Abstract
Advantageous inequality (AI) aversion, or paying at a personal cost to achieve equal reward distribution, represents a unique feature of human behavior. Here, we show that individuals have strong preferences for fairness in both disadvantageous (DI) and advantageous inequality (AI) situations, such that they alter others' payoff at a personal financial cost. At the neural level, we found that both types of inequality activated the putamen, orbitofrontal cortex, and insula, regions implicated in motivation. Individual difference analyses found that those who spent more money to increase others' payoff had stronger activity in putamen when they encountered AI and less functional connectivity between putamen and both orbitofrontal cortex and anterior insula. Conversely, those who spent more money to reduce others' payoff had stronger activity in amygdala in response to DI and less functional connectivity between amygdala and ventral anterior cingulate cortex. These dissociations suggest that both types of inequality are processed by similar brain areas, yet modulated by different neural pathways. Hum Brain Mapp 35:3290–3301, 2014. © 2013 Wiley Periodicals, Inc.
Keywords: inequality aversion, striatum, insula, amygdala, fMRI
INTRODUCTION
Inequity aversion, or the preference for fairness, is a response observed in many social animals. Chimpanzees, capuchin monkeys, and even dogs are sensitive to disadvantageous unfairness [Brosnan and De Waal, 2003; Range, et al., 2009; Tinklepaugh, 1928], suggesting that aversion to inequity is an evolutionary stable strategy. Humans, however, not only show strong aversion to inequity [Fehr and Schmidt, 1999], but also exhibit a capacity to feel similar emotions when others are treated unfairly [Lowwenstein, et al., 1989]. Despite the ubiquity of this phenomenon, few studies have investigated the sensitivity to advantageous inequity and its underlying neural mechanisms.
While chimpanzees show no evidence of sensitivity to advantageous inequality [Silk, et al., 2005], humans seem to consider other factors such as reputation management [Frith, et al., 2010], and their own current circumstances including whether they are winning or the financial status of the other player [Tricomi, et al., 2010]. A recent behavioral study found that individuals are willing to reduce or augment others' incomes at their personal costs even when there is no cooperative norm to advance [Dawes, et al., 2007]. It has also been shown that equal distribution is preferred to advantageous unequal distribution, which was preferred to disadvantageous unequal distribution in cooperative contexts [Messick and Sentis, 1985]. In other settings, advantageous inequity tends to produce as much satisfaction as equity [Lowwenstein, et al., 1989].
We used functional magnetic resonance imaging (fMRI) and a modified payoff distribution task in which two participants received the same payoff [fair equity condition (FE)] or different payoffs (advantageous inequality condition (AI): self > other, or disadvantageous inequality condition (DI): self < other), after equally contributing to a collective task (Fig. 1). Payoffs took the form of rewards or punishments. In this way, we sought to investigate the behavioral and neural responses to three types of distribution (i.e., AI, DI, and FE) in both win and loss domains (see Table 1). Importantly, subjects could not be punished for their response as the other player did not have ability to reward or punish the subject's responses. Thus, there was no consequence to their actions.
Figure 1.
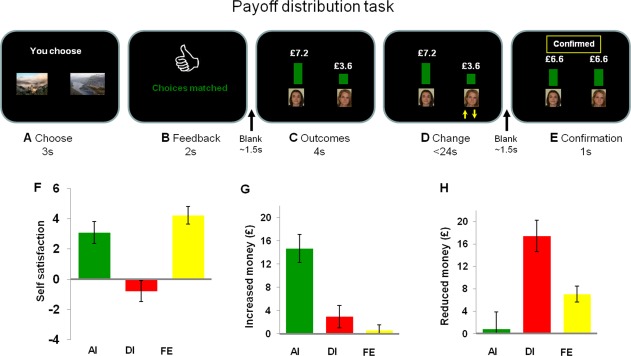
Experimental task design and behavioural results. A: In the payoffs distribution task, participants were required to choose one image. B: After the Choose stage, participants were informed whether their choices were matched or not and hence both win or both lose. C: The outcome for the participants and the outcome for their partners were presented. D: After the Outcome stage, participants could alter the partner's payoff at their personal costs. E: Participants pressed a third key when they finished changing. The final payoffs for both players were depicted. F: The self‐reported satisfaction toward outcomes across win and loss trails in AI, DI, and fair equal condition (FE). G: The increased money (total money spent to increase other's payoffs) in each condition. H: Reduced money (total money spent to decrease other's payoffs) in each condition.
Table 1.
The list of payoff pairs used in the payoff distribution task
| AI | FE | DI | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Win | Loss | Win | Loss | Win | Loss | ||||||
| Self | Other | Self | Other | Self | Other | Self | Other | Self | Other | Self | Other |
| 5.6 | 2.8 | −9.6 | −14.4 | 5.6 | 5.6 | −9.6 | −9.6 | 5.6 | 8.4 | −9.6 | −4.8 |
| 6.4 | 3.2 | −8.8 | −13.2 | 6.4 | 6.4 | −8.8 | −8.8 | 6.4 | 9.6 | −8.8 | −4.4 |
| 7.2 | 3.6 | −8 | −12 | 7.2 | 7.2 | −8 | −8 | 7.2 | 10.8 | −8 | −4 |
| 8 | 4 | −7.2 | −10.8 | 8 | 8 | −7.2 | −7.2 | 8 | 12 | −7.2 | −3.6 |
| 8.8 | 4.4 | −6.4 | −9.6 | 8.8 | 8.8 | −6.4 | −6.4 | 8.8 | 13.2 | −6.4 | −3.2 |
| 9.6 | 4.8 | −5.6 | −8.4 | 9.6 | 9.6 | −5.6 | −5.6 | 9.6 | 14.4 | −5.6 | −2.8 |
Each pair was presented once in block one and once in block two, respectively. The unit is £.
We reasoned that individuals' satisfaction with outcomes would be modulated by self‐other outcome difference, after controlling for the magnitude of their own reward, that is, participants receive the same amount of reward across different equality conditions. Thus, any effects in behavior and emotion should be explained by the relative reward between one's own reward and the other's reward but not by the amount of reward for oneself since the latter is identical across three main experimental conditions (i.e., AI, DI, and FE). Specifically, disadvantageous outcomes would be perceived negatively as being shown in a large number of studies both in humans and other animals [Brosnan and De Waal, 2003; Range, et al., 2009; Sanfey, et al., 2003; Tabibnia and Lieberman, 2007; Tinklepaugh, 1928]. Furthermore, being in advantageous situation may also produce negative emotions as discomfort may stem from being a target of threatening upward comparisons and the concern for being envied [Exline and Lobel, 1999]. We also predicted that participants would increase (i.e., costly giving) or reduce (i.e., costly taking) the payoffs of others in AI and DI condition, respectively [Dawes et al., 2007]. At the neural level, we reasoned that both types of inequality would activate brain regions sensitive to aversion, including insula and orbitofrontal cortex (OFC) [Calder et al., 2001; Rolls et al., 2001; Sanfey et al., 2003]. Further, we predicted that activation in reward related regions (e.g., ventral striatum) would be associated with the tendency to increase others' payoffs [Tricomi et al., 2010], whereas activation in aversion related regions (e.g., anterior insula) would be associated with the tendency to decrease others' payoffs [Calder et al., 2001; Phillips et al., 1997; Sanfey et al., 2003; Tabibnia and Lieberman, 2007].
