Abstract
Factor replacement therapy for the treatment of moderate to severe haemophilia A and B can be complicated by the production of inhibitory alloantibodies to factor VIII (FVIII) or factor IX. Treatment with the nanofiltered anti-inhibitor coagulant complex, Factor Eight Inhibitor Bypassing Activity (FEIBA NF), is a key therapeutic option for controlling acute haemorrhages in patients with high-titre inhibitors or low-titre inhibitors refractory to replacement therapy. Given the high risk for morbidity and mortality in haemophilia patients with inhibitors to FVIII or FIX, we conducted this Phase 3 prospective study to evaluate whether prophylaxis with FEIBA NF is a safe and effective treatment option. Over a 1-year period, 17 subjects were treated prophylactically (85 ± 15 U kg−1 every other day) while 19 subjects were treated on demand. The median (IQR) annualized bleeding rate (ABR) during prophylaxis was 7.9 (8.1), compared to 28.7 (32.3) during on-demand treatment, which amounts to a 72.5% reduction and a statistically significant difference in ABRs between arms (P = 0.0003). Three (17.6%) subjects (ITT) on prophylaxis experienced no bleeding episodes, whereas none treated on demand were bleeding episode-free. Total utilization of FEIBA NF for the treatment of bleeding episodes was significantly higher during on-demand therapy than prophylaxis (P = 0.0067). There were no differences in the rates of related adverse events between arms. This study demonstrates that FEIBA prophylaxis significantly reduces all types of bleeding compared with on-demand treatment, and the safety of prophylaxis is comparable to that of on-demand treatment.
Keywords: FEIBA, haemophilia A/B, inhibitors, on-demand, Prophylaxis
Introduction
The development of inhibitory alloantibodies to factor VIII (FVIII) or factor IX (FIX) is the most serious and challenging complication in haemophilia patients and a major cause of morbidity and mortality as bleeds are difficult to treat 1. The presence of inhibitors generally precludes the efficacious use of FVIII and FIX replacement therapy, especially in patients with high-responding and high-titre inhibitors, due to an anamnestic response. Bleeding in patients with high- or low-titre inhibitors refractory to replacement therapy is treated with bypassing agents such as anti-inhibitor coagulant complex (AICC) or activated recombinant factor VII (rFVIIa).
Prophylactic therapy with FVIII in severe haemophilia A patients without inhibitors has been shown to be highly beneficial in reducing bleeding episodes and is considered standard of care 1–3. Haemophilia A and B patients with inhibitors may also benefit from prophylactic therapy. Previous reports have shown that prophylaxis with AICC, Factor Eight Inhibitor Bypassing Activity (FEIBA) or rFVIIa reduces morbidity and improves general health 4–9 and health-related quality of life (HRQoL) 9–12. A recent prospective clinical study (PRO-FEIBA) with a crossover design showed that FEIBA prophylaxis over a 6-month period was associated with statistically significant reductions in all bleeding episodes (62%), haemarthroses (61%), and target joints (72%) in haemophilia A patients with high-titre inhibitors 13. This study was designed to compare the efficacy, safety and HRQoL of a prophylactic regimen of the nanofiltered FEIBA NF, to an on-demand regimen over a 12-month observation period.
Materials and methods
Patients
This study is registered at http://ClinicalTrials.gov (NCT00851721). The protocol was approved by the ethics committees of 17 participating institutions, and written consent was obtained from patients before enrolment.
Patients were eligible for participation in the study if they had haemophilia A or B with documented history of high-titre inhibitor (>5 BU) or low-titre inhibitor (≤5 BU) refractory to increased dosing of either FVIII or FIX for at least 12 months; were ≥4 and ≤65 years of age; were currently being treated on-demand with bypassing agents; had ≥12 bleeding episodes in the previous 12 months; and a negative human immunodeficiency virus (HIV) status, or if positive, with a stable CD4 count. Patients were excluded from the study if they had symptomatic liver disease; had platelet count <100 000 mL−1; were currently receiving ITI or prophylaxis; needed elective surgery; needed alpha-interferon or protease inhibitor use; or had previous thromboembolic events. Although the study was open-label, a blocked randomization scheme stratified by geography with 1:1 allocation to each arm (12 months ± 14 days of prophylactic treatment, or 12 months ± 14 days of on-demand treatment) was generated. The use of a centralized and blocked randomization scheme was to result in a balanced number of subjects in each treatment arm.
Study design
This was a Phase 3, randomized, multicentre, open-label, two-arm parallel study comparing prophylaxis to an on-demand regimen. The prophylactic dose and dosing regimen was based on a review of literature on the use of FEIBA in the prevention of bleeding in patients with haemophilia A with inhibitors to FVIII 5–7. The primary outcome was a reduction in the annualized bleeding rate (ABR) among subjects on prophylaxis as compared to those treated on-demand over a period of 12 months ± 14 days. Secondary outcome measures included the following: number of bleeding episodes in joints, target joints (i.e. ankles, knees, elbows and/or hips with ≥4 bleeds over 6 months), and other anatomical locations; occurrence of new target joints; pain, joint range of motion (ROM) and haemostatic efficacy; total FEIBA NF utilization and the number of infusions required to treat a bleeding episode; pharmacoeconomics and HRQoL; and safety.
