Abstract
Mouse epiblast stem cells (EpiSCs) display temporal differences in the upregulation of Mixl1 expression during the initial steps of in vitro differentiation, which can be correlated with their propensity for endoderm differentiation. EpiSCs that upregulated Mixl1 rapidly during differentiation responded robustly to both Activin A and Nodal in generating foregut endoderm and precursors of pancreatic and hepatic tissues. By contrast, EpiSCs that delayed Mixl1 upregulation responded less effectively to Nodal and showed an overall suboptimal outcome of directed differentiation. The enhancement in endoderm potency in Mixl1-early cells may be accounted for by a rapid exit from the progenitor state and the efficient response to the induction of differentiation by Nodal. EpiSCs that readily differentiate into the endoderm cells are marked by a distinctive expression fingerprint of transforming growth factor (TGF)-β signalling pathway genes and genes related to the endoderm lineage. Nodal appears to elicit responses that are associated with transition to a mesenchymal phenotype, whereas Activin A promotes gene expression associated with maintenance of an epithelial phenotype. We postulate that the formation of definitive endoderm (DE) in embryoid bodies follows a similar process to germ layer formation from the epiblast, requiring an initial de-epithelialization event and subsequent re-epithelialization. Our results show that priming EpiSCs with the appropriate form of TGF-β signalling at the formative phase of endoderm differentiation impacts on the further progression into mature DE-derived lineages, and that this is influenced by the initial characteristics of the cell population. Our study also highlights that Activin A, which is commonly used as an in vitro surrogate for Nodal in differentiation protocols, does not elicit the same downstream effects as Nodal, and therefore may not effectively mimic events that take place in the mouse embryo.
Keywords: endoderm differentiation, lineage propensity, TGF-β signalling, directed differentiation, epiblast stem cells, gastrulation
1. Introduction
In the postimplantation mouse embryo, the formation of the primitive streak (PS) heralds the beginning of gastrulation. Cells in the epiblast are recruited to the PS and, as they disengage from the neighbouring epithelial cells and ingress through the PS, they acquire a mesenchymal morphology [1]. Cells emerging from the PS are either incorporated into an expanding layer of mesenchymal cells (the mesoderm) or become integrated into the pre-existing visceral endoderm layer to form the definitive endoderm (DE) [2]. The DE, together with a subset of visceral endoderm cells, constitutes the precursors of the epithelial tissues of the fetal digestive tract and its associated organs [3]. Fate mapping studies have revealed that progenitors of the DE and mesoderm are localized to the anterior segment of the PS [4–6]. It is unclear, however, if the anterior primitive streak (APS) cells have a dual potential to contribute to mesoderm and endoderm (i.e. mesendoderm progenitors) [7], or are a mixed population of two types of germ layer progenitors.
In the gastrula embryo, cells in different segments of the PS may be subjected to graded levels of signalling activity, based on the expression pattern of the pathway genes and the loss-of-function phenotypes [8,9]. Nodal (a transforming growth factor (TGF)-β-related factor) activity is high in the APS, bone morphogenetic protein (BMP) activity peaks in the posterior PS and fibroblast growth factor (FGF) activity is higher in the middle region than other parts of the streak. High Nodal activity is required for the induction of the putative mesendoderm progenitors [10,11] and mutant embryos with enhanced Nodal activity gain more endoderm cells, whereas those with reduced or absent Nodal function are deficient in DE [8–10,12,13].
Epiblast stem cells (EpiSCs) are self-renewing multipotent cells that are derived from the epiblast and ectoderm of postimplantation mouse embryos at the pre-gastrulation to the late gastrulation stages [14–17]. These EpiSCs are maintained in vitro by culturing them in the presence of Activin A (another TGF-β-related factor) and FGF2 [18], reminiscent of the provision of Nodal and FGF signals at the APS of the embryo [9,19,20]. Irrespective of the developmental stage of origin, the established EpiSC lines are developmentally comparable to the ectoderm of the late-gastrula-stage mouse embryo with regard to their transcriptome. Furthermore, EpiSCs are enriched with gene transcripts that are expressed by APS cells [17], and when transplanted into the PS of a host embryo they display the range of cell fates and express the lineage markers that are characteristic of the descendants of APS cells [17,21]. These functional and genetic attributes of the EpiSCs point to the possibility that they are the in vitro counterpart of the APS cells and, therefore, would be an informative experimental model for studying lineage differentiation of the mouse epiblast and, in particular, the PS.
In this study, we investigated endoderm development in the context of the propensity of EpiSCs to differentiate to endodermal lineages, in response to TGF-β signalling induced by Nodal and Activin A. Our findings provide new insights into the role of Nodal signalling in the formation of the DE during mouse gastrulation.
2. Endoderm lineage propensity of the epiblast stem cells
Analysis of the transcriptome of EpiSCs revealed that while the gene expression profiles are globally similar among the established lines, they can be clustered into distinct subgroups according to the expression profile of genes that are characteristic of embryonic germ layers (endoderm, mesoderm and neurectoderm) [17]. By assaying the temporal pattern of expression of genes associated with germ layer formation in embryoid bodies (EBs) over a 4-day period, EpiSC lines were found to respond differently to the induction of differentiation. In particular, the temporal expression profile of Mixl1, a gene that is expressed in the PS and required for DE formation [22], varied across the set of EpiSCs analysed. Prior to differentiation, Mixl1 expression was comparable across all EpiSC lines analysed [17]. Upon differentiation, EpiSCs could be classified into three groups according to the pace at which Mixl1 expression is upregulated. A subset of EpiSC lines showed rapid upregulation of Mixl1 (termed Mixl1-early); a second group showed a much delayed upregulation of Mixl1 (Mixl1-late) and a third group (Mixl1-intermediate) showed peak expression of Mixl1 at a time point in between.
Our previous work has shown that cell lines in these three categories can be distinguished by the expression profiles of selected genes prior to differentiation [17], suggesting that the readiness to differentiate is influenced by their intrinsic molecular characteristics. Re-analysing the transcriptome of the undifferentiated EpiSCs with reference to their Mixl1-category revealed that the Mixl1-early EpiSCs showed higher expression of pluripotency and endoderm-related genes, whereas the Mixl1-late EpiSCs show higher expression of mesenchyme and neural-related genes [17]. EpiSCs of the three categories of Mixl1 expression pattern consistently showed different outcomes of differentiation. Mixl1-early EpiSCs express endoderm lineage markers at a higher level during in vitro differentiation within EBs and they generate teratomas with more abundant endoderm derivatives than Mixl1-intermediate and Mixl1-late EpiSCs [17].
In the embryo, Mixl1 is expressed in the PS and downregulated in DE cells [23,24]. The rapid changes in Mixl1 expression in differentiating Mixl1-early EpiSCs are therefore reminiscent of the in vivo situation where Mixl1 expression mirrors the specification of the endoderm progenitors, and the transition to DE is accompanied by the cessation of expression. The findings of the transcriptome analysis outlined above suggest that endoderm differentiation propensity of a cell line may be negatively correlated with its ability to undergo neural and mesoderm differentiation [21,25]. Mixl1-early EpiSCs may therefore be entrained with a molecular signature that primes them to differentiate into DE and this is reflected in the rapid switch between upregulation and downregulation of Mixl1, which is similar to the changes in Mixl1 expression during DE formation in vivo.