MATERIALS AND METHODS
Participants
Nineteen healthy volunteers (11 female, mean age and SD 24.8 ± 4.47) participated in return for payment. All participants were right‐handed, fluent speakers of English and screened for psychiatric or neurological problems. The study was authorized by the National Health Service Local Research Ethics Committee for Cambridge. All participants gave written, informed consent.
Stimuli
Landscape images (1020) were downloaded online, carefully divided into 510 pairs. In a pilot study, for each image pair, 10 individuals were asked to choose one image, which they think most people would choose. To minimize the predictability of one' choice in each image pair, we selected image pairs in which one particular image was selected by half of the ten individuals. Seventy‐two image pairs were used in the experiment without replacement.
Experimental Paradigm
Before the scanning session, participants were introduced to their partner (confederate). The participant was asked to stand against the wall and a picture of him/her was taken using a digital camera. Similarly, a picture of the confederate was taken in the presence of the participant. Then, both the participant and the confederate were given a comprehensive description of the tasks they would perform. The participants were then placed inside the MRI scanner and began playing the payoff distribution task. The same confederate was used for all participants in this study. Using the same confederate minimizes the confounding factors in confederates.
At the beginning of each trial, the participants were presented two landscape images and they were required to choose one image from the two by pressing the left or right key within 3 s (see Fig. 1). They were told that if both players chosen the same image, they both would win some monetary reward. Otherwise, they both would receive some monetary punishment. It was emphasized that the images position in their screen and the position in their partner's screen were independently determined. Thus, the image positions provided no useful information for improving performance. After the 3 s Choose stage, participants were informed whether their choices were matched or not. The 2 s Feedback stage was followed by a blank screen for 1–2 s (randomly jittered in the interval 1–2 s). The outcome for the participants and the outcome for their partners were presented for 4 s. Participants were explicitly told that the outcomes were determined by the program such that in the win condition, the program randomly chose one reward pair from a reward pair pool, while in the loss condition, it chose one loss pair from a loss pair pool. It was further emphasized that for the level of payments, it plays no role, who clicked faster when choosing the images. After the Outcome stage, two yellow arrows, one arrow pointing up and the other pointing down, were presented underneath the partner's photograph, indicating that the self‐paced Change stage began. During this stage, participants could alter the partner's payoff at their personal costs. Every fifty pence increase or decrease in the partner's payoff cost participants ten pence. The relative positions of the up‐arrow and down‐arrow informed participants the mapping between left/right keys and increase/decrease functions. For example, when the up‐arrow was on the left side of the down‐arrow, pressing the left key once in the win condition would increase the partner's reward by 50 pence and decrease participants' reward by 10 pence, whereas in the loss condition it would increase the partner's loss by 50 pence and decrease participants' payoff by 10 pence. The associated current payoffs were immediately updated as participants pressed left/right keys. Participants pressed a third key when they finished changing. The final payoffs for both players were depicted for 1 second, followed by a blank screen for 1–3 s (randomly jittered in the interval 1–3 s). Participants were told that only they had the option to change payoffs and their partner could only watch the Change stage.
The payoff distribution task was equally divided into two blocks with 36 trials each. This allows us to test any strategy changes during the experiment. Unbeknown to the participants, the outcomes were predetermined such that there were 36 win trials and 36 loss trials in total. The 36 win trials were divided into 12 AI trials in which the ratio of the partner's payoff to participants payoffs r = 1/2, 12 fair trials r = 1, and 12 DI trials r = 3/2; similarly, the 36 loss trials were equally divided into three experimental conditions (see Table 1 for the details of all payoffs). All experiment conditions were randomized across subjects. Participants' payoffs were identical in all three conditions and that it was the other player's payoffs that were varied to produce the inequities.
At the end of the experiment, one trial was randomly chosen to implement and all subjects were paid the base payment (£60) plus the amount they received in that trial. Prior to scanning, participants read written instructions describing the sequence of events, the payoff rules, and the details of the different stages. After the subjects had read the instructions, we checked they understood the instructions by means of several hypothetical questions. All subjects answered the questions correctly.
Postscan Questionnaires
After the end of the experiment, participants were asked to indicate how satisfied they felt about different types of outcomes using a 20 point scale (from −10 most unsatisfied to 10 most satisfied).
Image Acquisition
MRI scanning was conducted at the Medical Research Council Cognition and Brain Sciences Unit on a 3‐Tesla Tim Trio Magnetic Resonance Imaging scanner (Siemens, Germany) using a standard head‐coil system. Whole‐brain data were acquired with echo planar T2*‐weighted imaging (EPI), sensitive to BOLD signal contrast (48 sagittal slices, 3 mm‐thickness; TR = 2400 ms; TE = 25 ms; flip angle = 90°; FOV = 224 mm; voxel size: 3 × 3 × 3 mm). To allow for equilibration effects the first 3 volumes were discarded. T1 weighted structural images were acquired at a resolution of 1 × 1 × 1 mm.
Image Preprocessing
SPM5 software (http://www.fil.ion.ucl.ac.uk/spm/) was used for data analysis. The EPI images were sinc interpolated in time for correction of slice timing differences and realigned to the first scan by rigid body transformations to correct for head movements. Field maps were estimated from the phase difference between the images acquired at the short and long TE and unwrapped, employing the FieldMap toolbox. Field map and EPI imaging parameters were used to establish voxel displacements in the EPI image. Application of the inverse displacement to the EPI images served in the correction of distortions. Using linear and nonlinear transformations, and smoothing with a Gaussian kernel of full‐width‐half‐maximum 8‐mm, EPI and structural images were coregistered and normalized to the T1 standard template in MNI space [montreal neurological institute (MNI)–International Consortium for Brain Mapping]. Global changes were removed by high‐pass temporal filtering with a cut‐off of 128 s to remove low‐frequency drifts in signal.
