Summary
Ceramide serves as a central mediator in sphingolipid metabolism and signaling pathways, regulating many fundamental cellular responses. It is referred to as a “tumor suppressor lipid”, since it powerfully potentiates signaling events which drive apoptosis, cell cycle arrest, and autophagic responses. In the typical cancer cell, ceramide levels and signaling are usually suppressed by over-expression of ceramide-metabolizing enzymes or down-regulation of ceramide-generating enzymes. However, chemotherapeutic drugs as well as radiotherapy increase intracellular ceramide levels while exogenously treating cancer cells with short-chain ceramides leads to anti-cancer effects. All evidence currently points to the fact that the up-regulation of ceramide level is a promising anti-cancer target. In this review, we exhibited a full scroll of anti-cancer ceramide analogs as down-stream receptor agonists and ceramide metabolizing enzyme inhibitors.
Keywords: Ceramide, Sphingosine, Sphingolipid signaling pathway, Ceramidase, Glucosylceramide synthase, Anticancer agents, Enzyme inhibitors
Anti-cancer targets in the ceramide signaling pathway
Ceramide signaling pathway
There are two major pathways known to trigger the generation of ceramide. In the sphingomyelinase pathway, hydrolysis of plasma membrane sphingomyelin or lysosomal sphingomyelin by sphingomyelinases produces ceramide and phosphorylcholine. Exposure of cells to stress factors, such as pro-inflammatory cytokines, oxidative and nitrosative stress, UV- and γ-irradiation, or chemotherapeutic agents has been shown to rapidly activate sphingomyelinase activity, resulting in increased ceramide formation [1].
The de novo synthesis of ceramide begins with the condensation of palmitate and serine by serine palmitoyltransferase to yield 3-keto-dihydrosphingosine, which is, in turn, reduced to dihydrosphingosine, and acylated by dihydroceramide synthase to produce dihydroceramide. Finally, desaturation of dihydroceramide by dihydroceramide desaturase generates ceramide. The de novo synthesis of ceramide occurs in the endoplasmic reticulum (ER), and the newly formed ceramide does not enter cytosol immediately. On the contrary, it is subsequently transported to the Golgi apparatus by either vesicular trafficking or the ceramide transfer protein CERT. Once in the Golgi, ceramide is converted to sphingomyelin (SM) by sphingomyelin synthase on the luminal side of the Golgi, or to glucosylceramide (GluCer) by glucosylceramide synthase on the cytosolic surface of the Golgi, and in turn to the complex glycosphingolipids [2]. The de novo formation of ceramide can be induced by several factors including tumor necrosis factor-α, hypoxia, and certain chemotherapeutic agents [3, 4].
Ceramide can also be synthesized by re-utilizing free sphingosine that is derived from the degradation of glycosphingolipids and other complex sphingolipids. This recycling of sphingosine is termed the salvage pathway.
Ceramide and apoptosis
Whether through endogenous elevation of ceramide level or exogenous treatment with cell-permeable short-chain ceramides, a series of biological effects, denoted as apoptosis, cell cycle arrest, differentiation, and autophagy, can be triggered.
In the past two decades, numerous studies have focused on the ceramide-induced apoptosis pathway in the fields of physiology, biochemistry, pathophysiology, and pharmacology. A classic mitochondria-dependent apoptosis can be triggered by endogenous and exogenous ceramide signaling. An immediate downstream target of ceramide is known to be CAPPs (ceramide-activated Ser-Thr protein phosphatases), such as PP2A (protein phosphatase 2A). The effect of ceramide on CAPPs leads to inactivation of an anti-apoptotic kinase, AKT (serine/threonine-specific protein kinase or protein kinase B), through protein dephosphorylation [5]. Activation of PKCζ by ceramide has been implicated in the regulation of membrane potential, inhibition of AKT, and pro-apoptotic functions [6, 7]. JNK and p38 can also be activated by ceramide through apoptosis signal-regulating kinase 1 (ASK1). JNK and p38 increase the level of BAX, a pro-apoptotic protein in mitochondrial pathway, while p38 also contributes to the inactivation of AKT. Thus, the downgrading of AKT by several signals (PP2A, PKCζ, and p38) decreases the phosphorylation of BCL-2. At the same time, PP2A’s catalytic subunit (PP2Ac) also inhibits BCL-2 phosphorylation, leading to increased p53/BCL-2 binding. Finally, downgraded BCL-2 levels and a suppressed BCL-2/BAX ratio results in intrinsic apoptosis [8]. Cathepsin D is considered as a specific target for lysosomally generated ceramide, and is involved in both mitochondria-dependent and independent apoptosis pathways [9]. Therefore, ceramide analogs can act on the ceramide downstream targets, imitating ceramide-like apoptosis.
The permeability of mitochondria is the critical factor inducing the release of apoptotic proteins, such as cytochrome c, in turn leading to apoptosis. Ceramide can directly induce mitochondrial outer membrane permeabilization (MOMP), which is a key event in apoptotic signaling, through the formation of ceramide channels. This MOMP process is concomitant with and promoted by pro-apoptotic BAX translocation to mitochondria [10, 11]. This is why a group of positively charged ceramide analogs was developed to target ceramide-induced apoptosis [12, 13].
Ceramide metabolism and cancer
In vitro assays have shown that a short-chain ceramide molecule, C6-Cer, modestly activates the catalytic subunit of PP2A (PP2Ac), an immediate ceramide downstream target. In contrast, 4–5 double-bond saturated analog, C6-dhCer, inhibits PP2Ac. Further studies demonstrated the strict structural requirements for interaction of ceramide with PP2A (an amide group, a primary hydroxyl group, and a secondary hydroxyl group) [14]. However, many ceramide analogs that do not activate or even inhibit PP2A, do exhibit apoptotic activity in cancer cells (like D-e-MAPP and 3-keto-C6-ceramide) [15, 16]. Thus, other agents inducing apoptosis in ceramide signaling pathways are expected to exist.
Multiple enzymes are directly involved in regulating intracellular ceramide concentrations. These include ceramide-generating enzymes, such as ceramide synthase, cerebrosidase, sphingomyelinase, and ceramide-metabolizing enzymes, such as ceramidase, glucosylceramide synthase, sphingomyelin synthase, and ceramide kinase. Inhibition of glycosphingolipid biosynthesis or ceramide degradation gives rise to an elevation of endogenous ceramide level. Among these enzymes ceramidase (CDase) and glucosylceramide synthase (GCS) play important roles in regulating ceramide levels.
Ceramidase
Ceramidases hydrolyze the amide bond of ceramide producing sphingosine (Sph) and a fatty acid. According to their optimum activity pH, ceramidases are classified into acid [17], neutral [18, 19], and alkaline ceramidases [20]. They modulate the intracellular ceramide levels, especially regulating the lysosomal pool of ceramide (by acid CDase). A large amount of evidence supports the notion that CDase inhibitors serve as potential ceramide-increasing agents useful for cancer chemotherapy. Ceramide analogs such as B13 [21, 22] and AD2646 [23] can antagonize ceramidases, exhibiting interesting activities in cancer chemotherapy and have been widely used in research on ceramide signaling pathways.
Glucosylceramide synthase
Glucosylceramide synthase (GCS), also called UDP-glucose ceramide glucosyltransferase, is another important ceramide metabolizing enzyme. GCS, located on the cytosolic surface of the Golgi apparatus, catalyzes the conversion of ceramide into glucosylceramide [24]. Generally, up-regulation of GCS levels prevents the accumulation of a ceramide pool, which reduces ceramide-induced apoptosis in response to certain cytotoxic drugs [25–27]. On the other hand, evidence also shows a close relationship between GCS and P-glycoprotein (P-gp, an important multidrug-resistance protein). Overexpression of P-gp in resistant cancer cells is concomitant with high GCS expression [28, 29]. GCS inhibitor treatment in multidrug-resistant cancer cells down-regulates the expression of MDR1 (P-gp-encoding gene) [30]. Furthermore, drug-resistant cancer cells exposed to GCS inhibitors become sensitive to anticancer agents [30–32]. These results strengthen the notion that inhibition of GCS is a promising therapeutic strategy for combating multidrug-resistance, thus, promoting the design of ceramide analogs as GCS inhibitors.