3. Impact of TGF-β activity on endoderm differentiation
EpiSCs from different Mixl1-response groups respond differently to conditions that direct the differentiation of the stem cells to endoderm. Mixl1-early EpiSCs expressed higher levels of genes that signify the presence of gut endoderm cells, liver and pancreas than cells of Mixl1-intermediate or Mixl1-late categories [17]. The induction of differentiation was achieved by treating the EpiSCs with Activin A at a concentration higher than that for maintenance of the cell line. Activin A acts as a mesoderm inducer in Xenopus embryos [26–28] and as an inducer of endoderm differentiation of mouse and human pluripotent stem cells [29–34]. However, although genes encoding TGF-β receptors such as Acvr2a, Acvr2b and Acvr1b are expressed in the gastrula-stage mouse embryo, Inhba (for Activin A) is not expressed. It is generally considered that Activin receptors in vivo mediate the signalling activity of other TGF-β proteins, with Nodal being the most probable signalling factor. Consistent with this postulation, mutations of the receptors, co-receptor (Cripto), intracellular transducers (Smad2, -3 and -4) and Nodal proximal enhancer that impact adversely on the Nodal/Smad/Foxh1 pathway lead to defects in PS function and endoderm formation [10,13,35–38]. Furthermore, a comparison of the effect of Nodal and Activin A on the differentiation of mouse embryonic stem cells shows that the DE generated by Nodal activity is better able to colonize the embryonic foregut and differentiate into pancreatic cells in vivo [30]. This suggests that while Activin A signals through a similar molecular pathway to Nodal, it might not fully substitute the function of Nodal in endoderm differentiation.
In view of the developmental similarities between EpiSCs and PS cells, we tested whether Nodal and Activin A may have different effects on endoderm differentiation and if the response may also be correlated with the endoderm lineage propensity of the EpiSCs.
4. EpiSCs of different lineage propensity respond differently to Nodal and Activin A
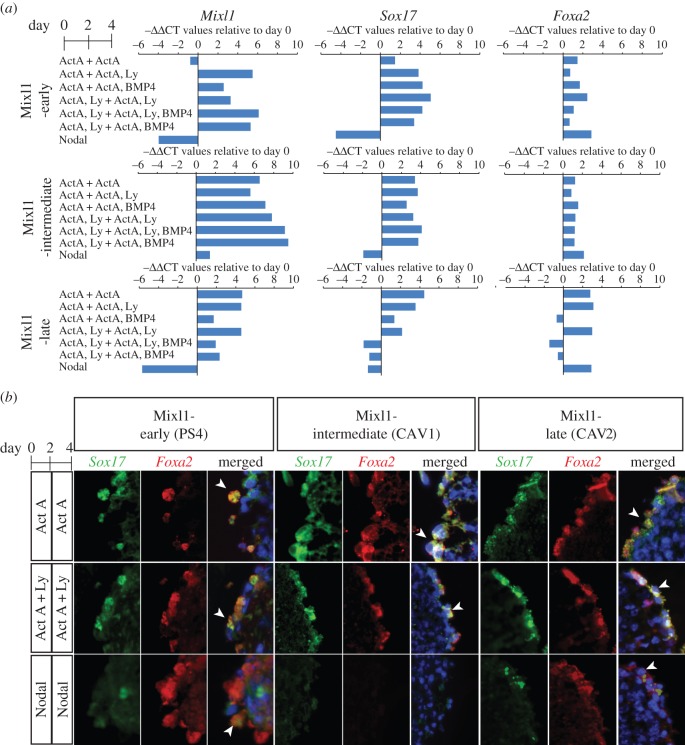
EpiSC lines of Mixl1-early (PS4), Mixl1-intermediate (CAV1) and Mixl1-late (CAV2, CAV4) groups were studied for differentiation to DE. EpiSC-derived EBs were cultured in medium supplemented with Nodal for 4 days, or with Activin A or Activin A + phosphoinositide 3-kinase (PI3K) inhibitor (Ly294002; Ly) for day 0–2 and then in various combinations of BMP4 and PI3K inhibitor for day 2–4 (figure 1a). Endoderm formation was assessed by qPCR analysis of the expression of mesendoderm progenitors (Mixl1) and DE (Sox17 and Foxa2) markers (figure 1a). In Mixl1-early EpiSCs, Mixl1 expression was low by day 4 when cultured in either Activin A or Nodal-supplemented medium, but Mixl1 expression remained high in all other conditions (Activin A + other factors). Sox17 was expressed in all Activin A cultures, but was reduced in Nodal culture. By contrast, Foxa2 expression was detected under all conditions and most strongly with Nodal. Mixl1-intermediate EpiSCs had elevated expression of all three markers in all Activin A conditions. However, in Nodal cultures, expression of Mixl1 was only increased slightly, Sox17 was reduced and Foxa2 was increased compared with day 0. Mixl1-late EpiSCs displayed variable responses to most Activin A + BMP4 conditions, though upregulated Mixl1, Sox17 and Foxa2 in Activin A and Activin A + Ly cultures. The expression pattern of these genes in response to Nodal was similar to that of Mixl1-early EpiSCs.
Figure 1.
Differentiation of Mixl1-early, Mixl1-intermediate and Mixl1-late EpiSCs. (a) Level of expression of Mixl1 (PS marker), Sox17 and Foxa2 (definitive endoderm marker) in day-4 EBs cultured under seven culture protocols. Gene expression level is presented as the negative value of the relative difference in threshold cycles (−ΔΔCT; normalized against Actb) relative to day 0 value and is the mean of triplicate cultures. Positive value, upregulation; negative value, downregulation. (b) Endoderm cells co-expressing Foxa2 and Sox17 (arrowheads) in day-4 EBs, visualized by immunofluorescence of Foxa2 (red) and Sox17 (green). (Online version in colour.)