Statistical Analysis
After preprocessing statistical analysis was performed using the general linear model. Analysis was carried out to establish each participant's voxel‐wise activation during actual outcomes epochs. Activated voxels in each experimental context were identified using an event‐related statistical model representing the eight experimental conditions: two conditions at the Feedback stage: winning, and losing, and six conditions at the Outcome stage : AI_win, FE_win, DI_win, AI_loss, FE_loss, DI_loss. Regressors were convolved with a canonical hemodynamic response function (HRF) and mean‐corrected. Six head‐motion parameters defined by the realignment were added to the model as regressors of no interest. Multiple linear regression was then run to generate parameter estimates for each regressor at every voxel. A random effects analysis (one‐sample t test) was performed to analyze data at a group level. Small volume correction (svc) was used on a priori regions of interest. Ventral anterior cingulate cortex (vACC) is defined as an 8 mm sphere centered at MNI [x = ±10, y = 42, z = −10] [Passamonti et al., 2008]. The caudate, putamen, amygdala, and anterior insula were defined using the corresponding AAL mask [Tzourio‐Mazoyer et al., 2007]. Each ROI region was separately used in svc. Activations in other areas are reported if they survive P < 0.001 uncorrected, cluster size k > 10. For display purposes, all images are depicted at P < 0.005.
Conjunction Analysis
To find out brain regions that are commonly activated across the experimental conditions, that is, DI and AI, we used Conjunction Null analysis implemented in SPM5 [Friston et al., 2005; Nichols et al., 2005]. The null hypothesis for “Conjunction Null Hypothesis” is that “not all subjects/contrasts activated this pixel.” If the conjunction results are significant, the null hypothesis is rejected and the conclusion is that “all subjects/contrasts activated this pixel.” The threshold for individual contrast is P < 0.005 uncorrected. A logical AND requires that all the comparisons in the conjunction are individually significant. The Conjunction Null analysis is stricter than Conjunction Global, which tests “the null hypothesis” that “no subject/contrast activated this pixel” and therefore rejecting the null hypothesis leads to the conclusion that “at least one subject/contrast activated this pixel.”
Psychophysiological Interaction (PPI)
PPI is used to access the physiological connectivity between two brain regions that is also varied with the psychological context [Friston et al., 1997]. Here, we were interested in connectivity that is modulated by the context of viewing AI versus FE, as well as the context of viewing DI versus FE, separately. Thus, it constitutes a PPI [Friston et al., 1997]. We sought to identify “target” regions that showed differential connectivity according to the context with a source region. The PPI was time‐locked to the outcome displays before the Change displays. Thus, it is unlikely to be influenced by other time periods, for example, Change or Conformation.
Separate connectivity analyses (PPIs) were carried out, using the left putamen, identified by the AI minus FE modulated by increased money, and the left amygdala, identified by the DI minus FE modulated by reduced money, as the sources in each. We used the 4 mm spheres for all subjects (centers for all subjects in the left putamen: MNI, [x = −18, y = 8, z = −2], which the maximal voxel for the AI minus FE modulated by the amount of increased money across participants; in the left amygdala: MNI, [x = −24, y = −2, z = −14], the maximal voxel for the DI minus FE modulated by the amount of decreased money across participants). The BOLD time series for each subject was deconvolved to estimate a “neuronal time series” for the source region, using the PPI deconvolution parameter defaults in SPM5 [Gitelman et al., 2003].
The psychophysiological interaction term (PPI regressor) was calculated as the element‐by‐element product of the neuronal time series in the source region and a vector coding for the main effect. This product was reconvolved by the canonical HRF. The model also included the main effect convolved by the HRF, the neuronal time series for each source, and the movement regressors as effects of no interest. PPI models were run, and contrast images were generated for positive and negative PPIs. The identified regions had greater or lesser coupling with the source regions according to the context of AI (or DI) versus FE condition. Moreover, we sought to identify target regions for which the change in connectivity with the source region varied as a function of behavioral performance (i.e., increased money or decreased money). We refer to this latter analysis examining the interaction with alternation amount as a “higher‐order PPI” [Passamonti et al., 2008, 2009]. Note, here we aggregated the amount taken or given over all the trials within the conditions as an overall index of taking or giving behavior. Similar results were also found using the frequency of taking and giving as an index of willingness to change. Since the frequency of change was significantly correlated with the amount of change for taking and giving choices (reported below), both indexes are valid.
RESULTS
Behavioral Data
Repeated‐measures analysis of variance (ANOVA), with valence (win/loss) and fairness (AI/FE/DI) as two independent factors, was conducted for behavioral data. Participants rated the win condition as more rewarding than the loss condition, F(1,17) = 20.29, P < 0.001. The main effect of fairness was significant, F(2,34) = 23.29, P < 0.001. Valence did not interact with fairness conditions (P > 0.20). Post hoc pair‐wise comparisons revealed that participants rated themselves as feeling less satisfied in the DI condition (−0.78 ± 0.63) than in the AI condition (3.08 ± 0.75), t 17 = 3.86, P < 0.001, or than in the FE (4.22 ± 0.96) condition, t 17 = 5.00, P < 0.001. AI did not differ from FE (P > 0.40).
Because the act of payoff alternation comes at a cost, purely selfish subjects will not change others' payoff in any treatment. Importantly, participants in our experiment did not know their partner personally and there is no overt obligation for the participants to increase their partners' payoff at their own costs. Nevertheless, across the win/loss domains, post hoc pair‐wise comparisons show that participants gave an average of £14.68 ± 2.40 (mean ± SE) reward to their partner in the AI condition, significantly more than the £2.94 ± 1.95 in DI condition (t 17 = 2.76, P = 0.014) or the 0.57 ± 0.98 in the FE conditions, t 17 = 4.54, P < 0.001. The latter two did not differ, P > 0.2 (Fig. 1). Participants took £17.40 ± 2.81 from their partners in the DI condition, significantly more than the £0.82 ± 3.05 in AI condition, t 17 = 2.91, P = 0.010, or the £7.06 ± 1.40 in the FE condition, t 17 = 3.21, P = 0.005. The latter two did not differ, P > 0.1. No significant main effect or interaction effect involving win/loss valence was found, P values > 0.2. The correlation between the amount given and the amount taken approached significant, r = −0.45, P = 0.062.
Similar analyses were conducted for the frequency of taking or giving. Participants gave reward to their partner on 48% ± 0.09 (mean ± SE) of trials in the AI condition, significantly more than the 1.4% ± 0.008 in DI condition (t 17 = 5.03, P < 0.001) or the 2.3% ± 0.016 in the FE conditions, t 17 = 5.21, P < 0.001. The latter two did not differ, P > 0.2. Participants took from their partners in 34% ± 0.082 trials in the DI condition, significantly more than the 4% ± 0.013 in AI condition, t 17 = 3.48, P = 0.003, or the 13% ± 0.028 in the FE condition, t 17 = 3.83, P = 0.001. The latter two did not differ, P = 0.16. No significant main effect or interaction effect involving win/loss valence was found, P values > 0.2. The correlation between the frequency of giving and the frequency of taking approached significant, r = −0.428, P = 0.076.