Ceramide kinase and ceramide-1-phospate
Phosphorylation of ceramide by the ceramide kinase (CerK) produces Ceramide-1-phospate (C1P) which plays an opposing physiological role compared to ceramide. C1P is a potent inhibitor of apoptosis, which blocks apoptosis through inhibition of acid sphingomyelinase and serine palmitoyltransferase in macrophages [33, 34]. C1P also stimulates cell proliferation through activation of the PI3- kinase/PKB, JNK and ERK1/2 pathways [35, 36]. Therefore, blocking ceramide kinase to decrease the generation of C1P is the third anti-cancer target of ceramide analogs as enzyme inhibitors.
Sphingosine kinase (SK), sphingosine-1-phospate (S1P), and S1P receptors
Sphingosine, the metabolite of ceramide generated by ceramidase, can be phosphorylated by sphingosine kinase (SK) to yield sphingosine-1-phospate (S1P), another important signaling molecule in the ceramide pathway. Sphingosine-1-phosphate (S1P) is implicated in many critical cellular processes. However, unlike ceramide, it promotes cell survival, proliferation, and migration, as well as angiogenesis and allergic responses [2].
S1P mediates cell survival through different pathways, such as an intracellular signaling mechanism (for example, regulation of BCL-2 family members) and an S1P receptor-dependent mechanism, referred to as “inside-out” signaling. In the former pathway, S1P can negatively mediate apoptosis by up-regulating the expression of anti-apoptotic proteins such as BCL-2 [37], while down-regulating the pro-apoptotic proteins BAX [37, 38]. It was also observed that exogenous S1P blocks the translocation of BAX to the mitochondria, which enhances the stability of mitochondrial membranes [39]. Furthermore, up-regulated expression of BCL-2 family members can increase SK1 (sphingosine kinase 1) levels, which in turn generates more S1P from sphingosine, resulting in S1P signal amplification [40, 41].
In the S1P receptor-dependent mechanism, S1P is secreted from the inside to the outside by the members of the ATP-binding cassette (ABC) family transporters [42], and binds to and signals through S1P receptors in an autocrine manner. To date, five S1P receptors have been identified, which are specific G protein-coupled receptors (GPCRs), designated as S1P1–5. Through these receptors, S1P regulates different cellular processes. The functions of S1P receptors are under investigation. Some results support that S1P1 and S1P3 mediate cell proliferation [43], migration [44, 45], invasion [46, 47], and angiogenesis [48].
S1P is interconvertible with ceramide, which is a negative mediator of apoptosis. It has been postulated that the ratio between S1P and ceramide determines cell fate. Targeting the conversion of ceramide to sphingosine 1-phosphate is a novel strategy for cancer therapy. Since phosphorylation of sphingosine by sphingosine kinase (SK) is the sole known source of S1P, SK inhibitors are promising cancer chemotherapeutics.
As described above, two main approaches to promote anti-cancer activity in the ceramide-sphingosine-S1P axis have been identified as 1) use of an exogenous supplement of ceramide to promote apoptosis, and 2) inhibition of ceramide metabolizing enzymes to regulate the ceramide/S1P rheostat. Since both of these can be achieved by employing ceramide analogs (mimics or derivatives), these types of compounds have attracted a lot of attention in the past few years. Herein, we will review each step to discovery and development of anti-cancer ceramide analogs.
Development of ceramide analogs
Structural characteristics of ceramide
Ceramide molecules contain a sphingoid long-chain base (sphingosine) backbone, linked to a fatty acid molecule through an amide bond. (Figure 2) In earlier studies, the importance of the 4,5-trans-double bond was emphasized, and treated as the mark of sphingosine backbone. This conclusion was based on the notion that targeting ceramide-downstream effectors is the sole purpose to design ceramide analogs. With the development of ceramide-metabolizing enzyme inhibitors, many non-4,5-double bond agents such as S18 [49], PDMP [50], and FTY720 were designed [51]. To date, the 2-amino-1,3-propanediol or 2-amino-propanol moieties are considered as common characteristics of ceramide analogs. These structural backbones are marked in red throughout this review.
Figure 2.
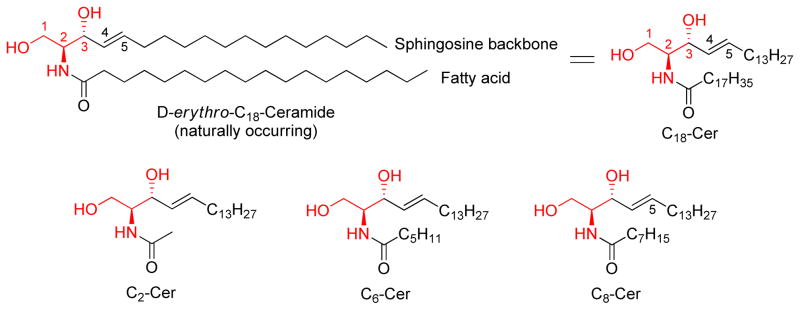
Structures of the naturally occurring ceramide, D-erythro-C18-ceramide (C18-Cer), and synthetic short-chain ceramides, C2-Cer, C6-Cer, and C8-Cer
In order to increase water-solubility, the long-chain fatty acid of native ceramide structure is often replaced by short-chain fatty acids (acetic, hexanoic and octanoic acids) to form C2-, C6-, and C8-ceramides (C2-, C6-, and C8-Cer). Exposure of cells to short-chain ceramides has lead to ceramide-like anti-cancer activity in many preclinical studies [5, 52–55]. Thus, in most studies short-chain ceramides, but not the naturally occurring ceramides, have been used as research tools.
Steorespecific apoptotic activity of short-chain ceramides
Since two chiral centers exist in the ceramide molecule, the natural D-erythro-ceramide has three stereoisomers, denoted as L-erythro-ceramide, D-threo-ceramide, and L-threo-ceramide. To investigate the relationship between stereochemistry and activity, four C2-Cer isomers and four C2-dihydroceramide (C2-dhCer) isomers were synthesized and evaluated in leukemia cells [56]. (Figure 3) The four isomers of C2-Cer were active in inhibition of cell growth and induction of apoptosis with modest differences in potency (ranking as follows: L-threo-C2-Cer > D-erythro-C2-Cer = L-erythro-C2-Cer > D-threo-C2-Cer). This observation suggested that the non-natural stereoisomer L-threo-C2-Cer was more pro-apoptotic than the natural stereoisomer D-erythro-C2-Cer. On the other hand, with C2-dihydroceramide (C2-dhCer) only the threo compounds were active in these assays, whereas the erythro compounds were completely inactive. Thus, lack of the 4,5-double bond lead to inactivity in erythro-C2-dhCer, but not in threo-C2-dhCer. This systematic study provided a basic view of the relationship between ceramide stereoisomers and pro-apoptotic activities. Stereospecific activity is a common feature for ceramide analogs, suggesting that the targets of ceramide analogs also own specific spatial configurations. A large number of ceramide analog studies involve the investigation of stereostructure-activity relationships [14, 57–62].
Figure 3.