Treatments
Prophylaxis dosing was 85 ±15 U kg−1 by i.v. bolus infusion every other day. On-demand dosing as well as dosing for the treatment of bleeding while on prophylaxis was dependent upon the type of bleeding and was at the discretion of the investigator (as described in the Supporting Information).
Clinical, pharmacoeconomic, and HRQoL assessments
Descriptions of bleeding episodes (including aetiology, severity and anatomical site), pharmacoeconomic data, adverse events (AEs) and FEIBA NF infusion details were recorded in subject diaries and verified by the investigator. Each episode may have included more than one joint or non-joint anatomical site. Haemostatic efficacy was assessed by the number of infusions used to treat each episode and by the subject's rating based on a 4-point ordinal scale (excellent, good, fair, or none; see full descriptions in Supporting Information). Joint ROM measurements and clinical chemistry testing were performed at screening, 6 months and termination.
Factor VIII and FIX inhibitor assessments (Nijmegen assay modification of the Bethesda assay 14), complete blood count and coagulation tests were performed every 3 months; clinically significant abnormal values were reported as AEs. Viral serology testing for HIV type 1 and 2, hepatitis A virus (HAV), hepatitis B virus (HBV), hepatitis C virus (HCV) and parvovirus B19 (B19V) was performed at baseline and termination.
Health-related quality of life was assessed at screening, 6 months and at termination using the EQ-5D 15, Haem-A-QoL 16, Haemo-QoL 17, and visual analogue scale (VAS) for general pain assessment 18,19.
Statistical analyses
The sample size calculation assumed that 30 subjects (15 per arm) would provide greater than 80% power to detect a reduction of 50% in ABRs during prophylaxis compared with on-demand therapy. To account for a 12% subject dropout, at least 34 subjects were planned for randomization.
Efficacy analyses were performed with two analysis sets: intent-to-treat (ITT), which included all subjects who were randomized (used for primary analysis); and per-protocol (PP), which included all subjects who completed the treatment period with ≥80% adherence to dose and frequency of dose. ABRs were transformed using the square root of the number of bleeding episodes observed (X = bleeds per year), X′ = √(X + 0.5) to stabilize the variance and align the sample distribution with the assumption of normality inherent in using the t-test. The difference in mean transformed ABRs was used to perform statistical tests and to generate P–values (two-sample, two-sided t-test with a significance level of 5%). To further assess differences in ABRs between regimens, a negative binomial mixed model was used to evaluate the number of bleeding episodes as the dependent variable with the logarithm of time as the offset variable and regimen as the independent variable. A repeated effect over time was included because each subject contributed multiple observations during their treatment period.
Secondary efficacy outcome measures were, for the most part, descriptively compared between arms for all subjects with at least one treated bleeding episode. In addition, HRQoL and general pain assessments and pharmacoeconomic parameters were descriptively compared between arms for all randomized subjects with any available assessments. Safety assessments were descriptively compared between arms for all subjects who received at least one infusion of FEIBA NF.
Results
Subjects
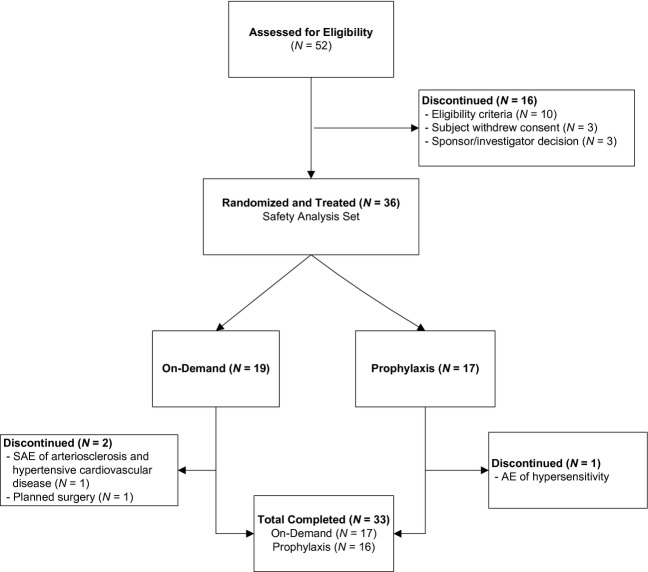
A total of 36 subjects were randomized and treated with FEIBA NF (17 in the prophylaxis arm and 19 in the on-demand arm) and they comprised the efficacy and HRQoL ITT and safety analysis sets. For details on subject disposition, refer to Fig.1.
Figure 1.
Subject disposition flow diagram.
All treated subjects were male and their median age was 23.5 years (range: 7–56). Overall demographic and baseline characteristics were similar between arms (Table1).
Table 1.
