Abstract
Background: Previous studies have shown that morphine consumption during pregnancy may delay embryo development or cause abnormal nervous system function. Objective: The present study focused on the effect of maternal morphine consumption on development of placenta and blood corticosteron concentration in addictive pregnant mothers.
Materials and Methods: 24 female rats, 170-200g weight, were used. The experimental groups after pregnancy received an oral dose of 0.05 mg/ml of morphine by tap water while the control group received only tap water. On 10th and 14th day of pregnancy, rats were anesthetized and placenta removed surgically, 1ml blood was collected from each pregnant mother from retro-orbital sinus, the concentration of blood corticosteron was determined by corticosteron Elisa kit after centrifugation. The fixed tissue was processed, sectioned and stained with hematoxylin and eosin. Placenta was studied microscopically according to the thickness of layers, area of blood cisterns, and the number of cells.
Results: Comparing the plasma corticosteron concentration of the treatment and the control groups, not only a severe increase in the treatment group was detected, but also the thickness of maternal and embryonic portions of the placenta at day 10th and 14th of gestation was different significantly (p≤0.05). Furthermore, an increase in number of cells in maternal and embryonic portion of placenta and a decrease in blood cistern area were demonstrated in both the experimental and the control groups.
Conclusion: The effects of morphine, including an increase in blood concentration of corticosteron, in dependent pregnant mothers were seen. Development of placenta in the experimental group was delayed.
Key Words: Placentafetalportion, Placentamaternalportion, Bloodcisterns, Morphine, Rat, Corticosteron.
Introduction
Dependence on addictive drugs spread all over the world and the side effects of addiction, it is necessary to study the function of drugs in animal trials especially in placenta. Many behavioral problems in addicted mother’s infants indicated the effects of opioids on embryo (1, 2). The majority of studies focused on the embryo, whereas they neglected to study the placenta as an important organ. Disruptive effects of consumption of opioids in human samples and laboratory animals were well conducted. The research showed that consumption of opiate materials by pregnant mothers cause delay in embryonic development and malfunctions, such as spinabifida (1, 3). In accordance to previous studies, high blood corticosteron concentration of pregnant mother attenuate placenta and embryo. The capacity of placenta for displacement and releasing food materials depends on the placenta’s shape, size and transferring factors. As morphine is small and imploring molecule, it can easily pass through blood barrier and placenta and then become effective on embryonic cells (4-6). As placenta in mammals is the most important part to exchange materials between embryonic and maternal blood, the size of the placenta is directly related to food material transporting (simple and active transport) (2, 4, 6). The morphine effects were presented with Mio, Kappa and Delta opioids receptors and activating of these receptors caused several changes, including decrease in the CAMP, an increase in output of -K+ ion and a decrease in input of Ca-ion (7, 8).
On the other hand, the ca-ion has important role in secretion of estrogen and progesterone hormone from placenta, stableness and embryonic development (9, 10). By progress of pregnancy, placenta can act as a gland that secrets progesterone, estrogen and other enzymes that are needed for embryonic development and considered as an alternative for ovary secretion hormones (6, 11). Therefore, morphine can interfere and causes dysfunction in placental secretion operating and delay in placental development (5, 10). Several experiments have shown that morphine administration cause the decrease of placenta weight in rabbits (12, 13). The presence of opioids receptors on the placental villi cells can affect placental function. On the other hand, because the placenta is a protective barrier, it can prevent the input or output of some materials. Placental barrier as a protective mechanism is often considered against pathogenic factors (2, 14, 15). Disorders in the development of placenta cause placental disability in exchanging endocrine and protective acts in embryo function (6). Corticosteron hormone stimulates morphine function (6, 9).
The importance of mother’s blood corticosteron concentration in placenta development and the effects of morphine on delay of placental development are the major reasons for the present research that consider the oral morphine administrations effect on the placenta of addictive mothers on 10th and 14th days of pregnancy.
Materials and methods
Female Wister rats (W: 170-200 g) were used. The animals were housed 2/cage to a temperature of (24 ±10C) and controlled environment with a 12-h light/dark cycle and were provided with food and water. This Experimental study was accomplished with financial support of Baqyiatallah (a.s.) University of Medical Sciences as a thesis project. All experiments were conducted in accordance with standard ethical guidelines and approved by the local ethical committee (The Baqiyatallah (a.s.) University of Medical Sciences Committee on the Use and Care of Animals, 86/143, Apr 15, 2007).