The amount given was significant correlated with the frequency of giving in AI condition, P = 0.96, P < 0.001 and the amount taken was significant correlated with the frequency of taking in DI condition, r = 0.93, P < 0.001. The behavioral patterns did not changed significantly across the two blocks, P values > 0.3. Since the amounts given to or taken from the confederate equal the net money participants spent multiplied by 5, we did not report the later. No significant correlation was found between reduced satisfaction and the taking/giving behaviors (P values > 0.1).
fMRI Results
Feedback phase: winning vs. losing
During the initial Feedback phase, winning minus losing activated bilateral putamen, posterior cingulate cortex, and superior frontal gyrus (see Table 2). These regions have been consistently implicated in reward processing in different fMRI tasks [Fannon et al., 2000; McClure et al., 2004].
Table 2.
Brain regions activated by winning minus losing feedback in the payoff distribution task
| Brain regions | Z scores | MNI Coordinates | ||
|---|---|---|---|---|
| X | Y | Z | ||
| Winning minus Losing | ||||
| L Putamen | 4.59a | −12 | 12 | −8 |
| R Putamen | 4.14a | 14 | 12 | −8 |
| R Posterior Cingulate Cortex | 3.57 | 4 | −34 | 34 |
| L Superior Frontal Gyrus | 4.17 | −16 | 14 | 56 |
| Losing minus winning | ||||
| N/A | N/A | N/A | ||
All values P < 0.001 unc.
P < 0.05 FWE‐corr svc.
Outcome phase: effects of AI and DI
A whole‐brain analysis showed that AI minus FE activated the right putamen, left lateral orbital frontal cortex (lOFC), left anterior insula, and other regions (Fig. 2). DI minus FE activated the left anterior insula, right putamen, left orbitofrontal cortex, and other regions. No significant differences were found between the AI and DI conditions in a priori regions of interest. To identify areas of activation overlap, a conjunction analysis was performed (P < 0.005, voxel‐level uncorrected for the AI and DI unfairness conditions to FE condition separately). Conjunction analysis revealed that the right putamen, anterior insula, and the left lOFC were activated in both types of inequality conditions (see Table 3).
Figure 2.
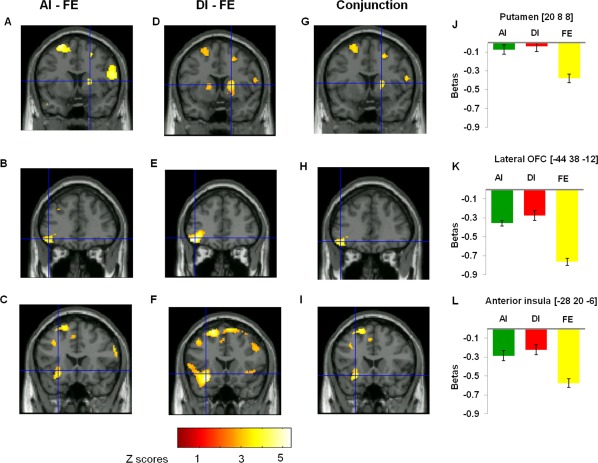
Main contrasts results. A–C: Right putamen left lateral OFC, and left anterior insula responses to AI minus FE contrast (AI‐FE). D – F: Right putamen, left lateral OFC, and left anterior insula responses to DI minus FE contrast (DI‐FE). G – I: The conjunction between (AI‐FE) and (DI‐FE). The plots represent the contrast estimates (beta parameters of the general linear model) in the conjunction analysis for the significant peaks (P < 0.05 after svc) for the right putamen (J), left lateral OFC (K), and anterior insula (L). For display purpose, images were thresholded at P < 0.001, uncorrected.
Table 3.
Brain regions activated in main contrasts in the payoff distribution task
| Brain Regions | Z scores | MNI Coordinates | ||
|---|---|---|---|---|
| X | Y | Z | ||
| AI minus FE | ||||
| R Inferior Frontal Gyrus | 3.78 | 62 | 16 | 28 |
| R Postcentral Gyrus | 3.29 | 54 | −26 | 40 |
| L Superior Frontal Gyrus | 3.57 | −26 | 8 | 66 |
| L Superior Patietal Cortex | 3.71 | −20 | −68 | 58 |
| L Lateral Orbitofrontal Cortex | 3.74a | −44 | 38 | −12 |
| R Putamen | 3.29a | 22 | 8 | 8 |
| L Anterior Insula | 3.68a | −28 | 20 | −12 |
| DI minus FE | ||||
| R Inferior Frontal Gyrus | 3.63 | 58 | 14 | 16 |
| L Superior Frontal Gyrus | 4.34 | −20 | 18 | 60 |
| R Superior Frontal Gyrus | 4.31 | 16 | 12 | 68 |
| R Precuneus | 4.15 | 16 | −62 | 56 |
| L Lateral Orbitofrontal cortex | 4.69a | −42 | 40 | −10 |
| R Putamen | 3.87a | 20 | 8 | 8 |
| L Anterior Insula | 3.44a | −30 | 18 | −6 |
| Conjunction of (AI – FE) and (DI – FE) | ||||
| L Superior Frontal Gyrus | 4.36 | −20 | 18 | 60 |
| L Inferior Parietal Cortex | 3.78 | −20 | −66 | 56 |
| L Lateral Orbitalfrontal Cortex | 3.74a | −44 | 38 | −12 |
| R Putamen | 3.34a | 20 | 8 | 8 |
| L Anterior Insula | 3.55a | −28 | 20 | −12 |
| FE – AI | ||||
| R Temporal Cortex | 5.86 | 10 | −80 | 2 |
| 4 | ||||
| FE – DI | ||||
| N/A | N/A | N/A | ||
| AI – DI | ||||
| N/A | N/A | N/A | ||
| DI – AI | ||||
| N/A | N/A | N/A | ||
All values P < 0.001 unc.
P < 0.05 FWE‐corr svc.
At the group‐level, we performed a whole‐brain analysis to identify areas that correlated with increased money in AI condition (or reduced money in the DI condition) at the time of outcome evaluation. For AI minus FE, the activity in left putamen (and also middle frontal gyrus and postcentral gyrus) predicted the amount participants spent to increase the other's payoff (see Fig. 3 and Table 4). For DI minus FE, the activity in left amygdala and right sub‐gyral predicted the amount participants spent to decrease other's payoff (see Fig. 3 and Table 4).
Figure 3.
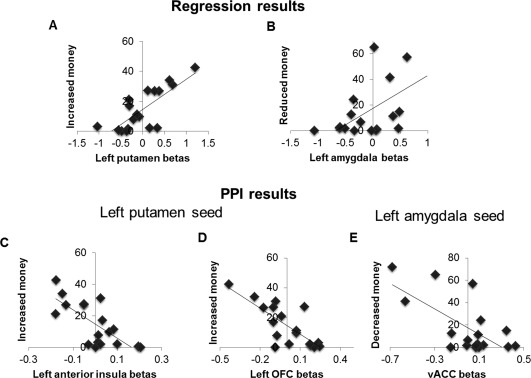
Regression results and PPI results. A: Left putamen activity predicted subsequent increased money in AI condition. B: Left amygdala activity predicted subsequent reduced money in DI condition. Higher‐order PPIs from the left putamen and left amygdala (seed regions). C: When viewing advantageous versus equal payoffs, subsequent increased money was negatively correlated with the change in connectivity between the left putamen and the left lateral OFC; (D) Similar negative correlation was found with the change in connectivity between the left putamen and left anterior insula; (E) When viewing disadvantageous versus equal payoffs, subsequent reduced money was negatively correlated with the change in connectivity between the left amygdala and the right vACC. For display purpose, images were thresholded at P < 0.001, uncorrected.