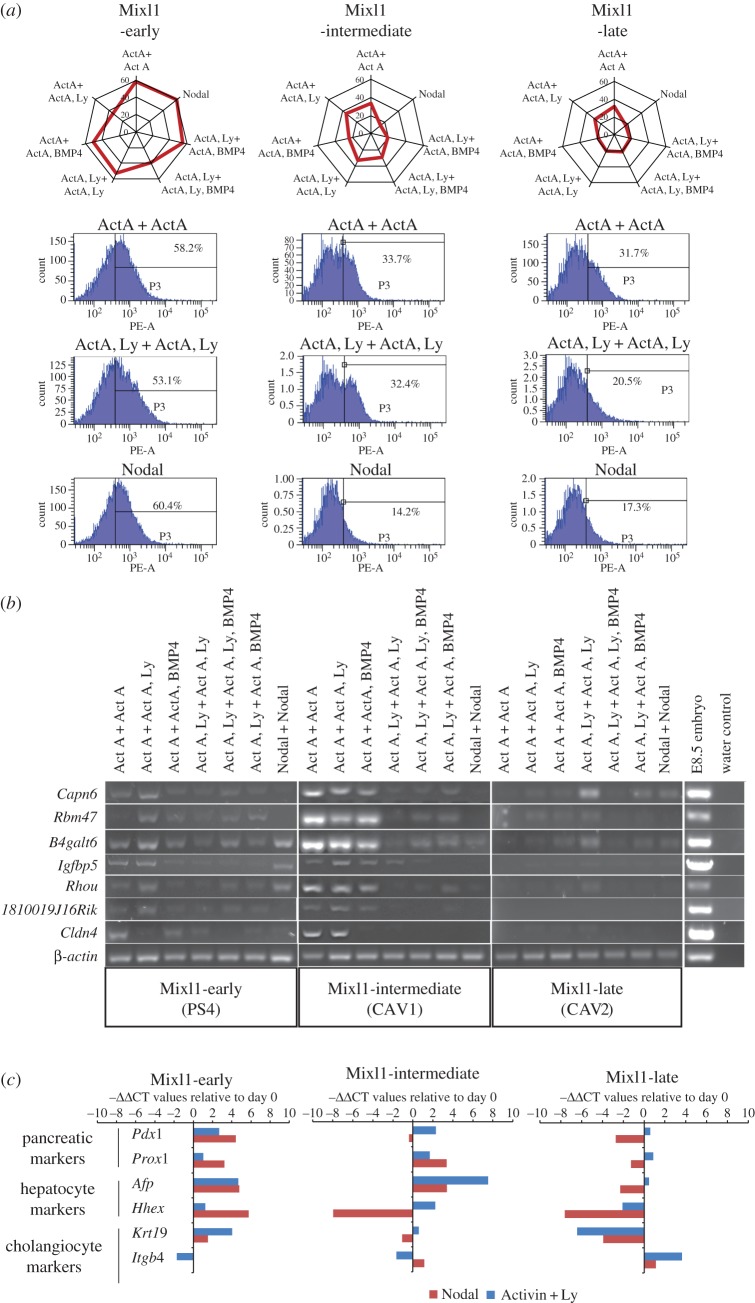
The EBs were further analysed by immunofluorescence to visualize cells that co-expressed Sox17 and Foxa2 (figure 1b, electronic supplementary material, figure S1), which are likely to be equivalent to endoderm cells. Foxa2+;Sox17+ cells were detected in the outer cell layers of EBs of Mixl1-early EpiSCs in all culture conditions. For Mixl1-intermediate EpiSCs, Foxa2+;Sox17+ cells could be detected in EBs differentiated under most combinations of Activin A and PI3K inhibitor but not in Nodal-supplemented culture. For Mixl1-late EBs, Foxa2+;Sox17+ cells were present in Activin A, Activin A + Ly (without BMP4) and Nodal-treated conditions. Endoderm-like cells were, therefore, induced by Activin A in EpiSCs of all three categories but only in Mixl1-early cells and, at a much lower abundance, in Mixl1-late cells following Nodal induction. To assess the efficiency of Activin A and Nodal induction of DE versus visceral endoderm-like cells, the enrichment of the CXCR4-positive population [39,40] in the EBs was quantified by FACS analysis (figure 2a, electronic supplementary material, figure S2). CXCR4 is expressed in embryonic germ layers, but not extraembryonic visceral endoderm. Mixl1-early EpiSCs showed the greatest enrichment of CXCR4-positive cells, whereas the Mixl1-intermediate and Mixl1-late EpiSCs responded modestly to induction by Activin and Activin + Ly, and weakly to Nodal (figure 2a).
Figure 2.
Differentiation of EpiSCs to endoderm lineages. (a) The level of enrichment of CXCR4-positive cells in day-4 EBs generated from EpiSCs of the three Mixl1 categories. Radar graphs (top row) show the data of all seven culture conditions (radial axis: level (%) of enrichment). Flow cytometry results and the % CXCR4-positive cells in the samples are shown for three conditions (rows 2–3) for each Mixl1-group. (b) PCR analysis of the expression of foregut endoderm genes in day-4 EBs cultured in seven conditions. Integrity of the cDNA sample was ascertained by PCR analysis of a housekeeping gene, β-actin. (c) Expression of Pdx1, Prox1 (pancreatic markers), Afp, Hhex (hepatocyte markers) and Krt19, Itgb4 (cholangiocyte markers) after 9-day directed differentiation of EpiSCs of the three Mixl1 categories. EpiSCs were cultured in either Activin A + Ly or Nodal at day 1–4. Gene expression level is presented as the negative value of the relative difference in threshold cycles (−ΔΔCT; normalized against Actb) relative to day 0 value. Positive value, upregulation; negative value, downregulation. (Online version in colour.)
The expression of other lineage markers in response to the various growth factors was also examined (electronic supplementary material, figure S3). Mixl1-late and Mixl1-intermediate lines showed high levels of Sox1 expression in response to Activin A treatments, indicative of the presence of neuroectoderm progenitors in EBs derived from these lines. Conversely, the Mixl1-early line showed either a relatively weak induction or downregulation of Sox1 in response to all the test conditions. Lines from all groups showed low-level expression of Pax6 suggesting the lack of differentiation of more advanced ectoderm derivatives. Expression of markers for pluripotency (Oct4 and Nanog) was reduced in all EpiSC lines but stayed relatively higher in Mixl1-late cells cultured under Activin A-containing conditions, which might indicate the persistence of undifferentiated stem cells. Other non-endoderm lineage markers, Meox1, T and Sox7 were generally expressed at similar levels among the cell lines, irrespective of culture conditions (electronic supplementary material, figure S3).
Overall, these results show that Mixl1-early EpiSCs respond effectively to both Nodal and Activin A by expressing endoderm markers, generating Sox17 and Foxa2-positive cells, and showing enrichment of CXCR4-expressing cells. Compared with Mixl1-early EpiSCs, Mixl1-intermediate and Mixl1-late EpiSCs respond poorly to Nodal and are less efficient in endoderm differentiation.
5. Nodal promotes differentiation of epiblast stem cells with enhanced endoderm propensity
The ability of the EpiSCs to differentiate into cells with foregut endoderm properties in day 4 EBs was assessed by the expression of seven validated markers (1810019J16Rik, B4galt6, Capn6, Cldn4 Igfbp5, Rbm47 and Rhou; figure 2b) that were identified as transcripts enriched in the foregut endoderm of early-somite-stage mouse embryos (electronic supplementary material, figure S4, and tables S1 and S2). In Mixl1-early EBs, various combinations of foregut endoderm markers could be detected in all culture conditions. In Mixl1-intermediate EpiSCs, foregut endoderm markers were expressed when only Activin A was present (i.e. without Ly) in the first 2 days of culture. Compared to the Mixl1-early EpiSCs, only a few foregut endoderm markers could be detected in the Mixl1-late EpiSC culture. Both Mixl1-intermediate and Mixl1-late EpiSCs responded poorly to Nodal in expressing foregut endoderm markers.
To test the differentiation potential of cells with DE and foregut endoderm characteristics, we developed a protocol in which EpiSCs were subjected to extended differentiation culture for a further 2 days in media supplemented with various combinations of FGF10, BMP4, retinoic acid (RA) and an inhibitor of Hedgehog signalling (cyclopamine, cyc), following 4 days with Activin A and Ly (electronic supplementary material, figure S5a). Cells were grown as EBs for the first 2 days, dissociated and subsequently grown in an adherent culture for the next 4 days. As an example, in the presence of FGF10 and cyc, Mixl1-intermediate EpiSCs expressed foregut markers (Tbx1, Pyy, Hnf1b, Hnf4a) and the lung and thyroid marker Nkx2.1, weakly expressed the intestine marker Cdx2 and downregulated the foregut progenitor marker Sox2 (electronic supplementary material, figure S5b). Further extending the culture (day 6–9, electronic supplementary material, figure S5a) in medium containing FGF10, RA and cyclopamine enhanced the expression of pancreatic endoderm and endocrine precursors markers Pdx1, Prox1 and Ngn3 (electronic supplementary material, figure S5c, and data not shown).
EpiSCs of the three Mixl1 categories were then compared after a 9-day culture period for the expression of markers of pancreatic cells (Pdx1, Prox1), hepatocytes (Afp, Hhex) and cholangiocytes (Krt19, Itgb4; figure 2c). Mixl1-early EpiSCs expressed pancreatic and hepatocyte markers more robustly when cultured in Nodal for the first 4 days of differentiation than in Activin A + Ly, but the two cholangiocyte markers responded differently to Nodal versus Activin A. Mixl1-intermediate EpiSCs cultured in Nodal did not show any consistent pattern of marker expression for the three endoderm lineages, except that the Activin A + Ly treated cells upregulated markers of pancreatic and liver cells. Only Itgb4 was upregulated in Mixl1-late EpiSC in either Activin A or Nodal conditions. All other markers were downregulated in Activin A + Ly culture (figure 2c), suggesting these EpiSCs may be predisposed for the cholangiocyte lineage.