In this study, prepared morphine sulphate from Iran TEMAD Co, was used orally. Twenty-four female Wistar rats were divided into four equal groups consisting of six animals each. The female rats were mated into 2 groups with one adult male rat. After pregnancy (with the observation of vaginal plug and existence of sperm in vagina), they were separated from male rats the next morning and kept in the same coed-groups. Thereafter (0 day of pregnancy), experimental group received a daily dose of 0.05mg/ml morphine (5 mg per 1000 ml potable water from city pipeline for 12 rats) (15). The amounts of oral morphine were 14ml/100gr rat body weight. Also, 1ml bloods were collected from retro-orbital sinus at 13th days of pregnancy. Blood samples were centrifuged, and plasma was extracted and keeps in -20OC. Finally plasma corticosterone was assessed by Rat Corticosterone ELISA kit (EIA-4164; DRG Instruments GmbH, Germany) in both experimental groups. In 10th and 14th days of pregnancy, the animals were anesthetized with chloroform and placenta were removed and transferred to 10% formalin solution for 10 days, then, placenta were put in tissue processing molding, then blocks were sagittaly sectioned with 5um thickness and serially by microtome (15, 16). These slices were put on slides and stained with hematoxylin and eosin methods (H&D). After staining, slides were studied microscopically.
Statistical analysis
Data were reported as mean± SEM. Differences between all groups were calculated by a one-way analysis of variance (ANOVA) and post-hoc Duncan test by using the SPSS/PC computer program (version 9.1). Statistical significance between the two measurements was determined by the two-tailed unpaired sample t-Test.
Result were considered statistically significant when p< 0.05. The thickness of portion placenta, blood cisterns surface, and number of cells in the experimental and control groups was measured with MOTIC software. The system used included a microscope connected to a computer and a monitor with software which could take photos from slides. Subsequently, the number of cells on each layer was counted randomly and compared with that of the experimental groups.
Results
Our results showed that the oral morphine consumption in pregnant rats increased the placenta concentration of corticosteron at day 13th of pregnancy in comparison with control group. In addition morphine administration in pregnant rats
placenta showed, thickness of maternal part has increased in 10th and 14th days placentae (Table I). In contrast, significant decrement in thickness of placenta in embryonic part was shown (Table I). Also, increment in cells number of placenta in maternal and embryonic part in experimental groups was assessed (Table I), thereby decrement in blood pools surface of placenta in maternal and embryonic part in 10th day experimental groups was shown in this study (Table I).
Table I.
Indicates effect of administration of oral morphine on placenta and plasma corticosterone concentration in rats
| Placenta/Day |
Maternal
thickness ( µm) |
Fetal thickness
( µm) |
Maternal cell
number (count/ unit) |
Fetal cell
number (count/ unit) |
Maternal
lacuna area ( µ ²) |
Fetal
Lacuna area (µ ²) |
Plasma concentration
(ml ) |
|---|---|---|---|---|---|---|---|
| Control 10th | 873.85 ±114 | 677.6 ±36 | 13 ±0.1 | 5±0.1 | 45307 ± 0.1 | 27046 ±0.1 | - |
| Experiment 10th | 1064.55 ±197* | 205.2 ±33* | 13 ±0.1** | 7 ± 0.1* | 20300 ± 1210** | 19525±491** | - |
| Control 14th | 574.92 ±26 | 1394.67±0 | 17±1 | 17 ± 0.5 | 21979.3 ±317 | 7970.54 ±164 | - |
| Experiment 14th | 2351 ±173*** | 533. ±0*** | 24±5** | 24±2* | 21935.5 ±242 | 8383.1 ±118 | - |
| Plasma Control 13th | - | - | - | - | - | - | 650 ± 45 |
| Plasma Experiment 13th | - | - | - | - | - | - | 1230 ±52** |
Result were considered statistically significant when p< 0.05. (Mean ± SEM).
Effect of administration of oral morphine on the10, 14th days of placenta portions thickness and cell number and lacuna area development furthermore, Plasma corticosterone concentration in rats which received oral morphine on the 13th day of pregnancy and comparison of control and experimental groups.