Table 4.
Regional brain responses to unfairness predicted subsequent payoff alternation (i.e., increased money and reduced money)
| Brain regions | Z scores | MNI Coordinates | ||
|---|---|---|---|---|
| X | Y | Z | ||
| AI minus FE modulated by increased money | ||||
| L Middle Frontal Gyrus | 4.13 | −22 | 0 | 68 |
| R Middle Frontal Gyrus | 4.09 | 26 | −2 | 56 |
| R Postcentral Gyrus | 4.62 | 60 | −20 | 38 |
| L Putamen | 3.73a | −22 | 8 | −2 |
| DI minus FE modulated by reduced money | ||||
| L Amygdala | 2.90a | −24 | −2 | −14 |
| R Superior Temporal Gyrus | 4.39 | 44 | −14 | −14 |
All values P < 0.001 unc.
P < 0.05 FWE‐corr svc.
Functional connectivity predicted payoff alternations
An initial whole‐brain analysis showed no significant changes in connectivity between the seed regions and other regions of the brain. Next, we examined whether any changes in connectivity might be modulated by variation in performance. Note that this distinct from the overall effect regardless of behavioral performance. For PPI comparing the AI and FE conditions showed that the amount of increased money across participants in the AI condition strongly modulated the change in connectivity between left putamen and left lOFC ([−24, 36, −8, peak z = 3.74, P < 0.05, svc] and left anterior insula ([−42, 4, 14, peak z = 3.41, P < 0.05, svc]; see Fig. 3). Individuals who spent more money to increase others' payoff showed a less positive change than those who spent less money. A separate PPI comparing the DI and FE conditions showed that the amount of reduced money strongly modulated the change in connectivity between amygdala and an area of vACC ([10, 50, −4, peak z = 3.01, svc]; see Fig. 3). Similarly, individuals who spent more money to decrease others' payoff had less positive values than those spent less money.
DISCUSSION
Our study investigated individuals' behavioral and brain responses to inequality, when they were in an advantageous or disadvantage position during inequity. That is, behavioral results show that individuals have strong preferences to equity in both reward allocation (e.g., monetary win) and liability distribution (e.g., monetary loss) such that they alter others' payoff toward more equal distribution, at their personal costs [Dawes et al., 2007]. They also reported less satisfaction to unequal distribution to equal division, either in AI or DI conditions. This suggests that both types of fairness are critical to social behavior and may relate to findings that positive reciprocal relationships promote continued cooperation [Nowak et al., 2009]. Another explanation might be that the positive emotions that go with AI, drive the individual to be nice [Nowak et al., 2008].
At the neural level, we found that both types of unfairness similarly activated the putamen at the outcome stage, which may signal the anticipatory pleasure of changing others' payoffs toward more equal distribution, as well as lOFC, and anterior insula, regions associated with aversion. These findings are surprising since intuitively advantageous outcomes, which are “good things” should be quite different from disadvantageous outcomes which are “bad things” in terms of the valence of outcomes. However, unequal outcomes should elicit mixed emotion in our tasks including the unpleasantness of in unequal situations and the expected pleasure of being able to make changes afterwards. Moreover, cognitive functions such as motor planning, working memory, cognitive controls, as well as social and affective functions all contribute to this complex outcome evaluation process. For example, the OFC is also involved in joint maintenance, manipulation, and monitoring of information in working memory [Barbey et al., 2011] and it may be activated by the larger amount of information to process in inequality conditions. Thus, it is important to examine the individual differences and to associate brain activity with certain behaviors to better understand the functional significance of the activity [Passamonti et al., 2008, 2009]. Individual difference analyses showed that individuals who spent more money to increase others' payoff had stronger activity in putamen when they encountered AI condition, as well as decreased functional connectivity between putamen and lOFC or anterior insula. Those who spent more money to reduce others' payoff had stronger activity in amygdala in response to DI, as well as less functional connectivity between amygdala and vACC. These dissociations suggest that while two types of inequality aversion are coded by similar brain areas, they possess different neural pathways.
We show that both AI and DI activated similar brain regions, including the anterior insula, lOFC, and putamen. Previous studies consistently found that insula encoded unfavorable disadvantageous unequal payoffs [Sanfey et al., 2003; Tabibnia and Lieberman, 2007] and unequal distribution among others [Civai et al., 2012], while the lOFC has been implicated in processing negative stimuli [Mobbs et al., 2006; O'Doherty et al., 2001]. Our results extend on previous fMRI studies by showing that AI elicited activity in regions implicated in aversion, similarly to those elicited by DI. One possibility is that the putamen encodes an unsigned prediction error on the other player's payoff relative to one's own reward rather than social inequality. Previous studies have found prediction error signals in putamen [Fannon et al., 2000; Hagger et al., 1999]. In our study, participants were fully aware of the unequal distribution situations and indeed unfairness trials (AI and DI trials) were more frequent than FE trails. Over the experiment, the prediction error account would predict that the error signals would be diminished after learning. Thus, simple prediction error account cannot fully explain the putamen signals in unfairness conditions. Nevertheless, it is still possible that social prediction error, which is the violation of one's social expectation based on fairness norms, activated the putamen. Given the unpredictability of reward distribution and the complex cognitive processes involved in the task, the prediction and prediction error may change dynamically across the experiment. A computational based approach may be used to capture the trial‐by‐trial changes in prediction and its neural correlates.
