Abstract
Multitasking (MT) constitutes engaging in two or more cognitive activities at the same time. MT‐training improves performance on untrained MT tasks and alters the functional activity of the brain during MT. However, the effects of MT‐training on neural mechanisms beyond MT‐related functions are not known. We investigated the effects of 4 weeks of MT‐training on regional gray matter volume (rGMV) and functional connectivity during rest (resting‐FC) in young human adults. MT‐training was associated with increased rGMV in three prefrontal cortical regions (left lateral rostral prefrontal cortex (PFC), dorsolateral PFC (DLPFC), and left inferior frontal junction), the left posterior parietal cortex, and the left temporal and lateral occipital areas as well as decreased resting‐FC between the right DLPFC and an anatomical cluster around the ventral anterior cingulate cortex (ACC). Our findings suggest that participation in MT‐training is as a whole associated with task‐irrelevant plasticity (i.e., neural changes are not limited to certain specific task conditions) in regions and the network that are assumed to play roles in MT as well as diverse higher‐order cognitive functions. We could not dissociate the effects of each task component and the diverse cognitive processes involved in MT because of the nature of the study, and these remain to be investigated. Hum Brain Mapp 35:3646–3660, 2014. © 2013 The Authors. Human Brain Mapping Published by Wiley Periodicals, Inc.
Keywords: dual task, multitasking, cognitive training, resting state, functional connectivity, gray matter, structure, plasticity
INTRODUCTION
Multitasking (MT) constitutes simultaneous engagement in two or more cognitive activities. The ability to perform multiple tasks at the same time is an important function of the central executive system [D'Esposito et al., 1995]. This ability is becoming increasingly important in modern everyday life [Erickson et al., 2007]. However, there appears to be an inherent limitation to an individual's ability to juggle through the increasingly large number of events in their daily lives [Erickson et al., 2007], and this capability is known to impair with age [Verhaeghen et al., 2003]. Damage to the prefrontal cortex (PFC) is associated with impaired MT performance [McDowell et al., 1997]. Neuroscientific findings showed that, like other externally directed attention‐demanding tasks such as working memory tasks, networks mainly consisting of the lateral frontal cortex and parts of the inferior and superior parietal lobes are activated during MT [Erickson et al., 2007; Fox et al., 2005]. However, areas of the lateral prefrontal cortex such as the dorsolateral PFC (DLPFC) and ventrolateral PFC (VLPFC) also play an important role in MT performance [Tachibana et al., 2012]. DLPFC is suggested to be involved in central executive processes. Although DLPFC may have multiple functions and executive processes have diverse processes, among them particularly relevant to MT was DLPFC's involvement in scheduling processes in complex tasks (“task management”) [Smith and Jonides, 1999]. On the other hand, an area of VLPFC, the inferior frontal junction (IFJ; in the vicinity of the junction of the inferior frontal sulcus and the inferior precentral sulcus) appears to deal with situations where multiple tasks interfere with each other [Herath et al., 2001].
Previous studies investigated the effects of MT‐training on cognitive functions and neural systems, and this training was shown to lead to improvements in untrained MT tasks [e.g., Bherer et al., 2008] as well as functional activity changes during MT in regions such as DLPFC [Dux et al., 2009; Erickson et al., 2007]. In a study by Erikson et al.'s [2007] study, activity was decreased in most of the areas involved in task performance, but increased in DLPFC. These changes suggest that adaptation to MT leads to increased efficiency in task execution as well as learning to rely on cognitive processes involving DLPFC, such as those described above [Erickson et al., 2007]. On the other hand, Dux et al.'s [2009] study showed that decrease in interference during caused by MT training is achieved by increasing the speed of information processing for all the subtasks in MT in IFJ.
However, to our knowledge, no previous studies have investigated the effect of MT‐training on neural mechanisms such as regional gray matter volume (rGMV) and functional connectivity at rest (resting‐FC). Therefore, we determined these effects in the present study. We hypothesized that MT‐training leads to changes in brain structure and MT‐irrelevant brain functions that involve DLPFC, which plays an important role in MT. Using morphological analyses, we could determine whether and to which locations the effects of MT‐training extend beyond task‐specific functional activation. By analyzing rest‐related neural mechanisms, we (a) determined how brain regions interact with other regions, both those to which they are structurally connected and those to which they are not, and (b) investigated the state of brain regions during cognitive processes involved during rest. However, these task‐irrelevant imaging paradigms cannot dissociate the neural processes involved in each task and each cognitive component during MT, and since it is practically difficult to set up diverse control intervention conditions for dissociating these effects, we focused on the effects of MT training as a whole on these neural measures in this study. Given that the ability to perform multiple tasks at the same time is an important function of the central executive system [D'Esposito et al., 1995] and that improvements in activities such as attention control, interference resolution, and task switching may underlie MT‐training‐related improvements in MT performance [Bherer et al., 2008], MT‐training may affect cognitive functions other than simply the ability to perform multiple tasks but such effects remain unknown. We therefore investigated the effects of MT‐training on diverse cognitive functions in an exploratory manner.
Using various psychological measures, such as non‐trained MT tasks, rGMV analysis using voxel‐based morphometry (VBM), and resting‐FC analyses, we investigated the effects of MT training on these variables. Subjects in the MT training group underwent a 4‐week intensive adaptive MT training program, whereas subjects in the control group received no interventions during the same period.
METHODS
Subjects
Eighty‐one healthy, right‐handed university or postgraduate students (59 men and 22 women; mean age, 21.2 years; SD, 1.9 years; range 18–26) participated in this study. Of these 81 subjects, 41 were assigned to a working memory (WM) training group for another study [Takeuchi et al., 2013], and the remaining 40 subjects were enrolled in the present study. The relatively larger number (41) of subjects assigned to the WM training group were involved in studies that are irrelevant to this study and one purpose of those studies that involves intra‐group analyses of polymorphism. All subjects had normal vision. They were recruited using advertisements on bulletin boards at Tohoku University or via email introducing the study. These advertisements and emails specified the unacceptable conditions in individuals with regard to participation in the study such as handedness, the existence of metal in and around the body, claustrophobia, the use of certain drugs, a history of certain psychiatric and neurological diseases, and previous participation in related experiments. We provided questionnaires to all potential experimental subjects for the assessment of psychiatric illnesses and recent drug use history. In the questionnaire, subjects were asked to provide a list of all drugs that they had recently used and diseases they had or have. None had a history of neurological or psychiatric illness. These assessments, made during recruitment and through questions after recruitment, were based on voluntary self‐report. Handedness was evaluated using the Edinburgh Handedness Inventory [Oldfield, 1971]. This study was conducted together with another intervention study that involved WM training [Takeuchi et al., 2013]. Both studies shared the subjects of the control group, psychological and neuroimaging outcome measures, training period, and training frequency. Groups of participants completed the pre‐ and post‐training MRI studies and psychological experiments during different predetermined experimental periods (e.g., one group participated in a 4‐week project starting from November 4th, another group participated in a 4‐week project starting from November 10th etc.). Participants were randomly assigned to an intervention group (WM or MT training group) or a passive control group. For the flowchart of this study, see Figure 1. The participants in the same intervention period were all assigned to the same training protocol group (WM or MT training group). This means that participants from the 4‐week period from November 4th (for example) were assigned to the WM training group if they were assigned to the intervention group, but participants from the 4‐week period from November 10th (for example) were assigned to the MT training group if they were assigned to the intervention group. The participants chose their period of participation, and they were not notified about the existence of two intervention groups before the experiment. The number of subjects in the MT training and control groups was similar, and MRI and psychological tests were performed in these groups as well as in the WM training group. The MT‐training group consisted of 20 participants (17 men and 3 women; mean age, 21.6 years; SD, 2.1 years; range 18–25). The control group consisted of 20 subjects (15 men and 5 women; mean age, 21.4 years; SD, 2.2 years; range 18–26). The MT‐training and control groups did not differ significantly (P > 0.1, two‐tailed t tests) in basic background characteristics such as age, sex, and score on Raven's Advanced Progressive Matrix [Raven, 1998]. The subjects who misunderstood the rules for the psychological measures, tended to fall asleep during the psychological tests, or could not participate in the psychological experiments as planned were excluded from the relevant analyses (which means, for example, if a subject misunderstood the rule of one task, then that subject was excluded from the analysis involving that task). One subject in the MT‐training group could not undergo MRI and did not complete the procedures related to the MRI experiments. The sex mismatch of the sample (male:female, >2:1) is likely to simply reflect the sex mismatch of the parent population (students of our university and maybe students who are willing to participate in this type of experiment). Given the sample size, it is difficult to quantify sex‐related differences in training effects in the present study, and these effects may or may not be affected by individual physiological and cognitive differences as well as sex differences. We believe that many reports of sex‐related differences fail to be replicated [for the case of language, see Wallentin, 2009] and that usually there are more individual differences than sex‐related differences [Baron‐Cohen, 2003]. Although there have been reports of sex‐related differences in dual tasking [Goddard et al., 1998], we are not aware of any robust sex‐related differences in the effects of dual task (MT) training. If there were any sex‐related differences in MT training effects, the results of this study were mix of the effects of two sexes and the results of this study must have been prone to represent the effects of males more strongly.
