Abstract
Discrepancies exist for some of the modified coagulation factors when assayed with different one-stage clotting and chromogenic substrate assay reagents. The aim of this study was to evaluate the performance of a recombinant factor VIII Fc fusion protein (rFVIIIFc), currently in clinical development for the treatment of severe haemophilia A, in a variety of one-stage clotting and chromogenic substrate assays in clinical haemostasis laboratories. Haemophilic plasma samples spiked with rFVIIIFc or Advate® at 0.05, 0.20 or 0.80 IU mL−1 were tested by 30 laboratories using their routine procedures and plasma standards. Data were evaluated for intra- and inter-laboratory variation, accuracy and possible rFVIIIFc-specific assay discrepancies. For the one-stage assay, mean recovery was 95% to 100% of expected for both Advate® and rFVIIIFc at 0.8 IU mL−1. Intra-laboratory percent coefficient of variance (CV) ranged from 6.3% to 7.8% for Advate®, and 6.0% to 10.3% for rFVIIIFc. Inter-laboratory CV ranged from 10% for Advate® and 16% for rFVIIIFc at 0.8 IU mL−1, to over 30% at 0.05 IU mL−1 for both products. For the chromogenic substrate assay, the average FVIII recovery was 107% ± 5% and 124% ± 8% of label potency across the three concentrations of Advate® and rFVIIIFc, respectively. Plasma rFVIIIFc levels can be monitored by either the one-stage or the chromogenic substrate assay routinely performed in clinical laboratories without the need for a product-specific rFVIIIFc laboratory standard. Accuracy by the one-stage assay was comparable to that of Advate®, while marginally higher results may be observed for rFVIIIFc when using the chromogenic assay.
Keywords: B-domain deleted, chromogenic substrate assay, factor VIII, field study, one-stage clotting assay
Introduction
Infusion of replacement clotting factor VIII (FVIII) is the mainstay of treatment for haemophilia A 1. Currently approved recombinant FVIII (rFVIII) products contain either full-length rFVIII, or a B-domain deleted (BDD) rFVIII. Monitoring FVIII levels is important for the diagnosis of haemophilia A, and for the assessment of FVIII activity post infusion, as well as to ensure adequate FVIII levels for haemostatic coverage during surgery and to determine trough FVIII levels in a prophylactic treatment setting. Assessment of plasma FVIII activity after infusion of these products is typically performed in clinical haemostasis laboratories using existing FVIII activity assays and commercially available assay standards, which are typically calibrated against normal plasma with a FVIII activity assigned against an international reference standard. The most common assay used to test for FVIII activity levels is the one-stage clotting assay, based on the activated partial thromboplastin time (aPTT). The FVIII chromogenic substrate assay is generally considered too expensive and labour intensive for routine use, although it is thought to be more precise than the one-stage assay 2,3.
It is widely acknowledged that FVIII activity discrepancies exist between the one-stage and chromogenic substrate assay for rFVIII products, either in direct measurements of the drug products or in patient samples post infusion 2,4,5. Substantial variability has been observed with the assay of high-purity factor products 2. For full-length products, rFVIII activity has been reported to be 20% lower with the one-stage assay compared with the chromogenic substrate assay; however, this difference can increase to up to 50% for the BDD-rFVIII ReFacto® (Pfizer Inc, New York, NY, USA) 6,7. The increased discrepancy with ReFacto® may be related to the phospholipid composition of the reagents in the one-stage assay 7. The development of a product-specific reference standard was introduced to enable more accurate assessment of ReFacto® activity 4,8–10. The problem appears to be more pronounced with ReFacto, as a field study of 30 clinical haemostasis laboratories with another BDD-rFVIII product in clinical development, N8, did not show the same degree of discrepancy between the two assays as previously observed with ReFacto®; rather, the average chromogenic substrate/one-stage ratio was dependent on the FVIII level and ranged from 0.68 at 3% FVIII to 1.30 at 90% FVIII 3. A small phase 1 study of a recombinant BDD porcine FVIII in development, OBI-1, has demonstrated discordance between assays; of the three patients who received OBI-1, the one-stage assay yielded higher results in two patients (15% and 45%) than the chromogenic substrate assay, and lower results in one patient (35%) 11. Further study will be required to determine if a product-specific standard will be helpful for assay of this porcine FVIII product.
The measurement of FVIII concentrations is further complicated by inter-laboratory variability due to the use of a wide selection of reagents and instruments, particularly with the one-stage clotting assay 4,5. Although recommendations have been made to standardize the assay of rFVIII activity to improve accuracy 12–14, the discordance and variability that still exist can potentially lead to misinterpretation of pharmacokinetic parameters and inaccurate dosing 2. As new factor products are developed for the treatment of haemophilia, it is important to assess the accuracy and precision of the current assays with these products, and to determine whether their activities can be assessed with the routinely used laboratory methods and standards.