Another possibility is that the putamen may encode the efficiency (i.e., maximize some overall good). A previous study found that the putamen activation was significantly correlated with the efficiency of distributing goods to others when the participant's own payoff was unaffected by others' payoff [Hsu et al., 2008]. However, since in all three conditions participants have the power to change other's payoff, but we they did so mainly in inequality conditions, this account would predict equivalent putamen activity in all three conditions. For example, participants could also increase other's payoff in equality condition in order to maximize their overall gains. Thus, FE and AI should both activate the putamen according to the efficiency account. One interesting research topic is whether the putamen also encodes efficiency when self‐interest is involved, like in our current design. In this study, giving in AI condition reduces self‐interest, increase others' payoff, decreases inequity, and increases efficiency (i.e., total reward), whereas taking in DI condition reduces self‐interest, decrease others' payoff, decrease inequity, and decrease efficiency. Future studies may use factorial design to disentangle the neural correlates of these factors. An alternative interpretation of our results is that putamen activity signals the pleasure of giving or taking money away from others. For example, in the DI condition, putamen activity may indicate the pleasure of punishment [de Quervain et al., 2004; Singer et al., 2006], whereas in AI condition, putamen activity may indicate the pleasure of giving money to others and promoting social equality [Moll et al., 2006]. Indeed, in the AI condition, subsequent connectivity analysis showed negative coupling between putamen and lOFC‐insula network, suggesting that these regions have different functional significance in response to unfairness. Finally, the putamen is also involved in motor preparation and may reflect the planning of motor responses (e.g., changing others' payoff) in unfair conditions [Jankowski et al., 2009]. If this is the case, we would observe similar correlation between behavioral changes and putamen activity in both the AI and DI condition. However, such correlation was only significant in the AI condition, suggesting that the putamen activity cannot simply be explained by motor planning. Although our data do not allow us to directly test these three hypotheses, the significant correlations between putamen signals and behavioral performance (increased money in AI condition) do support the emotion account. It also worth mentioning that substantial past literature suggests that the putamen may vary functionally along its dorsal‐ventral/anterior‐posterior axes, since the putamen subregions tend to correspond to different “loops” with respect to the projections that they receive from/send to other parts of the brain [Alexander et al., 1986; Zhou et al., 2008]. The posterior putamen connects to the primary motor cortex and the supplementary motor area, whereas the anterior putamen exhibits patterns of connectivity with dorsolateral frontal regions and posterior parietal cortex, and the rostral ACC [Di Martino et al., 2008; Postuma and Dagher, 2006]. It is possible that the posterior putamen activated by inequality main effect also reflects general motor function and the anterior putamen activated by the correlation analysis is more associated with more high‐level social motives.
Amygdala‐vACC Pathway in DI
Previous studies found that activity in the insula responding to DI predicted subsequent taking behavior (e.g., rejection the unfair offer) [Sanfey et al., 2003; Tabibnia and Lieberman, 2007]. Only two recent studies found a connection between amygdala activity and inequality aversion [Gospic et al., 2011; Haruno and Frith, 2010]. In one study, activity in dorsal amygdala and the reward difference between self and the other was positively correlated in pro‐social individuals, but negatively correlated in individualists, measured by the social value orientation questionnaire [Haruno and Frith, 2010]. In another study, the rejection of a disadvantageous unfair proposal was associated with a higher activity in the right amygdala. The amygdala responses to unfair offers, together with the behavioral rejection rate, was diminished by benzodiazepine treatment [Gospic et al., 2011]. Our study found that amygdala activation in response to DI predicted costly taking (i.e., reduced money). Further, the functional connectivity between amygdala and vACC predicted reduction amount. Functional connections between the amygdala and vACC have been studied extensively [Beaver et al., 2008; Ghashghaei et al., 2007; Passamonti et al., 2008], and the functional interaction between these two regions is thought to be essential in emotional control, particularly during aversive events. Individual who spent more money to reduce others' reward may face the conflict between self‐utility and the desire to bring others down. The weaker functional connectivity between amygdala and vACC in high‐punishment individuals may indicate that these people have difficulty suppressing the negative emotion in amygdala in the DI condition.
Putamen‐Insula‐OFC Pathway in AI
At the group level, individual difference analysis found that activity in the left putamen predicted the participants' inclination to increase the other player's money in AI condition, whereas amygdala activation predicted reduced money in DI condition. Previous neuroimaging studies have shown that charitable contributions activate the striatum, as well as more anterior sectors of the prefrontal cortex [Moll et al., 2006]. A recent fMRI study found that individuals in advantage position (paid $ 50) showed stronger activity in striatum for monetary transferred to others than to self, whereas individuals in disadvantage position (paid $0) showed opposite effect [Tricomi et al., 2010]. Together, these studies suggest that the warm feeling derived from giving drives people to give/donate at their own costs in order to maintain social fairness norms.
Functional connectivity between putamen and insula‐lOFC was also negatively correlated with giving behaviors. One explanation is that the insula and lOFC may signal the negative emotion elicited by advantage unfairness. Envy appears to be an unpleasant, and hostile emotion that often prompts aggressive behaviors [Smith and Kim, 2007] and disliking others' wealth leads people to pay to destroy the envied person's money [van de Ven et al., 2009; Zizzo and Oswald, 2001]. On the other hand, when people are in AI condition, they could expect others to become envious and the destructive effects. People who do not like to cause such negative feelings in others may be distressed by their advantage position [van de Ven et al., 2009]. A recent study indicates that the insula is associated with the guilt feelings of betraying others' trust [Howard, 1998]. Anticipating the pleasure of giving (reducing guilt) may activate the putamen. By increasing others' payoffs, participants may also reduce the distress thereby resulting in reduced functional connectivity between putamen, insula, and lOFC). A previous study found that stronger ACC activation to envy was related to stronger striatum activation to schadenfreude in the later stage [Takahashi et al., 2009]. It is worth noting that the envy and schzdenfreude were experienced at different stages in that study. However, in our study, participants may experience both emotions within the same time frame. Thus, this may induce the competitive between these two emotions and thus produce reduce insula/LOFC‐putamen connectivity. Another study has shown that the insular cortex activations are not only significantly associated with egalitarian behavior inside the scanner, but also significantly correlated with self‐reported measure of egalitarianism and egalitarian behavior measured outside the scanner using a series of dictator games [Dawes et al., 2012]. Our findings show functional connectivity between insula and other brain regions predict egalitarian behaviors.
Advantageous/disadvantageous inequity not only violates the equity principle but also implies a higher/lower status than in an equitable state. Status‐related motives may also contribute to behavioral and neural responses in our study. Previous studies have shown that subjective socioeconomic status predicts human ventral striatal responses to social status information [Ly et al., 2011] and brain responses to superiority and inferiority are dissociable, even in the absence of explicit competition [Zink et al., 2008]. Thus, our results may also be explained by different social status manipulation in the AI and DI conditions. A recent study showed that AI activated insular regions, suggesting that the simultaneous weighing of equity and status concerns induces higher cognitive demands [Fliessbach et al., 2012]. Future studies may manipulate status in inequity experiments independently from monetary distributions in order to dissociate these effects.