In summary, the outcome of the directed differentiation of the EpiSCs shows that the immediacy in the activation of Mixl1 is associated with an enhanced propensity for endoderm differentiation and receptivity to TGF-β signalling. The Mixl1-early EpiSCs respond effectively to the inductive activity of both Nodal and Activin A and generate foregut endoderm-like cells that are competent to differentiate into more advanced cell types. Induction by Nodal or Activin A at the formative phase of the endoderm lineage has a critical influence on the outcome of differentiation: Nodal promotes the expression of pancreas and liver cell markers, whereas Activin A promotes the expression of the cholangiocyte marker Krt19, indicating that cells have acquired characteristics of the bile duct epithelium. The Mixl1-intermediate EpiSCs respond to Activin A more effectively than to Nodal, demonstrated by the generation of cells displaying foregut endoderm properties. These cells, however, are not competent to differentiate into cells with consistent endoderm characteristics. Mixl1-late EpiSCs do not respond effectively to either TGF-β factors to form DE.
6. Innate TGF-β signalling activity in epiblast stem cells
(a). Implications for directed differentiation of pluripotent stem cells
For directed differentiation of pluripotent cells to endoderm tissues such as hepatocytes and pancreatic endocrine cells, the road-tested strategy has been to drive differentiation first to DE-like cells, followed by enrichment of cells that display properties of the posterior foregut endoderm. This intermediate cell type is then subjected to culture conditions that promote the differentiation towards the hepatic or pancreatic tissue lineages [33]. A variety of in vitro culture protocols have been devised for the directed differentiation of pluripotent stem cells to endoderm derivatives. These protocols vary in parameters such as the formulation of the basic culture medium, the combination of supplements used and the dosage and timing at which they are delivered to the differentiating cells. Of particular interest is that Activin A and Nodal, two TGF-β superfamily factors, have been reported to elicit similar signalling responses and can induce differentiation of embryonic stem cells to DE-like cells with very similar molecular phenotypes [30,34], but Nodal-treated cells display greater functional capacity than their Activin A counterpart to differentiate into functionally competent pancreatic endoderm cells [30]. In our study, we observed different responses of EpiSCs to Nodal and Activin A during differentiation in culture. There is mounting evidence that in pluripotent stem cells, lineage-specific genes are co-expressed with genes of the genetic network that maintain the cells at the pluripotent state [41], which could signify that the cells are already poised to undergo lineage-specific differentiation. Some lineage-specific genes are known to play a critical role in specifying the tissue lineage. The expression of some of these lineage specifiers is regulated by pluripotency-related genes such as Oct4 and Sox2, and enforced expression of these genes can substitute for Oct4 and Sox2 in reprogramming somatic cells to pluripotency [42]. For example, Gata3 can efficiently substitute for Oct4 and Gmnn can substitute for Sox2 in the generation of induced pluripotent stem cells (iPSCs) [42]. The authors proposed a ‘seesaw’ model whereby mesendoderm lineage specifiers dampen the upregulation of ectoderm genes induced by Sox2 while ectoderm specifiers attenuate the elevation of mesendoderm genes induced by Oct4. It is postulated that a balance in the counteracting effects of lineage specifiers contributes to both the induction and maintenance of pluripotency. It is also possible that slight deviations from balanced activity of lineage-specific genes might not affect pluripotency, but could confer a lineage bias in the undifferentiated cells that is revealed upon differentiation. Recent studies of iPSCs have revealed that these stem cells harbour genetic and epigenetic variations which may impact on their differentiation potential and the phenotype of the differentiated cells [43]. Collectively, these findings highlight that the efficacy of directed differentiation could be influenced by the individual characteristics of the pluripotent stem cells, which determine their response to the induction of differentiation.
Our results show that the EpiSC lines are inherently different in their endoderm differentiation potential as revealed by the immediacy of Mixl1 activation and the enhanced expression of endoderm lineage-specific genes in the parental EpiSCs. Activin/Nodal signalling activates different transcriptional responses in human embryonic stem cells or endoderm progenitors that either promote the maintenance of pluripotency or drive cell differentiation [32]. The enhanced capacity of Mixl1-early EpiSCs for endoderm differentiation may be underpinned by the rapid emergence of Mixl1-expressing progenitors of the endoderm lineage and this may be the population that responds effectively to the induction of differentiation by the Activin/Nodal signals.
(b). Different downstream activity of Nodal and Activin A in epiblast stem cells
Transcriptome analysis has revealed that undifferentiated EpiSCs express genes that are characteristic of the APS [17]. In view of the contrast in endoderm differentiation propensity among EpiSCs, we examined the pattern of expression of PS genes in the three Mixl1 categories. Mixl1-early EpiSCs expressed a larger set of PS genes at a higher level than EpiSCs of the other two Mixl1 categories (electronic supplementary material, figure S6a). The Mixl1-early EpiSCs expressed higher levels of transcripts encoding members of embryonic patterning, morphogenesis and Wnt signalling pathways when compared with Mixl1-intermediate EpiSCs. They were enriched for genes related to cell–extracellular matrix interaction, cell proliferation and metabolism and growth factor activity when compared with Mixl1-late EpiSCs (electronic supplementary material, figure S6b). Mixl1-early EpiSCs therefore display more robust gene expression indicative of the propensity for germ layer differentiation.