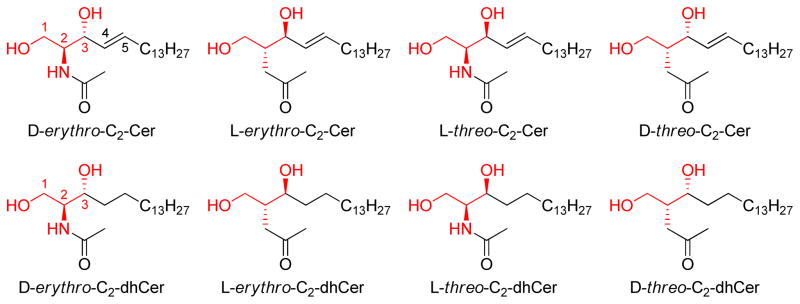
Structures of the four stereoisomers of C2-Cer and the four stereoisomers of C2-dhCer studied by [56]
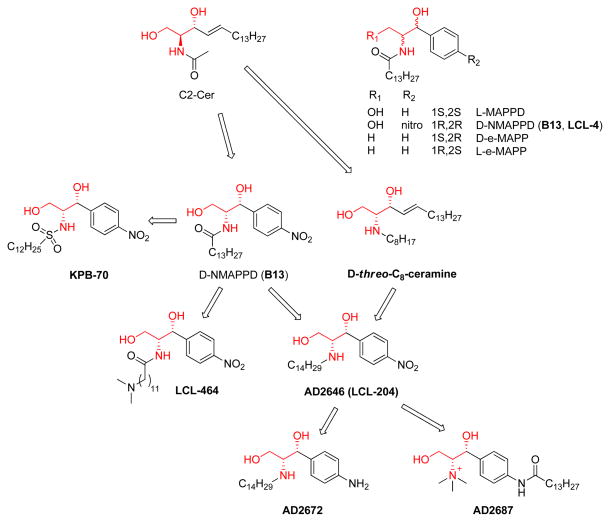
Discovery and development of B13 (inducing apoptosis and inhibiting ceramidase)
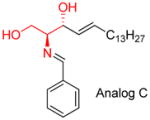
In 1992, a group of phenyl modified ceramide analogs (L-MAPPD, D-NMAPPD, D-e-MAPP), was reported [57]. (Figure 4) The idea of this design approach might have been derived from a previous modification of GCS inhibitors (discussed below) [63]. In the original paper, this group of analogs showed ceramide-like anti-proliferation activity in human myelocytic leukemia HL-60 cells. Very interestingly, this activity had stereospecific requirements. The L-isomer L-e-MAPP was found to be inactive, while De-MAPP was soon reported to be a neutral ceramidase inhibitor in vitro and induced a concentration-and time-dependent growth suppression accompanied by an arrest in the G0/G1 phase of the cell cycle [15]. In that paper, the explanation provided for the inactivity of L-e-MAPP was that L-e-MAPP is metabolized by alkaline ceramidase to an extent similar to that seen with C16-ceramide. However, years later, D-NMAPPD (later named as B13, Figure 4) was found to be an acid ceramidase inhibitor, and induce apoptosis in several cancer cell lines [21, 22, 64]. Thus, D-e-MAPP and D-NMAPPD (B13) not only up-regulated ceramide levels in cultured cells but also potently induced apoptosis [1].
Figure 4.
The B13-family of ceramide analogs
Almost at the same time, ceramide’s amide group proved to be not required for apoptosis, and the replacement of the carbonyl group by methylene group substantially decreased the time required for cells to die, with maximum DNA fragmentation occurring at 6 h as opposed to the 18 h required by D-erythro-C8-Cer [58]. The most potent compound (D-threo-C8-ceramine) in this group is shown in Figure 4. According to the structural features of B13 (D-NMAPPD) and D-threo-C8-ceramine, a group of ceramide analogs, including AD2646 (its hydrochloric salt was called LCL-204 by another group, Figure 4) AD2672 and AD2687, was created [65]. When these compounds were applied to HL-60 cells, they inhibited the biosynthesis of sphingomyelin (SM) and glycosphingolipids and induced apoptosis. AD2687 (trimethyl sphingosine analog) induced cell death at lower concentrations. An investigation of mechanisms involved found that these compounds were able to kill leukemic cells through distinct pathways implicating caspase activation and mitochondrial events [23, 66].
Structural optimization of B13-like ceramides has been ongoing. The newest B13-like ceramide analogs are LCL-464 [67, 68] and KPB-27 [69], which showed potent inhibition of ceramidase as well as ceramide-like anti-cancer activities.
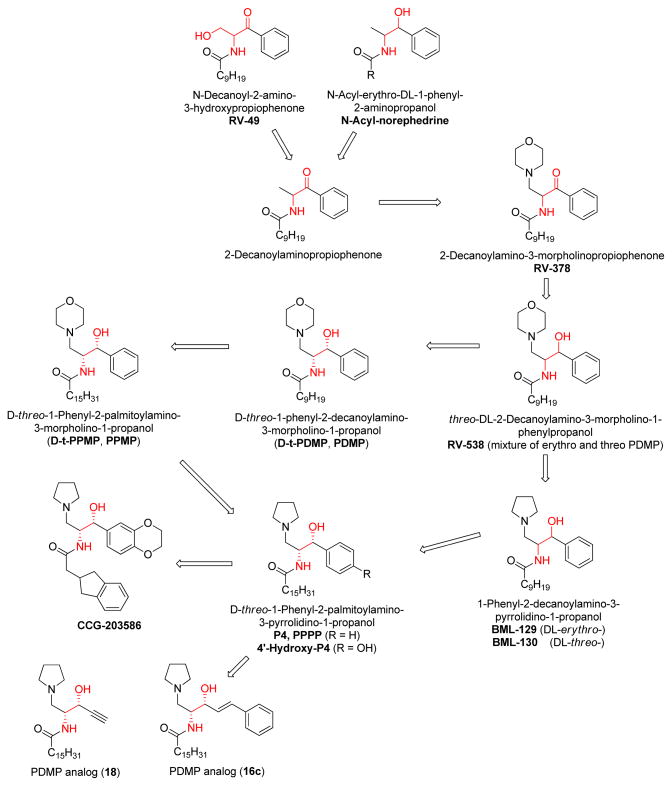
Glucosylceramide synthase (GCS) inhibitors (PDMP family)
The investigation of glucosylceramide synthase Inhibitors was initiated prior to that of ceramidase inhibitors. The original purpose of these studies was to treat Gaucher’s Disease, a genetic lack of adequate β-glucosidase activity [63]. The precursors of this family of compounds are N-decanoyl-2-amino-3-hydroxypropiophenone (RV-49, a 3-ketone-4-phenyl-ceramide) and N-acyl-norephedrine (a 1-dehydroxy-4-phenyl-ceramide). (Figure 5) Using RV-49 and N-acyl-norephedrine, 2-decanoylaminopropiophenone was yielded with a higher inhibition potency for glucosylceramide synthase than the lead compounds. Introducing a morphorlinyl group into the 1-position produced a non-competitive GCS inhibitor, RV-378 (Figure 5). It inactivated the enzyme, probably by covalent reaction with the enzyme’s active site. Reduction of RV-378 at 3-ketone formed the more potent analog RV-583 that is a competitive GCS inhibitor [63, 70]. When discovered, RV-583 was originally a mixture of four stereoisomers. After determination of each isomer, RV-583 got a new name PDMP (Figure 5). It was found that only the D-threo-PDMP (1S,2R) is active against GCS [71]. Shortly after, it was observed that D-threo-PDMP caused growth inhibition and ceramide accumulation in cultured rabbit skin fibroblasts [72]. Replacement of the morphorlinyl group of RV-583 by a pyrrolidinyl group produced a pair of erythro- and threo- isomers (BML-129 and BML-130). Both of these compounds showed very effective growth inhibition of several kinds of cancer cells [73]. This was the first time that GCS inhibitors were linked to anti-cancer activities. Moreover, only threo-isomer BML-130 inhibited GCS in MDCK cell homogenates, while erythro-isomer BML-129 did not. Then, PPMP, PPPP, and 4′-hydroxy-P4 were prepared based on the structural modification of D-threo-PDMP and BML-129/130 [50, 74, 75], leading to the discovery of the most potent GCS inhibitor, 4′-hydroxy-P4, at the end of the 20th century. In 2006, a group of PDMP-family analogs were reported by Hillaert et al. Among these, compounds 16c and 18 (Figure 5) were derived from PPPP with the replacement of backbone phenyl group, and showed a comparable inhibitory potency as PDMP [76]. The newest PDMP-family member is CCG-20358 (Figure 5), with a bulky N-acyl group to increase the rigidity of the whole molecule. This compound (CCG-20358) inhibited GCS in the nM range, and in an in vivo assay in mice led to a dose-dependent decrease in brain glucosylceramide contents by intraperitoneal injection [77].
Figure 5.