Figure 1.
Flow of participants through the study. The recruited subjects were assigned to groups in a nonarbitrary manner as described in Methods. Other than the subjects excluded in the figure, there were several instances in which subjects were excluded from analyses involving certain measures for various reasons specifically related to those measures (such as misunderstanding the rules of the measures).
In accordance with the Declaration of Helsinki (1991), written informed consent was obtained from each subject. This study was approved by the Ethics Committee of Tohoku University.
Procedure
The MT‐training program consisted of in‐house developed Borland C++ programs consisting of six computerized tasks. The subjects undertook ∼4 weeks (27 days) of training, which lasted 20–60 min day−1 in most cases (for the average number of sessions completed, see the Training data subsection of the Results). However, the total time depended on the level and time between trials. The length of training varied because we did not control for the amount of training by time in order to avoid a situation where subjects completed a task without actually performing the task. Instead, task completion was based on the number of trials each subject completed (in other words, we controlled the length of the task based on how much subjects did the tasks). Thus, subjects could not finish a task without performing it no matter how much time had passed. Further, subjects took more time to complete a task when they did not feel well or when they were not motivated to complete the task. The log of the performance and the time taken was recorded for each trial. Therefore, we were able to determine the duration and the time at which each subject completed training. The subjects used the program provided to them on their personal computers. They were recommended to undertake MT‐training daily; two training sessions for a week were conducted in the laboratory. When they could not perform the tasks because of computer problems or illness, the subjects were allowed to miss the MT‐training session. They were also allowed to undertake MT‐training more than once a day. When the subjects attended the laboratory sessions, they stayed in the laboratory until the training session was completed and went home immediately after the session for the day ended. In the laboratory, they just completed the training sessions in the laboratory by themselves and were free to decide when they would next attend a laboratory session. However, they were instructed on how to perform the training tasks on the first training day, and this lasted for less than 20 min. The subjects were asked to come to the laboratory two times a week to ensure that they were completing the training and could perform the tasks properly at the level recorded in the log.
Performances in each block (a period when stimuli were presented sequentially) were logged in a computer file, and occasionally, the subjects were asked to mail the logs for compliance verification. MRI scanning and psychological tests were performed immediately before and after the 4‐week training period. In other words, pre‐training MRI scans and psychological tests were performed on day 1, training was provided from day 2 to day 28, and post‐training MRI scans and psychological tests were performed on day 29. The experimenter provided training feedback to the subjects as necessary.
Training Tasks
Six MT‐training tasks were presented during each training session. In all of these tasks, a certain type of stimulus was presented successively and randomly, and in each trial, the subjects had to push multiple buttons on a keyboard that corresponded to the stimuli presented before the next trial (the next stimulus) was initiated. In all tasks, a block (a period when stimuli were presented sequentially) consisted of 24 trials. Three of the tasks involved auditory tasks alone and were like dichotic (or multicotic) listening tasks, but dichotic listening and divided attention are considered multitasks [Green and Vaid, 1986; Hiscock, 1986]. The six MT‐training tasks were as follows: [A] An auditory–auditory dual task in which auditory stimuli are randomly presented in a dichotic manner. Numbers (1, 2, 3, or 4) are presented in Japanese to the left ears of the subjects. Only the first syllable is presented (i, ni, sa, or yo instead of i‐chi, ni, sa‐n, or yo‐n) such that the entire stimulus is presented despite the fast presentation rate. The subjects have to push “S” on the keyboard when they register stimulus 1, “D” for 2, “F” for 3, and “G” for 4. Japanese letters (a, i, u, or e) are presented to their right ears, and they have to push “H” on the keyboard when they register stimulus a, “J” for i, “K” for u, and “L” for e. [B] An auditory–visual dual task in which in each trial, one auditory stimulus (1, 2, 3, or 4) is presented in English to both ears as well as one visual stimulus (a mark in one of four locations in a vertical row). The subjects have to push “S” on the keyboard when they register stimulus 1, “D” for 2, “F” for 3, and “G” for 4. For the visual stimuli, the subjects have to push “H” for the leftmost stimulus, “J” for the stimulus immediately after the first, then “K” and “L” for the last two stimuli. For these first two tasks, performance is scored using the lower score (number of correct responses) of the two dual tasks. [C] An auditory–auditory–auditory triple task in which in each trial, three auditory stimuli are presented. English numbers (1, 2, 3, or 4) are presented to the left ears of the subjects, and they have to push the keys corresponding to these numbers outlined for the task [A]. English letters (a, b, c, or d) are presented to the right ears of the subjects, and they have to push “H” when they register stimulus a, “J” for b, “K” for c, and “L” for d. Finally, an English number (9 or 10) is presented to both ears; the subjects have to push “V” if they register “9” but not if they register “10.” [D] An auditory–auditory–visual arithmetic triple task in which in each trial, two auditory stimuli are presented in a dichotic manner and one equation is presented onscreen. The tasks for the left and right ear stimuli are the same as those used in the task [C]. Finally, the onscreen equation is presented as one digit plus one digit plus an answer. In ∼50% of the trials, the presented equations are correct but they are incorrect (the correct answer plus or minus one) in the remainder. For these stimuli, the subjects have to push “V” if the equation is correct and not if it is incorrect. [E] An auditory–auditory–auditory–auditory quad task in which in each trial, four auditory stimuli are presented (one sound to the left ear, one to the right ear, and one to both ears but the volume of sound presented to the left ear is twice as that presented to the right ear and vice versa). The tasks for the sound registered in the left and right ears alone are the same as those used in the task [C]. The task for the combined stimulus that is louder in the left ear is the same as that in the task [C]. Finally, the task for the combined stimulus that is louder in the right ear involves English letters (x or y). The subjects have to push “N,” if they hear “x” and not if they hear “y.” [F] An auditory–auditory–visual arithmetic–visuospatial quad task in which in each trial, two auditory stimuli are presented in a dichotic manner and one equation is presented onscreen. In approximately half of the trials, a mark is presented in either one of four locations in the corner of the screen, and in the other half of the trials, the mark is not presented in any of the four locations. The tasks for the left and right ear stimuli and for the visually presented equations are the same as those used in the task [D]. When the mark described above is presented onscreen, the subjects have to push “N.” For tasks C, D, E, and F, performance is scored as the number of trials in which the subjects respond correctly to all of the stimuli. A task ended after the subjects had performed the task six times (completed six blocks). In all six training tasks, difficulties (stimulus presentation rates) were modulated based on the subjects' performances in each block. The stimulus presentation rate was modulated by multiplying by 0.99 or (100/99), i.e., the subjects' performance in each task was expressed as X in a certain block and the stimulus presentation rate was A in that block, when X was 0–6, in the next block, the stimulus presentation rate became A(0.99)4, when X was 7–9, in the next block, the stimulus presentation rate became A(0.99)10−X, when X was 10–12, in the next block, the stimulus presentation rate did not change, and when X was 13–24, then in the next block, the stimulus presentation rate became A(100/99)X−12. The subjects were then allowed to advance to the next task. Training was completed for the day once the subjects had completed all tasks. As the task level was modulated as described above, the difficulty of each training task at certain presentation speed can be expressed as (initial presentation rate) (100/99)Y and here Y is the task level of certain presentation speed). We calculated how each subjects in the MT group improved during training period by summing the (highest level at which subjects achieved performance of X > 12 in the last 3 training sessions—highest level at which subjects achieved performance of X > 12 in the last 3 training sessions) of each task. This value was used to investigate the association between neural changes following MT‐training and performance improvement of MT‐training.
Multiple (and occasionally heterogeneous) training programs [e.g., Hogarty et al., 2004; Klingberg et al., 2002] are commonly observed in this type of study of cognitive training. This procedure is, as a general rule, supposed to strengthen transfer effects [Goldstone, 1998; Sweller et al., 1998], but it may also make it difficult to observe the effects of each training program, which is the trade‐off we choose.