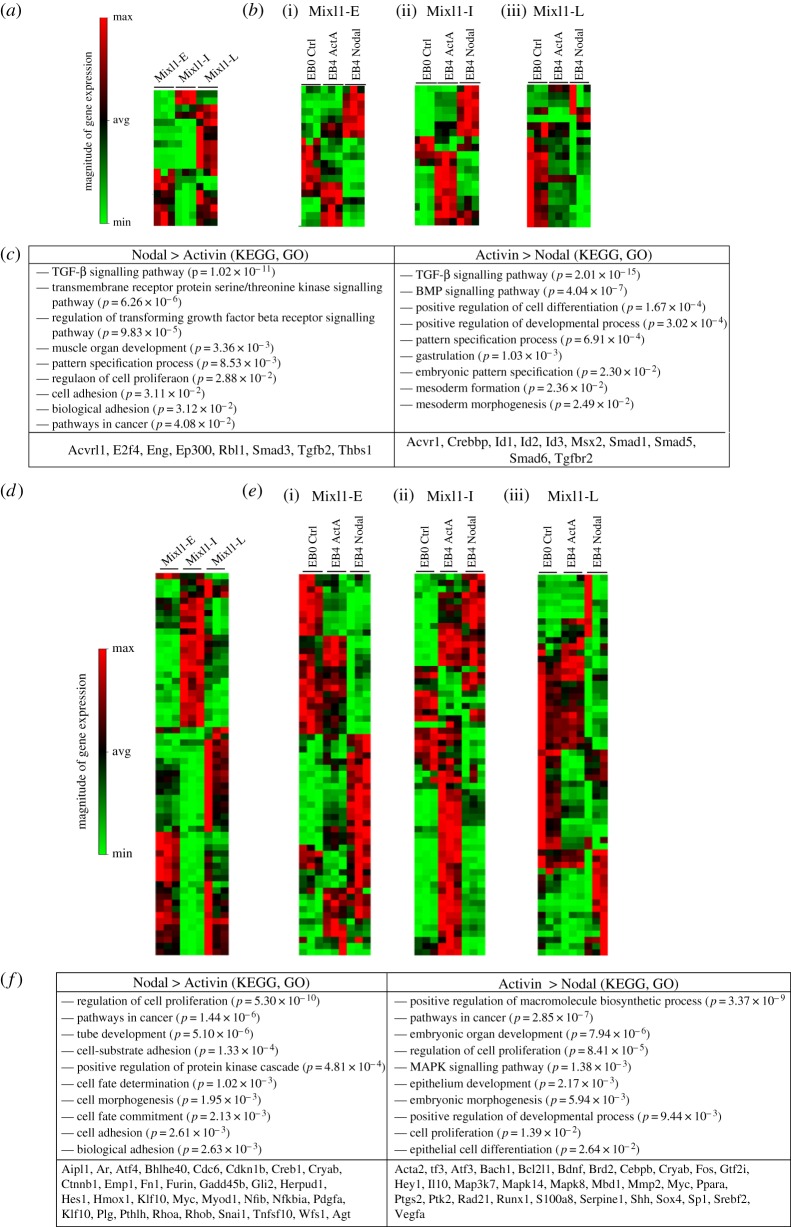
The differences in the outcome of Activin A and Nodal induced differentiation among the EpiSCs point to the inherent differences in their receptivity to TGF-β signalling. A comparison of the expression profiles of genes encoding TGF-β signalling pathway components (ligands, receptors and transducers) reveals that EpiSCs of different Mixl1 expression categories expressed different sets of genes before differentiation (figure 3a). The expression of different sets of TGF-β response genes (figure 3d) further showed that EpiSCs of different lineage propensity might be primed differentially to activate different signalling pathways during differentiation. Mixl1-early and Mixl1-intemediate EpiSCs activated different pathways in response to Nodal and Activin during differentiation, but Mixl1-late EpiSCs downregulated most TGF-β-associated genes (figure 3b). The expression levels of selected pathway genes (electronic supplementary material, figure S7a) also varied among the three groups of EpiSCs. In the Mixl1-early EpiSCs, Nodal activity engaged the activation of pathway genes of TGF-β signalling. Somewhat surprisingly, Activin A treatment elicited higher levels of expression of genes that mediate or are targets of BMP signalling than Nodal treatment (figure 3c).
Figure 3.
The expression profile of TGF-β signalling pathway components and response genes in the EpiSCs. (a) Heat map showing different expression patterns of pathway components in undifferentiated (day 0) Mixl1-early, Mixl1-intermediate and Mixl1-late EpiSCs. (b)(i)–(iii) Heat maps showing the different response of (i) Mixl1-early, (ii) Mixl1-intermediate and (iii) Mixl1-late EpiSC-derived embryonic bodies cultured for 4 days in Activin A- or Nodal-supplemented medium, compared with the expression profile at day 0 of culture. (For gene lists of the heat maps, see electronic supplementary material, table S4). (c) Functional annotation of genes that are significantly upregulated in cells of Nodal or Activin A cultures based on clustered GO terms using DAVID. Examples of upregulated genes are listed separately for Nodal and Activin A treatment in Mixl1-early EpiSCs. (d) Heat map showing different expression patterns of response genes in undifferentiated (day 0) Mixl1-early, Mixl1-intermediate and Mixl1-late EpiSCs. (e)(i)–(iii) Heat maps showing the different expression patterns of downstream genes of (i) Mixl1-early, (ii) Mixl1-intermediate and (iii) Mixl1-late EpiSC-derived embryonic bodies cultured for 4 days in Activin A- or Nodal-supplemented medium, compared with the expression profile at day 0 of culture. (For gene lists of the heat maps, see electronic supplementary material, table S4). (f) Functional annotation of genes that are differentially upregulated in Mixl1-early EpiSCs of Nodal or Activin A cultures. Examples of upregulated genes are listed separately for Nodal and Activin A treatment. The order that genes are listed on the heat map is determined by the clustering of the expression data, which were normalized against that of housekeeping genes: Actb, B2M, Gapdh, Hsp90ab1 and Gusb. Mixl1-E, Mixl1-early; Mixl1-I, Mixl1-intermediate; Mixl1-L, Mixl1-late; EB0, day-0 embryoid bodies; EB4, day-4 embryoid bodies; ctrl, control; ActA, Activin A. (Online version in colour.)
To examine whether the ability to respond to TGF-β signalling in undifferentiated EpiSCs could have entrained the cells in their response to TGF-β signalling during differentiation, the expression of responding genes was examined in cells after 4 days of differentiation as EBs. Our data (Heat maps: figure 3e(i)–(iii); selected gene set: electronic supplementary material, figure S7b) clearly show that all three groups of EpiSCs responded differently to Nodal and Activin A. Activin A treatment resulted in the upregulation of genes associated with the BMP pathway, most notably in Mixl1-early cells (e.g. Crebbp, Msx2, Smad6, Id1, Id2 and Acvr1), and the MAPK pathway (e.g. Mapka and Map3k7), and genes associated with cell fate decision (e.g. Pdgfa, Ctnnb1 and Hes1) (figure 3f). In addition, Activin A promoted higher expression of epithelial markers (and their upstream genes, e.g. Id2, Id3, Fn1, Mmp3, Rhob and Ifrd1) than Nodal and weaker expression of mesenchymal markers (e.g. Snai1, Rhoa and Fn1). KEGG and GO analysis of the tested TGF-β targets also shows that Activin A engaged the pathways associated with epithelial cell differentiation and development, as well as proliferation and embryonic organ development (31 genes, see figure 3f). By contrast, Nodal elicited the activity of a different set of pathway genes (TGF-β: E2f4, Eng, Rbl1, Ep300, Tgfb2, Smad3, Acvrl1; Nodal subtilisin-like convertase: Furin; Wnt: Ctnnb1) [44]. Nodal also upregulated genes that promote cell–substrate interaction, cell adhesion (Rhoa, Rhob, Snai1, Ctnnb1, Myc, Cdc6), the acquisition of mesenchymal phenotype (Rhoa, Snai1, Myod1, Aipl1, Hes1) and cell motility [13,45], but downregulated genes associated with an epithelial phenotype (e.g. Id2, Id3 (this study); Cdh1) [46]).