Subject characteristics
|
N (%) of Treated Subjects |
|||
|---|---|---|---|
| All (36 subjects) | On-demand (19 subjects) | Prophylaxis (17 subjects) | |
| Age | |||
| ≥7 and <12 years | 5 (13.9) | 2 (10.5) | 3 (17.6) |
| ≥12 and <16 years | 4 (11.1) | 2 (10.5) | 2 (11.8) |
| ≥16 years of age | 27 (75.0) | 15 (78.9) | 12 (70.6) |
| Race | |||
| White | 29 (80.6) | 14 (73.7) | 15 (88.2) |
| Asian | 3 (8.3) | 2 (10.5) | 1 (5.9) |
| Black or African American | 2 (5.6) | 2 (10.5) | 0 (0.0) |
| Other | 2 (5.6) | 1 (5.3) | 1 (5.9) |
| Ethnicity | |||
| Non-hispanic or non-latino | 32 (88.9) | 17 (89.5) | 15 (88.2) |
| Hispanic or Latino | 4 (11.1) | 2 (10.5) | 2 (11.8) |
| Haemophilia type | |||
| Haemophilia A | 33 (91.7) | 17 (89.5) | 16 (94.1) |
| Haemophilia B | 3 (8.3) | 2 (10.5) | 1 (5.9) |
| Severity of haemophilia | |||
| Moderate | 3 (8.3) | 2 (10.5) | 1 (5.9) |
| Severe | 33 (91.7) | 17 (89.5) | 16 (94.1) |
| Number of target joints | |||
| 0 | 9 (25.0) | 5 (26.3) | 4 (23.5) |
| 1 | 14 (38.9) | 8 (42.1) | 6 (35.3) |
| 2–3 | 8 (22.2) | 4 (21.1) | 4 (23.5) |
| ≥4 | 5 (13.9) | 2 (10.5) | 3 (17.6) |
Median (minimum–maximum) weight-adjusted dose per infusion was 81 U kg−1 (72–91) in the prophylaxis arm and 70 U kg−1 (50–106) in the on-demand arm, and the dose for treating breakthrough bleeding during prophylaxis was 82 U kg−1 (48–97). The median (minimum–maximum) total number of units administered per subject was 904 354 U (35 432–1 384 714) in the prophylaxis arm and 305 740 U (46 640–714 913) in the on-demand arm. Thus, total product usage in the prophylaxis arm was 3.0-fold greater than usage in the on-demand arm.
Primary outcome
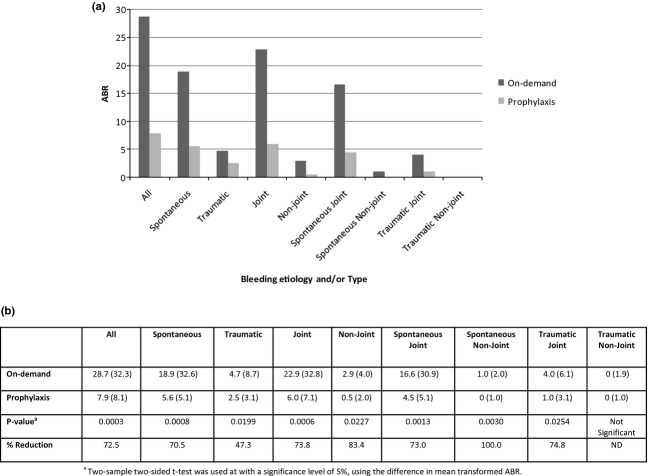
During the 12-month study period, 196 bleeding episodes occurred during prophylaxis and 629 occurred during on-demand therapy. Three of the 17 (17.6%) prophylaxis subjects (ITT analysis set) were bleeding episode-free during the study; of these, two completed the study (12 months) and one was in study for 2.5 weeks. None of the 19 on-demand subjects were bleeding episode-free. The median (IQR) ABR for the on-demand arm was 28.7 (32.3) compared to 7.9 (8.1) for the prophylaxis arm, which amounts to a 72.5% reduction and a statistically significant difference in ABRs between arms (P = 0.0003, see Fig.2). These results support the primary objective of this study.
Figure 2.
Comparison of ABRs (ITT analysis set). (a) Median ABRs during treatment regimens. (b) Median (IQR) ABRs and per cent reductions during Prophylaxis vs. On-demand therapies.
To further confirm these results, an evaluation of the influence of treatment regimen on the number of bleeding episodes over time revealed an increased incidence of bleeding associated with the on-demand regimen (negative binomial mixed effects model; coefficient = 0.9993, standard error = 0.2945, P = 0.0007).
When analysed by bleeding aetiology and/or type, statistically significant differences between on-demand and prophylaxis regimens were observed for spontaneous (P = 0.0008), traumatic (P = 0.0199), joint (P = 0.0006), and non-joint (P = 0.0227) bleeding episodes, as well as spontaneous-joint (P = 0.0013), spontaneous-non-joint (P = 0.0030) and traumatic-joint bleeding episodes (P = 0.0254). Median (IQR) ABRs and relative per cent reductions in ABRs for the prophylaxis arm compared with the on-demand arm are shown in Fig.2.
In the prophylaxis arm, median (IQR) ABRs were higher during the first 6 months of treatment than the last 6 months of treatment (8.0 [13.5] vs. 5.9 [19.1]; not significant).
