Abstract
During mouse embryogenesis, diffusible growth factors, i.e. fibroblast growth factors, Wnt, bone morphogenetic protein and Hedgehog family members, emanating from localized areas can travel through the extracellular space and reach their target cells to specify the cell fate and form tissue architectures in coordination. However, the mechanisms by which these growth factors travel great distances to their target cells and control the signalling activity as morphogens remain an enigma. Recent studies in mice and other model animals have revealed that heparan sulfate proteoglycans (HSPGs) located on the cell surface (e.g. syndecans and glypicans) and in the extracellular matrix (ECM; e.g. perlecan and agrin) play crucial roles in the extracellular distribution of growth factors. Principally, the function of HSPGs depends primarily on the fine features and localization of their heparan sulfate glycosaminoglycan chains. Cell-surface-tethered HSPGs retain growth factors as co-receptors and/or endocytosis mediators, and enzymatic release of HSPGs from the cell membrane allows HSPGs to transport or move multiple growth factors. By contrast, ECM-associated HSPGs function as a reservoir or barrier in a context-dependent manner. This review is focused on our current understanding of the extracellular distribution of multiple growth factors controlled by HSPGs in mammalian development.
Keywords: diffusible growth factors, heparan sulfate proteoglycans, mouse, extracellular matrix, embryogenesis, shedding
1. Introduction
Diffusible growth factors, including fibroblast growth factors (FGF), Wnt, bone morphogenetic protein (BMP), transforming growth factor-β (TGF-β) and Hedgehog (Hh) family members, regulate anterior–posterior, left–right and dorsoventral patterning in the mammalian embryos by controlling cell proliferation, cell re-arrangement, migration and cell death [1–5]. These factors are secreted from spatially and temporally restricted areas such as local signalling centres and are considered to control cell behaviours in the target regions or tissues in a concentration-dependent manner. However, our knowledge about how growth factors travel through the extracellular space, arrive precisely at target cells and transmit signalling within cells at the appropriate level is still limited [6–11]. Recent observations in mice and other model animals deficient for biosynthesis of heparan sulfate proteoglycans (HSPGs) have identified the importance of HSPGs that are localized on the cell surface and in the extracellular matrix (ECM) for the extracellular distribution of growth factors during early embryonic patterning. The roles of HSPGs appear to depend primarily on the fine structures and location—i.e. the cell surface or ECM—of their heparan sulfate (HS) glycosaminoglycan chains. In this review, we focus on recent advances in the distribution of diffusible growth factors and consequent signalling activation played by cell-surface-tethered and ECM-associated HSPGs during early embryogenesis by integrating the relevant models in mice and other animals.
2. The structures of biosynthesized heparan sulfate proteoglycans are highly heterologous and complex
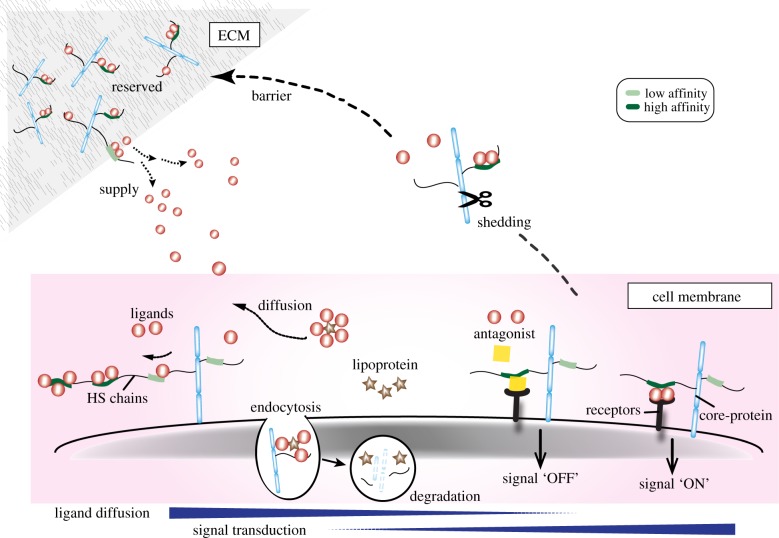
HSPGs are composed of a specific core-protein covalently linked with a few HS chains which have highly heterogeneous polysaccharides with respect to molecular mass, disaccharide construction and sulfation patterns when compared with proteins or nucleic acids (reviewed in [12–17]). HSPGs are further divided into three major groups depending on their core-protein structure, i.e. transmembrane type (e.g. syndecans), glycerophosphatidylinositide (GPI)-anchored type (e.g. glypicans) and secreted ECM type (e.g. perlecan, agrin and collagen type XVIII; figures 1 and 2). The former two types of HSPGs are generally localized at the cell surface, but are sometimes cleaved by a sheddase (e.g. a proteinase, heparanase or Notum, a member of the α/β-hydrolase superfamily with similarity to pectin acetylesterases releasing GPI-anchored glypicans from the cell surface), so that the detached forms of the cell-surface-tethered HS are also distributed in the ECM (figures 1 and 2). The latter ECM type of HSPGs is directly secreted and localized in the ECM including the basement membrane (basal lamina).
Figure 1.
Schematic of divergent roles of HSPGs in distribution and signal transduction of growth factors. The roles of HSPGs in distribution of multiple growth factors depend on the localizations of HSPGs. The HS chains are covalently liked to core-proteins: the transmembrane type (e.g. syndecans), GPI-anchored type (e.g. glypicans) and secreted type (e.g. perlecan and agrin). The former two types are located on the cell surface, and the latter one is in the ECM including basement membrane (basal lamina). Thus, the localization of HS chains is dependent on the types of attached core-proteins. On occasion, however, HSPGs cleaved with proteases, heparanase or Notum by shedding, are detached from the cell surface and ECM. Principally, the roles of HSPGs in distribution and signal transduction of growth factors appear to depend on their localizations. The cell-surface-tethered HSPGs play roles as co-receptors and/or endocytosis mediators, whereas ECM-associated HSPGs act as a reservoir or barrier. In addition, released HSPGs by shedding with a protease, heparanase or Notum, promote the transport or movement.
Figure 2.
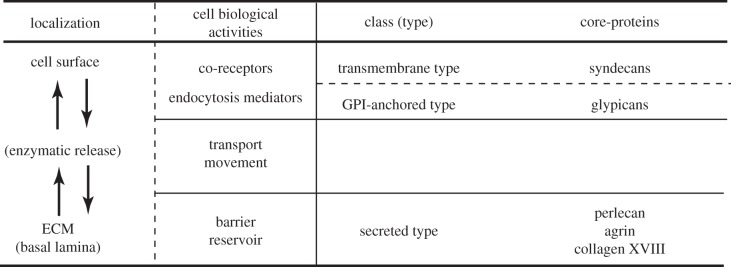
Summary of localizations of HSPGs and their cellular functions for growth factors. Localizations of HSPGs are closely linked to their cellular functions for growth factors. Their localizations can be modified by enzymatic cleavage with shedding.