Several limitations of the current study are worth mentioning. First, the outcome stage was followed the match/mismatch feedback stage and the BOLD signals associated with outcomes may be confounded by the residual signals from the feedback stage. However, the interaction between outcome valence (win/loss) and equality conditions was not significant both at the behavioral level and the neural level. Thus, our main findings of the inequality effects cannot simply be explained by the match/mismatch signals in the proceeding stage. Second, our design does not allow us to investigate the effect of the degree of unfairness. The ratio of the reward difference was kept constant in our study. Although both the absolute reward difference and the reward for self were changed across trials, the two factors were highly correlated and cannot be dissociated in the current design. In addition, it is unclear whether the degree of unfairness is determined by the ratio of the difference outcomes (e.g., the absolute difference between the reward for self and the reward for the other divided by the reward for self) or the absolute difference. However, the main focus of this study is to compare the two different inequality conditions while controlling for the amount of reward for oneself and the degree of unfairness. Third, although participants were only required to pay attention to the distribution outcomes and only made their responses at the Change stage, it is still possible that complicated emotional and cognitive processes, such deciding whether to give up pay to change the outcome and anticipating the final outcome, may be involved in the Outcome stage. However, disentangle such the emotional and cognitive processes, as well as experiencing and anticipating processes, could be very difficult in a social decision task. In real‐life situations, noting the unequal pay is always accomplished with negative emotion, cognitive reappraisal, planning to forgive or revenge, and anticipating the future events. The key findings in our study are two “opposite” types of inequality activated similar brain network. Indeed, our individual analysis also revealed that different brain regions were associated with individual differences in different subsequent behavior (taking or giving). Thus, our neuroimaging data should be interpreted as reflecting a complex outcome evaluation process involving both emotional and cognitive components rather than a ‘pure’ evaluation process. In this study, we cannot rule out that the observed brain activity may simply reflect nonsocial cognitive functions, such as motor planning and working memory. Ideally, it would be good to include a non‐social version of the task as a control condition, for example, a computer agency. However, previous studies have shown that people may treat computers as social agency and respond to unfairness in computer conditions [Sanfey et al., 2003]. Thus, a neutral situation that involves no social emotions is difficult to achieve. Fourth, the number of our sample size limits our ability to examine other interesting individual differences such as gender effect. Future studies could also examine the possible gender difference with a larger sample size. Finally, our findings are correlational and do not support causal links between brain regions and certain behaviors or emotions. Other research methods such as transcranial magnetic stimulation and lesion patient studies are needed to further test our conclusions and to establish strong brain‐behavior relationships.
Altruistic punishment (i.e., costly taking) and altruistic reward (i.e., costly giving) are crucial in the evolution of human cooperation [Fehr and Fischbacher, 2003]. One argument for prosocial components of advantageous inequity is that it increases the chance of future cooperative interactions. Indeed, while social exchange games have shown that punishment drives cooperation, so do positive interactions between subjects [Nowak et al., 2009]. Because the act of payoff alternation comes at a cost and participants in our experiment did not know their partner personally, their behaviors cannot be simply explained by selfish incentives to benefit from cooperation in the future. The preference for equality persisted across gain and loss domain, and regardless of one's advantage or disadvantage position, suggesting that it is a robust phenomenon. However, equality preference is just one of human motives that guide human behaviors. It may or may not be the strongest motive in humans. Other motives such as greed may still drive humans.
Our studies identified separate neural substrates associated with altruistic punishment and altruistic reward in response to DI and AI, respectively. This provides insight into why prosocial punishment and prosocial helping both seem to be such pervasive and fundamental features of human social exchange.
Conflict of Interest: All authors have no conflict of interest.
REFERENCES
- Alexander GE, DeLong MR, Strick PL (1986): Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 9:357–381. [DOI] [PubMed] [Google Scholar]
- Barbey AK, Koenigs M, Grafman J (2011): Orbitofrontal contributions to human working memory. Cereb Cortex 21:789–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaver JD, Lawrence AD, Passamonti L, Calder AJ (2008): Appetitive motivation predicts the neural response to facial signals of aggression. J Neurosci 28:2719–2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosnan SF, De Waal FB (2003): Monkeys reject unequal pay. Nature 425:297–299. [DOI] [PubMed] [Google Scholar]
- Calder AJ, Lawrence AD, Young AW (2001): Neuropsychology of fear and loathing. Nat Rev Neurosci 2:352–363. [DOI] [PubMed] [Google Scholar]
- Civai C, Crescentini C, Rustichini A, Rumiati RI (2012): Equality versus self‐interest in the brain: Differential roles of anterior insula and medial prefrontal cortex. Neuroimage 62:102–112. [DOI] [PubMed] [Google Scholar]
- Dawes CT, Fowler JH, Johnson T, McElreath R, Smirnov O (2007): Egalitarian motives in humans. Nature 446:794–796. [DOI] [PubMed] [Google Scholar]
- Dawes CT, Loewen PJ, Schreiber D, Simmons AN, Flagan T, McElreath R, Bokemper SE, Fowler JH, Paulus MP (2012): Neural basis of egalitarian behavior. Proc Natl Acad Sci USA 109:6479–6483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Quervain DJF, Fischbacher U, Treyer V, Schelthammer M, Schnyder U, Buck A, Fehr E (2004): The neural basis of altruistic punishment. Science 305:1254–1258. [DOI] [PubMed] [Google Scholar]
- Di Martino A, Scheres A, Margulies DS, Kelly AM, Uddin LQ, Shehzad Z, Biswal B, Walters JR, Castellanos FX, Milham MP (2008): Functional connectivity of human striatum: a resting state FMRI study. Cereb Cortex 18:2735–2747. [DOI] [PubMed] [Google Scholar]
- Exline JJ, Lobel M (1999): The perils of outperformance: Sensitivity about being the target of a threatening upward comparison. Psychol Bull 125:307–337. [DOI] [PubMed] [Google Scholar]
- Fannon D, Riley E, Doku V, O'Ceallaigh S, Tennakoon L, Chitnis X, McGovern D, Sharma T (2000): Cognitive impairment predicted by the degree of premorbid deterioration in first episode schizophrenia. Schizophr Res 41:45. [Google Scholar]
- Fehr E, Fischbacher U (2003): The nature of human altruism. Nature 425:785–791. [DOI] [PubMed] [Google Scholar]
- Fehr E, Schmidt KM (1999): A theory of fairness, competition, and cooperation. Q J Econ 114:817–868. [Google Scholar]
