transfus. 2012;10:169–73. doi: 10.2450/2012.0174-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Witkop M, Lambing A, Divine G, Kachalsky E, Rushlow D, Dinnen J. A national study of pain in the bleeding disorders community: a description of haemophilia pain. Haemophilia. 2012;18:e115–9. doi: 10.1111/j.1365-2516.2011.02709.x. [DOI] [PubMed] [Google Scholar]
- 17.Choiniere M, Melzack R. Acute and chronic pain in hemophilia. Pain. 1987;31:317–31. doi: 10.1016/0304-3959(87)90161-8. [DOI] [PubMed] [Google Scholar]
- 18.Ceponis A, Glass CS, Von Drygalski A. Rapid, high resolution joint ultrasound reveals frequent discrepancies in the clinical management of painful joint and tissue events in symptomatic adults with hemophilia [abstract] Blood. 2012;120:625. [Google Scholar]