The HS chain has a long linear backbone of repeating disaccharide units incorporating N-acetylglucosamine and glucuronic acid (reviewed in [12–18]). HS chains are assembled on serine residues in core-proteins by a series of glycosyltransferases and modification enzymes in the Golgi. Notably, HS elongation takes place by adding 25–100 repeating disaccharide units, and is catalysed by Ext1 and Ext2 proteins. Following chain elongation, extensive modification reactions are carried out by four families of sulfotransferases and one epimerase. GlcNAc N-deacetylase/N-sulfotransferases deacetylate N-acetylglucosamine and add sulfate to generate N-sulfated glucosamine. Some glucuronic units undergo 2-O sulfation by 2-O sulfotransferase and selected glucosamine residues are 6-O sulfated by a 6-O sulfotransferase. Because these modification reactions of HS biosynthesis occur in clusters along HS chains, the modified domains are separately segmented as N-acetylated (NA), N-sulfated (NS) and mixed (NA/NS) domains [19,20]. Moreover, because these modification reactions proceed incompletely, HS chains carry highly divergent disaccharide structures. The resultant complex structures might play unique roles in specific affinity for multiple growth factors and their related receptors, as described in the next section.
3. Binding of heparan sulfate proteoglycans to growth factors is mediated by the fine features of heparan sulfate chains
As HS chains are consecutively sulfated by N-, 2-O-, 6-O, 3-O- sulfotransferases in the Golgi (see above), the consequent modified sulfated patterns of HS at the overall level as well as in local domains appear to modulate the affinity for multiple growth factors [21,22–27]. In addition, sulfated HS chains are further partially desulfated by extracellular endosulfatases mostly at the cell surface but not in the Golgi [26]. Extensively sulfated HS chains strongly facilitate the formation of ternary complexes with FGF and FGF receptor (FGFR) [25] and, consequently, promote FGF signalling activity, e.g. distinct sulfate patterns modulate different FGF10-mediated gland developmental processes such as proliferation, duct elongation, bud expansion and differentiation [21–24]. Conversely, desulfation of HS chains via these endosulfatases downregulates FGF-mediated signalling activity [27–29]. FGF signalling is strongly disrupted by: Hs2st; by Hs6st double mutations, where the entire sulfation level is severely reduced; or by overexpression of 6-O sulfatase, which removes 6-O sulfate groups [30]. Moreover, the overall size or length of HS disaccharide units appears to affect the binding activity of FGF; shorter chains can form a ternary complex with FGF–FGFR more efficiently than longer chains [21,31]. Thus, these fine HS features including sulfated patterns, and the overall length of HS chains seem to modulate the binding activity and signalling activation for growth factors.
Desulfation of HS by 6-O-endosulfatases Sulf1 and Sulf2 promotes the complex formation of Wnt ligands and Frizzled receptors [26]. 6-O desulfation reduces the ability of HS chains of glypican 1 to bind to Wnt ligands. Because 6-O-sulfated HS binds to Wnt with a high affinity and competes with the binding of Wnt to Frizzled receptors, 6-O desulfation activity would convert the HSPGs to a low affinity binding state for Wnt ligands. Thus, removal of Wnt from HS chains via desulfation facilitates the complex formation of Wnt–Frizzled receptor indirectly, and thereby enhances Wnt signalling [32]. Consistent with this notion, Sulf1 may stabilize gradient formation of Wnt ligands by controlling the stability and distribution of Wnt. Given that Sulf1 appears to be induced by Wnt signalling itself and then to repress Wnt signalling activation, Sulf1 can act as a feedback loop, possibly by stabilizing the shape of the Wnt gradient [33].
The binding affinity of HS for other HS-interacting proteins is also affected by their fine sulfated patterns. Noggin, an extracellular BMP antagonist, binds efficiently to heavily sulfated heparin/HS carrying N-, 6-O- and 2-O-sulfates [34]. Given that Sulf1 selectively removes sulfate groups from the 6-O position of HS within the most highly sulfated S domains but not within the NA/NS domains [34], the 6-O desulfation activity results in the release of Noggin from the HS chains and consequently upregulates the BMP signalling within these cells [34]. These findings suggest that fine features of HS can modulate the binding activity of other HS-interacting proteins, including BMP-bound antagonists (also see below).
In addition, the spatio-temporal changes of fine structures of HS chains can further modulate the distribution of growth factors and their signalling activity during morphogenesis [35,36]. Elongation and modifications of HS chains appear to be controlled spatially and temporally to some extent. Modification enzymes are expressed in a cell- or tissue-specific manner and the resultant sulfated location and overall level within HS chains are neither uniform nor complete [37,38]. Indeed, expression studies with several monoclonal antibodies recognizing different HS structures such as sulfation have indicated that the fine structures of cell-surface-tethered HS chains are also different from those of ECM-associated chains [35,36,39]. Thus, the spatio-temporally controlled diversity of HS fine structures can modulate the distribution and signalling activity of growth factors during development [18,39].
4. Divergent functions of heparan sulfate proteoglycans in the distribution of growth factors depend on localizations of heparan sulfate chains
The cell biological roles of HSPGs in the distribution of growth factors appear to be dependent on localizations of HSPGs; syndecans and glypicans are localized at the cell surface as transmembrane type and GPI-anchored type, respectively, whereas perlecan and agrin are secreted and localized in the ECM (figures 1 and 2). Furthermore, the locations of these HSPGs are modified by enzymatic cleavages with sheddases (figures 1 and 2). Their distinct localizations permit the divergent, but unique roles of HSPGs in the regulation of distribution of diffusible growth factors as described below in detail (figure 2).
(a). Cell-surface-tethered heparan sulfate proteoglycans retain growth factors as co-receptors and/or endocytosis mediators
Cell-surface-tethered HSPGs contribute to the stable retention of multiple growth factors and signal transduction as co-receptors (figure 2). The roles of HSPGs in the formation of one of these growth factors, FGF–FGFR, have been extensively studied for years [40–42]. In the early mouse embryo, both the local retention and signalling activation of FGF depend on cell surface HSPGs. Given that the Ext1/Ext2 complex catalyses HS chain elongation and Ext2 transcripts are prominent in the extraembryonic ectoderm, HS expression of cell-surface-tethered HSPGs is increased during extraembryonic ectoderm development [39]. While the Fgf4 and Fgf8 genes are transcribed in the epiblast and visceral endoderm, their protein products are mostly localized on the cell surface of the extraembryonic ectoderm but not evident in the epiblast or visceral endoderm [39]. Although the extracellular route of diffused FGF proteins travelling from epiblast cells to the cell surface of the extraembryonic ectoderm is still unknown, FGF proteins appear to be stably co-localized with cell-surface-tethered HSPGs such as transmembrane-type syndecan-1 as well as the FGFR2 protein, but are unlikely to co-localize with ECM-associated HSPGs such as perlecan in the ECM, particularly the basement membrane (basal lamina) [39]. Moreover, the cell surface retention of FGF4 and FGF8 proteins and FGF signalling activity in the extraembryonic ectoderm is specifically lost in Ext2-deficient embryos in which HS disaccharides are not synthesized but FGFR2 expression on the cell surface is evident [39]. Thus, together with the chimaeric studies in Ext2-deficient embryos, these results led the authors to propose that cell-surface-tethered HSPGs rather than ECM-associated HSPGs are crucial for the stable and local retention of FGF ligands to the FGFR and subsequent activation of FGF signalling in extraembryonic ectoderm cells [39]. Consistent with these observations, during the mouse peri-implantation stages, the highly dynamic expression patterns of HS chains are largely matched with the FGF signalling activity, marked by the diphosphorylated form of extracellular signal-regulated kinase [39,43] (figure 3); in particular, both activities are prominent in trophectoderm-derived tissues, including the extraembryonic ectoderm.