7. The role of Nodal in gene regulation and cell behaviour during endoderm formation
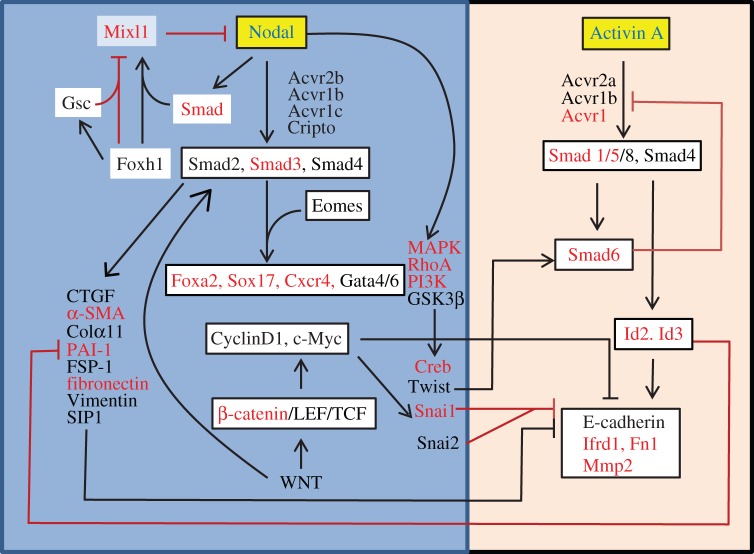
The association between rapid upregulation of Mixl1 expression and enhanced endoderm lineage propensity, and the differential response of the lineage competent EpiSCs to induction of differentiation by Nodal and Activin A have highlighted a molecular paradigm of Mixl1 and TGF-β function in endoderm formation. The initial activation of Mixl1 in differentiating EpiSCs is reminiscent of the expression of Mixl1 first in the PS, and subsequent deactivation mimics the loss of Mixl1 expression in the DE in vivo. Mixl1 is reputed to act as a transcriptional regulator in the Nodal downstream pathway via Gata and SoxF factors to regulate endoderm formation [47–52]. That the loss of Mixl1 activity leads to a failure to generate DE in the embryo suggests that activation of Mixl1 in the progenitor population is critical for the formation of the endoderm. In addition, constitutive expression of Mixl1 in differentiating mouse embryonic stem cells promotes endoderm differentiation at the expense of haematopoietic mesoderm [53]. It is therefore plausible that Mixl1 activity is required for the cell fate decision process for the specification of the endoderm lineage. Mixl1 expression can be activated by TGF-β/Nodal activity [54] through the interaction of Smad and Foxh1 with the proximal response element in the Mixl1 promoter [55]. Loss of Foxh1 results in expanded Mixl1 expression in the mouse gastrula [55] while loss of Mixl1 leads to expansion of Nodal-lacZ expression [23]. Nodal signalling therefore is involved in both the feedforward and feedback regulatory loop that regulates Mixl1 activity (figure 4). The timely response to Nodal-dependent regulation of Mixl1 expression in EpiSCs ([46] and data not shown) may be the critical factor of the lineage differentiation propensity of these stem cells. Delayed activation of Mixl1 expression in the Mixl1-intermediate and Mixl1-late EpiSCs may underpin the reduced efficiency of DE differentiation compared with Mixl1-early cells. This is reflected in the weaker activation of pathway components and targets following Nodal stimulation, and the comparatively reduced efficiency of directed differentiation to advanced endoderm cell types.
Figure 4.
A model of the molecular cascade downstream of Nodal and Activin A signalling in EpiSCs. Nodal activates Mixl1 activity via Smad and Foxh1 transactivation to induce mesendoderm progenitors in the PS. Mixl1 in turns represses Nodal expression. Foxh1 and Gsc cooperatively repress Mixl1 expression. Nodal signals via the TGF-β–Smad2/3/4 pathway and activates MAPK/RhoA/PI3K signalling. The downstream activity of these pathways dismantles the epithelial phenotype of the epiblast cells, and promotes the acquisition of a transitory mesenchymal cell state during gastrulation. Smad factors, along with Eomes, promote expression of endoderm genes (Sox17, Foxa2, Cxcr4, Gata). It is postulated that the acquisition of a mesenchymal phenotype is a pre-requisite step of the allocation of cells to the endoderm lineage in the embryo, which can be enhanced by Wnt/β-catenin signalling activity. Activin A, in contrast to Nodal, upregulates some components of the Smad-mediated cascade that promote via Id proteins the maintenance of an epithelial cell state. The omission of the mesenchymal state during Activin A induced differentiation may hamper the generation of competent endoderm cells that can differentiate into more advanced cell types. Genes identified as differentially expressed by microfluidic qPCR are shown in red. (Online version in colour.)
A comparison of the expression of pathway components and TGF-β response genes in EpiSCs reveals that Activin A results in greater expression of some components and targets of the BMP–Smad1/5/6/8 pathway [56] than Nodal signalling (figure 4). Of interest is that the molecular cascade activated by Activin A may be involved with maintenance of an epithelial cell phenotype, whereas Nodal seems to promote the transition to the mesenchyme phenotype. The Nodal cascade may receive input from the Wnt signalling pathway, like in human embryonic stem cells [57], to initiate Smad2/3 activation, which together with PI3K activity counteracts Activin A/Smad1/5 downstream activity. This scenario of signalling activity may point to a potential role of Nodal in the transition of cellular state during the ingression of the epiblast cells in the PS and the emergence of the nascent mesenchyme in which the endoderm progenitors reside transiently. Cells in the APS experience strong Nodal activity [56], which potentially promotes cell ingression movement and activates downstream genes that influence cell fate decisions [58–60]. Nodal negatively regulates Mixl1 in the APS by the Foxh1–Gsc complex that recruits the repressive histone deacetylase, and enforced Gsc expression in EBs suppresses Foxh1-dependent Mixl1 expression [61] The repression of Nodal-dependent Mixl1 activity may coincide with the onset of Gsc expression at mid-gastrulation and the allocation of Gsc+/Foxa2+/Sox17+ DE. Downstream of Nodal, Smad2/3 interacts with Eomes to activate the transcriptional network for endoderm formation ([46], fig. 4). It is therefore likely that Nodal plays separate roles in regulating the Mixl1-dependent transcriptional regulatory activity for the specification of the endoderm lineage and the transition of cell state (epithelial to mesenchymal and back to epithelial) to facilitate the generation of the DE.
Supplementary Material
Supplementary Material
Supplementary Material
Funding statement
Our work is supported by the National Health and Medical Research Council of Australia (grants 632667 and 1022498) and Mr James Fairfax. Y.K. is supported by the Manpei Suzuki Diabetes Foundation fellowship and the research fellowship of Uehara Memorial Foundation. P.P.L.T. is a Senior Principal Research Fellow of the National Health and Medical Research Council of Australia (grant no. 1003100).