- Fliessbach K, Phillipps CB, Trautner P, Schnabel M, Elger CE, Falk A, Weber B (2012): Neural responses to advantageous and disadvantageous inequity. Front Human Neurosci 6:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ (1997): Psychophysiological and modulatory interactions in neuroimaging. Neuroimage 6:218–229. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Penny WD, Glaser DE (2005): Conjunction revisited. Neuroimage 25:661–667. [DOI] [PubMed] [Google Scholar]
- Frith CD, Tennie C, Frith U (2010): Reputation management in the age of the world‐wide web. Trends Cogn Sci 14:482–488. [DOI] [PubMed] [Google Scholar]
- Ghashghaei H, Hilgetag CC, Barbas H (2007): Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage 34:905–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitelman DR, Penny WD, Ashburner J, Friston KJ (2003): Modeling regional and psychophysiologic interactions in fMRI: The importance of hemodynamic deconvolution. Neuroimage 19:200–207. [DOI] [PubMed] [Google Scholar]
- Gospic K, Mohlin E, Fransson P, Petrovic P, Johannesson M, Ingvar M (2011): Limbic justice‐amygdala involvement in immediate rejection in the Ultimatum Game. PLoS Biol 9:e1001054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagger C, Friedman L, Kenny J, Hyde J, Buckley P (1999): Cognitive impairment in substance‐abusing patients with schizophrenia. Schizophr Res 36:133. [Google Scholar]
- Haruno M, Frith CD (2010): Activity in the amygdala elicited by unfair divisions predicts social value orientation. Nat Neurosci 13:160–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard R (1998): Cognitive impairment in late life schizophrenia: A suitable case for treatment? Int J Geriatr Psych 13:400–404. [DOI] [PubMed] [Google Scholar]
- Hsu M, Anen C, Quartz SR (2008): The right and the good: Distributive justice and neural encoding of equity and efficiency. Science 320:1092–1095. [DOI] [PubMed] [Google Scholar]
- Jankowski J, Scheef L, Huppe C, Boecker H (2009): Distinct striatal regions for planning and executing novel and automated movement sequences. Neuroimage 44:1369–1379. [DOI] [PubMed] [Google Scholar]
- Lowwenstein G, Thompson L, Bazerman MH (1989): Social utility and decision making in interpersonal contexts. J Pers Soc Psychol 57:426–441. [Google Scholar]
- Ly M, Haynes MR, Barter JW, Weinberger DR, Zink CF (2011): Subjective socioeconomic status predicts human ventral striatal responses to social status information. Curr Biol 21:794–797. [DOI] [PubMed] [Google Scholar]
- McClure SM, Laibson DI, Loewenstein G, Cohen JD (2004): Separate neural systems value immediate and delayed monetary rewards. Science 306:503–507. [DOI] [PubMed] [Google Scholar]
- Messick DM, Sentis KP (1985): Estimating social and nonsocial utility functions from ordinal data. Eur J Soc Psychol 15:389–399. [Google Scholar]
- Mobbs D, Weiskopf N, Lau HC, Featherstone E, Dolan RJ, Frith CD (2006): The Kuleshov effect: The influence of contextual framing on emotional attributions. Soc Cogn Affect Neurosci 1:95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll J, Krueger F, Zahn R, Pardini M, de Oliveira‐Souza R, Grafman J (2006): Human fronto‐mesolimbic networks guide decisions about charitable donation. Proc Natl Acad Sci USA 103:15623–15628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB (2005): Valid conjunction inference with the minimum statistic. Neuroimage 25:653–660. [DOI] [PubMed] [Google Scholar]
- Nowak MA, Dreber A, Rand DG, Fudenberg D (2008): Winners don't punish. Nature 452:348–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak MA, Rand DG, Dreber A, Ellingsen T, Fudenberg D (2009): Positive interactions promote public cooperation. Science 325:1272–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C (2001): Abstract reward and punishment representations in the human orbitofrontal cortex. Nat Neurosci 4:95–102. [DOI] [PubMed] [Google Scholar]
- Passamonti L, Rowe JB, Ewbank M, Hampshire A, Keane J, Calder AJ (2008): Connectivity from the ventral anterior cingulate to the amygdala is modulated by appetitive motivation in response to facial signals of aggression. Neuroimage 43:562–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passamonti L, Rowe JB, Schwarzbauer C, Ewbank MP, von dem Hagen E, Calder AJ (2009): Personality predicts the brain's response to viewing appetizing foods: The neural basis of a risk factor for overeating. J Neurosci 29:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Young AW, Senior C, Brammer M, Andrew C, Calder AJ, Bullmore ET, Perrett DI, Rowland D, Williams SC, Gray JA, David AS (1997): A specific neural substrate for perceiving facial expressions of disgust. Nature 389:495–498. [DOI] [PubMed] [Google Scholar]
- Postuma RB, Dagher A (2006): Basal ganglia functional connectivity based on a meta‐analysis of 126 positron emission tomography and functional magnetic resonance imaging publications. Cereb Cortex 16:1508–1521. [DOI] [PubMed] [Google Scholar]
- Range F, Horn L, Viranyi Z, Huber L (2009): The absence of reward induces inequity aversion in dogs. Proc Natl Acad Sci USA 106:340–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, O'Doherty J, Kringelbach ML, Hornak J, Andrews C (2001): Abstract reward and punishment representations in the human orbitofrontal cortex. Nat Neurosci 4:95–102. [DOI] [PubMed] [Google Scholar]
- Sanfey AG, Rilling JK, Aronson JA, Nystrom LE, Cohen JD (2003): The neural basis of economic decision‐making in the Ultimatum Game. Science 300:1755–1758. [DOI] [PubMed] [Google Scholar]
- Silk JB, Brosnan SF, Vonk J, Henrich J, Povinelli DJ, Richardson AS, Lambeth SP, Mascaro J, Schapiro SJ (2005): Chimpanzees are indifferent to the welfare of unrelated group members. Nature 437:1357–1359. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O'Doherty JP, Stephan KE, Dolan RJ, Frith CD (2006): Empathic neural responses are modulated by the perceived fairness of others. Nature 439:466–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RH, Kim SH (2007): Comprehending envy. Psychol Bull 133:46–64. [DOI] [PubMed] [Google Scholar]
- Tabibnia G, Lieberman MD (2007): Fairness and cooperation are rewarding: Evidence from social cognitive neuroscience. Ann N Y Acad Sci 1118:90–101. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Kato M, Matsuura M, Mobbs D, Suhara T, Okubo Y (2009): When your gain is my pain and your pain is my gain: neural correlates of envy and schadenfreude. Science 323:937–939. [DOI] [PubMed] [Google Scholar]
- Tinklepaugh OL (1928): An experimental study of representative factors. J Comp Psychol 197–236. [Google Scholar]
- Tricomi E, Rangel A, Camerer CF, O'Doherty JP (2010): Neural evidence for inequality‐averse social preferences. Nature 463:1089–1091. [DOI] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer N, Herve PY, Mazoyer B (2007): Neuroanatomy: Tool for functional localization, key to brain organization. Neuroimage 37:1059–1060; discussion 1066‐8. [DOI] [PubMed] [Google Scholar]
- van de Ven N, Zeelenberg M, Pieters R (2009): Leveling up and down: the experiences of benign and malicious envy. Emotion 9:419–429. [DOI] [PubMed] [Google Scholar]
- Zhou FW, Matta SG, Zhou FM (2008): Constitutively active TRPC3 channels regulate basal ganglia output neurons. J Neurosci 28:473–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink CF, Tong Y, Chen Q, Bassett DS, Stein JL, Meyer‐Lindenberg A (2008): Know your place: Neural processing of social hierarchy in humans. Neuron 58:273–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zizzo DJ, Oswald A (2001): Are people willing to pay to reduce others' income? Ann Economie et de Statistique 63:39–65. [Google Scholar]