Figure 3.
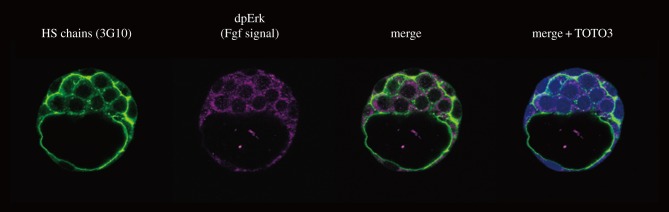
Expression of HS chains of HSPGs and FGF signalling activation at preimplantation stage. Expression of HS chains examined by the 3G10 monoclonal antibody is observed in the trophectoderm as well as in the inner cell mass at E3.5. FGF signalling activation is monitored by immunohistochemistry with the polyclonal antibody against the dephosphorylated form of Erk (dp-Erk). Expression of dp-Erk is found in the cytoplasm of HS chain-positive cells. The detailed materials and methods are described previously [39].
Similarly, cell surface HSPGs appear to contribute to retention of other growth factors [44–53], although the crystal structures of ligand and receptor complexes remain to be elucidated. The heparin-binding domain in the N-terminal basic moiety of BMP proteins seems to be crucial for local retention of BMP ligands as well as their biological activity [54,55]. Consistently, in the HS-deficient limb mesenchyme, both the BMP protein distribution and subsequent BMP signalling-active domains become more broad and diffused [47]. More specifically, interactions between HS and BMP on the cell surface are required to recruit BMP type II receptor subunits to BMP type I receptor complexes for signalling activation [49]. This suggests that some of the cell surface HSPGs may function to catalyse the active ligand–receptor complex formation rather than just stabilize the BMP–receptor complex. Glypicans, cell surface- and GPI-anchored-type HSPGs, can facilitate the complex formation of growth factors, Wnt and Hh and their respective receptors for signalling activation [51,52,56,57]. Glypican 3 directly interacts with both Wnt ligands and their receptor Frizzled through its glycosaminoglycan chains [56]. Similarly, glypican 5 also binds to both Hh and its receptor Ptc1 through its glycosaminoglycan chains [57]. These findings are in good agreement with the previous notion that the Drosophila glypican dally-like is essential for the extracellular distribution of Wingless [58]. Although the role of HSPGs in Wnt, Shh and BMP signalling during early mammalian embryogenesis remains to be shown [39,59], the above lines of evidence together with cellular and developmental processes in other model animals suggest the possible involvement of HSPGs in other signalling pathways apart from FGF in early mouse embryogenesis. To address these issues more explicitly, it is necessary to examine whether the local distribution of growth factors is correctly matched with cell-surface-tethered HSPGs at the single-cell level in wild-type and HS-deficient cells.
Cell surface HSPGs can also alter the distribution of growth factors by trapping other interacting proteins for growth factors and thereby generating sharp gradients of signalling activity. Gradients of BMP signalling activity are considered to be established by a number of inhibitory binding proteins for BMP, such as Chordin and Noggin [60]. Chordin is thought to readily diffuse in tissues, thereby forming gradients of BMP inhibition that result in reciprocal gradients of BMP signalling. Notably, retention of Chordin to the cell surface in the mouse embryo is dependent on cell-surface-tethered HSPGs (e.g. syndecans), but not on ECM-associated HSPGs (e.g. perlecan) [61]. Moreover, mammalian Twisted Gastrulation, the secreted protein that enhances Chordin inhibitory activity on BMP, so that it sharpens BMP signalling gradients by acting as a cofactor, is able to interact with heparin only after heparin is pre-bound to Chordin and/or BMP-4. Therefore, Chordin–HSPG interactions might be prerequisite for the antagonism of BMP signalling by Chordin as well as the retention of Chordin to the cell surface [61]. Similarly, HS chains are also crucial for the binding of Noggin to the cell surface [34; also see below]. These several lines of evidence suggest that in addition to interactions between HS and growth factors, interactions between HS and diffusible antagonists of growth factors contribute to the extracellular distribution of growth factors during embryogenesis (figure 2).
Consistent with the notion that HSPGs play a crucial role in the stable retention of multiple growth factors, precise transmission electron microscopy and photothermal heterodyne imaging have revealed that the spatial distribution of the FGF2 protein around the cell surface is highly divergent, ranging from several nm to several μm, which can be considered to correspond to the length from HS chains (40–160 nm) to the entire cell size [62]. This finding may support the idea that FGF proteins are localized on the cell surface by translocating FGF-binding sites of HS chains.
Cell surface HSPGs also mediate endocytosis of the complex of growth factors and their receptors, although the exact role of HSPGs in the cell-surface receptor for diverse macromolecular cargo is still controversial [63] (figure 2). Core-protein moieties of transmembrane-type HSPGs, syndecans, can contribute to endocytosis of the FGF–FGFR complex [64]. Syndecans are composed of three distinct domains that can interact with several proteins and participate in different functions: an extracellular domain linking to the HS chains, a transmembrane domain for self-clustering, and a cytoplasmic domain for interacting with multiple proteins for signal transduction, cell migration and macropinocytosis [65]. Among these three domains, the cytoplasmic domain appears to promote FGF signalling activity by endocytosis of the FGF–FGFR complex [64]. Therefore, the efficiency of endocytosis of FGF ligands or FGF–FGFR complexes can be translated into FGF signalling activation as recently proposed [66].
Glypicans, which are GPI-anchored-type HSPGs, can also contribute to endocytosis of the ligand–receptor complex. Glypicans antagonize the effect of a BMP type I receptor, which is able to downregulate BMP signalling by receptor-mediated endocytosis of BMP. This suggests that glypicans may regulate the local retention of BMP at the cell surface, signalling activity by disrupting receptor-mediated internalization and degradation of the BMP–receptor complex [67]. Endocytosis of glypicans from the apical surface of Hh-receiving cells involves internalization of the complex of Hh and its receptor Patched [68]. The co-internalization of glypican with the Hh–Patched complex is dynamin-dependent and necessary for strong Hh signalling. In addition, Wingless, a fly Wnt homologue, is secreted apically in the epithelium, and the apicobasal trafficking of the glypican allows transcytosis of Wnt ligands for spreading along the basolateral compartment. Thus, endocytosis through cell-surface-tethered HSPGs, i.e. glypicans, may be a common regulatory mechanism of both Hh and Wnt ligands for signalling action [68,69].