Fc fusion, an established technology, has been used for the development of a long-lasting rFVIII product. Recombinant FVIII Fc fusion protein (rFVIIIFc) consists of a BDD-rFVIII molecule that is genetically fused to the Fc domain of human immunoglobulin G1 with no intervening linker sequence 15 (Fig.1). rFVIIIFc is produced in human embryonic kidney cells (HEK293) to provide human glycosylation patterns and high expression levels, with no added human- or animal-derived materials 15.
Figure 1.
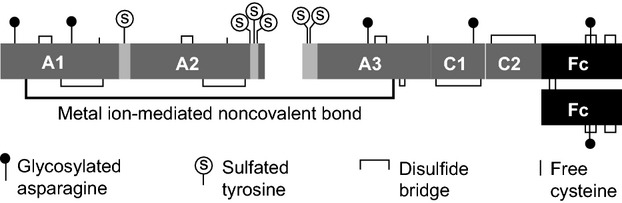
Structure of rFVIIIFc. rFVIIIFc consists of a single B-domain deleted FVIII covalently fused to an Fc dimer with no intervening linker, resulting in a 220-kDa protein 15. Specific activity of rFVIIIFc ranges from approximately 8000 to 10 000 IU mg−1 (1760–2200 IU nmol−1), which is comparable to the specific activity for Advate®, reported at 1120–2800 IU nmol−1 24. rFVIIIFc, recombinant factor VIII Fc fusion protein.
The Fc domain provides protection from degradation through an endogenous pathway, mediated by the neonatal Fc receptor, resulting in prolonged half-life of the clotting factor 16,17. In preclinical studies, rFVIIIFc demonstrated approximate two-fold prolonged half-life and an approximate two-fold prolonged duration of prophylactic efficacy in haemophilia A animal models 15. In a phase 1/2a human clinical study, rFVIIIFc had a 1.5- to 1.7-fold increase in plasma half-life compared with full-length rFVIII (Advate®) 18. The phase 3 pivotal study of rFVIIIFc (A-LONG) in previously treated subjects at least 12 years of age demonstrated safety of rFVIIIFc with effective control and prevention of bleeding in prophylaxis regimens dosed once to twice a week 19. A phase 3 study in previously treated paediatric patients with severe haemophilia A is ongoing (ClinicalTrials.gov identifier NCT01458106).
Previous biochemical characterization has shown that the posttranslational FVIII modifications and in vitro functionality of rFVIIIFc are comparable with those of other rFVIII products, with the exception of human glycosylation patterns in rFVIIIFc. The specific activity of rFVIIIFc is equivalent to that of native FVIII (on a molar basis) and the clotting and chromogenic substrate assays demonstrated similar results 20. The objective of this global comparative field study was to evaluate the performance of rFVIIIFc with a variety of one-stage clotting and chromogenic substrate assay reagents and instruments in clinical haemostasis laboratories. Haemophilic plasma samples spiked with either rFVIIIFc or a comparator were tested by the laboratories using their routine procedures and commercially available plasma or pooled normal plasma calibrated against the World Health Organization (WHO) international reference plasma for FVIII. Advate® was chosen as a comparator because this is the most widely used commercial product.
Materials and methods
Field study kits
Field study kits were prepared and shipped to the participating laboratories (see Acknowledgements). Lyophilized rFVIIIFc was reconstituted in distilled water, and commercial Advate® (obtained from Novis Pharmaceuticals, Miami, FL, USA) was reconstituted with sterile water, each to approximately 200 IU mL−1. Congenital haemophilic plasma (HRF Inc, Raleigh, NC, USA) with no detectable FVIII activity (<0.005 IU mL−1) was spiked with either Advate® or rFVIIIFc at nominal concentrations of 0.05, 0.20 or 0.80 IU mL−1, based on the label potency for Advate® and the one-stage clotting activity rFVIIIFc drug product. Based on the chromogenic label potency for this lot of rFVIIIFc, the three spike levels of rFVIIIFc were 0.054, 0.22 or 0.87 IU mL−1, which are the values used for determination of accuracy in this study. Individual aliquots (1 mL) were prepared and vials were capped with a colour-coded screw cap. Each kit contained 18 vials: three aliquots of each of the three concentrations for both Advate®-spiked and rFVIIIFc-spiked plasma to permit assays to be conducted in triplicate.
Study design
Participating laboratories (n = 30) in seven countries received field study samples. All clinical laboratories were instructed to analyse samples using their in-house standard reagents and routine assay procedure for haemophilia A patient samples. The clinical haemostasis laboratories received the three sets of blinded samples to be tested using the one-stage clotting assay on three separate days. Eleven of these laboratories also performed the chromogenic substrate assay for FVIII in triplicate on additional sets of samples. Each laboratory provided procedural data on type and source of reagents and substrate plasma used, instrument employed, number of dilutions performed in each assay, type of diluent used, source of calibrators, laboratory certification and type of proficiency testing conducted. Laboratories provided raw data and FVIII activities calculated from in-house standard curves, and provided the final results to Biogen Idec for analysis.