Evolution of PDMP-family ceramide analogs (targeting glucosylceramide synthase, GCS)
Derivatives of sphingosine as sphingosine kinase (SK) inhibitors
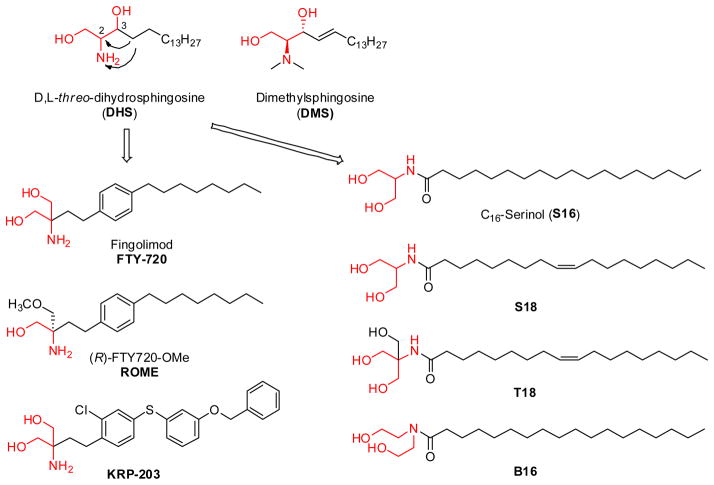
DHS and DMS
As a sphingosine derivative, D,L-threo-dihydrosphingosine (DHS or safingol, Figure 6) was the first identified sphingosine kinase (SK) inhibitor. DHS was shown to be a competitive inhibitor for SK1, and a substrate for SK2 [61]. Shortly after, N,N-dimethylsphingosine (DMS) was proved to be more potent than DHS [78], and served as a competitive inhibitor for both SK1 and SK2 [79, 80]. DMS induces apoptosis in both human epidermoid carcinoma KB-3-1 and its multidrug-resistant (MDR) subclone KB-C2 cells in vitro [81]. Human colonic carcinoma cell lines HT29, HRTI8, MKN74, and COLO205 were shown to be more susceptible to apoptosis upon addition of DMS, with potency comparable to C2-Cer [82]. DHS and DMS have since been intensively used in studies involving ceramide metabolism. However, neither DHS nor DMS were a specific sphingosine kinase inhibitor, since they also inhibited protein kinase C [83] and ceramide kinase [84].
Figure 6.
Sphingosine-derivative sphingosine kinase inhibitors
FTY720 (Fingolimod)
When the long-chain hydrophobic group of sphingosine was shifted from the 3-position to the 2-position, a new group of sphingosine derivatives, 2-substituted 2-amino-1,3-propanediols, was developed. Among these analogs, FTY720 (2-amino-2-(2-(4-octylphenyl)ethyl)-1,3-propanediol hydrochloride, Figure 6) has been the most investigated. This compound is known for its immunosuppressive properties. However, FTY720 is also able to induce growth arrest and apoptosis of various cancer cell lines, including human prostate cancer cells [51], bladder cancer cells [85], renal cancer cells [86], pancreatic cancer cells [87], breast cancer cells, colon cancer cells [88], and brain tumor stem cells [89]. The in vivo assays showed that FTY720 inhibited tumor growth and metastasis [90], and reduced tumor vascularization and angiogenesis [91]. The pro-apoptotic effect of FTY720 was shown to be associated with mitochondria-dependent activation [51, 92] as well as inhibition of SK1 [93]. It is very interesting that phosphorylation of FTY720 by SK2 produces a biologically active molecule, FTY720-phosphate (FTY720-P) [94, 95] that could bind to and activate four of the five S1P receptors, S1P1,3–5 [96]. Although FTY720-P is an S1P1 receptor agonist, it leads to endocytosis and proteasomal degradation of the S1P1 receptor in T lymphocytes [97], thereby preventing their egress from lymph nodes [97]. It is unclear whether the anti- tumor activity of FTY720 is partly from down-regulating S1P1 receptor. However, the structural optimization of FTY720 is in progress [98].
ROME
Since FTY720 is a substrate of SK2, it was O-mono-methylated, forming (S) and (R)-FTY720-OMe (ROME) to avoid metabolism by SK2 (Figure 6). The inhibitory activity of ROME was stereospecific. (R)-FTY720-OMe specifically inhibited SK2, and not SK1, while (S)-FTY720-OMe failed to inhibit SK2. Prolonged treatment of HEK 293 cells with (R)-FTY720-OMe induced a reduction in SK2 expression and inhibited DNA synthesis in HEK 293 cells [60]. Treatment of MCF-7 cells with ROME prevented actin enrichment into lamellipodia in response to S1P, suggesting that metastasis could be inhibited. As a SK2-selective inhibitor, (R)-FTY720 methyl ether increased sphingosine and decreased S1P levels, but had no effect on ceramide levels and did not induce apoptosis in LNCaP cells [99].
KRP-203
Recently, a FTY720-like immunosuppressant, KRP-203 (Figure 6) has been reported to be a S1P1 receptor-selective agonist in contrast to FTY720 [100]. Both of the compounds had a similar high affinity for the S1P1 receptor with an ED50 in the nM range. However, for S1P3 the FTY720-P displayed an ED50 of 1.74 nM whereas KRP-203-P had an ED50 of>1 mM [101]. KRP-203 proved to not only regulate T-cell responses but also those of B cell. The anticancer activity of KRP-203 is under investigation.
S16, S18, and T18
In contrast to FTY720, modification of 2-amino-1,3-propanediol on the amino group produced amidated 2-amino-1,3-propanediol derivatives (S16, B16, S18, and T18, Figure, 6). S16 was the first compound of this group. Incubation of neuroblastoma cells with C16-serinol (S16) increased the concentration of endogenous ceramide by 50–80% and caused apoptosis in rapidly dividing low-density cells but not in confluent cells. Further studies indicated that apoptosis in neuroblastoma cells induced by C16-serinol was at least partially mediated by activation of PKCζ, an important regulator in ceramide-induced apoptosis pathway (shown in Figure 1) [102]. Structural optimization of S16 created a new generation 2-amino-1,3-propanediol, S18 (N-(2-hydroxy-1-(hydroxymethyl)ethyl)-oleoylamide, Figure 6), which induced apoptosis in a variety of neuronal and non-neuronal cancer lines. S18 triggered pro-apoptotic signaling pathways without prior elevation of endogenous ceramide, suggesting that it probably replaced ceramide to activate downstream effectors [49]. However, unfortunately the SK inhibition activity of this group has not been investigated.
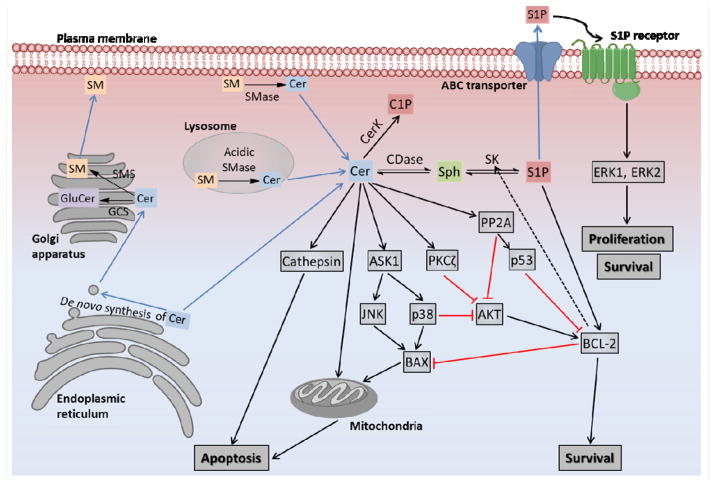
Figure 1.
Ceramide metabolism and signaling pathways and their anti-cancer targets. Cer, ceramide; CDase, ceramidase; Sph, sphingosine; SK, sphingosine kinase; S1P, sphingosine-1-phosphate; CerK, ceramide kinase; C1P, ceramide-1-phosphate; SMS, sphingomyelin synthase; SM, sphingomyelin; GCS, glucosylceramide synthase; GluCer, glucosylceramide; SMase, sphingomyelinase; ASK1, Apoptosis signal-regulating kinase 1; JNK, c-Jun N-terminal kinase; BAX, BCL-2–associated X protein; PP2A, protein phosphatase 2A; AKT, serine/threonine-specific protein kinase; BCL-2, B-cell lymphoma 2; ERK, extracellular-signal-regulated kinase; ABC transporter, ATP-binding cassette transporter.
Other SK inhibitors, such as SKI I,II,V [103], B-5354 [104], F-12509A, and S-15183a,b [104] do not belong to the ceramide structure system, and they are not discussed here.