A post hoc analysis compared the number of bleeding episodes in subjects who reported ≥12 historical episodes during a 12-month, prestudy period of on-demand therapy with bypassing agents with the number of episodes during the 12-month study period. A ≥50% reduction in the number of bleeding episodes was observed in 12 of 16 prophylaxis subjects vs. 2 of 19 on-demand subjects, which is consistent with the PRO-FEIBA study's definition of a good responder 13. The median per cent change for all bleeds between arms was statistically significant in favour of prophylactic therapy (−14.1% vs. −64.4%, P = 0.0001).
Secondary outcomes
Occurrence of new target joints
A new target joint was defined as an ankle, knee, elbow or hip joint in which ≥4 bleeding episodes occurred in the same joint within a 6 month period during the study and was not a target joint at study initiation. The occurrence of new target joints was substantially lower in the prophylaxis arm than in the on-demand arm; 7 new target joints in 5/17 [29.4%] prophylaxis subjects [range per subject: 1–2] vs. 23 new target joints in 11/19 [57.9%] on-demand subjects [range per subject: 1–6] (Fig.3).
Figure 3.
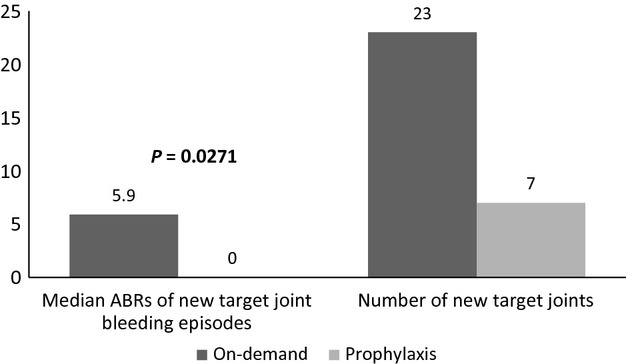
New target joints and associated bleeding episodes.
Incidence of bleeding episodes by site
The majority of bleeding episodes occurred in joints (572 during on-demand therapy and 171 during prophylaxis) vs. other non-joint anatomical locations (57 during on-demand therapy and 25 during prophylaxis). In the on-demand arm, 243/572 (42.48%) joint bleeding episodes occurred in new target joints, while in the prophylaxis arm, 81/171 (47.37%) occurred in existing target joints. The median (IQR) ABR for new target joints was higher in the on-demand arm (5.9 [12.9]) than in the prophylaxis arm (0 [4.1]); this difference was statistically significant (P = 0.0271).
Treatment of bleeding
The proportions of bleeding episodes treated with 1–2, 3, or ≥4 infusions were similar between arms, as were the proportion of treatments rated excellent/good, fair, and none. The majority (78.5%) of all bleeding episodes were treated with 1–2 infusions and the majority (87.1%) of treatments were rated by the subject as having excellent or good haemostatic efficacy at the 24 ± 1 h post infusion (Table2). The median (IQR) total utilization of FEIBA NF to treat bleeding episodes was significantly higher during on-demand therapy (4049.7 [5083.9] U kg−1) than prophylaxis (1524.9 [2590.2] U kg−1) (P = 0.0067).
Table 2.
Haemostatic efficacy of treatment
| All | On-demand | Prophylaxis | |
|---|---|---|---|
| Number of infusions to treat bleeding episodes (%) | |||
| 1–2 | 78.5 | 78.0 | 80.3 |
| 3 | 9.4 | 10.0 | 7.5 |
| ≥4 | 12.1 | 12.0 | 12.1 |
| Bleeding episode efficacy ratings at 24 ± 1 h (%) | |||
| Excellent/good | 87.1 | 90.2 | 75.7 |
| Fair | 6.0 | 5.8 | 6.9 |
| None | 0.1 | 0.2 | 0 |
| Not done | 6.5 | 3.9 | 16.2 |
| Not available | 0.3 | 0 | 1.2 |
h, hour(s).
HRQoL and ROM
There were no statistically significant differences between the prophylaxis and on-demand arms for the HRQoL and ROM results when controlling for multiple comparisons; the study was not statistically powered to show a difference for these outcome measures due to the small sample size and limited observation period offered by the 12-month study (ROM). Subjects in the prophylaxis arm reported mean (±SD) improvement in HRQoL as measured by EQ-5D Index and EQ-VAS scores (0.08 [±0.26] and 15.7 [±18.68] respectively) at termination, which can be considered clinically meaningful (minimally important difference threshold = 0.07 points and 7.0 points respectively 20,21), while subjects in the on-demand arm did not (−0.01 [±0.25] and 5.8 [±21.30] respectively). Compared to on-demand, prophylaxis subjects had greater mean (±SD) improvement in Haem-A-QoL total score (9.5 [±12.77] vs. 6.1 [±15.41]) and in 8 of 10 Haem-A-QoL domains, greater mean (±SD) reduction in general pain scores (23.2 [±46.61] vs. 5 [±28.70], a 3.6-fold reduction), and lower mean (±SD) number of days lost from school/work due to bleeding episodes (8.8 [±14.42], vs. 16.4 [±25.76]). The number of hospitalizations for bleeding episodes was similar between arms. ROM in the three key joints (ankles, knees and elbows) was maintained or improved over the course of the study in both arms.