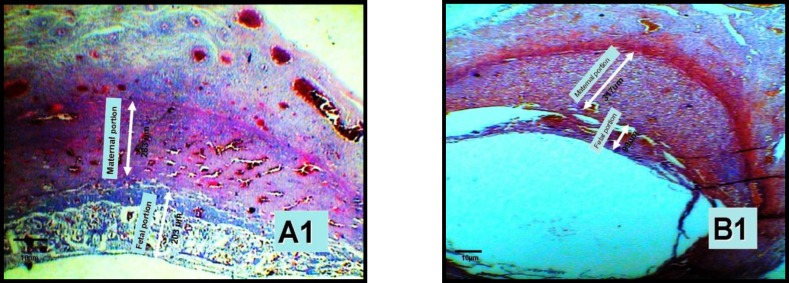
Figure 1.
Changes in thickness of the placenta portion in experimental(B1) and control(A1) group in the 10-day old placenta by ×40 (two arrowheads).
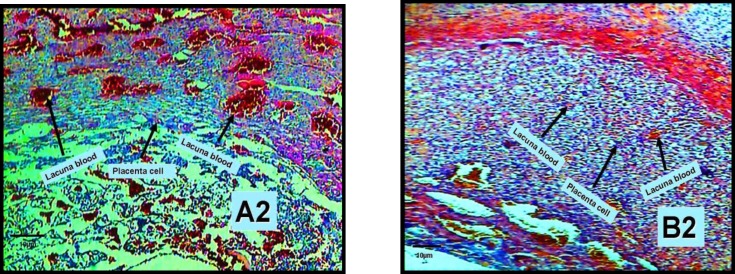
Figure 2.
Morphologic changes on 10-day old placenta in experimental group (B2) indicates placenta cells and blood lacuna of placenta shuch as indicates placenta cells and blood lacuna in control group (A2) in 10 days placenta with zoom ×100, (a one head arrow).
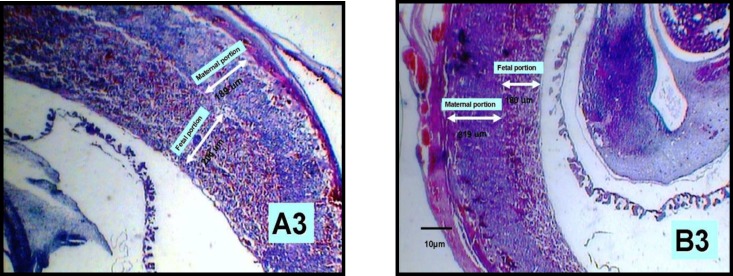
Figure 3.
The changes of thickness in placenta layer in 14-day old placenta in experimental(B3) and control(A3) groups zoomed ×40 ( two heads arrow).
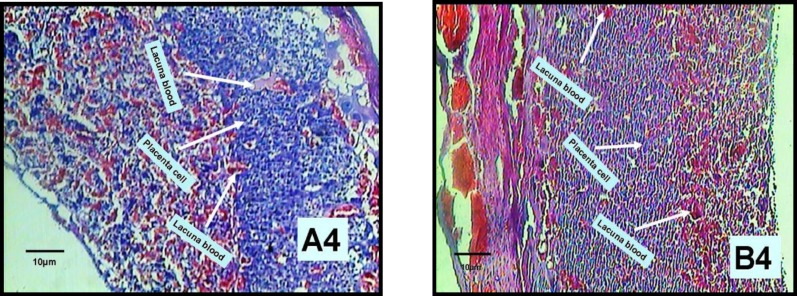
Figure 4.
The changes of the blood cisterns and the number of placenta portion cells in 14-day old placenta in experimental (B4) and control (A4) groups, zoomed ×100 (one head arrow).
Discussion
Results of the present study showed that morphine consumption during pregnancy can cause delay in the development of embryonic and maternal portion of placenta. On pregnant mother, concentration of corticosteron at blood plasma will increase at second period of pregnancy so we measured corticosteron concentration at 13th days after pregnancy (17, 18). In agreement with this subject, our data also indicated the oral morphine administration increased blood plasma corticosteron concentration on addictive pregnant mothers (Table I). Increase in corticosteron density of the blood plasma of the pregnant female mice can justify the side effects in embryos of these mothers. In addition the results of these studies were consistent with previous ones, which indicated that cortisol administration could delay differentiation of placental cells (1, 5). Regarding the fact that embryonic development is the result of placental natural function and due to the essential function of that is the hormone secretion and material exchange, any disorder in normal function of fetal and maternal portion of placenta can cause abnormality on embryo growth (3, 16, 19).