Data and analysis
Manual data entries were cross-checked against source documentation for 100% accuracy. Results were analysed for intra- and inter-laboratory variation; accuracy in terms of measured vs. assigned (label) concentration, and measured vs. consensus (laboratory mean) concentration; relative variation and accuracy for rFVIIIFc vs. Advate®; relative variation and accuracy for rFVIIIFc and Advate® vs. type of reagent (e.g. silica-based vs. ellagic acid activator) and instrumentation (e.g. optical vs. mechanical clot detection); and correlation between chromogenic substrate and one-stage assay results for rFVIIIFc and Advate® at each drug level. Statistical evaluations to assess for correlations with particular assay reagents, standards, instrumentation or methodology included Student's t-test and analysis of variance using GraphPad Prism 5 software (GraphPad Software Inc, San Diego, CA, USA).
Outliers
An apparent mix-up between a 0.8 and a 0.2 IU mL−1 Advate sample in one laboratory resulted in outliers for the corresponding one-stage assay results and these two test results were excluded from the analysis. Among the chromogenic substrate assay results, three unrelated, individual test results (two for Advate and one for rFVIIIFc) were considered outliers at greater than three standard deviations from the sample means and were not included in the data analysis.
Results
Laboratory assays
One-stage clotting assay
An array of one-stage assay reagents and critical steps in methodology were employed by the 30 participating laboratories. aPTT activator reagents that were used included ellagic acid (8 laboratories), silica (19 laboratories), kaolin (2 laboratories) and polyphenols (1 laboratory). Congenital FVIII-deficient plasma was used by 10 laboratories and FVIII-depleted plasma by 20 laboratories, of which 14 used a source of plasma that was presumed to contain less than 30% of normal levels of von Willebrand factor (VWF). The number of sample dilutions tested in the assay also varied by laboratory, ranging from 1 to 4 dilutions. Six different commercial sources of reference plasmas were used by 29 laboratories, while one laboratory prepared their own pooled normal plasma from 50 donors and calibrated it against to the World Health Organization's international standard for FVIII.
Chromogenic substrate assay
The chromogenic substrate assay kits utilized in the study were specified by 10 of the 11 participating laboratories and included five kits overall: Biophen FVIII:C (Hyphen Biomed, Neuville-Sur-Oise, France), Factor VIII Chromogenic Assay kit (Siemens Healthcare Diagnostics, Deerfield, IL, USA), Technochrom® FVIII:C (Technoclone GmbH, Vienna, Austria) and Coatest® and Coamatic® (Chromogenix Technologies, Milan, Italy).
One-stage clotting assay
Study samples contained Advate® at concentrations of 0.05, 0.20 and 0.80 IU mL−1, and rFVIIIFc at concentrations of 0.054, 0.22 and 0.87 IU mL−1. The mean FVIII activity (n = 3 independent tests per level) measured by the 30 laboratories was calculated as percentage of expected (nominal) activity in each sample (Table1; Fig.2). At the highest concentration (0.80 or 0.87 IU mL−1), the average spike recovery by the one-stage clotting assay in all 30 clinical laboratories (90 test results per dose level) was 95% to 100% of expected for both Advate® and rFVIIIFc. At the 0.20 IU mL−1 level, the average one-stage assay result was approximately 10% higher than expected for either product. This relative overestimation of FVIII activity increased to approximately 20% at the 0.05 IU mL−1 level.
Table 1.
Comparable mean spike recovery for rFVIIIFc and Advate® by the one-stage clotting assay
| Label activity, IU mL−1 | Mean recovery, % of label (n = 29–30 per level) | Mean intra-laboratory % CV (n = 3 per laboratory) | Inter-laboratory % CV (n = 30) | |
|---|---|---|---|---|
| Advate® | 0.80 | 96.7 | 6.3 | 10 |
| 0.20 | 110.2 | 7.8 | 19 | |
| 0.05 | 118.1 | 7.4 | 34 | |
| rFVIIIFc | 0.87 | 94.6 | 6.0 | 16 |
| 0.22 | 106.0 | 8.7 | 17 | |
| 0.054 | 115.7 | 10.3 | 31 |
Conducted in 30 laboratories.
CV, coefficient of variance; rFVIIIFc, recombinant factor VIII Fc fusion protein.
Figure 2.