(b). Heparan sulfate proteoglycans released by shedding can transport or move growth factors by modulating their distribution
Enzymatic cleavage of HSPGs tethered to the cell surface by proteinases, heparanases or Notum gives rise to the release of HSPGs from the cell membrane, which can facilitate growth factor dispersal or movement through the extracellular space (reviewed in [14,16,18]) (figures 1 and 2). A secreted serine protease, HtrA1, can cleave cell surface-tethered HSPGs, including biglycan, syndecan 4 and glypican 4, and spread HS as well as dermatan sulfate for long-range FGF signalling activation during mesoderm formation, and neuronal differentiation in Xenopus [70]. In early mouse embryos, the non-cell-autonomous roles of HSPGs in FGF signalling activation appear to be dependent on serine proteases during extraembryonic ectoderm development [39]. Given that proper FGF signalling activation is blocked by specific inhibitors for serine proteases but neither by inhibitors specific for actin polymerization nor inhibitors for several other types of proteolysis, the spread of FGF signalling activation in the extraembryonic ectoderm would probably involve serine protease-dependent cleavage of HSPGs rather than actin-dependent cytoneme- and transcytosis-mediated processes [39]. These findings support the hypothesis that shedding, i.e. cleaving HSPGs by serine proteases, allows for diffusion by releasing cell-surface-bound FGF or FGF–FGFR complexes together with HS chains, and consequently directs FGF signalling activation in a cell non-autonomous manner.
Cleavage of HS chains by endoglycosidases such as a heparanase promotes FGF dispersal. With heparanases, the complex of HS chains and growth factors detached from core-proteins can be diffused at a long distance. This process will enhance signalling activation primarily by altering the distribution of HS chains and FGF. Indeed, heparanase-released HS chains become more bioactive than the original and native HS chains covalently linked to core-proteins [71,72]; heparanase releases FGF10 from perlecan in the basement membrane (basal lamina) and promotes FGF signalling for branching morphogenesis [21,72].
Notum, which releases the GPI-anchored glypicans from the cell surface, can modulate the distribution of growth factors in a context-dependent manner. For Wnt, loss and gain of Notum alter the gradient of Wnt ligands in the extracellular space by detaching glypicans from the cell surface, thereby leading to increased and reduced activity in Drosophila [73]. By contrast, for Hh signalling, Notum appears to promote internalization of Hh together with glypicans and its Patched receptor and to activate signalling at a higher level [74,75]. Thus, release of glypicans with Notum provides a switch from low- to high-level signalling by releasing the ligand from the cell surface and promoting internalization of the ligand–receptor complex.
HSPGs are also suggested to be involved in extracellular spreading of the Hh family members via interaction with lipoprotein particles [76] (figure 2). Hh can act directly as long-range morphogens, and their activity is closely linked to the formation of freely diffusible multi-mers from the lipidated, cell-surface-tethered monomer. Notably, HSPGs can interact with lipoprotein particles, which participate in morphogen spreading for Hh signalling in Drosophila [76]. More specifically, membrane-associated glypicans recruit lipoprotein particles to the membrane, and remain associated with these particles after they are released from the membrane by cleavage of the GPI anchor of glypicans. In addition, shifted, the orthologue of the human Wnt inhibitory factor, which co-localizes Hh and interacts with HSPGs in the ECM, is required for Hh stability and for lipid-modified Hh diffusion [77]. Thus, HSPGs can regulate Hh diffusion and stability through the shifted protein. Consistent with these findings, in mammalian cell cultures, the release of soluble and oligomerized Shh from the membrane is considered to be mediated by HS-dependent mechanisms [78–80]. These data suggest that HSPGs can influence lipid-linked growth factor signalling—at least in part—by binding to lipoproteins. Similarly, the extracellular movement of BMP family members appears to be mediated by cell-surface-tethered HSPGs. Decapentaplegic, Drosophila BMP, fails to move across cells with mutations for dally and dally-like, two Drosophila glypican members of transmembrane-type HSPGs [81]. Belenkaya et al. [81] have proposed a model in which BMP moves along the cell surface by restricted extracellular diffusion involving these glypicans but not dynamin-mediated endocytosis. Transmembrane-type HSPGs bind BMP via lipoprotein crossveinless d for BMP movement, probably as a part of the lipid–BMP–lipoprotein complex [82]. Although it is not certain that enzymatic cleavage of glypicans is required for the BMP movement, this process may be similar to the mechanism for the lipoprotein transport of Hh as described above. Thus, cell-surface-tethered HSPGs detached from the membrane appear to be involved in non-cell-autonomous spreading of multiple growth factors.
(c). Extracellular matrix-associated heparan sulfate proteoglycans control the dispersal of growth factors as a reservoir or barrier
ECM-associated HSPGs can contribute to the distribution of growth factors as a reservoir or barrier depending on their cellular context (figures 1 and 2). First, HSPGs are considered to trap diffused growth factors in the ECM as a reservoir and supply the growth factors for target cells on occasion. Second, they prevent passive diffusion of growth factors over longer distances, instead confining ligands to the vicinity of the produced cells. In the former case, although it remains uncertain whether ECM-associated HS chains can stabilize the FGF–FGFR complex formation directly or indirectly via sheddases, they are capable of enhancing FGF signalling to some extent. One of the ECM-associated HSPGs, perlecan, can form ternary complexes with FGF18 and FGFR3 in an HS-dependent manner and is essential for normal cartilage development [83]. Another ECM-associated HSPG, agrin, is involved in the formation of neuromuscular junctions and increases the activity of neurite outgrowth through the FGF2/FGFR-dependent pathway [84]. Together, these findings suggest that HSPGs play crucial roles in the stable and local retention of FGF as a co-receptor for FGF signalling activation. To give an example of the latter case, among FGF ligands, FGF9 and FGF20 can form homodimers reversibly, and their monomers have lower affinity to HS than their dimers. Accordingly, monomeric FGF can spread for a longer distance than dimeric FGF and consequently can activate signalling over a longer range than dimers [85,86]. Similarly, HS chains are essential to prevent FGF dispersal for lacrimal gland development, supporting the barrier function of ECM-associated HS in signalling activation as a barrier [87]. It can be assumed that differences in the binding affinity of FGF to HS underlie the different lengths of spreading, which consequently appear to give rise to the distinct biological activities of FGF. For instance, single amino acid conversion of FGF10 within the HS-binding domain into the corresponding residue of FGF7 reduces FGF10 binding to HS and allows FGF10 to become FGF7 with respect to diffusion characteristics and morphogenetic activity [88], i.e. this single-mutated FGF10 induces branching rather than induces elongation of epithelial buds like FGF7.