Blood flow is a vital factor on placental function and embryo growth. Morphologic studies have shown that if physiologic changes of spiral veins occur during pregnancy, trophoblast cells will attack placenta and blood flow will increase at this site and finally placental villi disrupting will appear (6, 20, 21). Morphologic and morphometric results have shown the oral morphine effect on both embryonic and maternal parts of placenta (Table I). Nowadays, it is stabilized that corticosteron concentration increases in blood of pregnant mother. Also our study indicated that oral morphine administration in pregnant mouse caused corticosteron secretion in experimental group (5, 10, 17). Fowden and Forhead declared, the increase of placing embryo and placenta in exposure of glococorticoides cause attenuation of embryo and placenta, and this will directly change the cell cycle from mitotic to differentiated state (5, 6, 10). Also corticosteron induces proliferation of cytotrophoblast cells of maternal and fetal portion of placenta (6, 17, 22) by stimulation of procytotrophoblastic cells to shortening of interphase (5, 17, 23), so cells do not have enough time for growth, protein synthesize, replication, and enough growth, and finally cause disorder in normal function of placental fetal cells (6, 7, 23), in contrast lead to late differentiation of placental cell and embryonic development (5, 7, 10, 19). Results of present research together with above data showed high level of corticosteron by morphine administration raised maternal portion thickness and number of these cells in placenta of day 10th and 14th of pregnancy. In addition morphine consumption has decreased more placenta fetal portion in contrast to maternal portion and this increment cause severs abnormality at embryo (Table I). The previous studies indicated oral morphine administration in the 9th day of pregnancy attenuate neural tube and neural plate evolution and development of frontal cortex in embryo were reduced in the 17th day of pregnancy (15, 16, 24, 25). The major role of placenta is material exchange between maternal blood and placenta and secretory substances such as steroids, peptides, cytokine and glycoproteins release from mother blood and enter to embryo by placenta. Otherwise because consumption and production of nutrition material were determined by placenta any disorder in functionality of fetal placental portion (5, 6, 11) cause delay in development of embryo and placenta. During pregnancy in parallel with vessel angiogenesis, thickness of fetal placenta portion will decrease, so material exchanges become more and finally morphine uptake increases and decrease in thickness of fetal placenta portion is the effect of this increment (14, 20, 23). So, speed of morphine transition and disruptive effects have direct ratio with decrease of fetal layer thickness. In present research, effect of morphine resulted decrease in abnormal thickness in embryonic unit of experimental group compared to control group in 10 and 14 day old placenta, and decrease of blood cisterns surface, also number of cell in both 10 and 14 day old placenta in experimental group compared to control group are consistent with increase of corticosteron concentration (Table I). The other researches of morphine effect on placenta fetal portions of both groups indicated, morphine causes decrease in blood cisterns area and these data not only are consistent with koulin’s and Doppler’s researches, but also they are valuable to research in human.
In other researches, it was shown; that the morphine consumption orally and by injection indicated the same effect (20, 26, 27). The effective factors on blood vessel contraction are corticosteron and opioides receptors on the membrane of placental cells (2, 3, 7), that located on placental villi and be contracted by morphine stimulation and resulted decrease in blooding, embryonic hypoxia and delay in embryonic development (20, 28) Embryonic portion of placenta basically give rise to cyncytiotrophoblast cells and these cells play important role in embryonic development through secretory function, like estrogen and progesterone hormone (10, 17) and disorder in the secretion of these cells cause delay in placental and embryonic development (6, 19) and can aggravate embryonic abortion.
According to studies, morphine administration caused embryonic abortion and decrease in babies’ weight in pregnant rabbits (12). In total, these results indicated oral morphine consumption causes the unusual increment in plasma corticosteron density and delay in placenta fetal and maternal portion development in Wistar rat but it was not identified if present study results are due to morphine or corticosteron effect or both of them. Although behavioral disorders in infants or embryonic abortion from addictive pregnant mothers need to be studied more.