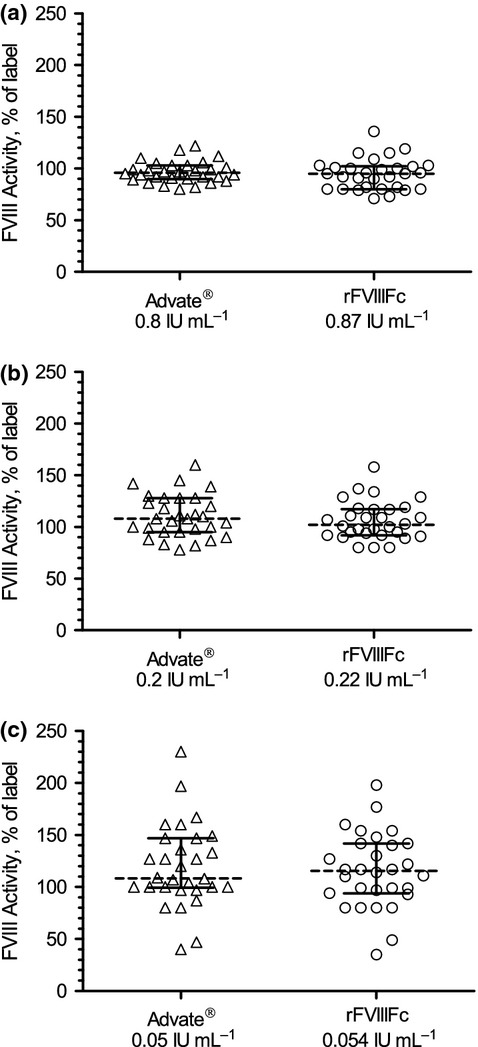
FVIII activity by one-stage clotting assay showing comparable activity at all dose levels for both rFVIIIFc and Advate®. (a) Advate® 0.8 IU mL−1, rFVIIIFc 0.87 IU mL−1; (b) Advate® 0.2 IU mL−1, rFVIIIFc 0.22 IU mL−1; (c) Advate® 0.05 IU mL−1, rFVIIIFc 0.054 IU mL−1. Box graphs represent median result, 25/75 percentile and minimum/maximum as percentage of label activity. Individual test results (n = 89–90) for each of the three FVIII levels were plotted. FVIII, factor VIII.
Intra-laboratory coefficient of variance (CV) for the one-stage assay was typically below 10% (Table1). The % CV for Advate® ranged from 6.3% to 7.8%, and for rFVIIIFc from 6.0% to 10.3%. Inter-laboratory % CV (comparing mean results from each laboratory) ranged from 10% for Advate® to 16% for rFVIIIFc at 0.80 and 0.87 IU mL−1, respectively, and to more than 30% for both products at 0.05 IU mL−1 (Table1). Some laboratories reported over two-fold higher or lower results for both products compared with the expected 0.05 IU mL−1 value (Fig.2). At each dose level, an approximately normal distribution of results was seen for both Advate® and rFVIIIFc (Fig.3). Laboratories that trended high (or low) for Advate® also reported correspondingly higher (or lower) than expected results for rFVIIIFc. This correlation was particularly significant at the 0.05 IU mL−1 (P < 0.05). The relative error in the estimated FVIII activity was not correlated with any obvious procedural or reagent differences in the one-stage clotting assays conducted by the laboratories. The VWF content of the substrate plasma may affect potency measurements of highly purified FVIII products in the one-stage assay 21. However, in our setting using spiked plasma samples, no statistically significant differences were observed with and without VWF in the substrate plasma (data not shown). Three dilutions are recommended when testing clinical samples to verify parallelism and detect potential inhibitors present in a sample 22. In this study using samples prepared in prescreened plasma, the four laboratories that tested only single dilutions or the three laboratories using two dilutions were not statistically different from the average.
Figure 3.
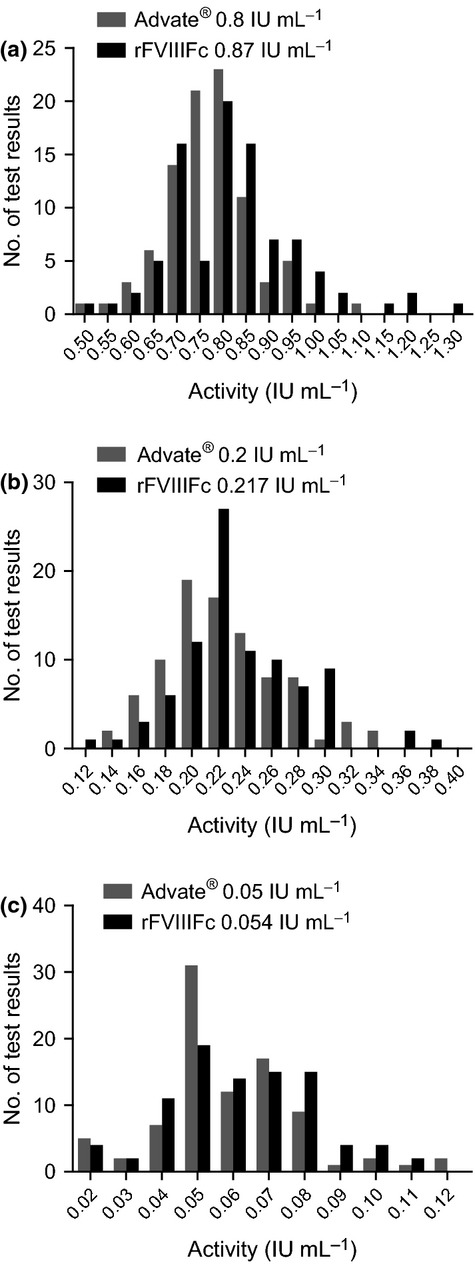
Distribution of one-stage clotting assay results by dose level showing an approximate normal distribution for both rFVIIIFc and Advate®. (a) Advate® 0.8 IU mL−1, rFVIIIFc 0.87 IU mL−1; (b) Advate® 0.2 IU mL−1, rFVIIIFc 0.22 IU mL−1; (c) Advate® 0.05 IU mL−1, rFVIIIFc 0.054 IU mL−1. rFVIIIFc, recombinant factor VIII Fc fusion protein.