Safety
Among the 36 treated subjects, no thromboembolic events or major safety issues were identified. Furthermore, an evaluation of laboratory markers of thrombogenicity did not reveal any apparent trends over time.
Twenty-three (23; 63.9%) subjects reported a total of 104 AEs, of which 74 (71.2%) in 17 (47.2%) subjects were non-serious AEs and 30 (28.8%) in 13 (36.1%) subjects were serious adverse events (SAEs; see Table3). One fatal SAE attributed to atherosclerotic and hypertensive cardiovascular disease was reported in a 51-year-old on-demand subject after 5 months in the study. This event was considered unlikely related to FEIBA NF due to the absence of temporal association (at least 11 days between treatment and last known contact before the reported death) with product exposure. One subject discontinued the study due to hypersensitivity reaction considered possibly related to FEIBA NF. The median (IQR) rates of related AEs per year were similar in the on-demand (0.000 [0.000]) and the prophylaxis (0.000 [0.979]) arm.
Table 3.
Serious adverse events and related non-serious adverse events
| Preferred term | On-demand (N = 19) |
Prophylaxis (N = 17) |
All (N = 36) | ||
|---|---|---|---|---|---|
| Number of AEs | N (%)* | Number of AEs | N (%)* | N (%)† | |
| Serious adverse events | |||||
| Abdominal wall haematoma | 0 | 0 (0.0) | 1 | 1 (5.9) | 1 (2.8) |
| Cholecystitis acute | 1 | 1 (5.3) | 0 | 0 (0.0) | 1 (2.8) |
| Haematoma infection | 1 | 1 (5.3) | 0 | 0 (0.0) | 1 (2.8) |
| Femoral neck fracture | 1 | 1 (5.3) | 0 | 0 (0.0) | 1 (2.8) |
| HBsAb positive | 1‡ | 1 (5.3) | 4§ | 4 (23.5) | 5 (13.9) |
| Arthropathy | 1 | 1 (5.3) | 0 | 0 (0.0) | 1 (2.8) |
| Haemarthrosis | 3 | 1 (5.3) | 8 | 2 (11.8) | 3 (8.3) |
| Muscle haemorrhage | 0 | 0 (0.0) | 1 | 1 (5.9) | 1 (2.8) |
| Haematuria | 1 | 1 (5.3) | 0 | 0 (0.0) | 1 (2.8) |
| Epistaxis | 1 | 1 (5.3) | 0 | 0 (0.0) | 1 (2.8) |
| Catheter removal | 1 | 1 (5.3) | 0 | 0 (0.0) | 1 (2.8) |
| Arteriosclerosis | 1 | 1 (5.3) | 0 | 0 (0.0) | 1 (2.8) |
| Haematoma | 0 | 0 (0.0) | 2 | 1 (5.9) | 1 (2.8) |
| Haemorrhage | 1 | 1 (5.3) | 0 | 0 (0.0) | 1 (2.8) |
| Hypertensive crisis | 1 | 1 (5.3) | 0 | 0 (0.0) | 1 (2.8) |
| Related non-serious adverse events | |||||
| Headache | 20 | 1 (5.3) | 0 | 0 (0.0) | 1 (2.8) |
| Dizziness | 1 | 1 (5.3) | 0 | 0 (0.0) | 1 (2.8) |
| Hypersensitivity | 0 | 0 (0.0) | 1 | 1 (5.9) | 1 (2.8) |
| Hypotension | 0 | 0 (0.0) | 1 | 1 (5.9) | 1 (2.8) |
| Rash | 0 | 0 (0.0) | 1 | 1 (5.9) | 1 (2.8) |
AE, adverse event; N (%), number of subjects (% of subjects); HBsAb, hepatitis B surface antibody.
Per cent relative to total number of subjects exposed to FEIBA NF within each arm.
Per cent relative to total number of subjects exposed to FEIBA NF.
This SAE was considered related to administration of FEIBA NF.
Three of these four SAEs were considered related to administration of FEIBA NF (two by investigators and one by sponsor).
Of the subjects evaluated for changes in inhibitor classification (i.e. from low to high titre or from high to low titre) at screening and termination, the majority (23/31) of haemophilia A subjects and 2/3 haemophilia B subjects did not change their classification. One on-demand subject and two prophylaxis subjects had high-titre inhibitors at screening that changed to low-titre inhibitors by termination. Two subjects in the on-demand arm (2.3–6.1 BU and 4.9–79.6 BU) and one in the prophylaxis arm (3.7–9.5 BU) had a rise in inhibitor levels from low to high titre. In three subjects, two haemophilia A and one haemophilia B subject, a low-titre inhibitor at screening was no longer detectable at study termination.