Multifarious modifications in the recent decade
Over the past ten years, there have been a variety of approaches to the design of new ceramide analogs. According to the modification sites, these design approaches can be classified into 1) sphingosine backbone modification, 2) N-terminal side chain modification, and 3) mixed structural modification.
It is known that the sphingosine backbone is more important than the N-terminal side-chain for the pro-apoptotic activity of ceramide. The purpose of backbone optimization is to discover a better pharmacophore than 3-hydroxy-4-ene (the main active moiety of ceramide), which can more effectively activate ceramide downstream effectors, inducing apoptosis. On the basis of the reported results, both the 3-hydroxy and the 4,5- double bond are replaceable. A relatively rigid structure with a conjugated system (such as a conjugated diene, an enone, or an aromatic ring) in the sphingosine backbone improves the pro-apoptotic activities [16, 64, 105]. The following table provides information on a number of ceramide analogs and their activities.
Unlike the backbone modification, the N-terminal side-chain modification focuses on the metabolic properties of ceramide, such as water/lipid solubility [118]. Modifying the N-terminal amide group could avoid ceramide analog metabolism by ceramidase [59, 119] or inhibit ceramidase metabolizing endogenous ceramide [120]. A major success of N-terminal side-chain modification was the discovery of positively charged ceramide analogs (LCL124, LCL29, and LCL30, etc) [118]. These analogs own a pyridinium functional group, which dramatically increases the water-solubility of the whole molecule. Furthermore these positively charged ceramide analogs can target negatively charged intracellular compartments, and accumulate mainly in mitochondria-, and nuclei-enriched fractions, in turn increasing mitochondrial membrane permeability and triggering the mitochondrial apoptosis cascade. These compounds are displayed in table 2 with other N-terminal side-chain modified ceramides.
Table 2.
Ceramide N-terminal side-chain modification, structures, and activities
| Name and structure | Activity | Reference |
|---|---|---|
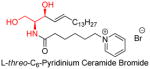
|
|
[12, 118, 121] |
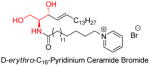
|
|
[13, 122, 123] |
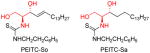
|
|
[124] |
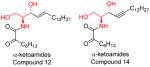
|
|
[120] |
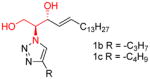
|
|
[119] |
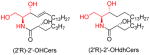
|
|
[59] |
|
|
[125] |
The mixed modification on both chains of ceramide has been reported only in the past couple of years. However, the limited information available points to the fact that 1-position modification highly correlates with the inhibition of sphingosine kinase (SK) and glycosylceramide synthase (GCS).
Future Perspective
To date, thousands of potential anti-cancer ceramide analogs have been synthesized and evaluated aiming at the diverse targets in the ceramide signaling pathway. Since ceramide is in the center of sphingolipid metabolism, its analogs are intrinsically involved in multiple mechanisms of action. Sometimes, multiple mechanisms point in the same direction, and we can obtain multi-action anticancer agents. However, most often, selective agents are more desirable. As in the case of any other anti-cancer agents, improving the selectivity of the agent for a certain target over other targets, for a certain organ over other organs, and for tumor cells over normal cells, are the best approaches to develop new ceramide analogs. Thus, mechanism investigation is as important as activity screening for these chemicals and a multi-evaluation system around ceramide-related targets is desired. In recent years, the crystal structures of some ceramide targets, such as sphingosine kinase [132], neutral ceramidase [133], and protein phosphatase 2A (PP2A) [134] have been published. These developments prompted a change in the approach to the design of ceramide analogs from ligand-based optimization to ligand/protein-based optimization, and accelerated the development of ceramide-based anti-cancer agents.
Table 1.
Ceramide backbone modification, structures, and activities
| Name and structure | Activity | Reference |
|---|---|---|
|
|
[106] |
|
|
[107, 108] |
|
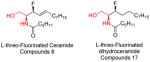
|
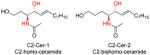
|
[105] |
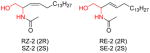
|
|
[109] |
|
|
|
[110] |
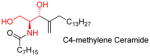
|
|
[111] |
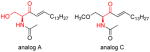
|
|
[16] |
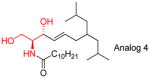
|
|
[112] |
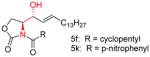
|
|
[113] |
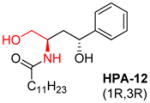
|
|
[114–116] |
|
|
|
[117] |
Table 3.
Other modifications on ceramide, structures, and activities
| Name and structure | Activity | Reference |
|---|---|---|
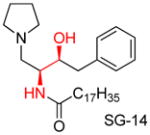
|
|
[126] |
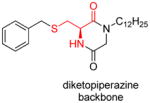
|
|
[127] |
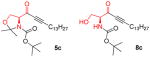
|
5c
8C
|
[128] |
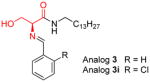
|
|
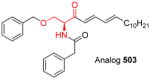
[129–131] |
|
|
Unpublished data from authors’ lab |
Executive Summary.
Anti-cancer target system surrounding ceramide
Activating CAPPs (ceramide-activated Ser-Thr protein phosphatases)
Inhibiting ceramidase, glycosylceramide synthase, and ceramide kinase
Inhibiting sphingosine kinase and sphingosine-1-phospate receptor
Structure-activity relationship of anti-cancer ceramide analogs
Stereospecific activity is a common feature for ceramide analogs
Sphingosine backbone modification by rigid groups increasing apoptotic activity
1-Position modification leading to the inhibition of glucosylceramide synthase
Introducing positively charged groups increases mitochondrial membrane permeability and triggers the mitochondrial apoptosis cascade
Acknowledgments
We thank Louisiana Cancer Research Consortium (LCRC), DoD Award W81XWH-11-1-0105, and AREA Award Number R15CA159059 from the National Cancer Institute for their financial support leading to our fruitful inter-institutional collaboration.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the above funding agencies.