5. Concluding remarks
The many lines of evidence discussed above suggest that cell-surface-tethered HSPGs primarily contribute to the local retention of growth factors at the cell surface and activate signalling, whereas ECM-associated HSPGs contribute to the extracellular distribution of growth factors as a reservoir or barrier. In addition, HSPGs enzymatically released by shedding provide further functions of HS chains, such as transport or movement of growth factors. By means of highly divergent HS structures, these HSPGs together with shedding will act as mediators linking extracellular microenvironments to the cellular machinery which senses and transmits the outside signal in a context-dependent manner. However, the precise mechanisms by which HSPGs regulate the distribution and signalling activity of many growth factors during early mammalian patterning are not fully understood. Complex networks of the extracellular distribution of multiple growth factors and consequent signalling outputs for cellular behaviours will be clarified by discovering the precise functions of HSPGs in the early mouse embryo.
Acknowledgements
We apologize to the many authors whose works we were unable to cite owing to space restrictions, and thank Kayo Shimokawa for her technical support.
Funding statement
Our work is supported in part by a grant-in-aid for Scientific Research (B) from Japan Society for the Promotion of Science and a grant-in-aid for Scientific Research on Innovative Areas from the Ministry of Education, Culture, Sports, Science and Technology, Japan and by grants from the Takeda Science Foundation and the Uehara Foundation.
References
- 1.Arnold SJ, Robertson EJ. 2009. Making a commitment: cell lineage allocation and axis patterning in the early mouse embryo. Nat. Rev. Mol. Cell Biol. 10, 91–103. ( 10.1038/nrm2618) [DOI] [PubMed] [Google Scholar]
- 2.Hamada H, Meno C, Watanabe D, Saijoh Y. 2002. Establishment of vertebrate left–right asymmetry. Nat. Rev. Genet. 3, 103–113. ( 10.1038/nrg732) [DOI] [PubMed] [Google Scholar]
- 3.Rossant J, Tam PP. 2009. Blastocyst lineage formation, early embryonic asymmetries and axis patterning in the mouse. Development 136, 701–713. ( 10.1242/dev.017178) [DOI] [PubMed] [Google Scholar]
- 4.Ribes V, Briscoe J. 2009. Establishing and interpreting graded Sonic Hedgehog signaling during vertebrate neural tube patterning: the role of negative feedback. Cold Spring Harb. Perspect. Biol. 1, a002014 ( 10.1101/cshperspect.a002014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caspary T, Anderson KV. 2003. Patterning cell types in the dorsal spinal cord: what the mouse mutants say. Nat. Rev. Neurosci. 4, 289–297. ( 10.1038/nrn1073) [DOI] [PubMed] [Google Scholar]
- 6.Inomata H, Shibata T, Haraguchi T, Sasai Y. 2013. Scaling of dorsal–ventral patterning by embryo size-dependent degradation of Spemann's organizer signals. Cell 153, 1296–1311. ( 10.1016/j.cell.2013.05.004) [DOI] [PubMed] [Google Scholar]
- 7.Shilo BZ, Haskel-Ittah M, Ben-Zvi D, Schejter ED, Barkai N. 2013. Creating gradients by morphogen shuttling. Trends Genet. 29, 339–347. ( 10.1016/j.tig.2013.01.001) [DOI] [PubMed] [Google Scholar]
- 8.Bökel C, Brand M. 2013. Generation and interpretation of FGF morphogen gradients in vertebrates. Curr. Opin. Genet. Dev. 23, 415–422. ( 10.1016/j.gde.2013.03.002) [DOI] [PubMed] [Google Scholar]
- 9.Hironaka K, Morishita Y. 2012. Encoding and decoding of positional information in morphogen-dependent patterning. Curr. Opin. Genet. Dev. 22, 553–561. ( 10.1016/j.gde.2012.10.002) [DOI] [PubMed] [Google Scholar]
- 10.Gradilla AC, Guerrero I. 2013. Hedgehog on the move: a precise spatial control of Hedgehog dispersion shapes the gradient. Curr. Opin. Genet. Dev. 23, 363–373. ( 10.1016/j.gde.2013.04.011) [DOI] [PubMed] [Google Scholar]
- 11.Rogers KW, Schier AF. 2011. Morphogen gradients: from generation to interpretation. Annu. Rev. Cell Dev. Biol. 27, 377–407. ( 10.1146/annurev-cellbio-092910-154148) [DOI] [PubMed] [Google Scholar]
- 12.Perrimon N, Bernfield M. 2000. Specificities of heparan sulphate proteoglycans in developmental processes. Nature 404, 725–728. ( 10.1038/35008000) [DOI] [PubMed] [Google Scholar]
- 13.Bishop JR, Schuksz M, Esko JD. 2007. Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature 446, 1030–1037. ( 10.1038/nature05817) [DOI] [PubMed] [Google Scholar]
- 14.Bülow HE, Hobert O. 2006. The molecular diversity of glycosaminoglycans shapes animal development. Annu. Rev. Cell Dev. Biol. 22, 375–407. ( 10.1146/annurev.cellbio.22.010605.093433) [DOI] [PubMed] [Google Scholar]
- 15.Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME. (eds). 2009. Essentials of glycobiology, 2nd edn Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. [PubMed] [Google Scholar]
- 16.Yan D, Lin X. 2009. Shaping morphogen gradients by proteoglycans. Cold Spring Harb. Perspect. Biol. 1, a002493 ( 10.1101/cshperspect.a002493) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sarrazin S, Lamanna WC, Esko JD. 2011. Heparan sulfate proteoglycans. Cold Spring Harb. Perspect. Biol. 3, a004952 ( 10.1101/cshperspect.a004952) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsuo I, Kimura-Yoshida C. 2013. Extracellular modulation of fibroblast growth factor signaling through heparan sulfate proteoglycans in mammalian development. Curr. Opin. Genet. Dev. 23, 399–407. ( 10.1016/j.gde.2013.02.004) [DOI] [PubMed] [Google Scholar]
- 19.Maccarana M, Sakura Y, Tawada A, Yoshida K, Lindahl U. 1996. Domain structure of heparan sulfates from bovine organs. J. Biol. Chem. 271, 17 804–17 810. ( 10.1074/jbc.271.30.17804) [DOI] [PubMed] [Google Scholar]
- 20.Murphy KJ, Merry CL, Lyon M, Thompson JE, Roberts IS, Gallagher JT. 2004. A new model for the domain structure of heparan sulfate based on the novel specificity of K5 lyase. J. Biol. Chem. 279, 27 239–27 245. ( 10.1074/jbc.M401774200) [DOI] [PubMed] [Google Scholar]