Psychological Outcome Measures
A battery of neuropsychological tests and questionnaires was administered before and after MT‐training. As described in the Introduction, we investigated the effects of MT training on diverse cognitive functions in an exploratory manner, and we did not necessarily prepare a rationale for the inclusion of all of these measures, although all of the cognitive measures did assess cognitive functions of important domains. If there are no beneficial effects of MT training, that is one answer to this purpose and those findings contribute to the science of cognitive training. Generally, the following battery was used in our previous studies [Takeuchi et al., 2011b, 2013]. This battery included the following tests. [A] Raven's Advanced Progressive Matrices [Raven, 1998], a non‐verbal reasoning task. For the details of how this test was performed see our previous work [Takeuchi et al., 2010b]. [B] Bochumer Matrizen‐Test [Hossiep et al., 1999] in which the task is performed groupwise and as described in Jaeggi et al. [2008]. [C] A (computerized) digit span task, a verbal WM task. For the detail of this task, see Takeuchi et al. [2011c]. [D] A (computerized) visuospatial WM task. For the detail of this task, see Takeuchi et al. [2013]. [E] Tanaka B‐type intelligence test [Tanaka et al., 2003], a non‐verbal mass intelligence test used for third‐year junior high school and older examinees, does not include story problems but uses figures, single numbers, and letters as stimuli. In all subtests, the subjects have to solve as many problems as possible before a certain time (a few minutes). For the details of these subtests, see Takeuchi et al. [2013]. [F] The Stroop task (Hakoda's version) [Hakoda and Sasaki, 1990; Takeuchi et al., 2012c], which measures response inhibition and impulsivity. Hakoda's version is a matching‐type Stroop task requiring subjects to check whether their chosen answers are correct, unlike the traditional oral naming Stroop task. The test consists of two control tasks (Word‐Color task and Color‐Word task), a Stroop task, and a reverse‐Stroop task. In this study, we used the Word‐Color and Color‐Word tasks as measures of simple PS and the Stroop and reverse‐Stroop tasks as measures of inhibition. In the Word‐Color task, a color name (e.g., “red”) is presented in the leftmost of six columns. The other five columns are painted with five colors, and subjects have to check the column whose color corresponds to the color name in the leftmost column. In the Color‐Word task, the leftmost of six columns is painted with a color and the five other columns contain color names. The subjects have to check the column with the name corresponding to the color painted in the leftmost column. In the reverse Stroop task, in the leftmost of six columns, a color name is printed in another color (e.g., “red” is printed in blue letters) and the other five columns are painted in five different colors. The subjects have to check the column whose color corresponds to the color name in the leftmost column. In the Stroop task, in the leftmost of six columns, a color name is printed in another color (e.g., “red” is printed in blue letters) and the other five columns contain color names. The subjects have to check the column with the name of the color in which the word in the leftmost column is printed. In each task, the subjects have to complete as many of the exercises as possible in 1 min. [G] Arithmetic tasks, similar to the ones constructed by Grabner et al. [2007], measured multiplication performance consisting of two forms of one‐digit times one‐digit multiplication problems (a simple arithmetic task with numbers between 2 and 9) and two forms of two‐digit times two‐digit multiplication problems (a complex arithmetic task with numbers between 11 and 19). The two forms of each task are the same, but the numbers used in the problems are ordered differently. Each form of the simple and complex arithmetic tasks have to be completed in 30 and 60 s, respectively. [H] Kyodai SX test's subtests for numerical factors. This task is considered to measure the ability for complex arithmetic reasoning ability. For the detail of this task, see Takeuchi et al. [2013]. [I] The SA creativity test [Society_For_Creative_Minds, 1969], which measures creativity through divergent thinking, involves three types of tasks (generate unique ways of using typical objects, imagine desirable functions for ordinary objects, and imagine the consequences of unimaginable things happening). The SA test scores the four 4 dimensions of the creative process (fluency, originality, elaboration, and flexibility) [Takeuchi et al., 2010b]. Here the sum of the graded scores of the four dimensions was used for analysis. [J] The shortened Japanese version [Yokoyama, 2005] of the Profile of Mood States (POMS) [McNair et al., 1992] measures participants' moods. In our study, it was used to measure each participant's mood on the day of the experiment [Takeuchi et al., 2011a] and in the preceding week. The POMS fatigue subscale score on the day of the experiment was also analyzed to determine whether MT training induced fatigue on the day of the experiment.
Several questionnaires designed to assess the traits or states of the subjects were collected but are not described in this study. A tester blinded to the groups performed all neuropsychological assessments.
Statistical Analysis of Group‐Level Behavioral Data
Behavioral data were analyzed using SPSS 16.0 (SPSS, Chicago, IL). Because the superiority (or beneficial effects) of training was our primary interest, in our behavioral analysis, test–retest changes in the MT‐training group were compared to those in the control group using one‐tailed one‐way ANCOVAs with the differences between pre‐ and post‐test measures as dependent variables and the pre‐test scores as independent variables (P < 0.05). In ANCOVAs performed in this type of study, practice effects in cognitive measurements are controlled by comparing changes in the group of interest with changes in the control group. We employed ANCOVAs instead of repeated measure ANOVAs to control the effects of pre‐test scores. Statistical experts strongly recommend to use ANCOVA instead of repeated measure ANOVA in this type of study design [Dimitrov and Rumrill, 2003]. With randomized designs, the purpose of ANCOVA is to reduce error variance, whereas with non‐randomized designs (or with analyses involving substantial pre‐existing group differences), ANCOVA is used to adjust the post‐test means for pretest differences among groups [Dimitrov and Rumrill, 2003]. One might recommend that post‐test scores be used instead of the differences between pre‐ and post‐test measures. However, in fact, when the pretest scores are included as covariates, the two analyses return the same statistical value. Unlike in the previous study involving WM training [Takeuchi et al., 2011d], one‐tailed ANCOVA was used to measure creativity. This is because while creativity and WM have certain opposing characteristics [for the detailed discussion about this matter, see Takeuchi et al., 2011c], creativity has been suggested to be associated with a wider attention span [Mendelsohn, 1976], and thus, MT‐training was hypothesized to lead to enhanced creativity. Alternatively, while MT is an essential component of WM [Baddeley et al., 1991], increased WM capacity is associated with selective attention [Engle et al., 1999a]. Thus, two‐tailed ANCOVAs were applied to the tests of WM. Furthermore, we applied one‐tailed ANCOVA to test whether MT training increased the POMS fatigue subscale score because we were interested in determining whether MT training induced fatigue.
Image Acquisition and Analysis
MRI data acquisition was conducted using a 3‐T Philips Intera Achieva scanner. Using a MPRAGE sequence, high‐resolution T1‐weighted structural images (240 × 240 matrix, TR = 6.5 ms, TE = 3 ms, FOV = 24 cm, 162 slices, 1.0‐mm slice thickness) were obtained. For the resting state functional MRI (fMRI), 34 transaxial gradient‐echo images (64 × 64 matrix, TR = 2,000 ms, TE = 30 ms, flip angle = 70°, FOV = 24 cm, 3.75‐mm slice thickness) covering the entire brain were acquired using an echo‐planar sequence. For this scan, 160 functional volumes were obtained while the subjects were at rest. During resting state scanning, the subjects had to close their eyes but not move, sleep, or think about anything. Instructions similar to ours (eyes closed, not to think of anything in particular) have been used in representative studies of resting FC conducted by a number of other laboratories [Damoiseaux et al., 2006; Greicius et al., 2003]. The heterogeneity in terms of the presence of low‐frequency fluctuations in the brain and networks of resting FC are very similar during the eyes‐closed condition compared with the eyes‐open condition [Fransson, 2005]. We attempted to ensure that the subjects did not sleep during scans by recommending them to sleep before the MRI session and requesting that them to not sleep during the resting fMRI scan.
The resting‐state scanning was performed at the end of the MRI session. Furthermore, three images with no diffusion weighting (b value = 0 s mm−2) (b = 0 images) were obtained from 37 subjects and single b = 0 image was obtained from two subjects in the control group, using a spin‐echo EPI sequence (TR = 10,293 ms, TE = 55 ms, FOV =22.4 cm, 2 × 2 × 2 mm3 voxels, 60 slices). The mean image from the three b = 0 images (for the 37 subjects) or the single b = 0 image (for the 2 subjects) was then used for preprocessing of the imaging data. Our study subjects also participated in other studies or projects. Only some of the MRI scanning performed in this study have been described here. Because the subjects of both the training and control groups completed these procedures and since they were exactly the same across for all subjects, these procedures could not affect training‐related differences between the groups.
Preprocessing and Analysis of Structural Data
VBM, which is a method for the in vivo study of human brain structures that can detect changes in regional gray matter caused by training [Driemeyer et al., 2008; Ilg et al., 2008], was used to investigate the effect of MT‐training on brain structures. Preprocessing of the morphological data was performed using the VBM2 software, an extension of SPM2. T1‐weighted structural images of the pre‐training scan and those of the post‐training scan were independently normalized and segmented using templates created in a previous study [Takeuchi et al., 2010b] and modulated. We used the default parameter setting and normal VBM2 protocols in these procedures. For details, see our previous study [Takeuchi et al., 2011b, 2011d]. Segmentation using SPM5/VBM5 did not work properly to our T1 weighted structural image and the extensive details of the failure of segmentation using SPM5/VBM5 and possible reasons were provided in our previous study [Takeuchi et al., 2012b, 2012c]. Subsequently, all images were smoothed by convolving them with an isotropic Gaussian Kernel of 12‐mm full‐width at half maximum (FWHM). Finally, the signal change in rGMV between the pre‐ and post‐intervention images was computed at each voxel for each participant. For this computation, we included only voxels with GMV values of >0.10 in both the pre‐ and post‐training MRI scans to avoid possible partial volume effects around the borders between gray matter (GM) and white matter as well as between GM and CSF. The resulting maps representing the rGMV change between the pre‐ and post‐training MRI experiments (rGMV post − rGMV pre) were then forwarded to the group level analysis.