Bibliography
- 1.Huwiler A, Zangemeister-Wittke U. Targeting the conversion of ceramide to sphingosine 1-phosphate as a novel strategy for cancer therapy. Crit Rev Oncol Hematol. 2007;63(2):150–159. doi: 10.1016/j.critrevonc.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 2.Takabe K, Paugh SW, Milstien S, Spiegel S. “Inside-out” signaling of sphingosine-1-phosphate: therapeutic targets. Pharmacol Rev. 2008;60(2):181–195. doi: 10.1124/pr.107.07113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huwiler A, Kolter T, Pfeilschifter J, Sandhoff K. Physiology and pathophysiology of sphingolipid metabolism and signaling. Biochim Biophys Acta. 2000;1485(2–3):63–99. doi: 10.1016/s1388-1981(00)00042-1. [DOI] [PubMed] [Google Scholar]
- 4.Ogretmen B, Hannun YA. Biologically active sphingolipids in cancer pathogenesis and treatment. Nat Rev Cancer. 2004;4(8):604–616. doi: 10.1038/nrc1411. [DOI] [PubMed] [Google Scholar]
- 5.Zhou H, Summers SA, Birnbaum MJ, Pittman RN. Inhibition of Akt kinase by cell-permeable ceramide and its implications for ceramide-induced apoptosis. J Biol Chem. 1998;273(26):16568–16575. doi: 10.1074/jbc.273.26.16568. [DOI] [PubMed] [Google Scholar]
- 6.Bourbon NA, Sandirasegarane L, Kester M. Ceramide-induced inhibition of Akt is mediated through protein kinase Czeta: implications for growth arrest. J Biol Chem. 2002;277(5):3286–3292. doi: 10.1074/jbc.M110541200. [DOI] [PubMed] [Google Scholar]
- 7.Wang G, Silva J, Krishnamurthy K, Tran E, Condie BG, Bieberich E. Direct binding to ceramide activates protein kinase Czeta before the formation of a pro-apoptotic complex with PAR-4 in differentiating stem cells. J Biol Chem. 2005;280(28):26415–26424. doi: 10.1074/jbc.M501492200. [DOI] [PubMed] [Google Scholar]
- 8.Deng X, Gao F, May WS. Protein phosphatase 2A inactivates Bcl2’s antiapoptotic function by dephosphorylation and up-regulation of Bcl2-p53 binding. Blood. 2009;113(2):422–428. doi: 10.1182/blood-2008-06-165134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heinrich M, Neumeyer J, Jakob M, et al. Cathepsin D links TNF-induced acid sphingomyelinase to Bid-mediated caspase-9 and -3 activation. Cell Death Differ. 2004;11(5):550–563. doi: 10.1038/sj.cdd.4401382. [DOI] [PubMed] [Google Scholar]
- 10.Lee H, Rotolo JA, Mesicek J, et al. Mitochondrial ceramide-rich macrodomains functionalize Bax upon irradiation. PLoS One. 2011;6(6):e19783. doi: 10.1371/journal.pone.0019783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Von Haefen C, Wieder T, Gillissen B, et al. Ceramide induces mitochondrial activation and apoptosis via a Bax-dependent pathway in human carcinoma cells. Oncogene. 2002;21(25):4009–4019. doi: 10.1038/sj.onc.1205497. [DOI] [PubMed] [Google Scholar]
- 12**.Beckham TH, Lu P, Jones EE, et al. LCL124, a Cationic Analog of Ceramide, Selectively Induces Pancreatic Cancer Cell Death by Accumulating in Mitochondria. J Pharmacol Exp Ther. 2013;344(1):167–178. doi: 10.1124/jpet.112.199216. Positively charged ceramide analog LCL124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dahm F, Bielawska A, Nocito A, et al. Mitochondrially targeted ceramide LCL-30 inhibits colorectal cancer in mice. Br J Cancer. 2008;98(1):98–105. doi: 10.1038/sj.bjc.6604099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chalfant CE, Szulc Z, Roddy P, Bielawska A, Hannun YA. The structural requirements for ceramide activation of serine-threonine protein phosphatases. J Lipid Res. 2004;45(3):496–506. doi: 10.1194/jlr.M300347-JLR200. [DOI] [PubMed] [Google Scholar]
- 15.Bielawska A, Greenberg MS, Perry D, et al. (1S,2R)-D-erythro-2-(N-myristoylamino)-1-phenyl-1-propanol as an inhibitor of ceramidase. J Biol Chem. 1996;271(21):12646–12654. doi: 10.1074/jbc.271.21.12646. [DOI] [PubMed] [Google Scholar]
- 16.Azuma H, Ijichi S, Kataoka M, et al. Short-chain 3-ketoceramides, strong apoptosis inducers against human leukemia HL-60 cells. Bioorg Med Chem. 2007;15(8):2860–2867. doi: 10.1016/j.bmc.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 17.Bernardo K, Hurwitz R, Zenk T, et al. Purification, characterization, and biosynthesis of human acid ceramidase. J Biol Chem. 1995;270(19):11098–11102. doi: 10.1074/jbc.270.19.11098. [DOI] [PubMed] [Google Scholar]
- 18.El Bawab S, Roddy P, Qian T, Bielawska A, Lemasters JJ, Hannun YA. Molecular cloning and characterization of a human mitochondrial ceramidase. J Biol Chem. 2000;275(28):21508–21513. doi: 10.1074/jbc.M002522200. [DOI] [PubMed] [Google Scholar]
- 19.Tani M, Okino N, Mitsutake S, Tanigawa T, Izu H, Ito M. Purification and characterization of a neutral ceramidase from mouse liver. A single protein catalyzes the reversible reaction in which ceramide is both hydrolyzed and synthesized. J Biol Chem. 2000;275(5):3462–3468. doi: 10.1074/jbc.275.5.3462. [DOI] [PubMed] [Google Scholar]
- 20.Okino N, Tani M, Imayama S, Ito M. Purification and characterization of a novel ceramidase from Pseudomonas aeruginosa. J Biol Chem. 1998;273(23):14368–14373. doi: 10.1074/jbc.273.23.14368. [DOI] [PubMed] [Google Scholar]
- 21.Raisova M, Goltz G, Bektas M, et al. Bcl-2 overexpression prevents apoptosis induced by ceramidase inhibitors in malignant melanoma and HaCaT keratinocytes. FEBS Lett. 2002;516(1–3):47–52. doi: 10.1016/s0014-5793(02)02472-9. [DOI] [PubMed] [Google Scholar]
- 22.Samsel L, Zaidel G, Drumgoole HM, et al. The ceramide analog, B13, induces apoptosis in prostate cancer cell lines and inhibits tumor growth in prostate cancer xenografts. Prostate. 2004;58(4):382–393. doi: 10.1002/pros.10350. [DOI] [PubMed] [Google Scholar]
- 23.Granot T, Milhas D, Carpentier S, et al. Caspase-dependent and -independent cell death of Jurkat human leukemia cells induced by novel synthetic ceramide analogs. Leukemia. 2006;20(3):392–399. doi: 10.1038/sj.leu.2404084. [DOI] [PubMed] [Google Scholar]
- 24.Ichikawa S, Sakiyama H, Suzuki G, Hidari KI, Hirabayashi Y. Expression cloning of a cDNA for human ceramide glucosyltransferase that catalyzes the first glycosylation step of glycosphingolipid synthesis. Proc Natl Acad Sci U S A. 1996;93(10):4638–4643. doi: 10.1073/pnas.93.10.4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song M, Zang W, Zhang B, Cao J, Yang G. GCS overexpression is associated with multidrug resistance of human HCT-8 colon cancer cells. Journal of experimental & clinical cancer research : CR. 2012;31:23. doi: 10.1186/1756-9966-31-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu YY, Han TY, Giuliano AE, Cabot MC. Expression of glucosylceramide synthase, converting ceramide to glucosylceramide, confers adriamycin resistance in human breast cancer cells. The Journal of biological chemistry. 1999;274(2):1140–1146. doi: 10.1074/jbc.274.2.1140. [DOI] [PubMed] [Google Scholar]
- 27.Liu YY, Yu JY, Yin D, et al. A role for ceramide in driving cancer cell resistance to doxorubicin. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2008;22(7):2541–2551. doi: 10.1096/fj.07-092981. [DOI] [PubMed] [Google Scholar]
- 28.Liu YY, Gupta V, Patwardhan GA, et al. Glucosylceramide synthase upregulates MDR1 expression in the regulation of cancer drug resistance through cSrc and beta-catenin signaling. Molecular cancer. 2010;9:145. doi: 10.1186/1476-4598-9-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gouaze-Andersson V, Yu JY, Kreitenberg AJ, Bielawska A, Giuliano AE, Cabot MC. Ceramide and glucosylceramide upregulate expression of the multidrug resistance gene MDR1 in cancer cells. Biochimica et biophysica acta. 2007;1771(12):1407–1417. doi: 10.1016/j.bbalip.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gouazé V, Liu YY, Prickett CS, Yu JY, Giuliano AE, Cabot MC. Glucosylceramide synthase blockade down-regulates P-glycoprotein and resensitizes multidrug-resistant breast cancer cells to anticancer drugs. Cancer Res. 2005;65(9):3861–3867. doi: 10.1158/0008-5472.CAN-04-2329. [DOI] [PubMed] [Google Scholar]