Chromogenic substrate assay
A total of 11 laboratories performed the FVIII chromogenic substrate assay. FVIII recovery by this method is shown in Fig.4 and Table2. The mean FVIII recovery was 107% ± 5% of label potency for Advate® across the three concentrations. For rFVIIIFc, the mean FVIII recovery by chromogenic activity was 124% ± 8% across the three concentrations. Intra-laboratory % CV for the chromogenic substrate assay ranged from 4.4% to 12.5% for Advate®, and from 2.9% to 8.3% for rFVIIIFc (Table2). Inter-laboratory CV was comparable for both products (Table2).
Figure 4.
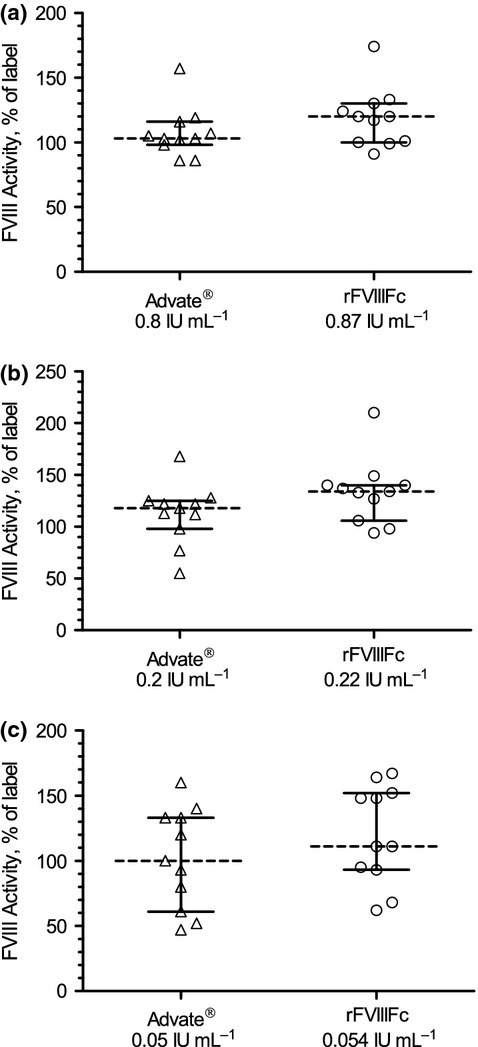
FVIII activity by chromogenic substrate assay showing a comparable distribution at all dose levels for rFVIIIFc and Advate®. (a) Advate® 0.8 IU mL−1, rFVIIIFc 0.87 IU mL−1; (b) Advate® 0.2 IU mL−1, rFVIIIFc 0.22 IU mL−1; (c) Advate® 0.05 IU mL−1, rFVIIIFc 0.054 IU mL−1. Box graphs represent median result, 25/75 percentile and minimum/maximum chromogenic substrate assay result as percentage of nominal (label) activity. Individual test results (n = 33) for each of the three FVIII levels were plotted. rFVIIIFc, recombinant factor VIII Fc fusion protein.
Table 2.
Minor increase in spike recovery for rFVIIIFc compared with Advate® by the chromogenic substrate assay
| Label activity, IU mL−1 | Mean recovery, % of label (n = 10 or 11 per level) | Mean intra-laboratory % CV (n = 3 per laboratory) | Inter-laboratory % CV (n = 11) | |
|---|---|---|---|---|
| Advate® | 0.80 | 108.1 | 4.4 | 18 |
| 0.20 | 112.4 | 11.7 | 26 | |
| 0.05 | 101.8 | 12.5 | 38 | |
| rFVIIIFc | 0.87 | 119.0 | 2.9 | 19 |
| 0.22 | 133.4 | 7.0 | 23 | |
| 0.054 | 120.2 | 8.3 | 31 |
Conducted in 11 laboratories.
CV, coefficient of variance; rFVIIIFc, recombinant factor VIII Fc fusion protein.