In the group level imaging analysis, we tested for group‐wise differences in rGMV change. We used a factorial design option in SPM5. In these analyses, the effects of the interventions, which were estimated by comparing changes in pre‐ to post‐test measures as described above, were compared between the groups at each voxel with total GMV in the pre‐measurement as a covariate. The level of statistical significance was set at P < 0.05, corrected at the non‐isotropic adjusted cluster level [Hayasaka et al., 2004] with an underlying voxel‐level of P < 0.0025. Non‐isotropic adjusted cluster‐size tests can and should be applied when cluster size tests are applied to non‐stationary data (i.e., not uniformly smooth), such as VBM data [Hayasaka et al., 2004].
In addition, we investigated whether pre‐existing differences in rGMV (in the preintervention scan) existed between the MT‐training and control groups at the whole brain level using ANOVA without any covariates.
Preprocessing and Statistical Analysis of Functional Connectivity Data
Preprocessing and analysis of the functional connectivity data were performed using SPM5 implemented in Matlab. Before analysis, BOLD images from the pre‐ and post‐training scans were corrected for slice timing, re‐aligned, and re‐sliced to fit the mean BOLD images from the pre‐training scan, which means that both the pretraining and post‐training BOLD images were aligned with the third image. Subsequent normalizing procedures were performed as described [Takeuchi et al., 2011c] using the b = 0 image from the pre‐training scan. They were then smoothed (8‐mm full‐width half‐maximum).
Individual‐level statistical analyses were performed using a general linear model. We removed low‐frequency fluctuations with a high‐pass filter cut‐off value of 128 s (1/128 Hz). Slow signal drifts with a period longer than this, probably not based on brain activities were removed by this value. We did not use a low‐pass filter and serial correlations in the BOLD signal were accounted for by a first‐degree autoregressive correction. Several sources of spurious variances and their temporal derivatives were then regressed by putting these variances into the following regressors: (i) six parameters obtained by a rigid body correction of head motion and (ii) the whole brain signal averaged over a whole brain mask. Such a regression procedure removes fluctuations unlikely to be involved in specific regional correlations. Correlation maps were produced by extracting the average BOLD time course from a seed region and then computing the correlation coefficient between that time course and the time course from all other brain voxels. In the present study, we examined correlations associated with the right DLPFC, which is related to the a priori hypothesis and showed structural changes in gray matter analysis. The right DLPFC seed region was defined previously [Song et al., 2008] using WFU_PickAtlas (http://fmri.wfubmc.edu/cms/software). We defined the right DLPFC seed region by intersecting BA46, the right middle frontal gyrus, and gray matter in WFU_PickAtlas and then resliced the generated regions into the same spatial resolution as the preprocessed fMRI images (3 × 3 × 3 mm3).
In individual‐level analysis, contrast images representing changes in resting‐FC with the seed regions following the 27‐day intervention period and those before the intervention were estimated for each subject after pre‐processing. These images were then subjected to group analysis.
In group‐level imaging analysis, we tested for group‐wise differences in changes in resting‐FC with the seed region across the whole brain. We performed voxel‐wise ANCOVAs with the differences in each measure between the pre‐ and post‐scan values at each voxel as dependent variables and the prescan values at each voxel as independent variables. This analysis was performed using biological parametrical mapping (BPM) [Casanova et al., 2007] implemented in SPM5 and images representing prescan resting‐FC and changes in resting‐FC. The rationale for using BPM in this manner was to correct for the effects of pre‐intervention imaging measures on a voxel‐by‐voxel basis as was the case for ANCOVA with the psychological measures. This analysis using BPM was not applied to rGMV analysis because BPM does not handle the nonisotropic adjusted cluster‐size test, which was used in the abovementioned rGMV analysis. Each imaging analysis was performed using the data of 19 subjects in the MT group and 19 subjects in the control group.
Regions with significance were inferred using cluster‐level statistics [Friston et al., 1996] implemented in SPM5. Only clusters with a P < 0.05, after correction for multiple comparisons at cluster size with a voxel‐level cluster‐determining threshold of P < 0.0025 uncorrected, were considered statistically significant in this analysis.
Investigation of Associations Between MT Performance Changes and Neural Changes
We next investigated whether there was an association between MT performance changes and neural changes where the effects of MT training were observed through simple regression analyses. We used the sum of improvement in the level in each MT task performance, as calculated above, and extracted the mean value of the pre‐ to post‐training changes in neural measures (rGMV or resting FC) in each of the significant clusters identified above.
RESULTS
Training Data
Subjects in the MT‐training group completed an average of 25.65 sessions (standard deviation (SD): 1.81 sessions) and at least 21 sessions during the 27‐day intervention period. This SD as well as the average of 25.65 sessions across the 27‐day period indicates that the number of training sessions was well controlled. The level of performance (defined by the shortest interstimulus interval (ISI) for tasks in which the subjects achieved a certain level of performance in one block and a shortened ISI in the following block) in all six MT‐trained tasks was significantly increased in the last three training sessions compared with the first three training sessions (paired t test, P < 0.001, Table 1).
Table 1.
The average of all subjects' highest performances (the shortest interstimulus interval (ISI) of the tasks in which subjects achieved a certain level of performance in that block and shortened ISI in the following block) in multitasking (MT)‐trained tasks among the first and last three training sessions
| First three sessions (ms) | Last three sessions (ms) | |
|---|---|---|
| Auditory–auditory dual task | 1621 ± 70 | 857 ± 34 |
| Auditory–visual dual task | 970 ± 36 | 592 ± 17 |
| Auditory–auditory–auditory triple task | 3496 ± 206 | 1117 ± 55 |
| Auditory–auditory–visual arithmetic triple task | 2171 ± 76 | 985 ± 36 |
| Auditory‐auditory–auditory–auditory quad task | 6116 ± 357 | 1990 ± 193 |
| Auditory–auditory–visual arithmetic–visuospatial quad task | 2640 ± 121 | 1213 ± 52 |
Data obtained from two subjects who could not achieve a certain level of performance in an auditory–auditory–auditory–auditory quad task during the first three sessions were removed from the calculation of the average in this task.
Effect of MT‐Training on Behavioral Measures
To determine the effects of MT‐training on behavioral measures in an exploratory manner, we performed analysis of covariance (ANCOVA) of differences using pre‐ and post‐test measures as dependent variables and pre‐test scores as independent variables. This exploratory analyses of behavioral cognitive measures showed that compared with the no‐intervention (control) group, the MT‐training group had a significantly greater pre‐ to post‐test increase in performance on a Word‐Color task (P = 0.037), Stroop task (P = 0.012), and creativity test (P = 0.050) as well as a trend towards greater pre‐ to post‐test increase in performance on a Color‐Word task (P = 0.071) (Table 2).
Table 2.