- 21.Patel VN, et al. 2008. Specific heparan sulfate structures modulate FGF10-mediated submandibular gland epithelial morphogenesis and differentiation. J. Biol. Chem. 283, 9308–9317. ( 10.1074/jbc.M709995200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pan Y, Carbe C, Powers A, Zhang EE, Esko JD, Grobe K, Feng GS, Zhang X. 2008. Bud specific N-sulfation of heparan sulfate regulates Shp2-dependent FGF signaling during lacrimal gland induction. Development 135, 301–310. ( 10.1242/dev.014829) [DOI] [PubMed] [Google Scholar]
- 23.Qu X, et al. 2011. Lacrimal gland development and Fgf10-Fgfr2b signaling are controlled by 2-O- and 6-O-sulfated heparan sulfate. J. Biol. Chem. 286, 14 435–14 444. ( 10.1074/jbc.M111.225003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pye DA, Vives RR, Turnbull JE, Hyde P, Gallagher JT. 1998. Heparan sulfate oligosaccharides require 6-O-sulfation for promotion of basic fibroblast growth factor mitogenic activity. J. Biol. Chem. 273, 22 936–22 942. ( 10.1074/jbc.273.36.22936) [DOI] [PubMed] [Google Scholar]
- 25.Escobar Galvis ML, et al. 2007. Transgenic or tumor-induced expression of heparanase upregulates sulfation of heparan sulfate. Nat. Chem. Biol. 3, 773–778. ( 10.1038/nchembio.2007.41) [DOI] [PubMed] [Google Scholar]
- 26.Dhoot GK, Gustafsson MK, Ai X, Sun W, Standiford DM, Emerson CP., Jr 2001. Regulation of Wnt signaling and embryo patterning by an extracellular sulfatase. Science 293, 1663–1666. ( 10.1126/science.293.5535.1663) [DOI] [PubMed] [Google Scholar]
- 27.Settembre C, Arteaga-Solis E, McKee MD, de Pablo R, Al Awqati Q, Ballabio A, Karsenty G. 2008. Proteoglycan desulfation determines the efficiency of chondrocyte autophagy and the extent of FGF signaling during endochondral ossification. Genes Dev. 22, 2645–2650. ( 10.1101/gad.1711308) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Otsuki S, Hanson SR, Miyaki S, Grogan SP, Kinoshita M, Asahara H, Wong CH, Lotz MK. 2010. Extracellular sulfatases support cartilage homeostasis by regulating BMP and FGF signaling pathways. Proc. Natl Acad. Sci. USA 107, 10 202–10 207. ( 10.1073/pnas.0913897107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang S, Ai X, Freeman SD, Pownall ME, Lu Q, Kessler DS, Emerson CP., Jr 2004. QSulf1, a heparan sulfate 6-O-endosulfatase, inhibits fibroblast growth factor signaling in mesoderm induction and angiogenesis. Proc. Natl Acad. Sci. USA 101, 4833–4838. ( 10.1073/pnas.0401028101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kamimura K, Koyama T, Habuchi H, Ueda R, Masu M, Kimata K, Nakato H. 2006. Specific and flexible roles of heparan sulfate modifications in Drosophila FGF signaling. J. Cell Biol. 174, 773–778. ( 10.1083/jcb.200603129) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jastrebova N, Vanwildemeersch M, Lindahl U, Spillmann D. 2010. Heparan sulfate domain organization and sulfation modulate FGF-induced cell signaling. J. Biol. Chem. 285, 26 842–26 851. ( 10.1074/jbc.M109.093542) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ai X, Do AT, Lozynska O, Kusche-Gullberg M, Lindahl U, Emerson CP., Jr 2003. QSulf1 remodels the 6-O sulfation states of cell surface heparan sulfate proteoglycans to promote Wnt signaling. J. Cell Biol. 162, 341–351. ( 10.1083/jcb.200212083) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kleinschmit A, Koyama T, Dejima K, Hayashi Y, Kamimura K, Nakato H. 2010. Drosophila heparan sulfate 6-O endosulfatase regulates Wingless morphogen gradient formation. Dev. Biol. 345, 204–214. ( 10.1016/j.ydbio.2010.07.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Viviano BL, Paine-Saunders S, Gasiunas N, Gallagher J, Saunders S. 2004. Domain-specific modification of heparan sulfate by Qsulf1 modulates the binding of the bone morphogenetic protein antagonist Noggin. J. Biol. Chem. 279, 5604–5611. ( 10.1074/jbc.M310691200) [DOI] [PubMed] [Google Scholar]
- 35.Allen BL, Rapraeger AC. 2003. Spatial and temporal expression of heparan sulfate in mouse development regulates FGF and FGF receptor assembly. J. Cell Biol. 163, 637–648. ( 10.1083/jcb.200307053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.David G, Bai XM, Van der Schueren B, Cassiman JJ, Van den Berghe H. 1992. Developmental changes in heparan sulfate expression: in situ detection with mAbs. J. Cell Biol. 119, 961–975. ( 10.1083/jcb.119.4.961) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Higginson JR, Thompson SM, Santos-Silva A, Guimond SE, Turnbull JE, Barnett SC. 2012. Differential sulfation remodelling of heparan sulfate by extracellular 6-O-sulfatases regulates fibroblast growth factor-induced boundary formation by glial cells: implications for glial cell transplantation. J. Neurosci. 32, 15 902–15 912. ( 10.1523/JNEUROSCI.6340-11.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nagamine S, et al. 2012. Organ-specific sulfation patterns of heparan sulfate generated by extracellular sulfatases Sulf1 and Sulf2 in mice. J. Biol. Chem. 287, 9579–9590. ( 10.1074/jbc.M111.290262) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shimokawa K, Kimura-Yoshida C, Nagai N, Mukai K, Matsubara K, Watanabe H, Matsuda Y, Mochida K, Matsuo I. 2011. Cell surface heparan sulfate chains regulate local reception of FGF signaling in the mouse embryo. Dev. Cell 21, 257–272. ( 10.1016/j.devcel.2011.06.027) [DOI] [PubMed] [Google Scholar]
- 40.Yayon A, Klagsbrun M, Esko JD, Leder P, Ornitz DM. 1991. Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell 64, 841–848. ( 10.1016/0092-8674(91)90512-W) [DOI] [PubMed] [Google Scholar]
- 41.Schlessinger J, Plotnikov AN, Ibrahimi OA, Eliseenkova AV, Yeh BK, Yayon A, Linhardt RJ, Mohammadi M. 2000. Crystal structure of a ternary FGF–FGFR–heparin complex reveals a dual role for heparin in FGFR binding and dimerization. Mol. Cell 6, 743–750. ( 10.1016/S1097-2765(00)00073-3) [DOI] [PubMed] [Google Scholar]
- 42.Pellegrini L, Burke DF, von Delft F, Mulloy B, Blundell TL. 2000. Crystal structure of fibroblast growth factor receptor ectodomain bound to ligand and heparin. Nature 407, 1029–1034. ( 10.1038/35039551) [DOI] [PubMed] [Google Scholar]