An unexpected finding in this study was the HBsAb positive results in seven subjects at termination. In three of these subjects, there was a history of HBV vaccination or prior HBV infection. Of the remaining four subjects, two reverted back to an HBsAb negative titre 2 months after the study completion. All four subjects were tested for HBcAb, HBsAg and HBV DNA by PCR and were found to be negative, indicating an absence of HBV infection. On the basis of extensive analysis of the immunoglobulin content of FEIBA NF, the titres of HBsAb in the retention plasma pools of lots tested and the detection level of the HBsAb assay, we believe that passive transfer of HBsAb from FEIBA NF provides a highly plausible explanation for the positive serology in some subjects. No subject manifested signs of an active HBV infection.
Discussion
The prophylactic regimen of FEIBA NF in haemophilia A and B subjects with inhibitors yielded a statistically significant and clinically relevant reduction in the number of all bleeding episodes when compared in a prospective manner to the on-demand regimen. In particular, the data showed reductions in the rate of bleeding episodes of all aetiologies and types during prophylaxis vs. on-demand therapy, with the exception of traumatic non-joint bleeding episodes. A secondary analysis (negative binomial mixed effects model) further confirmed an increased incidence of bleeding was associated with the on-demand regimen. Moreover, median (IQR) ABRs in the prophylaxis arm were higher during the first 6 months of treatment than the last 6 months of treatment (8.0 [13.5] vs. 5.9 [19.1]), suggesting that longer duration of prophylaxis may further reduce bleeding in some patients.
Overall, our primary outcome data complements the results of the recent prospective PRO-FEIBA study. However, the shorter duration of observation in PRO-FEIBA may not have accounted for possible seasonal effects (6 months vs. 12 months), showing a smaller reduction in the number of all bleeding episodes (62% vs. 72.5%) during prophylaxis. While the demographic characteristics of respective study populations were similar, efficacy results presented here should be interpreted in the light of differences in study designs (i.e. parallel vs. crossover). Interestingly, the interval between prophylactic dosing in both studies (every 48–72 h) exceeded the pharmacodynamic half-life of FEIBA as determined by thrombin generation assays (approximately 6 h) 22, and both prophylactic regimens were shown to be effective.
As expected for subjects in this study, the majority (90%) of bleeding episodes occurred in joints. Of the haemarthroses that occurred in subjects treated prophylactically, most were in existing target joints, while the majority of those in subjects treated on-demand were in new target joints. Thus, a statistically significant difference in the ABR in new target joints was observed between arms. Importantly, the development of new target joints was less common in the prophylaxis arm than on-demand arm. Therefore, we hypothesize that prophylaxis could prevent repeated joint bleeding and the development of new target joints, which is particularly promising for inhibitor patients with absent or minimal joint damage who start long-term primary prophylaxis at an early age 7,23,24.
The haemostatic efficacy of the treatment of bleeding episodes was similar among regimens, which is consistent with the results of FVIII usage in haemophilia A subjects without inhibitors 25–27.
Total FEIBA usage during the every-other-day prophylaxis regimen was comparable to usage during the thrice-weekly regimen of the PRO-FEIBA study in haemophilia A subjects with FVIII inhibitors (3.0- vs. 2.4-fold greater than on-demand usage) 13, and even to prophylactic FVIII usage in haemophilia A subjects without inhibitors (2.4- to 3.1-fold greater than on-demand usage) 28. The increased usage costs associated with FEIBA NF prophylaxis should be weighed against the benefits of this treatment option as reported in this study and the literature (reduction in bleeding episodes, morbidity, improvement in general health and HRQoL) 4–7,10–13.
While this study was not powered to show statistically significant differences in HRQoL and ROM, a greater tendency for improvement was noted in subjects during prophylaxis with respect to: EQ-5D Index and VAS scores; Haem-A-QoL total score; scores for 8 of 10 Haem-A-QoL domains; general pain scores; and number of days lost from work or school due to bleeding episodes. Furthermore, ROM for the three key joints (ankles, knees and elbows) was maintained or improved over the course of the study in both arms. This study was not designed to address long-term effects of prophylaxis on the progression of joint disease.
FEIBA has two viral inactivation steps in its manufacturing process, minimizing any potential risks of hepatitis viral transmission. Detailed monitoring of HBV serology and PCR testing implemented throughout this study detected four HBsAb positive cases, which were attributed to passive transfer of antibodies from plasma used in the manufacture of FEIBA. This interpretation is fully consistent with the current extensive HBV vaccination history of plasma donors. The passive transfer of these antibodies is transitory (which can explain the reversion to HBsAb negative titre in two of four subjects) and is based on the half-life of IgG and continued administration of high doses of FEIBA NF or other plasma products. These four cases represent transient hepatitis B surface antibody seroconversion and not viral transmission (active HBV infection).
There were also no thromboembolic events reported and no notable trends in thrombotic markers observed during the study further supporting the safety profile of FEIBA NF in terms of potential viral infections and thromboses.
Conclusion
A prophylactic regimen with FEIBA NF was shown to be effective and safe in the reduction in bleeding, including bleeding in joints and target joints and development of new target joints in patients with persistent high-titre inhibitors and low-titre inhibitors refractory to FVIII or FIX treatment. Taken together, this study and that of Leissinger et al. 13 provide basis for optimism that the therapy of patients with inhibitors can be significantly improved with prophylaxis leading to better orthopaedic and overall outcomes.