Comparison of one-stage and chromogenic substrate assay methods
Both the intra- and inter-laboratory CVs were generally comparable for the chromogenic substrate and the one-stage assays (Tables1 and 2). However, better dose linearity was observed for the chromogenic substrate assay than for the one-stage clotting assay, with no disproportionate overestimation at low FVIII levels by the chromogenic assay. The ratio of one-stage to chromogenic assay results in the 11 laboratories varied from 0.5 to 1.8 for Advate and the mean difference was not statistically significant (Table3). For rFVIIIFc, individual one-stage to chromogenic assay ratios ranged from 0.6 to 2.6 (Table3). In the case of rFVIIIFc, the mean difference in results from the two assays was statistically significant at the 0.87 and 0.22 IU mL−1 levels (P < 0.05).
Table 3.
Ratio of chromogenic substrate to one-stage assay results depend on FVIII level
| Label activity, IU mL−1 | Mean ratio CS (n = 11 labs)/OS (n = 30 labs) | Mean CS/OS ratio at individual labs (n = 11*) | Range of CS/OS ratios at individual labs (n = 11*) | |
|---|---|---|---|---|
| Advate® | 0.80 | 1.12 | 1.12 | 0.81–1.78 |
| 0.20 | 1.02 | 1.07 | 0.48–1.61 | |
| 0.05 | 0.86 | 1.00 | 0.58–1.60 | |
| rFVIIIFc | 0.87 | 1.26 | 1.27 | 0.87–2.18 |
| 0.22 | 1.26 | 1.37 | 0.59–2.63 | |
| 0.054 | 1.04 | 1.24 | 0.65–2.08 |
rFVIIIFc, recombinant factor VIII Fc fusion protein; CS, chromogenic substrate assay; OS, one-stage clotting assay.
Eleven of 30 participating laboratories performed both the one-stage and chromogenic substrate assays.
Discussion
Discrepancies between the one-stage and chromogenic substrate assays for the assessment of FVIII activity in post infusion samples have been widely reported, in addition to considerable inter-laboratory variability due to the array of reagents and instrumentation used 2,4,5. Such discordance can have implications for pharmacokinetic assessments and may result in inaccurate dosing of clotting factors 2. The aim of the current field study was to evaluate the accuracy and precision of the one-stage clotting and chromogenic substrate assays for rFVIIIFc and Advate® using a variety of assay reagents and instruments commonly used in clinical haemostasis laboratories.
Our field study results for Advate® were in good agreement with those reported previously by Viuff et al. 3 from a similar field study conducted in 36 laboratories, which compared N8 to Advate®. For rFVIIIFc, the FVIII activity assigned by the chromogenic potency release assay was used to determine spike recovery in the clinical assays. Despite the array of reagents and procedures used by the participating laboratories, the precision and accuracy were comparable for rFVIIIFc and Advate® using the one-stage clotting assay, the method most commonly used to assess post infusion FVIII activity. As with N8 described by Viuff et al. 3, the absence of the B domain in rFVIIIFc resulted on average in 20% to 30% higher activity measurements by the chromogenic assays compared with the one-stage assay. However, in individual laboratories, the relative activity observed by the two assays varied greatly (Table3). Further studies using clinical samples may be needed to confirm these assay discrepancies and to determine whether the increased chromogenic activity observed for rFVIIIFc has a noticeable clinical impact in the background of the already high inter-laboratory variability.
At low levels of Advate® or rFVIIIFc, most laboratories in the current field study overestimated the FVIII activity in the one-stage clotting assay using the plasma reference standard, with the extent of disparity decreasing as the FVIII level increased. This observation has previously been reported 3,14. Although the cause is unknown, it is unlikely related to the structure of the FVIII molecule, as it was observed with both BDD and full-length FVIII 3,14. Possibly, synthetic differences between the recombinant products evaluated here against the plasma FVIII reference standards may contribute to this discrepancy. In contrast to previous studies 3,4,14, the relative error in estimated FVIII activity in this study did not correlate with any apparent procedural or reagent differences used by the laboratories. Due to the relative overestimation of the one-stage activity at low levels, the ratio of chromogenic substrate to one-stage activity was thus dependent on the FVIII level in samples. In general, overestimation at low levels of rFVIIIFc and Advate® should not be of clinical importance in surgery, where high levels of FVIII activity are maintained, but it may result in overestimated trough levels for patients on prophylaxis. A significant discrepancy between the one-stage and chromogenic substrate assays has not been observed during potency assignment of N8 23 or rFVIIIFc (unpublished data). Assays used for FVIII potency assignments differ from the clinical tests in two important ways. First, the calibrators used are typically in-house product standards with a potency assigned against the current WHO international FVIII concentrate standard (plasma-derived FVIII), and second, the assay range for potency assignment may be comparatively narrow to ensure parallelism against the standard. In support of the current field study, a recent phase 1/2a clinical study indicated that rFVIIIFc can be monitored using standard clinical assays calibrated against FVIII reference plasma 18. Using commercially available reagents and calibration against normal human plasma standards, a good correlation between the one-stage and chromogenic substrate assays was observed in this clinical study; correlation coefficients of 0.94 and 0.95 were obtained for 151 samples following Advate® dosing and for 185 samples following rFVIIIFc dosing respectively 18.