- 31.Sietsma H, Veldman RJ, Kolk D, et al. 1-phenyl-2-decanoylamino-3-morpholino-1-propanol chemosensitizes neuroblastoma cells for taxol and vincristine. Clin Cancer Res. 2000;6(3):942–948. [PubMed] [Google Scholar]
- 32.Dijkhuis AJ, Klappe K, Jacobs S, et al. PDMP sensitizes neuroblastoma to paclitaxel by inducing aberrant cell cycle progression leading to hyperploidy. Molecular cancer therapeutics. 2006;5(3):593–601. doi: 10.1158/1535-7163.MCT-05-0457. [DOI] [PubMed] [Google Scholar]
- 33.Granado MH, Gangoiti P, Ouro A, Arana L, Gomez-Munoz A. Ceramide 1-phosphate inhibits serine palmitoyltransferase and blocks apoptosis in alveolar macrophages. Biochimica et biophysica acta. 2009;1791(4):263–272. doi: 10.1016/j.bbalip.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 34.Gomez-Munoz A, Kong JY, Salh B, Steinbrecher UP. Ceramide-1-phosphate blocks apoptosis through inhibition of acid sphingomyelinase in macrophages. Journal of lipid research. 2004;45(1):99–105. doi: 10.1194/jlr.M300158-JLR200. [DOI] [PubMed] [Google Scholar]
- 35.Gangoiti P, Granado MH, Wang SW, Kong JY, Steinbrecher UP, Gomez-Munoz A. Ceramide 1-phosphate stimulates macrophage proliferation through activation of the PI3-kinase/PKB, JNK and ERK1/2 pathways. Cellular signalling. 2008;20(4):726–736. doi: 10.1016/j.cellsig.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 36.Gangoiti P, Bernacchioni C, Donati C, et al. Ceramide 1-phosphate stimulates proliferation of C2C12 myoblasts. Biochimie. 2012;94(3):597–607. doi: 10.1016/j.biochi.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sauer B, Gonska H, Manggau M, et al. Sphingosine 1-phosphate is involved in cytoprotective actions of calcitriol in human fibroblasts and enhances the intracellular Bcl-2/Bax rheostat. Pharmazie. 2005;60(4):298–304. [PubMed] [Google Scholar]
- 38.Avery K, Avery S, Shepherd J, Heath PR, Moore H. Sphingosine-1-phosphate mediates transcriptional regulation of key targets associated with survival, proliferation, and pluripotency in human embryonic stem cells. Stem Cells Dev. 2008;17(6):1195–1205. doi: 10.1089/scd.2008.0063. [DOI] [PubMed] [Google Scholar]
- 39.Jürgensmeier JM, Xie Z, Deveraux Q, Ellerby L, Bredesen D, Reed JC. Bax directly induces release of cytochrome c from isolated mitochondria. Proc Natl Acad Sci U S A. 1998;95(9):4997–5002. doi: 10.1073/pnas.95.9.4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bektas M, Jolly PS, Müller C, Eberle J, Spiegel S, Geilen CC. Sphingosine kinase activity counteracts ceramide-mediated cell death in human melanoma cells: role of Bcl-2 expression. Oncogene. 2005;24(1):178–187. doi: 10.1038/sj.onc.1208019. [DOI] [PubMed] [Google Scholar]
- 41.Pyne NJ, Pyne S. Sphingosine 1-phosphate and cancer. Nat Rev Cancer. 2010;10(7):489–503. doi: 10.1038/nrc2875. [DOI] [PubMed] [Google Scholar]
- 42.Mitra P, Oskeritzian CA, Payne SG, Beaven MA, Milstien S, Spiegel S. Role of ABCC1 in export of sphingosine-1-phosphate from mast cells. Proc Natl Acad Sci U S A. 2006;103(44):16394–16399. doi: 10.1073/pnas.0603734103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Calise S, Blescia S, Cencetti F, Bernacchioni C, Donati C, Bruni P. Sphingosine 1-phosphate stimulates proliferation and migration of satellite cells: role of S1P receptors. Biochim Biophys Acta. 2012;1823(2):439–450. doi: 10.1016/j.bbamcr.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 44.Wang F, Van Brocklyn JR, Hobson JP, et al. Sphingosine 1-phosphate stimulates cell migration through a G(i)-coupled cell surface receptor. Potential involvement in angiogenesis. J Biol Chem. 1999;274(50):35343–35350. doi: 10.1074/jbc.274.50.35343. [DOI] [PubMed] [Google Scholar]
- 45.Sugimoto N, Takuwa N, Okamoto H, Sakurada S, Takuwa Y. Inhibitory and stimulatory regulation of Rac and cell motility by the G12/13-Rho and Gi pathways integrated downstream of a single G protein-coupled sphingosine-1-phosphate receptor isoform. Mol Cell Biol. 2003;23(5):1534–1545. doi: 10.1128/MCB.23.5.1534-1545.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hsu A, Zhang W, Lee JF, et al. Sphingosine-1-phosphate receptor-3 signaling up-regulates epidermal growth factor receptor and enhances epidermal growth factor receptor-mediated carcinogenic activities in cultured lung adenocarcinoma cells. Int J Oncol. 2012;40(5):1619–1626. doi: 10.3892/ijo.2012.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim ES, Kim JS, Kim SG, Hwang S, Lee CH, Moon A. Sphingosine 1-phosphate regulates matrix metalloproteinase-9 expression and breast cell invasion through S1P3-Gαq coupling. J Cell Sci. 2011;124(Pt 13):2220–2230. doi: 10.1242/jcs.076794. [DOI] [PubMed] [Google Scholar]
- 48.English D, Welch Z, Kovala AT, et al. Sphingosine 1-phosphate released from platelets during clotting accounts for the potent endothelial cell chemotactic activity of blood serum and provides a novel link between hemostasis and angiogenesis. FASEB J. 2000;14(14):2255–2265. doi: 10.1096/fj.00-0134com. [DOI] [PubMed] [Google Scholar]
- 49.Bieberich E, Hu B, Silva J, et al. Synthesis and characterization of novel ceramide analogs for induction of apoptosis in human cancer cells. Cancer Lett. 2002;181(1):55–64. doi: 10.1016/s0304-3835(02)00049-6. [DOI] [PubMed] [Google Scholar]
- 50.Nicholson KM, Quinn DM, Kellett GL, Warr JR. Preferential killing of multidrug-resistant KB cells by inhibitors of glucosylceramide synthase. Br J Cancer. 1999;81(3):423–430. doi: 10.1038/sj.bjc.6690711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang JD, Takahara S, Nonomura N, et al. Early induction of apoptosis in androgen-independent prostate cancer cell line by FTY720 requires caspase-3 activation. Prostate. 1999;40(1):50–55. doi: 10.1002/(sici)1097-0045(19990615)40:1<50::aid-pros6>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 52.Stover T, Kester M. Liposomal delivery enhances short-chain ceramide-induced apoptosis of breast cancer cells. J Pharmacol Exp Ther. 2003;307(2):468–475. doi: 10.1124/jpet.103.054056. [DOI] [PubMed] [Google Scholar]
- 53.Stover TC, Sharma A, Robertson GP, Kester M. Systemic delivery of liposomal short-chain ceramide limits solid tumor growth in murine models of breast adenocarcinoma. Clin Cancer Res. 2005;11(9):3465–3474. doi: 10.1158/1078-0432.CCR-04-1770. [DOI] [PubMed] [Google Scholar]
- 54.Zolnik BS, Stern ST, Kaiser JM, et al. Rapid distribution of liposomal short-chain ceramide in vitro and in vivo. Drug Metab Dispos. 2008;36(8):1709–1715. doi: 10.1124/dmd.107.019679. [DOI] [PubMed] [Google Scholar]
- 55.Morad SA, Levin JC, Shanmugavelandy SS, et al. Ceramide--antiestrogen nanoliposomal combinations--novel impact of hormonal therapy in hormone-insensitive breast cancer. Mol Cancer Ther. 2012;11(11):2352–2361. doi: 10.1158/1535-7163.MCT-12-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56***.Bielawska A, Crane HM, Liotta D, Obeid LM, Hannun YA. Selectivity of ceramide-mediated biology. Lack of activity of erythro-dihydroceramide. J Biol Chem. 1993;268(35):26226–26232. Stereospecific activity of ceramide analogs. [PubMed] [Google Scholar]