Acknowledgments
The authors recognize the investigators who participated in the study: T.J. Lissitchkov (Bulgaria); M. Timofeeva, S. Sadkov, and F. Perina (Russia); S. Zupancic-Salek (Croatia); A. Skotnicki and A. Klukowska (Poland); M. Serban and L. Rusen (Romania); L. Boggio and A. Schmaier (USA); M. Shima and M. Taki (Japan); and M.S. Renni (Brazil). The following Baxter employees made significant contributions: L. Patrone for critical review; J. Dyck-Jones, R. Dwarakanath, J. Doralt and M. Sharkhawy for report preparation.
Author contributions
JP, VM, OS, and SV Antunes performed the study and critically reviewed the manuscript. WYW, ST, BE, and NG-B designed the study, analysed and interpreted data and critically reviewed the manuscript. NG-B performed the statistical analyses. AG interpreted data and prepared the manuscript.
Disclosures
N. Guzmán-Becerra, A. Grigorian, S. Tangada, B. Ewenstein, and W. Y. Wong are employees of Baxter Healthcare Corporation, the sponsor of the study. S. V. Antunes received honoraria from Baxter Healthcare Corporation, Bayer HealthCare Pharmaceuticals, Grifols Brasil Ltda. and Novo Nordisk. V. Mamonov, J. Phillips and O. Stasyshyn received honoraria or research funding from Baxter Healthcare Corporation.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Table S1. On-demand dosing guidelines.
Table S2. Rating scale for treatment of bleeding episodes.
References
- 1.Srivastava A, Brewer AK, Mauser-Bunschoten EP, et al. Treatment Guidelines Working Group. The World Federation of Hemophilia. Guidelines for the management of hemophilia. Haemophilia. 2013;19:e1–14. doi: 10.1111/j.1365-2516.2012.02909.x. [DOI] [PubMed] [Google Scholar]
- 2.Berntorp E, Boulyjenkov V, Brettler D, et al. Modern treatment of haemophilia. Bull World Health Organ. 1995;73:691–701. [PMC free article] [PubMed] [Google Scholar]
- 3.National Hemophilia Foundation. National Hemophilia Foundation (NHF); 2007. Recommendation concerning prophylaxis (Regular administration of clotting factor concentrate to prevent bleeding); Report No.: MASAC 179. [Google Scholar]
- 4.DiMichele D, Negrier C. A retrospective postlicensure survey of FEIBA efficacy and safety. Haemophilia. 2006;12:352–62. doi: 10.1111/j.1365-2516.2006.01284.x. [DOI] [PubMed] [Google Scholar]
- 5.Hilgartner MW, Makipernaa A, DiMichele DM. Long-term FEIBA prophylaxis does not prevent progression of existing joint disease. Haemophilia. 2003;9:261–8. doi: 10.1046/j.1365-2516.2003.00771.x. [DOI] [PubMed] [Google Scholar]
- 6.Valentino LA. FEIBA prophylaxis for patients with haemophilia and inhibitors. Haemophilia. 2006;12(Suppl. 5):26–31. [Google Scholar]
- 7.Leissinger CA, Becton DL, Ewing NP, Valentino LA. Prophylactic treatment with activated prothrombin complex concentrate (FEIBA©) reduces the frequency of bleeding episodes in paediatric patients with haemophilia A and inhibitors. Haemophilia. 2007;13:249–55. doi: 10.1111/j.1365-2516.2007.01442.x. [DOI] [PubMed] [Google Scholar]
- 8.Valentino LA. The benefits of prophylactic treatment with APCC in patients with haemophilia and high-titre inhibitors: a retrospective case series. Haemophilia. 2009;15:733–42. doi: 10.1111/j.1365-2516.2009.01980.x. [DOI] [PubMed] [Google Scholar]
- 9.Konkle BA, Ebbesen LS, Erhardtsen E, et al. Randomized, prospective clinical trial of recombinant factor VIIa for secondary prophylaxis in hemophilia patients with inhibitors. J Thromb Haemost. 2007;5:1904–13. doi: 10.1111/j.1538-7836.2007.02663.x. [DOI] [PubMed] [Google Scholar]
- 10.Hoots WK, Ebbesen LS, Konkle BA, et al. Secondary prophylaxis with recombinant activated factor VII improves health-related quality of life of haemophilia patients with inhibitors. Haemophilia. 2008;14:466–75. doi: 10.1111/j.1365-2516.2008.01654.x. Novoseven (F7HAEM-1505) Investigators. [DOI] [PubMed] [Google Scholar]
- 11.Ekert H, Brewin T, Boey W, Davey P, Tilden D. Cost-utility analysis of recombinant factor VIIa (NovoSeven) in six children with long-standing inhibitors to factor VIII or IX. Haemophilia. 2001;7:279–85. doi: 10.1046/j.1365-2516.2001.00502.x. [DOI] [PubMed] [Google Scholar]
- 12.Scalone L, Mantovani LG, Mannucci PM, Gringeri A COCIS Study Investigators. Quality of life is associated to the orthopaedic status in haemophilic patients with inhibitors. Haemophilia. 2006;12:154–62. doi: 10.1111/j.1365-2516.2006.01204.x. [DOI] [PubMed] [Google Scholar]