Acknowledgment
This study has been financially sponsored by Neuroscience Research Center, Baqyiatallah (a.s) University of Medical Sciences, Tehran, Iran.
References
- 1.Kopecky EA, Simone C, Knie B, Koren G. Transfer of morphine across the human placenta and its interaction with naloxone. Life Sci. 1999;65:2359–2371. doi: 10.1016/s0024-3205(99)00503-2. [DOI] [PubMed] [Google Scholar]
- 2.Ornoy A, Michailevskaya V, Lukashov I, Bar-Hamburger R, Harel S. The developmental outcome of children born to heroin-dependent mothers, raised at home or adapted. Child Abuse Negl. 1996;20:385–396. doi: 10.1016/0145-2134(96)00014-2. [DOI] [PubMed] [Google Scholar]
- 3.Wilson JT, Chritie MJ, Manzoni O. Cellular and synaptic adaptations mediating opioid dependence. Physiol Rev. 2001;81:299–343. doi: 10.1152/physrev.2001.81.1.299. [DOI] [PubMed] [Google Scholar]
- 4.Wang JF, Wang ZY, Wu N, Yan HT, Li J. Effects of aquaporin4 deficiency on opioid receptors characteristics in naive and chronic morphine-treated mice. Neurosci Lett. 2009;457:111–114. doi: 10.1016/j.neulet.2009.03.060. [DOI] [PubMed] [Google Scholar]
- 5.Wallace JM, Aitken RP, Milne JS, Hay WW Jr. Nutritionally mediated placental growth restriction in the growing adolescent: consequenes for the fetus. Biol Reprod. 2004;71:1055–1062. doi: 10.1095/biolreprod.104.030965. [DOI] [PubMed] [Google Scholar]
- 6.Fowden AL, Forhead AJ, Coan PM, Burton GJ. The placenta and intrauterine programming. J Neuroendocrinol. 2008;20:439–450. doi: 10.1111/j.1365-2826.2008.01663.x. [DOI] [PubMed] [Google Scholar]
- 7.Fowden AL, Ward JW, Wooding FP, Forhead AJ, Constancia M. Programming placental nutrient transport capacity. J Physiol. 2006;572:5–15. doi: 10.1113/jphysiol.2005.104141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fabian G, Bozo B, Szikszay M, Horvath G, Coscta CJ, Szucs M. Chronic Morphine-Induced Changes in μ-Opioid Receptors and G Proteins of Different Subcellular Loci in Rat brain. J Pharmacol Exp Ther. 2002;302:774–780. doi: 10.1124/jpet.102.036152. [DOI] [PubMed] [Google Scholar]
- 9.Sargeant TJ, Day DJ, Miller JH, Steel RW. Acute in utero morphine exposure slows G2 / M phase transition in radial glial and basal progenitor cells in the dorsal telencephalon of the E15.5 embryonic mouse. Eur J Neurosci. 2008;28:1060–1067. doi: 10.1111/j.1460-9568.2008.06412.x. [DOI] [PubMed] [Google Scholar]
- 10.Khalili M, Semnanian S, Fatholahi Y. Caffeine increases paragigantocellularis neuronal firing rate and induces withdrawal signs in morphine dependent rats. Eur J Pharmacol. 2001;412:239–245. doi: 10.1016/s0014-2999(01)00718-x. [DOI] [PubMed] [Google Scholar]
- 11.Fwden AL, Forhead AJ. Endocrine mechanisms of intrauterine programming. Reproduction. 2004;127:515–526. doi: 10.1530/rep.1.00033. [DOI] [PubMed] [Google Scholar]
- 12.Sadler IW. Langman’s Medical Embryology. 9thEd. Philadelphia: Lippincott William & Wilkins; 2006. [Google Scholar]
- 13.Roloff DW, Howatt WF, Kanto WP Jr, Borker RC Jr. Morphine administration to pregnant rabbits: effect on fetal growth and lung development. Addict Dis. 1975;2:369–379. [PubMed] [Google Scholar]
- 14.Reynolds LP, Borowicz PP, Vonnahme KA, Johnson ML, Grazul-Bilska AT, Wallace JM, et al. Animal models of placental angiogenesis. Placenta. 2005;26:689–708. doi: 10.1016/j.placenta.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 15.McCrabb GJ, EganAR , Hosking BJ. Maternal under nutrition during mid-pregnancy in sheep. Placental size and its relationship to calcium transfer during late pregnancy. Br J Nutr. 1991;65:157–168. doi: 10.1079/bjn19910077. [DOI] [PubMed] [Google Scholar]