Pre‐ and post‐test scores for psychological measures (mean ± standard error of mean)
| MT‐traininga | Control | Planned contrast | P valueb | Effect size (d)c | |||
|---|---|---|---|---|---|---|---|
| Pre‐test scores | Post‐test scores | Pre‐test scores | Post‐test scores | ||||
| Nonverbal reasoning | |||||||
| RAPMd (score) | 29.1 ± 0.9 | 31.6 ± 0.8 | 29.1 ± 0.9 | 31.2 ± 0.9 | MT‐training > control | 0.242 | 0.232 |
| BOMATe (score) | 8.63 ± 0.45 | 9.26 ± 0.67 | 7.72 ± 0.57 | 9.72 ± 0.54 | MT‐training > control | 0.861 | 0.370 |
| WM | |||||||
| Digit span (score) | 38.2 ± 1.2 | 39.7 ± 1.7 | 35.6 ± 1.4 | 36.7 ± 1.6 | Two‐tailed | 0.738 | 0.114 |
| Visuospatial WM (score) | 29.1 ± 1.0 | 30.0 ± 0.8 | 27.9 ± 1.0 | 30.2 ± 0.9 | Two‐tailed | 0.270 | −0.372 |
| Intelligence test with speeded tasks | |||||||
| Tanaka‐B type intelligence test | 114.4 ± 2.7 | 123.8 ± 2.6 | 112.4 ± 2.1 | 120.3 ± 2.8 | MT‐training > control | 0.230 | 0.246 |
| Simple processing speed | |||||||
| Word‐Color task (items) | 70.2 ± 1.9 | 75.3 ± 2.3 | 71.6 ± 1.3 | 74.1 ± 1.5 | MT‐training > control | 0.037 | 0.609 |
| Color‐Word task (items) | 48.8 ± 1.7 | 53.4 ± 1.5 | 52.1 ± 1.7 | 54.4 ± 1.7 | MT‐training > control | 0.071 | 0.507 |
| Inhibition (interference resolution) | |||||||
| Reverse Stroop task (items) | 58.0 ± 2.2 | 63.2 ± 2.2 | 56.8 ± 2.0 | 61.1 ± 2.1 | MT‐training > control | 0.281 | 0.193 |
| Stroop task (items) | 46.1 ± 1.3 | 49.6 ± 1.6 | 47.6 ± 1.8 | 47.9 ± 1.7 | MT‐training > control | 0.012 | 0.779 |
| Arithmetic | |||||||
| Simple arithmetic (items) | 32.5 ± 1.3 | 32.9 ± 1.4 | 31.8 ± 1.2 | 33.0 ± 1.4 | MT‐training > control | 0.769 | −0.245 |
| Complex arithmetic (items) | 6.11 ± 0.32 | 6.47 ± 0.66 | 6.72 ± 0.55 | 7.25 ± 0.72 | MT‐training > control | 0.527 | −0.023 |
| Complex mathematic | |||||||
| Numerical factor in Kyodai SX test | 11.6 ± 0.5 | 12.9 ± 0.6 | 11.3 ± 0.5 | 12.3 ± 0.5 | MT‐training > control | 0.234 | 0.242 |
| Creativity | |||||||
| SA creativity test (total grade) | 25.1 ± 1.1 | 27.5 ± 1.1 | 28.8 ± 1.3 | 27.3 ± 1.2 | MT‐training > control | 0.050 | 0.591 |
| Fatigue | |||||||
| POMS fatigue subscale score on the day of the experiment | 2.80 ± 2.91 | 3.05 ± 2.86 | 5.26 ± 3.75 | 5.16 ± 4.56 | MT training > controlf | 0.948 | −0.535 |
Multitasking‐training
One‐way analysis of covariances with test–retest differences in psychological measures as dependent variables and pre‐test scores of the psychological measures as covariates
Effect size estimates were calculated using Cohen's d.
Raven's Advanced Progressive Matrices.
Bochumer Matrizen‐Test
Higher score indicates higher fatigue, and we tested whether MT training increased fatigue on the day of the experiment.
Furthermore, the MT training group did not have a significantly greater pre‐ to post‐test increase in the POMS fatigue subscale score on the day of the experiment (P = 0.948) (Table 2). This indicates that MT training did not induce lasting fatigue.
Effect of MT‐Training on Regional Gray Matter Structure
VBM analysis revealed that compared with the control group, the MT training group showed a statistically significant greater increase in rGMV around the left IFJ, left lateral rostral PFC (LRPFC), right DLPFC, and in an anatomical cluster that extended into the left posterior parietal region, left lateral occipital lobe, and a cluster in the left temporal region (Fig. 2, Table 3).
Figure 2.
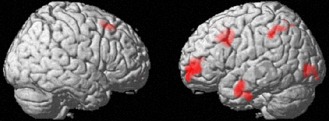
Effect of MT‐training on rGMV. The results are shown with P < 0.05, corrected for multiple comparisons at the non‐isotropic adjusted cluster‐level with an underlying voxel‐level of P < 0.0025, uncorrected. Compared with the control group (no‐intervention), the MT‐training group showed an increase in rGMV of the left IFJ, left LRPFC, right DLPFC, an anatomical cluster that extended into the left posterior parietal region, an area in the left lateral occipital lobe, and an area in the left lateral temporal lobe.
Table 3.
MT‐training‐related regional gray matter volume (rGMV) increases compared with no intervention (control) (post‐MT rGMV − pre‐MT rGMV) − (post‐control rGMV − pre‐control rGMV)
| Area | MNI coordinates | T score | Corrected P value (cluster) | |||
|---|---|---|---|---|---|---|
| X | y | z | ||||
| Inferior frontal gyrus/Middle frontal gyrus/Precentral gyrus (IFJ) | L | −48 | 11 | 39 | 4.61 | <0.001 |
| Superior frontal gyrus/Middle frontal gyrus/Medial frontal gyrus (DLPFC) | R | 14 | 28 | 48 | 4.54 | <0.001 |
| Middle occipital gyrus/Inerior occipital gyrus | L | −47 | −87 | 0 | 4.45 | <0.001 |
| Middle frontal gyrus/Superior frontal gyrus (LRPFC) | L | −31 | 63 | −13 | 4.38 | <0.001 |
| Middle temporal gyrus/Inferior temporal gyrus | L | −60 | −2 | −22 | 4.32 | 0.001 |
| Inferior parietal lobule/Superior parietal lobule/Precuneus/Postcentral gyrus | L | −63 | −42 | 43 | 4.20 | <0.001 |
No training‐related decreases in rGMV were observed.
IFJ, inferior frontal junction; LRPFC, lateral rostral prefrontal cortex; DLPFC, dorsolateral prefrontal cortex.
Furthermore, whole‐brain ANOVA showed no significant regional differences in rGMV between the MT‐training and control groups before the intervention (prescan; P > 0.3, corrected for multiple comparisons).
Effect of MT‐Training on Resting‐FC with the Right DLPFC
Next, we compared changes in resting‐FC with the right DLPFC (the seed region), which plays an important role in MT, in the MT‐training and control groups. We found a statistically significant MT‐training‐related (MT‐training group vs. control group) decrease in pre‐ to post‐test measures of resting‐FC between the right DLPFC (the seed region) and an anatomical cluster that spread around the ventral anterior cingulate cortex (ACC) (Fig. 3, x, y, z = −6, 30, −3; t = 4.55; P = 0.018, corrected for multiple comparisons at the cluster level with a cluster‐determining threshold of P < 0.0025, uncorrected). This cluster largely belonged to areas that had negative resting FC with the right DLPFC (one‐sample t test; P < 0.05, corrected for the false discovery rate). This means that the decrease in resting FC between these two areas corresponds to an increase in anticorrelation [Fox et al., 2005].
Figure 3.
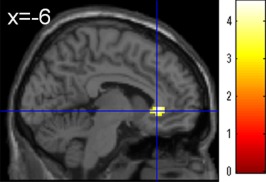
Effect of MT‐training on resting‐FC between the right DLPFC and the rest of the brain. Decrease in resting‐FC with the right DLPFC in the MT‐training group compared with the control group (P < 0.05, corrected for multiple comparisons at the cluster‐level with an underlying voxel‐level of P < 0.0025, uncorrected). Compared with the control group (no‐intervention), the MT‐training group showed a decrease in resting‐FC between the right DLPFC and anatomical cluster that spread around the ventral ACC.
Associations Between Neural Changes and MT Performance Changes
We next investigated the association between MT performance changes and neural changes where the effects of MT training were observed through simple regression analyses. The correlation between the sum of improvement in the level in each MT task performance, as calculated above, and the mean value of the pre‐ to post‐training changes in neural measures (rGMV or resting FC) in each significant cluster was tested. A significant positive correlation was observed between MT performance improvement and the mean increase in rGMV of a significant cluster in the right DLPFC (r = 0.542, P = 0.017). There were no other significant results. When the amount of training was included as a covariate in these correlation analyses (standardized partial regression coefficient (β) = 0.547; P = 0.020), this correlation remained significant and all other results remained insignificant. This finding may again suggest the importance of DLPFC [Erickson et al., 2007] in MT training‐related improvements and support an association between neural adaptation and behavioral improvement. However, the sample size is small, and this result is not strong considering the number of significant clusters. Therefore, this finding should be taken with caution.
DISCUSSION
To our knowledge, the present study is the first to reveal the effects of MT‐training as a whole on rGMV and resting‐FC. MT‐training was associated with (a) increases in rGMV in the right DLPFC, left IFJ, left LRPFC, left posterior parietal region, left lateral temporal and left occipital areas and (b) decreases in resting‐FC between the right DLPFC and an anatomical cluster around the ventral ACC. Our findings suggest that participation in MT‐training is as a whole associated with task‐irrelevant plasticity (i.e., neural changes are not limited to certain specific task conditions) in regions and the network that are assumed to play roles in MT as well as diverse higher‐order cognitive functions. Exploratory behavioral analysis revealed that MT‐training was associated with marginally significant or a trend toward improvements in performance on several non‐MT cognitive tests.