- 13.Leissinger C, Gringeri A, Antmen B, et al. Anti-inhibitor coagulant complex prophylaxis in hemophilia with inhibitors. N Engl J Med. 2011;365:1684–92. doi: 10.1056/NEJMoa1104435. [DOI] [PubMed] [Google Scholar]
- 14.Verbruggen B, Novakova I, Wessels H, Boezeman J, van den Berg M, Mauser-Bunschoten E. The Nijmegen modification of the Bethesda assay for factor VIII: C inhibitors: improved specificity and reliability. Thromb Haemost. 1995;73:247–51. [PubMed] [Google Scholar]
- 15.Hurst NP, Kind P, Ruta D, Hunter M, Stubbings A. Measuring health-related quality of life in rheumatoid arthritis: validity, responsiveness and reliability of EuroQol (EQ-5D) Br J Rheumatol. 1997;36:551–9. doi: 10.1093/rheumatology/36.5.551. [DOI] [PubMed] [Google Scholar]
- 16.Gringeri A, Mantovani L, Mackensen SV. Quality of life assessment in clinical practice in haemophilia treatment. Haemophilia. 2006;12(Suppl. 3):22–9. doi: 10.1111/j.1365-2516.2006.01257.x. [DOI] [PubMed] [Google Scholar]
- 17.von Mackensen S, Bullinger M Haemo-Qol Group. Development and testing of an instrument to assess the quality of life of children with haemophilia in Europe (Haemo-QoL) Haemophilia. 2004;10(Suppl. 1):17–25. doi: 10.1111/j.1355-0691.2004.00875.x. [DOI] [PubMed] [Google Scholar]
- 18.Choiniere M, Amsel R. A visual analogue thermometer for measuring pain intensity. J Pain Symptom Manage. 1996;11:299–311. doi: 10.1016/0885-3924(95)00204-9. [DOI] [PubMed] [Google Scholar]
- 19.Beyer JE, Aradine CR. Patterns of pediatric pain intensity: a methodological investigation of a self-report scale. Clin J Pain. 1987;3:130–41. [Google Scholar]
- 20.Walters SJ, Brazier JE. Comparison of the minimally important difference for two health state utility measures: EQ-5D and SF-6D. Qual Life Res. 2005;14:1523–32. doi: 10.1007/s11136-004-7713-0. [DOI] [PubMed] [Google Scholar]
- 21.Pickard AS, Neary MP, Cella D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual Life Outcomes. 2007;5:70. doi: 10.1186/1477-7525-5-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Varadi K, Negrier C, Berntorp E, et al. Monitoring the bioavailability of FEIBA with a thrombin generation assay. J Thromb Haemost. 2003;1:2374–80. doi: 10.1046/j.1538-7836.2003.00450.x. [DOI] [PubMed] [Google Scholar]
- 23.Escuriola Ettingshausen C, Kreuz W. Early long-term FEIBA prophylaxis in haemophilia A patients with inhibitor after failing immune tolerance induction: a prospective clinical case series. Haemophilia. 2010;16:90–100. doi: 10.1111/j.1365-2516.2009.02116.x. [DOI] [PubMed] [Google Scholar]
- 24.Aledort LM, Haschmeyer RH, Pettersson H. A longitudinal study of orthopaedic outcomes for severe factor-VIII-deficient haemophiliacs. The Orthopaedic Outcome Study Group. J Intern Med. 1994;236:391–9. doi: 10.1111/j.1365-2796.1994.tb00815.x. [DOI] [PubMed] [Google Scholar]
- 25.Blanchette VS, Shapiro AD, Liesner RJ, et al. Plasma and albumin free recombinant factor VIII (rAHF-PFM): pharmacokinetics, efficacy and safety in previously treated pediatric patients. J Thromb Haemost. 2008;6:1319–26. doi: 10.1111/j.1538-7836.2008.03032.x. [DOI] [PubMed] [Google Scholar]
- 26.Shapiro A, Gruppo R, Pabinger I, et al. Integrated analysis of safety and efficacy of a plasma and albumin-free recombinant factor VIII (rAHF-PFM) from six clinical studies in patients with hemophilia A. Expert Opin Biol Ther. 2009;9:273–83. doi: 10.1517/14712590902729392. [DOI] [PubMed] [Google Scholar]
- 27.Valentino LA, Mamonov V, Hellmann A, et al. The Prophylaxis Study Group. A randomized comparison of two prophylaxis regimens and a paired comparison of on-demand and prophylaxis treatments in hemophilia A management. J Thromb Haemost. 2012;10:359–67. doi: 10.1111/j.1538-7836.2011.04611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gringeri A, Lundin B, von Mackensen S, Mantovani L, Mannucci PM ESPRIT Study Group. A randomized clinical trial of prophylaxis in children with hemophilia A (the ESPRIT Study) J Thromb Haemost. 2011;9:700–10. doi: 10.1111/j.1538-7836.2011.04214.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. On-demand dosing guidelines.
Table S2. Rating scale for treatment of bleeding episodes.