- 43.Corson LB, Yamanaka Y, Lai KM, Rossant J. 2003. Spatial and temporal patterns of ERK signaling during mouse embryogenesis. Development 130, 4527–4537. ( 10.1242/dev.00669) [DOI] [PubMed] [Google Scholar]
- 44.Koziel L, Kunath M, Kelly OG, Vortkamp A. 2004. Ext1-dependent heparan sulfate regulates the range of Ihh signaling during endochondral ossification. Dev. Cell 6, 801–813. ( 10.1016/j.devcel.2004.05.009) [DOI] [PubMed] [Google Scholar]
- 45.Norton WH, Ledin J, Grandel H, Neumann CJ. 2005. HSPG synthesis by zebrafish Ext2 and Extl3 is required for Fgf10 signalling during limb development. Development 132, 4963–4973. ( 10.1242/dev.02084) [DOI] [PubMed] [Google Scholar]
- 46.Kraushaar DC, Rai S, Condac E, Nairn A, Zhang S, Yamaguchi Y, Moremen K, Dalton S, Wang L. 2012. Heparan sulfate facilitates FGF and BMP signaling to drive mesoderm differentiation of mouse embryonic stem cells. J. Biol. Chem. 287, 22 691–22 700. ( 10.1074/jbc.M112.368241) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matsumoto Y, Matsumoto K, Irie F, Fukushi J, Stallcup WB, Yamaguchi Y. 2010. Conditional ablation of the heparan sulfate-synthesizing enzyme Ext1 leads to dysregulation of bone morphogenic protein signaling and severe skeletal defects. J. Biol. Chem. 285, 19 227–19 234. ( 10.1074/jbc.M110.105338) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fischer S, Filipek-Gorniok B, Ledin J. 2011. Zebrafish Ext2 is necessary for Fgf and Wnt signaling, but not for Hh signaling. BMC Dev. Biol. 11, 53 ( 10.1186/1471-213X-11-53) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kuo WJ, Digman MA, Lander AD. 2010. Heparan sulfate acts as a bone morphogenetic protein coreceptor by facilitating ligand-induced receptor hetero-oligomerization. Mol. Biol. Cell 21, 4028–4041. ( 10.1091/mbc.E10-04-0348) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dejima K, Kanai MI, Akiyama T, Levings DC, Nakato H. 2011. Novel contact-dependent bone morphogenetic protein (BMP) signaling mediated by heparan sulfate proteoglycans. J. Biol. Chem. 286, 17 103–17 111. ( 10.1074/jbc.M110.208082) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Witt RM, et al. 2013. Heparan sulfate proteoglycans containing a glypican 5 core and 2-O-sulfo-iduronic acid function as Sonic Hedgehog co-receptors to promote proliferation. J. Biol. Chem. 288, 26 275–26 288. ( 10.1074/jbc.M112.438937) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yan D, Wu Y, Yang Y, Belenkaya TY, Tang X, Lin X. 2010. The cell-surface proteins Dally-like and Ihog differentially regulate Hedgehog signaling strength and range during development. Development 137, 2033–2044. ( 10.1242/dev.045740) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsuda M, et al. 1999. The cell-surface proteoglycan Dally regulates Wingless signalling in Drosophila. Nature 400, 276–280. ( 10.1038/22336) [DOI] [PubMed] [Google Scholar]
- 54.Ruppert R, Hoffmann E, Sebald W. 1996. Human bone morphogenetic protein 2 contains a heparin-binding site which modifies its biological activity. Eur. J. Biochem. 237, 295–302. ( 10.1111/j.1432-1033.1996.0295n.x) [DOI] [PubMed] [Google Scholar]
- 55.Ohkawara B, Iemura S, ten Dijke P, Ueno N. 2002. Action range of BMP is defined by its N-terminal basic amino acid core. Curr. Biol. 12, 205–209. ( 10.1016/S0960-9822(01)00684-4) [DOI] [PubMed] [Google Scholar]
- 56.Capurro M, Martin T, Shi W, Filmus J. 2014. Glypican-3 binds to Frizzled and plays a direct role in the stimulation of canonical Wnt signaling. J. Cell Sci. 127, 1565–1575. ( 10.1242/jcs.140871) [DOI] [PubMed] [Google Scholar]
- 57.Li F, Shi W, Capurro M, Filmus J. 2011. Glypican-5 stimulates rhabdomyosarcoma cell proliferation by activating Hedgehog signaling. J. Cell Biol. 192, 691–704. ( 10.1083/jcb.201008087) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baeg GH, Lin X, Khare N, Baumgartner S, Perrimon N. 2001. Heparan sulfate proteoglycans are critical for the organization of the extracellular distribution of Wingless. Development 128, 87–94. [DOI] [PubMed] [Google Scholar]
- 59.García-García MJ, Anderson KV. 2003. Essential role of glycosaminoglycans in Fgf signaling during mouse gastrulation. Cell 114, 727–737. ( 10.1016/S0092-8674(03)00715-3) [DOI] [PubMed] [Google Scholar]
- 60.De Robertis EM, Kuroda H. 2004. Dorsal-ventral patterning and neural induction in Xenopus embryos. Annu. Rev. Cell Dev. Biol. 20, 285–308. ( 10.1146/annurev.cellbio.20.011403.154124) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jasuja R, Allen BL, Pappano WN, Rapraeger AC, Greenspan DS. 2004. Cell-surface heparan sulfate proteoglycans potentiate Chordin antagonism of bone morphogenetic protein signaling and are necessary for cellular uptake of Chordin. J. Biol. Chem. 279, 51 289–51 297. ( 10.1074/jbc.M408129200) [DOI] [PubMed] [Google Scholar]
- 62.Duchesne L, Octeau V, Bearon RN, Beckett A, Prior IA, Lounis B, Fernig DG. 2012. Transport of fibroblast growth factor 2 in the pericellular matrix is controlled by the spatial distribution of its binding sites in heparan sulfate. PLoS Biol. 10, e1001361 ( 10.1371/journal.pbio.1001361) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Christianson HC, Belting M. 2013. Heparan sulfate proteoglycan as a cell-surface endocytosis receptor. Matrix Biol. 35, 51–55. ( 10.1016/j.matbio.2013.10.004) [DOI] [PubMed] [Google Scholar]
- 64.Elfenbein A, Lanahan A, Zhou TX, Yamasaki A, Tkachenko E, Matsuda M, Simons M. 2012. Syndecan 4 regulates FGFR1 signaling in endothelial cells by directing macropinocytosis. Sci. Signal. 5, ra36 ( 10.1126/scisignal.2002495) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lambaerts K, Wilcox-Adelman SA, Zimmermann P. 2009. The signaling mechanisms of syndecan heparan sulfate proteoglycans. Curr. Opin. Cell Biol. 21, 662–669. ( 10.1016/j.ceb.2009.05.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nowak M, Machate A, Yu SR, Gupta M, Brand M. 2011. Interpretation of the FGF8 morphogen gradient is regulated by endocytic trafficking. Nat. Cell Biol. 13, 153–158. ( 10.1038/ncb2155) [DOI] [PubMed] [Google Scholar]