VBM results showed that MT‐training as a whole led to increases in rGMV in regions involved in MT in various ways. Increases in rGMV were observed in the left RLPFC, right DLPFC, left IFJ, and left posterior parietal region, which all play a role in MT. As described in the Introduction section, the right DLPFC may be involved in in scheduling processes in complex tasks (“task management”) [Smith and Jonides, 1999]. IFJ has been suggested to be involved in interference control at various levels (e.g., perceptual, motor, and/or cognitive) during MT [Stelzel et al., 2008]. LRPFC mediates the human ability to hold and represent multiple pieces of information from different sources and/or to integrate these during MT [Rubens and Zanto, 2011; Wendelken et al., 2012]. Finally, posterior parietal regions are involved in spatial attention shifts [Yantis et al., 2002] and are activated during MT [Erickson et al., 2007]. MT‐training‐related changes have previously been observed in this region [Erickson et al., 2007]. Thus, these results may be interpreted as supporting the notion that MT‐training leads to an increase in rGMV in regions involved in MT. Furthermore, during multitasking, the network consisting of the lateral frontal cortex and parts of the parietal lobe is recruited [Erickson et al., 2007]. This network is involved in a wide variety of externally directed attention‐demanding tasks [Fox et al., 2005], and the structure of this network is suggested to be associated with fluid intelligence [Jung et al., 2010]. Thus, the rGMV changes may be interpreted as structural plasticity in this important network, and the implications have been discussed below. On the other hand, rGMV increases were also observed in the left lateral temporal and left occipital areas. Processing speed training has previously been shown to increase rGMV and other neural changes in contingent areas [Takeuchi and Kawashima, 2012; Takeuchi et al., 2011b]. However, neural changes in PFC and parietal cortices have not been observed in this previous study [Takeuchi and Kawashima, 2012; Takeuchi et al., 2011b]. Thus, these changes may reflect neural adaptation at the level of processing of auditory, language, and visual perception during MT training [Takeuchi et al., 2011b].
The decrease in resting‐FC between the right DLPFC and ventral ACC may reflect conditions in which cognitive resources are easily reallocated; this reallocation may be mediated by augmentation of the function of the right DLPFC through MT‐training. Certain regions show positively synchronized brain activity during rest (positive correlations between the activities of these regions) and form functional networks [Fox et al., 2005]. DLPFC is the key node of this type of functional network, which consists of regions that are consistently activated during cognitive tasks [Fox et al., 2005]. On the other hand, the ventral ACC and contingent medial PFC form the key node of another functional network (the default mode network or DMN), which consists of regions that are consistently deactivated during cognitive tasks [Fox et al., 2005]. Activities of these two networks during rest show spontaneous anticorrelations [Fox et al., 2005], i.e., when one network is activated, the other network is deactivated. Thus, the present finding of resting‐FC analysis, which shows a training‐related decrease in resting‐FC between the right DLPFC and ventral ACC, corresponds to an increase in anticorrelations between the two regions. Deactivation of DMN during cognitive tasks is considered to reflect the reallocation of cognitive resources from the network active at rest (DMN) to the network actively involved in the task (the network involving DLPFC) [McKiernan et al., 2003]. Thus, the increased anticorrelations between the right DLPFC and ventral ACC may reflect conditions in which cognitive resources are reallocated from one network to the other. Consistently decreased anticorrelations between the DLPFC and medial PFC have been observed in patients with schizophrenia and their relatives during rest as well as while performing working memory (WM) tasks, and this is considered to reflect inefficient use of attentional resources [Whitfield‐Gabrieli et al., 2009]. The changes observed in this study may be mediated by the attention shift function of the right DLPFC [Kondo et al., 2004] (described above) and may suggest that augmentation of this function is even extended to neural processes during rest.
MT‐training‐related improvements in behavioral performance were observed in a few untrained non‐MT cognitive tasks but not in non‐verbal reasoning tasks. First, MT‐training as a whole led to a significant improvement in creativity. It has long been acknowledged that a widened span of attention that allows concurrent processing of different information sources is important for creativity [Mendelsohn, 1976]. Thus, one speculation is that because MT‐training requires concurrent processing of multiple information sources, attentional capacity for processing multiple stimuli is increased and thus creativity is enhanced. The Stroop task was also associated with improvements in MT‐training‐related performance that may have resulted from increased efficiency of cognitive interference resolution, which is important for MT‐training tasks and Stroop performance. The observed changes in IFJ and DLPFC may underlie these improvements, considering the role and contribution of these regions in Stroop performance [Brass et al., 2005]. On the other hand, the observed improvements in simple processing speed (PS) tasks (significant improvement in the Word‐Color task as well as a trend towards improvement in the Color‐Word task) may be related to the increased speed of cognitive processing at the higher‐order level caused by MT‐training [Dux et al., 2009]. Training on dual WM tasks in which subjects have to perform two WM tasks concurrently affects performance on a non‐verbal reasoning task more than training on a single WM task [Dash et al., 2010]. This finding raises the following question: does training on dual tasks or MT itself affect performance on non‐verbal reasoning tasks? MT‐training did not lead to significant improvements in performance on non‐verbal reasoning tasks in this study but this result may be observed because of a lack of statistical power in our analysis. This lack of significance in non‐verbal reasoning measures occurred despite the presence of MT‐training‐induced increase in rGMV in the frontoparietal areas as well as the association between cortical structures in the frontoparietal areas and fluid intelligence [Jung and Haier, 2007]. Our previous study on working memory training using mental calculations [Takeuchi et al., 2011d] also showed the effects of training on rGMV of the frontoparietal areas but failed to show the effects of the training on non‐verbal reasoning measures [Our other study used more typical working memory training paradigms [Takeuchi et al., 2013] and showed divided results (marginal significance in one non‐verbal reasoning measure but no significance in another nonverbal reasoning measure), while showing rGMV changes in frontoparietal networks]. The effect of dual WM tasks on non‐verbal reasoning measures itself is a matter of substantial controversy as different studies have shown different results, and the reasons for the differences remain unclear [Jaeggi et al., 2008; Melby‐Lervåg and Hulme, 2012; Redick et al., 2013]. Thus, like the improvement of performance of WM, which is associated with performance of non‐verbal reasoning measures [Engle et al., 1999b] didn't always lead to improvement of performance of non‐verbal reasoning measures, the changes in rGMV in the frontoparietal areas may not cause (at least not always in a detectable manner) improvement in non‐verbal reasoning performance. Thus, the critical factor for improving nonverbal reasoning performance, which is a strong indicator of general intelligence, remains to be revealed.
There were a few limitations to this study. First, with regard to behavioral data analysis, corrections for multiple comparisons were not performed and the statistical values were marginal when significant or a trend toward training‐related effects were observed. However, this statistical procedure is standard in studies of this kind [e.g., Jaeggi et al., 2008; Klingberg et al., 2002, 2005; Mahncke et al., 2006; Redick et al., 2013; Takeuchi et al., 2011b, 2011d] and is appropriate considering the exploratory nature of the behavioral data analysis performed in this study. However, if we include the contrasts between MT training vs. WM‐training, the statistical tests for psychological procedures in this study involved 28 statistical comparisons. If we apply the two‐sharpened methods of false discovery rate procedures [Benjamini et al., 2006] to these tests, only three statistically significant results and one tendency were observed [visuospatial WM (WM training > MT‐training; P = 0.0003 corrected), digit span (WM training > MT‐training; P = 0.0004, corrected), Color‐Word task (MT training > WM‐training; P = 0.037 corrected), and Stroop task (MT training > control, P = 0.084, corrected)]. In case of studies of WM training, even when statistically strong training effects are observed on certain measures in one study, the effects are sometimes not observed in another and the reasons for this observation remain unclear [Takeuchi et al., 2010a]. Thus, the behavioral results should be interpreted with caution until replicated. The next limitation of this study relates to the complex training protocols used [Jaeggi et al., 2008; Tang et al., 2007], which are commonly observed in this type of study whether the training is related to WM [Jaeggi et al., 2008], video games [Green and Bavelier, 2003], or meditation [Tang et al., 2007]. These studies typically have none of the strict control groups or conditions that are present in normal fMRI studies. Thus, the present study did not even try to reveal any MT training‐specific effect compared with that of other complex or simple cognitively demanding tasks such as simple processing speed training. The results obtained may not be specific to MT training as is the case with almost all imaging studies of cognitive training. If one denies the meaning of this study for this very reason, then almost all neuroimaging studies of cognitive training conducted till date and this entire field itself should be denied. Although it would be statistically very challenging, it would be interesting to disentangle the multiple complex cognitive training protocols and investigate MT training‐specific effects. For further limitations of this study, and the incongruence between the present longitudinal intervention studies and cross‐sectional correlation studies of cognitive functions, see our Supporting Information Discussion.