In summary, despite the methodological variables that have been observed in laboratory assays, these data demonstrated that post infusion plasma rFVIIIFc levels can be monitored in patients by either the one-stage or chromogenic substrate assays routinely performed in clinical coagulation laboratories. By the one-stage assay, the accuracy is comparable to Advate®, while marginally higher results may be seen by the chromogenic assay, and neither assay requires a product-specific rFVIIIFc laboratory standard.
Acknowledgments
Editorial and writing support was provided by Samantha Taylor, PhD, of UBC-Envision Group and funded by Biogen Idec. The following laboratories participated in this study: Addenbrooke's Hospital, Cambridge, UK; Basingstoke & North Hampshire Hospital, Basingstoke, UK; Beth Israel Deaconess Medical Center, Boston MA, USA; Blood Center of Wisconsin, Milwaukee, WI, USA; Boston Medical Center, Boston MA, USA; Children's Hospital of Michigan, Detroit MI, USA; CHU Sainte-Justine, Montreal, Canada; Cleveland Clinic, Cleveland OH, USA; Duke University Medical Center, Durham, NC, USA; Esoterix Colorado Coagulation, Englewood, CO, USA; Hamilton Health Sciences Laboratory Reference Centre, Ontario, Canada; Hemocentro de Campinas, Campinas, Brazil; Hemostasis Reference Laboratory, Hamilton, Ontario, Canada; Hospital of the University of Pennsylvania, Philadelphia, PA, USA; ITxM Diagnostics, Pittsburgh, PA, USA; Louisiana Coagulation Laboratory, Covington, LA, USA; Massachusetts General Hospital, Boston MA, USA; Medical University of Vienna, Austria; Oxford University Hospital, Oxford, UK; Puget Sound Blood Center, Seattle WA, USA; Queen's University, Kingston, Canada; Quest Diagnostics Nichols Institute, San Juan Capistrano, CA, USA; Tufts Medical Center, Boston, MA, USA; University Medical Center, Utrecht, Netherlands; University Medical Centre St Radboud, Nijmegen, Netherlands; University of Colorado Health Sciences Center, Denver CO, USA; University of Texas Southwestern Medical Center, Dallas TX, USA; Vanderbilt University Medical Center, Nashville TN, USA; Westmead Hospital, Westmead, Australia.
Author contributions
JMS, GDK and BAK designed the research study, NM, BMV and SB provided administrative study support and quality-check functions and JMS, YB, BAK and GFP analysed the data. All authors reviewed the results and contributed to writing the paper.
Disclosures
This research was funded by Biogen Idec. JMS, NM, SB, YB, GDK, and GFP are employees of and hold equity interest in Biogen Idec. BMV was an employee of Biogen Idec at the time of this research. BAK is an employee of Puget Sound Blood Center and has received research funding from Biogen Idec.
References
- 1.Coppola A, Di CM, Di MM, et al. Treatment of hemophilia: a review of current advances and ongoing issues. J Blood Med. 2010;1:183–95. doi: 10.2147/JBM.S6885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lusher JM, Hillman-Wiseman C, Hurst D. In vivo recovery with products of very high purity–assay discrepancies. Haemophilia. 1998;4:641–5. doi: 10.1046/j.1365-2516.1998.440641.x. [DOI] [PubMed] [Google Scholar]
- 3.Viuff D, Barrowcliffe T, Saugstrup T, Ezban M, Lillicrap D. International comparative field study of N8 evaluating factor VIII assay performance. Haemophilia. 2011;17:695–702. doi: 10.1111/j.1365-2516.2010.02481.x. [DOI] [PubMed] [Google Scholar]
- 4.Ingerslev J, Jankowski MA, Weston SB, Charles LA. Collaborative field study on the utility of a BDD factor VIII concentrate standard in the estimation of BDDr Factor VIII:C activity in hemophilic plasma using one-stage clotting assays. J Thromb Haemost. 2004;2:623–8. doi: 10.1111/j.1538-7836.2004.00657.x. [DOI] [PubMed] [Google Scholar]
- 5.Barrowcliffe TW, Raut S, Sands D, Hubbard AR. Coagulation and chromogenic assays of factor VIII activity: general aspects, standardization, and recommendations. Semin Thromb Hemost. 2002;28:247–56. doi: 10.1055/s-2002-32658. [DOI] [PubMed] [Google Scholar]
- 6.Hubbard AR, Weller LJ, Bevan SA. A survey of one-stage and chromogenic potencies in therapeutic factor VIII concentrates. Br J Haematol. 2002;117:247–8. doi: 10.1046/j.1365-2141.2002.3406_1.x. [DOI] [PubMed] [Google Scholar]