- 16.Kazemi M, Azarnia M, Sahraei H, Bahadoran H, Saeidabadi S. Oral morphine consumption delayed lateral ventricles and chroid plexus in Wistar rat embryos. Kowsar Medical Journal. 2009;14:11–20. [Google Scholar]
- 17.Sadraie SH, Kaka GR, Sahraei H, Dashtnavard H, Bahadoran H, Mofid M, et al. Effects of maternal oral administration of morphine sulfate on developing rat fetal cerebrum: A morphometrical evaluation. Brain Res. 2008;48:123–132. doi: 10.1016/j.brainres.2008.09.052. [DOI] [PubMed] [Google Scholar]
- 18.Ward JW, Wooding FB, Fowden AL. The effect of cortisol on the binucleate cell population in the ovine placenta during late gestation. Placenta. 2002;23:451–464. doi: 10.1053/plac.2002.0834. [DOI] [PubMed] [Google Scholar]
- 19.Fowden AL, Li J, Forhead AJ. Glucocorticoids and the prcparation for life after birth:are there long trme cones qunces of the life insurance. Proc Nutr Soc. 1998;57:113–122. doi: 10.1079/pns19980017. [DOI] [PubMed] [Google Scholar]
- 20.Redmer DA, Wallace JM, Reynolds LP. Effect of nutrient intake during pregnancy on fetal and placental growth and vascular development. Domest Anim Endocrinal. 2004;27:199–217. doi: 10.1016/j.domaniend.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 21.Collins LR, Hall RW, Dajani NK, Wendel PJ, Lowery CL, Kay HH. Prolonged morphine exposure in utero causes fetal and placental vasoconstriction: a case report. J Matern Fetal Neonatal Med. 2005;17:417–421. doi: 10.1080/14767050500123996. [DOI] [PubMed] [Google Scholar]
- 22.Donnelly L, Campling G. Functions of the placenta. Anaesthesia & Intensive Care Medicine. 2008;9:124–127. [Google Scholar]
- 23.Glasel JA. The effects of morphine on cell proliferation. Prog Drug Res. 2000;55:33–80. doi: 10.1007/978-3-0348-8385-6_2. [DOI] [PubMed] [Google Scholar]
- 24.Wu LY, Chen JF, Tao PL, Huang EY. Attenuation by dextromethorphan on the higher liability to morphine-induced reward, caused by prenatal exposure of morphine in rat offspring. J Biomed Sci. 2009;16:106. doi: 10.1186/1423-0127-16-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nasiraei Moghadam S, Bahadoran H, Saeedabady S, Shams J, Sahraei H. Oral administration of morphine delays neural plat development in rat embryos. Physiology and Pharmacology. 2008;159:12–17. [Google Scholar]
- 26.Nasiraei-Moghadam S, Sahraei H, Bahadoran H, Sadooghi M, Salimi SH, Kaka GR, et al. Effects of maternal oral morphine consumption on neural tube development in Wistar rats. Brain Res Dev Brain Res. 2005;159:12–17. doi: 10.1016/j.devbrainres.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 27.Dugoff L, Lynch AM, Cioffi-Ragan D, Hobbins JC, Schultz LK, Malone FD, et al. First trimester uterine artery Doppler abnormalities predict subsequent intrauterine growth restriction. Am J Obstet Gynecol. 2005;193:1208–1212. doi: 10.1016/j.ajog.2005.06.054. [DOI] [PubMed] [Google Scholar]
- 28.Ahmed MS, Schoof T, Zhou DH, Quarles C. Kappa opioid receptors of human placental villi modulate acetylcholine release. Life sci. 1989;45:2383–2393. doi: 10.1016/0024-3205(89)90001-5. [DOI] [PubMed] [Google Scholar]
- 29.Penninga L, Longo LD. Ovine placentome morphology:effect of high altitude,long-term hypoxia. Placenta. 1998;19:187–193. doi: 10.1016/s0143-4004(98)90008-x. [DOI] [PubMed] [Google Scholar]