Related to this limitation, this project also involved a WM training group [Takeuchi et al., 2013]. MT and WM overlap in some respects in terms of brain activation patterns and the potential cognitive processes involved [Erickson et al., 2007; Takeuchi et al., 2010a, 2012a]. It would be interesting to compare MT training and WM training, and this comparison may reveal some specific and common effects of the training. We therefore compared the effects between these groups. For more details on the methods used and the results, please see Supporting Information and Supporting Information Fig. 1 and Supporting Information Table 1. Similar to some WM training in other laboratories [Chein and Morrison, 2011; Dash et al., 2010; Jaeggi et al., 2008], the WM training tasks used in this experiment included dual WM tasks and complex WM paradigms. Therefore, our WM training and MT training tasks were likely to share cognitive components of (a) MT or divided attention, management of complex tasks, shifting attention, attention to perceptual components, cognitive control, and other central executive components such as interference resolution more or less. On the other hand, WM training was likely to specifically involve cognitive components of (b) memory or maintenance of attention on the same information or maintenance of the same information over a long period, whereas MT training might have involved greater (c) interference resolution and divided attention, speeded components, and rapid changes in the focus of attention that did not require the maintenance of the same information over a long period. In summary, the measures that showed the significant effects of the (MT training group–control group) analysis, but that did not show the significant effects of the (MT training group–WM training group) analysis included the Word‐Color task, Stroop task, and Creativity task and all of the rGMV changes that were shown in the main text. These effects might have been caused by the cognitive components of (a), but these interpretations are based on results that did not show significant group differences (since we cannot show significantly “same” effects), and thus weak. Therefore, these interpretations should be viewed with caution. Measures that showed the significant and substantial effects of the (MT training group–control group) analysis and the significant and substantial effects of the (MT training group–WM training group) analysis included the Color‐Word task and the change (reduction) in resting FC between the right DLPFC and the ventral ACC. These effects may be best understood as the effects of cognitive components (c) which were specific to or more strongly associated with MT training. Specifically, considering the functions the Color‐Word task is measuring, the performance change in this task may be caused by the effects of the speed components of MT training. Further, considering the relevance of the attentional reallocation of this resting FC (as discussed above), among the cognitive components of (c), the recruitment of a more rapid change in the focus of attention might have caused this change in resting FC. For other patterns and details, please see the Supporting Information. However, these interpretations are limited by the differences between the groups. Future studies are therefore needed to confirm the speculations arising from these results.
MT plays a key role in human central executive functions, and the PFC regions (DLPFC, IFJ, and LRPFC), ACC, the part of the parietal lobe involved in MT, and the frontoparietal network consisting of these areas are associated with diverse higher‐order cognitive functions [Burgess et al., 2005; Derrfuss et al., 2004; Fuster, 2006]. Thus, the neural changes in these areas and the network caused by MT training as a whole, have implications in relation to the plasticity of human higher‐order cognitive functioning as well as the application of MT‐training in areas such as education.
ACKNOWLEDGMENTS
The authors thank Yuki Yamada for operating the MRI scanner, Haruka Nouchi for conducting the psychological tests, the subjects, and all the other colleagues in IDAC, Tohoku University for their support. The authors thank Enago (http://www.enago.jp) for the English language review.
Supporting information
Supporting Information Figure 1.
REFERENCES
- Baddeley AD, Bressi S, Della S (1991): The decline of working memory in Alzheimer's disease: A longitudinal study. Brain 114:2521. [DOI] [PubMed] [Google Scholar]
- Baron‐Cohen S (2003): The Essential Difference: The Truth About the Male and Female Brain. New York: Perseus Books Group. [Google Scholar]
- Benjamini Y, Krieger AM, Yekutieli D (2006): Adaptive linear step‐up procedures that control the false discovery rate. Biometrika 93:491–507. [Google Scholar]
- Bherer L, Kramer AF, Peterson MS, Colcombe S, Erickson K, Becic E (2008): Transfer effects in task‐set cost and dual‐task cost after dual‐task training in older and younger adults: Further evidence for cognitive plasticity in attentional control in late adulthood. Exp Aging Res 34:188–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brass M, Derrfuss J, Forstmann B, Cramon D (2005): The role of the inferior frontal junction area in cognitive control. Trends Cogn Sci 9:314–316. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Simons JS, Dumontheil I, Gilbert SJ (2005): The gateway hypothesis of rostral prefrontal cortex (area 10) function In: Duncan J, Philips L, McLeod P, editors. Measuring the Mind: Speed, Control, and Age. Oxford: Oxford University Press; p 217. [Google Scholar]
- Casanova R, Srikanth R, Baer A, Laurienti PJ, Burdette JH, Hayasaka S, Flowers L, Wood F, Maldjian JA (2007): Biological parametric mapping: A statistical toolbox for multimodality brain image analysis. Neuroimage 34:137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chein JM, Morrison AB (2011): Expanding the mind's workspace: Training and transfer effects with a complex working memory span task. Psychonom Bull Rev 17:193–199. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Detre JA, Alsop DC, Shin RK, Atlas S, Grossman M (1995): The neural basis of the central executive system of working memory. Nature 378:279–281. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts S, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF (2006): Consistent resting‐state networks across healthy subjects. Proc Natl Acad Sci USA 103:13848–13853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash D, McNab F, Klingberg T (2010): Effect of dual working memory training on a reasoning task—An fMRI study 16th Annual Meeting of the Organization for Human Brain Mapping. Barcelona, Spain. [Google Scholar]
- Derrfuss J, Brass M, Yves von Cramon D (2004): Cognitive control in the posterior frontolateral cortex: Evidence from common activations in task coordination, interference control, and working memory. Neuroimage 23:604–612. [DOI] [PubMed] [Google Scholar]
- Dimitrov DM, Rumrill J, Phillip D (2003): Pretest‐posttest designs and measurement of change. Work J Prevent Assess Rehabil 20:159–165. [PubMed] [Google Scholar]
- Driemeyer J, Boyke J, Gaser C, Büchel C, May A (2008): Changes in gray matter induced by learning—Revisited. PLoS One 3:e2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dux PE, Tombu MN, Harrison S, Rogers BP, Tong F, Marois R (2009): Training improves multitasking performance by increasing the speed of information processing in human prefrontal cortex. Neuron 63:127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engle RW, Kane MJ, Tuholski SW. 1999a. Individual Differences in Working Memory Capacity and What They Tell us About Controlled Attention, General Fluid Intelligence, and Functions of the Prefrontal Cortex Models of Working Memory: Mechanisms of Active Maintenance and Executive Control. Cambridge: Cambridge University Press; pp 102–134. [Google Scholar]
- Engle RW, Tuholski SW, Laughlin JE, Conway AR (1999b): Working memory, short‐term memory, and general fluid intelligence: A latent‐variable approach. J Exp Psychol Gen 128:309–331. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Colcombe SJ, Wadhwa R, Bherer L, Peterson MS, Scalf PE, Kim JS, Alvarado M, Kramer AF (2007): Training‐induced functional activation changes in dual‐task processing: An FMRI study. Cerebral Cortex 17:192–204. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME (2005): The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA 102:9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P (2005): Spontaneous low‐frequency BOLD signal fluctuations: An fMRI investigation of the resting‐state default mode of brain function hypothesis. Hum Brain Mapp 26:15–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Holmes A, Poline JB, Price CJ, Frith CD (1996): Detecting activations in PET and fMRI: levels of inference and power. NeuroImage 4:223–235. [DOI] [PubMed] [Google Scholar]
- Fuster JM (2006): The Prefrontal Cortex: Anatomy, Physiology, and Neuropsychology of the Frontal Lobe. New York: Raven Press. [Google Scholar]
- Goddard L, Dritschel B, Burton A (1998): Gender differences in the dual‐task effects on autobiographical memory retrieval during social problem solving. Br J Psychol 89:611–627. [DOI] [PubMed] [Google Scholar]
- Goldstone RL (1998): Perceptual learning. Annu Rev Psychol 49:585–612. [DOI] [PubMed] [Google Scholar]
- Grabner RH, Ansari D, Reishofer G, Stern E, Ebner F, Neuper C (2007): Individual differences in mathematical competence predict parietal brain activation during mental calculation. Neuroimage 38:346–356. [DOI] [PubMed] [Google Scholar]
- Green A, Vaid J (1986): Methodological issues in the use of the concurrent activities paradigm. Brain Cogn 5:465–476. [DOI] [PubMed] [Google Scholar]