- 67.Akiyama T, Kamimura K, Firkus C, Takeo S, Shimmi O, Nakato H. 2008. Dally regulates Dpp morphogen gradient formation by stabilizing Dpp on the cell surface. Dev. Biol. 313, 408–419. ( 10.1016/j.ydbio.2007.10.035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gallet A, Staccini-Lavenant L, Thérond PP. 2008. Cellular trafficking of the glypican Dally-like is required for full-strength Hedgehog signaling and Wingless transcytosis. Dev. Cell 14, 712–725. ( 10.1016/j.devcel.2008.03.001) [DOI] [PubMed] [Google Scholar]
- 69.Gallet A. 2011. Hedgehog morphogen: from secretion to reception. Trends Cell Biol. 21, 238–246. ( 10.1016/j.tcb.2010.12.005) [DOI] [PubMed] [Google Scholar]
- 70.Hou S, Maccarana M, Min TH, Strate I, Pera EM. 2007. The secreted serine protease xHtrA1 stimulates long-range FGF signaling in the early Xenopus embryo. Dev. Cell 13, 226–241. ( 10.1016/j.devcel.2007.07.001) [DOI] [PubMed] [Google Scholar]
- 71.Reiland J, Sanderson RD, Waguespack M, Barker SA, Long R, Carson DD, Marchetti D. 2004. Heparanase degrades syndecan-1 and perlecan heparan sulfate: functional implications for tumor cell invasion. J. Biol. Chem. 279, 8047–8055. ( 10.1074/jbc.M304872200) [DOI] [PubMed] [Google Scholar]
- 72.Patel VN, et al. 2007. Heparanase cleavage of perlecan heparan sulfate modulates FGF10 activity during ex vivo submandibular gland branching morphogenesis. Development 134, 4177–4186. ( 10.1242/dev.011171) [DOI] [PubMed] [Google Scholar]
- 73.Giráldez AJ, Copley RR, Cohen SM. 2002. HSPG modification by the secreted enzyme Notum shapes the Wingless morphogen gradient. Dev. Cell 2, 667–676. ( 10.1016/S1534-5807(02)00180-6) [DOI] [PubMed] [Google Scholar]
- 74.Ayers KL, Gallet A, Staccini-Lavenant L, Thérond PP. 2010. The long-range activity of Hedgehog is regulated in the apical extracellular space by the glypican Dally and the hydrolase Notum. Dev. Cell 18, 605–620. ( 10.1016/j.devcel.2010.02.015) [DOI] [PubMed] [Google Scholar]
- 75.Ayers KL, Mteirek R, Cervantes A, Lavenant-Staccini L, Thérond PP, Gallet A. 2012. Dally and Notum regulate the switch between low and high level Hedgehog pathway signalling. Development 139, 3168–3179. ( 10.1242/dev.078402) [DOI] [PubMed] [Google Scholar]
- 76.Eugster C, Panáková D, Mahmoud A, Eaton S. 2007. Lipoprotein-heparan sulfate interactions in the Hh pathway. Dev. Cell 13, 57–71. ( 10.1016/j.devcel.2007.04.019) [DOI] [PubMed] [Google Scholar]
- 77.Gorfinkiel N, Sierra J, Callejo A, Ibañez C, Guerrero I. 2005. The Drosophila ortholog of the human Wnt inhibitor factor shifted controls the diffusion of lipid-modified Hedgehog. Dev. Cell 8, 241–253. ( 10.1016/j.devcel.2004.12.018) [DOI] [PubMed] [Google Scholar]
- 78.Dierker T, Dreier R, Petersen A, Bordych C, Grobe K. 2009. Heparan sulfate-modulated, metalloprotease-mediated sonic hedgehog release from producing cells . J. Biol. Chem. 284, 8013–8022. ( 10.1074/jbc.M806838200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dierker T, Dreier R, Migone M, Hamer S, Grobe K. 2009. Heparan sulfate and transglutaminase activity are required for the formation of covalently cross-linked hedgehog oligomers. J. Biol. Chem. 284, 32 562–32 571. ( 10.1074/jbc.M109.044867) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ohlig S, Pickhinke U, Sirko S, Bandari S, Hoffmann D, Dreier R, Farshi P, Götz M, Grobe K. 2012. An emerging role of Sonic hedgehog shedding as a modulator of heparan sulfate interactions. J. Biol. Chem. 287, 43 708–43 719. ( 10.1074/jbc.M112.356667) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Belenkaya TY, Han C, Yan D, Opoka RJ, Khodoun M, Liu H, Lin X. 2004. Drosophila Dpp morphogen movement is independent of dynamin-mediated endocytosis but regulated by the glypican members of heparan sulfate proteoglycans. Cell 119, 231–244. ( 10.1016/j.cell.2004.09.031) [DOI] [PubMed] [Google Scholar]
- 82.Chen J, Honeyager SM, Schleede J, Avanesov A, Laughon A, Blair SS. 2012. Crossveinless d is a vitellogenin-like lipoprotein that binds BMPs and HSPGs, and is required for normal BMP signaling in the Drosophila wing. Development 139, 2170–2176. ( 10.1242/dev.073817) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chuang CY, Lord MS, Melrose J, Rees MD, Knox SM, Freeman C, Iozzo RV, Whitelock JM. 2010. Heparan sulfate-dependent signaling of fibroblast growth factor 18 by chondrocyte-derived perlecan. Biochemistry 49, 5524–5532. ( 10.1021/bi1005199) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim MJ, Cotman SL, Halfter W, Cole GJ. 2003. The heparan sulfate proteoglycan agrin modulates neurite outgrowth mediated by FGF-2. J. Neurobiol. 55, 261–277. ( 10.1002/neu.10213) [DOI] [PubMed] [Google Scholar]
- 85.Harada M, et al. 2009. FGF9 monomer-dimer equilibrium regulates extracellular matrix affinity and tissue diffusion. Nat. Genet. 41, 289–298. ( 10.1038/ng.316) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kalinina J, et al. 2009. Homodimerization controls the fibroblast growth factor 9 subfamily's receptor binding and heparan sulfate-dependent diffusion in the extracellular matrix. Mol. Cell. Biol. 29, 4663–4678. ( 10.1128/MCB.01780-08) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Qu X, Pan Y, Carbe C, Powers A, Grobe K, Zhang X. 2012. Glycosaminoglycan-dependent restriction of FGF diffusion is necessary for lacrimal gland development. Development 139, 2730–2739. ( 10.1242/dev.079236) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Makarenkova HP, Hoffman MP, Beenken A, Eliseenkova AV, Meech R, Tsau C, Patel VN, Lang RA, Mohammadi M. 2009. Differential interactions of FGFs with heparan sulfate control gradient formation and branching morphogenesis. Sci. Signal. 2, ra55 ( 10.1126/scisignal.2000304) [DOI] [PMC free article] [PubMed] [Google Scholar]