References
- 1.Williams M, Burdsal C, Periasamy A, Lewandoski M, Sutherland A. 2012. Mouse primitive streak forms in situ by initiation of epithelial to mesenchymal transition without migration of a cell population. Dev. Dyn. 241, 270–283. ( 10.1002/dvdy.23711) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burtscher I, Lickert H. 2009. Foxa2 regulates polarity and epithelialization in the endoderm germ layer of the mouse embryo. Development 136, 1029–1038. ( 10.1242/dev.028415) [DOI] [PubMed] [Google Scholar]
- 3.Kwon GS, Viotti M, Hadjantonakis AK. 2008. The endoderm of the mouse embryo arises by dynamic widespread intercalation of embryonic and extraembryonic lineages. Dev. Cell. 15, 509–520. ( 10.1016/j.devcel.2008.07.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kinder SJ, Loebel DA, Tam PP. 2001. Allocation and early differentiation of cardiovascular progenitors in the mouse embryo. Trends Cardiovasc. Med. 11, 177–184. ( 10.1016/S1050-1738(01)00091-3) [DOI] [PubMed] [Google Scholar]
- 5.Kinder SJ, Tsang TE, Quinlan GA, Hadjantonakis AK, Nagy A, Tam PP. 1999. The orderly allocation of mesodermal cells to the extraembryonic structures and the anteroposterior axis during gastrulation of the mouse embryo. Development 126, 4691–4701. [DOI] [PubMed] [Google Scholar]
- 6.Kinder SJ, Tsang TE, Wakamiya M, Sasaki H, Behringer RR, Nagy A, Tam PP. 2001. The organizer of the mouse gastrula is composed of a dynamic population of progenitor cells for the axial mesoderm. Development 128, 3623–3634. [DOI] [PubMed] [Google Scholar]
- 7.Tada S, Era T, Furusawa C, Sakurai H, Nishikawa S, Kinoshita M, Nakao K, Chiba T. 2005. Characterization of mesendoderm: a diverging point of the definitive endoderm and mesoderm in embryonic stem cell differentiation culture. Development 132, 4363–4374. ( 10.1242/dev.02005) [DOI] [PubMed] [Google Scholar]
- 8.Lewis SL, Tam PP. 2006. Definitive endoderm of the mouse embryo: formation, cell fates, and morphogenetic function. Dev. Dyn. 235, 2315–2329. ( 10.1002/dvdy.20846) [DOI] [PubMed] [Google Scholar]
- 9.Tam PP, Loebel DA. 2007. Gene function in mouse embryogenesis: get set for gastrulation. Nat. Rev. Genet. 8, 368–381. ( 10.1038/nrg2084) [DOI] [PubMed] [Google Scholar]
- 10.Vincent SD, Dunn NR, Hayashi S, Norris DP, Robertson EJ. 2003. Cell fate decisions within the mouse organizer are governed by graded Nodal signals. Genes Dev. 17, 1646–1662. ( 10.1101/gad.1100503) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robertson EJ, Norris DP, Brennan J, Bikoff EK. 2003. Control of early anterior–posterior patterning in the mouse embryo by TGF-β signalling. Phil. Trans. R. Soc. Lond. B 358, 1351–1357; discussion 1357 ( 10.1098/rstb.2003.1332) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Y, Festing M, Thompson JC, Hester M, Rankin S, El-Hodiri HM, Zorn AM, Weinstein M. 2004. Smad2 and Smad3 coordinately regulate craniofacial and endodermal development. Dev. Biol. 270, 411–426. ( 10.1016/j.ydbio.2004.03.017) [DOI] [PubMed] [Google Scholar]
- 13.Lu CC, Robertson EJ. 2004. Multiple roles for Nodal in the epiblast of the mouse embryo in the establishment of anterior–posterior patterning. Dev. Biol. 273, 149–159. ( 10.1016/j.ydbio.2004.06.004) [DOI] [PubMed] [Google Scholar]
- 14.Brons IG, et al. 2007. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature 448, 191–195. ( 10.1038/nature05950) [DOI] [PubMed] [Google Scholar]
- 15.Tesar PJ, Chenoweth JG, Brook FA, Davies TJ, Evans EP, Mack DL, Gardner RL, McKay RD. 2007. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature 448, 196–199. ( 10.1038/nature05972) [DOI] [PubMed] [Google Scholar]
- 16.Osorno R, et al. 2012. The developmental dismantling of pluripotency is reversed by ectopic Oct4 expression. Development 139, 2288–2298. ( 10.1242/dev.078071) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kojima Y, et al. 2014. The transcriptional and functional properties of mouse epiblast stem cells resemble the anterior primitive streak. Cell Stem Cell 14, 107–120. ( 10.1016/j.stem.2013.09.014) [DOI] [PubMed] [Google Scholar]
- 18.Greber B, et al. 2010. Conserved and divergent roles of FGF signaling in mouse epiblast stem cells and human embryonic stem cells. Cell Stem Cell 6, 215–226. ( 10.1016/j.stem.2010.01.003) [DOI] [PubMed] [Google Scholar]
- 19.Crossley PH, Martin GR. 1995. The mouse Fgf8 gene encodes a family of polypeptides and is expressed in regions that direct outgrowth and patterning in the developing embryo. Development 121, 439–451. [DOI] [PubMed] [Google Scholar]
- 20.Sun X, Meyers EN, Lewandoski M, Martin GR. 1999. Targeted disruption of Fgf8 causes failure of cell migration in the gastrulating mouse embryo. Genes Dev. 13, 1834–1846. ( 10.1101/gad.13.14.1834) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsakiridis A, et al. 2014. Distinct Wnt-driven primitive streak-like populations reflect in vivo lineage precursors. Development 141, 1209–1221. ( 10.1242/dev.101014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tam PP, Khoo PL, Lewis SL, Bildsoe H, Wong N, Tsang TE, Gad JM, Robb L. 2007. Sequential allocation and global pattern of movement of the definitive endoderm in the mouse embryo during gastrulation. Development 134, 251–260. ( 10.1242/dev.02724) [DOI] [PubMed] [Google Scholar]
- 23.Hart AH, Hartley L, Sourris K, Stadler ES, Li R, Stanley EG, Tam PP, Elefanty AG, Robb L. 2002. Mixl1 is required for axial mesendoderm morphogenesis and patterning in the murine embryo. Development 129, 3597–3608. [DOI] [PubMed] [Google Scholar]
- 24.Wolfe AD, Downs KM. 2014. Mixl1 localizes to putative axial stem cell reservoirs and their posterior descendants in the mouse embryo. Gene Expr. Patterns 15, 8–20. ( 10.1016/j.gep.2014.02.002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bernemann C, Greber B, Ko K, Sterneckert J, Han DW, Arauzo-Bravo MJ, Scholer HR. 2011. Distinct developmental ground states of epiblast stem cell lines determine different pluripotency features. Stem Cells 29, 1496–1503. ( 10.1002/stem.709) [DOI] [PubMed] [Google Scholar]
- 26.Brown LE, King JR, Loose M. 2014. Two different network topologies yield bistability in models of mesoderm and anterior mesendoderm specification in amphibians. J. Theor. Biol. 353, 67–77. ( 10.1016/j.jtbi.2014.03.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dyson S, Gurdon JB. 1998. The interpretation of position in a morphogen gradient as revealed by occupancy of activin receptors. Cell 93, 557–568. ( 10.1016/S0092-8674(00)81185-X) [DOI] [PubMed] [Google Scholar]
- 28.Wilson PA, Melton DA. 1994. Mesodermal patterning by an inducer gradient depends on secondary cell-cell communication. Curr. Biol. 4, 676–686. ( 10.1016/S0960-9822(00)00152-4) [DOI] [PubMed] [Google Scholar]
- 29.Toivonen S, Lundin K, Balboa D, Ustinov J, Tamminen K, Palgi J, Trokovic R, Tuuri T, Otonkoski T. 2013. Activin A and Wnt-dependent specification of human definitive endoderm cells. Exp. Cell Res. 319, 2535–2544. ( 10.1016/j.yexcr.2013.07.007) [DOI] [PubMed] [Google Scholar]
- 30.Chen AE, Borowiak M, Sherwood RI, Kweudjeu A, Melton DA. 2013. Functional evaluation of ES cell-derived endodermal populations reveals differences between Nodal and Activin A-guided differentiation. Development 140, 675–686. ( 10.1242/dev.085431) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Teo AK, et al. 2012. Activin and BMP4 synergistically promote formation of definitive endoderm in human embryonic stem cells. Stem Cells 30, 631–642. ( 10.1002/stem.1022) [DOI] [PubMed] [Google Scholar]