- Green CS, Bavelier D (2003): Action video game modifies visual selective attention. Nature 423:534–537. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V (2003): Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc Natl Acad Sci USA 100:253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakoda Y, Sasaki M (1990): Group version of the stroop and reverse‐stroop test: The effects of reaction mode, order and practice. Kyoikushinrigakukenkyu (Educational Psychology Research) 38:389–394. [Google Scholar]
- Hayasaka S, Phan KL, Liberzon I, Worsley KJ, Nichols TE (2004): Nonstationary cluster‐size inference with random field and permutation methods. Neuroimage 22:676–687. [DOI] [PubMed] [Google Scholar]
- Herath P, Klingberg T, Young J, Amunts K, Roland P (2001): Neural correlates of dual task interference can be dissociated from those of divided attention: An fMRI study. Cereb Cortex 11:796–805. [DOI] [PubMed] [Google Scholar]
- Hiscock M (1986): Lateral eye movements and dual‐task performance In: Hannay JH, editor. Experimental Techniques in Human Neuropsychology. New York: Grune & Stratton; pp 264–308. [Google Scholar]
- Hogarty GE, Flesher S, Ulrich R, Carter M, Greenwald D, Pogue‐Geile M, Kechavan M, Cooley S, DiBarry AL, Garrett A (2004): Cognitive enhancement therapy for schizophrenia: Effects of a 2‐year randomized trial on cognition and behavior. Arch Gen Psychiatry 61:866–876. [DOI] [PubMed] [Google Scholar]
- Hossiep R, Turck DMH (1999): Bochumer Matrizentest: BOMAT‐Advanced‐Short Version. Gottingen: Hogrefe. [Google Scholar]
- Ilg R, Wohlschlager AM, Gaser C, Liebau Y, Dauner R, Woller A, Zimmer C, Zihl J, Muhlau M (2008): Gray matter increase induced by practice correlates with task‐specific activation: A combined functional and morphometric magnetic resonance imaging study. J Neurosci 28:4210–4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeggi SM, Buschkuehl M, Jonides J, Perrig WJ (2008): Improving fluid intelligence with training on working memory. Proc Natl Acad Sci USA 105:6829–6833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung RE, Haier RJ (2007): The parieto‐frontal integration theory (P‐FIT) of intelligence: Converging neuroimaging evidence. Behav Brain Sci 30:135–154. [DOI] [PubMed] [Google Scholar]
- Jung RE, Segall JM, Bockholt HJ, Flores RA, Smith SM, Chavez RS, Haier RJ (2010): Neuroanatomy of creativity. Hum Brain Mapp 31:398–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingberg T, Forssberg H, Westerberg H (2002): Training of working memory in children with ADHD. J Clin Exp Neuropsychol 24:781–791. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Fernell E, Olesen PJ, Johnson M, Gustafsson P, Dahlstr?m K, Gillberg CG, Forssberg H, Westerberg HLP (2005): Computerized training of working memory in children with ADHD—A randomized, controlled trial. J Am Acad Child Adolesc Psychiatry 44:177–186. [DOI] [PubMed] [Google Scholar]
- Kondo H, Osaka N, Osaka M (2004): Cooperation of the anterior cingulate cortex and dorsolateral prefrontal cortex for attention shifting. Neuroimage 23:670–679. [DOI] [PubMed] [Google Scholar]
- Mahncke HW, Connor BB, Appelman J, Ahsanuddin ON, Hardy JL, Wood RA, Joyce NM, Boniske T, Atkins SM, Merzenich MM (2006): Memory enhancement in healthy older adults using a brain plasticity‐based training program: A randomized, controlled study. Proc Natl Acad Sci USA 103:12523–12528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell S, Whyte J, D'Esposito M (1997): Working memory impairments in traumatic brain injury: Evidence from a dual‐task paradigm. Neuropsychologia 35:1341–1353. [DOI] [PubMed] [Google Scholar]
- McKiernan KA, Kaufman JN, Kucera‐Thompson J, Binder JR (2003): A parametric manipulation of factors affecting task‐induced deactivation in functional neuroimaging. J Cogn Neurosci 15:394–408. [DOI] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman LF (1992): Profile of Mood States. San Diego, California: Educational and Industrial Testing Service. [Google Scholar]
- Melby‐Lervåg M, Hulme C (2012): Is working memory training effective? A meta‐analytic review. Dev Psychol 49:270–291. [DOI] [PubMed] [Google Scholar]
- Mendelsohn GA (1976): Associative and attentional processes in creative performance. J Pers 44:341–369. [Google Scholar]
- Oldfield RC (1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9:97–113. [DOI] [PubMed] [Google Scholar]
- Raven J (1998): Manual for Raven's Progressive Matrices and Vocabulary Scales. Oxford: Oxford Psychologists Press. [Google Scholar]
- Redick TS, Shipstead Z, Harrison TL, Hicks KL, Fried DE, Hambrick DZ, Kane MJ, Engle RW. (2013): No evidence of intelligence improvement after working memory training: A randomized, placebo‐controlled study. J Exp Psychol Gen 142:359–379. [DOI] [PubMed] [Google Scholar]
- Rubens MT, Zanto TP (2011): Characterizing the involvement of rostrolateral prefrontal cortex in prospective memory. J Neurosci 31:9067–9069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, Jonides J (1999): Storage and executive processes in the frontal lobes. Science 283:1657–1661. [DOI] [PubMed] [Google Scholar]
- Society_For_Creative_Minds (1969): Manual of S‐A Creativity Test. Tokyo: Tokyo Shinri Corporation. [Google Scholar]
- Song M, Zhou Y, Li J, Liu Y, Tian L, Yu C, Jiang T (2008): Brain spontaneous functional connectivity and intelligence. Neuroimage 41:1168–1176. [DOI] [PubMed] [Google Scholar]
- Stelzel C, Kraft A, Brandt SA, Schubert T (2008): Dissociable neural effects of task order control and task set maintenance during dual‐task processing. J Cogn Neurosci 20:613–628. [DOI] [PubMed] [Google Scholar]
- Sweller J, Van Merrienboer JJG, Paas F (1998): Cognitive architecture and instructional design. Educ Psychol Rev 10:251–296. [Google Scholar]
- Tachibana A, Noah JA, Bronner S, Ono Y, Hirano Y, Niwa M, Watanabe K, Onozuka M (2012): Activation of dorsolateral prefrontal cortex in a dual neuropsychological screening test: An fMRI approach. Behav Brain Funct 8:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Kawashima R (2012): Effects of processing speed training on cognitive functions and neural systems. Rev Neurosci 23:289–301. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Kawashima R (2010a): Effects of working memory training on cognitive functions and neural systems. Rev Neurosci 21:427–450. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Sassa Y, Hashizume H, Sekiguchi A, Fukushima A, Kawashima R (2010b): Regional gray matter volume of dopaminergic system associate with creativity: Evidence from voxel‐based morphometry. Neuroimage 51:578–585. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Hashizume H, Sassa Y, Nagase T, Nouchi R, Kawashima R (2011a): Cerebral blood flow during rest associates with general intelligence and creativity. PLoS One 6:e25532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Hashizume H, Sassa Y, Nagase T, Nouchi R, Kawashima R (2011b): Effects of training of processing speed on neural systems. J Neurosci 31:12139–12148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Hashizume H, Sassa Y, Nagase T, Nouchi R, Kawashima R (2011c): Failing to deactivate: The association between brain activity during a working memory task and creativity. Neuroimage 55:681–687. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Sassa Y, Hashizume H, Sekiguchi A, Fukushima A, Kawashima R (2011d): Working memory training using mental calculation impacts regional gray matter of the frontal and parietal regions. PLoS One 6:e23175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Sugiura M, Sassa Y, Sekiguchi A, Yomogida Y, Taki Y, Kawashima R (2012a): Neural correlates of the difference between working memory speed and simple sensorimotor speed: An fMRI study. PLoS One 7:e30579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Nouchi R, Sekiguchi A, Kotozaki Y, Miyauchi CM, Yokoyama R, Iizuka K, Hashizume H, Nakagawa S (2012b): A voxel‐based morphometry study of gray and white matter correlates of a need for uniqueness. Neuroimage 63:1119–1126. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Sassa Y, Hashizume H, Sekiguchi A, Nagase T, Nouchi R, Fukushima A, Kawashima R (2012c): Regional gray and white matter volume associated with Stroop interference: Evidence from voxel‐based morphometry. Neuroimage 59:2899–2907. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Nouchi R, Hashizume H, Sekiguchi A, Kotozaki Y, Nakagawa S, Miyauchi CM, Sassa Y, Kawashima R (2013): Effects of working memory‐training on functional connectivity and cerebral blood flow during rest. Cortex 49:2106–2125. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Okamoto K, Tanaka H (2003): Manual of New Tanaka B Type Intelligence Test. Tokyo: Kaneko Syobo. [Google Scholar]
- Tang YY, Ma Y, Wang J, Fan Y, Feng S, Lu Q, Yu Q, Sui D, Rothbart MK, Fan M (2007): Short‐term meditation training improves attention and self‐regulation. Proc Natl Acad Sci 104:17152–17156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhaeghen P, Steitz DW, Sliwinski MJ, Cerella J (2003): Aging and dual‐task performance: A meta‐analysis. Psychol Aging 18:443–460. [DOI] [PubMed] [Google Scholar]
- Wallentin M (2009): Putative sex differences in verbal abilities and language cortex: A critical review. Brain Lang 108:175–183. [DOI] [PubMed] [Google Scholar]
- Wendelken C, Chung D, Bunge SA (2012): Rostrolateral prefrontal cortex: Domain‐general or domain‐sensitive? Hum Brain Mapp 33:1952–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield‐Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, Shenton ME, Green AI, Nieto‐Castanon A, LaViolette P (2009): Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first‐degree relatives of persons with schizophrenia. Proc Natl Acad Sci USA 106:1279–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yantis S, Schwarzbach J, Serences JT, Carlson RL, Steinmetz MA, Pekar JJ, Courtney SM (2002): Transient neural activity in human parietal cortex during spatial attention shifts. Nat Neurosci 5:995–1002. [DOI] [PubMed] [Google Scholar]
- Yokoyama K (2005): POMS Shortened Version (in Japanese). Tokyo: Kanekoshobo. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Figure 1.