- 7.Mikaelsson M, Oswaldsson U, Sandberg H. Influence of phospholipids on the assessment of factor VIII activity. Haemophilia. 1998;4:646–50. doi: 10.1046/j.1365-2516.1998.440646.x. [DOI] [PubMed] [Google Scholar]
- 8.Morfini M, Cinotti S, Bellatreccia A, Paladino E, Gringeri A, Mannucci PM. A multicenter pharmacokinetic study of the B-domain deleted recombinant factor VIII concentrate using different assays and standards. J Thromb Haemost. 2003;1:2283–9. doi: 10.1046/j.1538-7836.2003.00481.x. [DOI] [PubMed] [Google Scholar]
- 9.Pouplard C, Caron C, Aillaud MF, et al. The use of the new ReFacto AF Laboratory Standard allows reliable measurement of FVIII:C levels in ReFacto AF mock plasma samples by a one-stage clotting assay. Haemophilia. 2011;17:e958–62. doi: 10.1111/j.1365-2516.2011.02523.x. [DOI] [PubMed] [Google Scholar]
- 10.Santoro C, Iorio A, Ferrante F, et al. Performance of recalibrated ReFacto laboratory standard in the measurement of FVIII plasma concentration via the chromogenic and one-stage assays after infusion of recalibrated ReFacto (B-domain deleted recombinant factor VIII) Haemophilia. 2009;15:779–87. doi: 10.1111/j.1365-2516.2009.01983.x. [DOI] [PubMed] [Google Scholar]
- 11.Kempton CL, Abshire TC, Deveras RA, et al. Pharmacokinetics and safety of OBI-1, a recombinant B domain-deleted porcine factor VIII, in subjects with haemophilia A. Haemophilia. 2012;18:798–804. doi: 10.1111/j.1365-2516.2012.02789.x. [DOI] [PubMed] [Google Scholar]
- 12.Barrowcliffe TW. Factor VIII and factor IX Sub-Committee. Recommendations for the assay of high-purity factor VIII concentrates. Thromb Haemost. 1993;70:876–7. [PubMed] [Google Scholar]
- 13.Barrowcliffe TW, Hubbard AR, Kitchen S. Standards and monitoring treatment. Haemophilia. 2012;18(Suppl. 4):61–5. doi: 10.1111/j.1365-2516.2012.02831.x. [DOI] [PubMed] [Google Scholar]
- 14.Cinotti S, Paladino E, Morfini M. Accuracy of FVIII: C assay by one-stage method can be improved using hemophilic plasma as diluent. J Thromb Haemost. 2006;4:828–33. doi: 10.1111/j.1538-7836.2006.01880.x. [DOI] [PubMed] [Google Scholar]
- 15.Dumont JA, Liu T, Low SC, et al. Prolonged activity of a recombinant factor VIII-Fc fusion protein in hemophilia A mice and dogs. Blood. 2012;119:3024–30. doi: 10.1182/blood-2011-08-367813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghetie V, Ward ES. Multiple roles for the major histocompatibility complex class I- related receptor FcRn. Annu Rev Immunol. 2000;18:739–66. doi: 10.1146/annurev.immunol.18.1.739. [DOI] [PubMed] [Google Scholar]
- 17.Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol. 2007;7:715–25. doi: 10.1038/nri2155. [DOI] [PubMed] [Google Scholar]
- 18.Powell JS, Josephson NC, Quon D, et al. Safety and prolonged activity of recombinant factor VIII Fc fusion protein in hemophilia A patients. Blood. 2012;119:3031–7. doi: 10.1182/blood-2011-09-382846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahlangu J, Powell J, Ragni M, et al. Phase 3 Clinical Study of Recombinant Fc Fusion Factor FVIII (rFVIIIFc) Demonstrated Safety, Efficacy, and Improved Pharmacokinetics (A-LONG) Haemophilia. 2013;19:PO104. [Google Scholar]
- 20.Peters RT, Toby G, Lu Q, et al. Biochemical and functional characterization of a recombinant monomeric Factor VIII-Fc fusion protein. J Thromb Haemost. 2013;11:132–41. doi: 10.1111/jth.12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dawson NJ, Kemball-Cook G, Barrowcliffe TW. Assay discrepancies with highly purified factor VIII concentrates. Haemostasis. 1989;19:131–7. doi: 10.1159/000215905. [DOI] [PubMed] [Google Scholar]
- 22.Verbruggen B, Meijer P, Novakova I, Van HW. Diagnosis of factor VIII deficiency. Haemophilia. 2008;14(Suppl. 3):76–82. doi: 10.1111/j.1365-2516.2008.01715.x. [DOI] [PubMed] [Google Scholar]
- 23.Christiansen ML, Balling KW, Persson E, et al. Functional characteristics of N8, a new recombinant FVIII. Haemophilia. 2010;16:878–87. doi: 10.1111/j.1365-2516.2010.02333.x. [DOI] [PubMed] [Google Scholar]
- 24.Advate Prescribing Information. Westlake Village, CA: Baxter Healthcare Corporation; 2012. [Google Scholar]