- 32.Brown S, et al. 2011. Activin/Nodal signaling controls divergent transcriptional networks in human embryonic stem cells and in endoderm progenitors. Stem Cells 29, 1176–1185. ( 10.1002/stem.666) [DOI] [PubMed] [Google Scholar]
- 33.Loh KM, et al. 2014. Efficient endoderm induction from human pluripotent stem cells by logically directing signals controlling lineage bifurcations. Cell Stem Cell 14, 237–252. ( 10.1016/j.stem.2013.12.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vallier L, et al. 2009. Early cell fate decisions of human embryonic stem cells and mouse epiblast stem cells are controlled by the same signalling pathways. PLoS ONE 4, e6082 ( 10.1371/journal.pone.0006082) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gu Z, Nomura M, Simpson BB, Lei H, Feijen A, van den Eijnden-van Raaij J, Donahoe PK, Li E. 1998. The type I activin receptor ActRIB is required for egg cylinder organization and gastrulation in the mouse. Genes Dev. 12, 844–857. ( 10.1101/gad.12.6.844) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song J, Oh SP, Schrewe H, Nomura M, Lei H, Okano M, Gridley T, Li E. 1999. The type II activin receptors are essential for egg cylinder growth, gastrulation, and rostral head development in mice. Dev. Biol. 213, 157–169. ( 10.1006/dbio.1999.9370) [DOI] [PubMed] [Google Scholar]
- 37.Ding J, Yang L, Yan YT, Chen A, Desai N, Wynshaw-Boris A, Shen MM. 1998. Cripto is required for correct orientation of the anterior–posterior axis in the mouse embryo. Nature 395, 702–707. ( 10.1038/27215) [DOI] [PubMed] [Google Scholar]
- 38.Chu GC, Dunn NR, Anderson DC, Oxburgh L, Robertson EJ. 2004. Differential requirements for Smad4 in TGF-β-dependent patterning of the early mouse embryo. Development 131, 3501–3512. ( 10.1242/dev.01248) [DOI] [PubMed] [Google Scholar]
- 39.D'Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E, Baetge EE. 2005. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat. Biotechnol. 23, 1534–1541. ( 10.1038/nbt1163) [DOI] [PubMed] [Google Scholar]
- 40.Gu G, Wells JM, Dombkowski D, Preffer F, Aronow B, Melton DA. 2004. Global expression analysis of gene regulatory pathways during endocrine pancreatic development. Development 131, 165–179. ( 10.1242/dev.00921) [DOI] [PubMed] [Google Scholar]
- 41.Loh KM, Lim B. 2011. A precarious balance: pluripotency factors as lineage specifiers. Cell Stem Cell 8, 363–369. ( 10.1016/j.stem.2011.03.013) [DOI] [PubMed] [Google Scholar]
- 42.Shu J, Deng H. 2013. Lineage specifiers: new players in the induction of pluripotency. Genomics Proteomics Bioinform. 11, 259–263. ( 10.1016/j.gpb.2013.09.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liang G, Zhang Y. 2013. Genetic and epigenetic variations in iPSCs: potential causes and implications for application. Cell Stem Cell 13, 149–159. ( 10.1016/j.stem.2013.07.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ben-Haim N, Lu C, Guzman-Ayala M, Pescatore L, Mesnard D, Bischofberger M, Naef F, Robertson EJ, Constam DB. 2006. The nodal precursor acting via activin receptors induces mesoderm by maintaining a source of its convertases and BMP4. Dev. Cell 11, 313–323. ( 10.1016/j.devcel.2006.07.005) [DOI] [PubMed] [Google Scholar]
- 45.Yamamoto M, Saijoh Y, Perea-Gomez A, Shawlot W, Behringer RR, Ang SL, Hamada H, Meno C. 2004. Nodal antagonists regulate formation of the anteroposterior axis of the mouse embryo. Nature 428, 387–392. ( 10.1038/nature02418) [DOI] [PubMed] [Google Scholar]
- 46.Teo AK, Arnold SJ, Trotter MW, Brown S, Ang LT, Chng Z, Robertson EJ, Dunn NR, Vallier L. 2011. Pluripotency factors regulate definitive endoderm specification through eomesodermin. Genes Dev. 25, 238–250. ( 10.1101/gad.607311) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tam PP, Loebel DA, Tanaka SS. 2006. Building the mouse gastrula: signals, asymmetry and lineages. Curr. Opin. Genet. Dev. 16, 419–425. ( 10.1016/j.gde.2006.06.008) [DOI] [PubMed] [Google Scholar]
- 48.Whitman M. 2001. Nodal signaling in early vertebrate embryos: themes and variations. Dev. Cell 1, 605–617. ( 10.1016/S1534-5807(01)00076-4) [DOI] [PubMed] [Google Scholar]
- 49.Schier AF. 2003. Nodal signaling in vertebrate development. Annu. Rev. Cell Dev. Biol. 19, 589–621. ( 10.1146/annurev.cellbio.19.041603.094522) [DOI] [PubMed] [Google Scholar]
- 50.Tremblay KD. 2011. Inducing the liver: understanding the signals that promote murine liver budding. J. Cell Physiol. 226, 1727–1731. ( 10.1002/jcp.22409) [DOI] [PubMed] [Google Scholar]
- 51.Zorn AM, Wells JM. 2009. Vertebrate endoderm development and organ formation. Annu. Rev. Cell Dev. Biol. 25, 221–251. ( 10.1146/annurev.cellbio.042308.113344) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seguin CA, Draper JS, Nagy A, Rossant J. 2008. Establishment of endoderm progenitors by SOX transcription factor expression in human embryonic stem cells. Cell Stem Cell 3, 182–195. ( 10.1016/j.stem.2008.06.018) [DOI] [PubMed] [Google Scholar]
- 53.Lim SM, Pereira L, Wong MS, Hirst CE, Van Vranken BE, Pick M, Trounson A, Elefanty AG, Stanley EG. 2009. Enforced expression of Mixl1 during mouse ES cell differentiation suppresses hematopoietic mesoderm and promotes endoderm formation. Stem Cells 27, 363–374. ( 10.1634/stemcells.2008-1008) [DOI] [PubMed] [Google Scholar]
- 54.Labbe E, Silvestri C, Hoodless PA, Wrana JL, Attisano L. 1998. Smad2 and Smad3 positively and negatively regulate TGF-β-dependent transcription through the forkhead DNA-binding protein FAST2. Mol. Cell 2, 109–120. ( 10.1016/S1097-2765(00)80119-7) [DOI] [PubMed] [Google Scholar]
- 55.Hart AH, Willson TA, Wong M, Parker K, Robb L. 2005. Transcriptional regulation of the homeobox gene Mixl1 by TGF-β and FoxH1. Biochem. Biophys. Res. Commun. 333, 1361–1369. ( 10.1016/j.bbrc.2005.06.044) [DOI] [PubMed] [Google Scholar]
- 56.Bhargava V, Ko P, Willems E, Mercola M, Subramaniam S. 2013. Quantitative transcriptomics using designed primer-based amplification. Sci. Rep. 3, 1740 ( 10.1038/srep01740) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.James D, Levine AJ, Besser D, Hemmati-Brivanlou A. 2005. TGF-β/activin/nodal signaling is necessary for the maintenance of pluripotency in human embryonic stem cells. Development 132, 1273–1282. ( 10.1242/dev.01706) [DOI] [PubMed] [Google Scholar]
- 58.Gritsman K, Talbot WS, Schier AF. 2000. Nodal signaling patterns the organizer. Development 127, 921–932. [DOI] [PubMed] [Google Scholar]
- 59.Hoodless PA, Pye M, Chazaud C, Labbe E, Attisano L, Rossant J, Wrana JL. 2001. FoxH1 (Fast) functions to specify the anterior primitive streak in the mouse. Genes Dev. 15, 1257–1271. ( 10.1101/gad.881501) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yamamoto M, Meno C, Sakai Y, Shiratori H, Mochida K, Ikawa Y, Saijoh Y, Hamada H. 2001. The transcription factor FoxH1 (FAST) mediates Nodal signaling during anterior–posterior patterning and node formation in the mouse. Genes Dev. 15, 1242–1256. ( 10.1101/gad.883901) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Izzi L, Silvestri C, von Both I, Labbe E, Zakin L, Wrana JL, Attisano L. 2007. Foxh1 recruits Gsc to negatively regulate Mixl1 expression during early mouse development. EMBO J. 26, 3132–3143. ( 10.1038/sj.emboj.7601753) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.