- 57.Bielawska A, Linardic CM, Hannun YA. Ceramide-mediated biology. Determination of structural and stereospecific requirements through the use of N-acyl-phenylaminoalcohol analogs. J Biol Chem. 1992;267(26):18493–18497. [PubMed] [Google Scholar]
- 58.Karasavvas N, Erukulla RK, Bittman R, Lockshin R, Zakeri Z. Stereospecific induction of apoptosis in U937 cells by N-octanoyl-sphingosine stereoisomers and N-octyl-sphingosine. The ceramide amide group is not required for apoptosis. Eur J Biochem. 1996;236(2):729–737. doi: 10.1111/j.1432-1033.1996.00729.x. [DOI] [PubMed] [Google Scholar]
- 59.Szulc ZM, Bai A, Bielawski J, et al. Synthesis, NMR characterization and divergent biological actions of 2′-hydroxy-ceramide/dihydroceramide stereoisomers in MCF7 cells. Bioorg Med Chem. 2010;18(21):7565–7579. doi: 10.1016/j.bmc.2010.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lim KG, Sun C, Bittman R, Pyne NJ, Pyne S. (R)-FTY720 methyl ether is a specific sphingosine kinase 2 inhibitor: Effect on sphingosine kinase 2 expression in HEK 293 cells and actin rearrangement and survival of MCF-7 breast cancer cells. Cell Signal. 2011;23(10):1590–1595. doi: 10.1016/j.cellsig.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Buehrer BM, Bell RM. Inhibition of sphingosine kinase in vitro and in platelets. Implications for signal transduction pathways. J Biol Chem. 1992;267(5):3154–3159. [PubMed] [Google Scholar]
- 62.Usta J, El Bawab S, Roddy P, et al. Structural requirements of ceramide and sphingosine based inhibitors of mitochondrial ceramidase. Biochemistry. 2001;40(32):9657–9668. doi: 10.1021/bi010535k. [DOI] [PubMed] [Google Scholar]
- 63***.Vunnam RR, Radin NS. Analogs of ceramide that inhibit glucocerebroside synthetase in mouse brain. Chem Phys Lipids. 1980;26(3):265–278. doi: 10.1016/0009-3084(80)90057-2. Discovery of RV-583 as a competitive GCS inhibitor. [DOI] [PubMed] [Google Scholar]
- 64.Selzner M, Bielawska A, Morse MA, et al. Induction of apoptotic cell death and prevention of tumor growth by ceramide analogues in metastatic human colon cancer. Cancer Res. 2001;61(3):1233–1240. [PubMed] [Google Scholar]
- 65.Dagan A, Wang C, Fibach E, Gatt S. Synthetic, non-natural sphingolipid analogs inhibit the biosynthesis of cellular sphingolipids, elevate ceramide and induce apoptotic cell death. Biochim Biophys Acta. 2003;1633(3):161–169. doi: 10.1016/s1388-1981(03)00122-7. [DOI] [PubMed] [Google Scholar]
- 66.Holman DH, Turner LS, El-Zawahry A, et al. Lysosomotropic acid ceramidase inhibitor induces apoptosis in prostate cancer cells. Cancer Chemother Pharmacol. 2008;61(2):231–242. doi: 10.1007/s00280-007-0465-0. [DOI] [PubMed] [Google Scholar]
- 67.Bhabak KP, Kleuser B, Huwiler A, Arenz C. Effective inhibition of acid and neutral ceramidases by novel B-13 and LCL-464 analogues. Bioorg Med Chem. 2012 doi: 10.1016/j.bmc.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 68.Bai A, Szulc ZM, Bielawski J, et al. Synthesis and bioevaluation of omega-N-amino analogs of B13. Bioorg Med Chem. 2009;17(5):1840–1848. doi: 10.1016/j.bmc.2009.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bhabak KP, Arenz C. Novel amide- and sulfonamide-based aromatic ethanolamines: effects of various substituents on the inhibition of acid and neutral ceramidases. Bioorg Med Chem. 2012;20(20):6162–6170. doi: 10.1016/j.bmc.2012.08.031. [DOI] [PubMed] [Google Scholar]
- 70.Abe A, Inokuchi J, Jimbo M, et al. Improved inhibitors of glucosylceramide synthase. J Biochem. 1992;111(2):191–196. doi: 10.1093/oxfordjournals.jbchem.a123736. [DOI] [PubMed] [Google Scholar]
- 71.Inokuchi J, Radin NS. Preparation of the active isomer of 1-phenyl-2-decanoylamino-3-morpholino-1-propanol, inhibitor of murine glucocerebroside synthetase. J Lipid Res. 1987;28(5):565–571. [PubMed] [Google Scholar]
- 72.Uemura K, Sugiyama E, Tamai C, Hara A, Taketomi T, Radin NS. Effect of an inhibitor of glucosylceramide synthesis on cultured rabbit skin fibroblasts. J Biochem. 1990;108(4):525–530. doi: 10.1093/oxfordjournals.jbchem.a123236. [DOI] [PubMed] [Google Scholar]
- 73.Abe A, Radin NS, Shayman JA, et al. Structural and stereochemical studies of potent inhibitors of glucosylceramide synthase and tumor cell growth. J Lipid Res. 1995;36(3):611–621. [PubMed] [Google Scholar]
- 74.Maurer BJ, Melton L, Billups C, Cabot MC, Reynolds CP. Synergistic cytotoxicity in solid tumor cell lines between N-(4-hydroxyphenyl)retinamide and modulators of ceramide metabolism. J Natl Cancer Inst. 2000;92(23):1897–1909. doi: 10.1093/jnci/92.23.1897. [DOI] [PubMed] [Google Scholar]
- 75.Lee L, Abe A, Shayman JA. Improved inhibitors of glucosylceramide synthase. J Biol Chem. 1999;274(21):14662–14669. doi: 10.1074/jbc.274.21.14662. [DOI] [PubMed] [Google Scholar]
- 76.Hillaert U, Boldin-Adamsky S, Rozenski J, Busson R, Futerman AH, Van Calenbergh S. Synthesis and biological evaluation of novel PDMP analogues. Bioorg Med Chem. 2006;14(15):5273–5284. doi: 10.1016/j.bmc.2006.03.048. [DOI] [PubMed] [Google Scholar]
- 77.Larsen SD, Wilson MW, Abe A, et al. Property-based design of a glucosylceramide synthase inhibitor that reduces glucosylceramide in the brain. J Lipid Res. 2012;53(2):282–291. doi: 10.1194/jlr.M021261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yatomi Y, Ruan F, Megidish T, Toyokuni T, Hakomori S, Igarashi Y. N,N-dimethylsphingosine inhibition of sphingosine kinase and sphingosine 1-phosphate activity in human platelets. Biochemistry. 1996;35(2):626–633. doi: 10.1021/bi9515533. [DOI] [PubMed] [Google Scholar]
- 79.Kohama T, Olivera A, Edsall L, Nagiec MM, Dickson R, Spiegel S. Molecular cloning and functional characterization of murine sphingosine kinase. J Biol Chem. 1998;273(37):23722–23728. doi: 10.1074/jbc.273.37.23722. [DOI] [PubMed] [Google Scholar]
- 80.Liu H, Sugiura M, Nava VE, et al. Molecular cloning and functional characterization of a novel mammalian sphingosine kinase type 2 isoform. J Biol Chem. 2000;275(26):19513–19520. doi: 10.1074/jbc.M002759200. [DOI] [PubMed] [Google Scholar]
- 81.Shirahama T, Sweeney EA, Sakakura C, et al. In vitro and in vivo induction of apoptosis by sphingosine and N, N-dimethylsphingosine in human epidermoid carcinoma KB-3-1 and its multidrug-resistant cells. Clin Cancer Res. 1997;3(2):257–264. [PubMed] [Google Scholar]
- 82.Sweeney EA, Sakakura C, Shirahama T, et al. Sphingosine and its methylated derivative N,N-dimethylsphingosine (DMS) induce apoptosis in a variety of human cancer cell lines. Int J Cancer. 1996;66(3):358–366. doi: 10.1002/(SICI)1097-0215(19960503)66:3<358::AID-IJC16>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 83.Igarashi Y, Hakomori S, Toyokuni T, et al. Effect of chemically well-defined sphingosine and its N-methyl derivatives on protein kinase C and src kinase activities. Biochemistry. 1989;28(17):6796–6800. doi: 10.1021/bi00443a002. [DOI] [PubMed] [Google Scholar]
- 84.Sugiura M, Kono K, Liu H, et al. Ceramide kinase, a novel lipid kinase. Molecular cloning and functional characterization. J Biol Chem. 2002;277(26):23294–23300. doi: 10.1074/jbc.M201535200. [DOI] [PubMed] [Google Scholar]
- 85.Azuma H, Takahara S, Horie S, Muto S, Otsuki Y, Katsuoka Y. Induction of apoptosis in human bladder cancer cells in vitro and in vivo caused by FTY720 treatment. J Urol. 2003;169(6):2372–2377. doi: 10.1097/01.ju.0000064938.32318.91. [DOI] [PubMed] [Google Scholar]
- 86.Ubai T, Azuma H, Kotake Y, et al. FTY720 induced Bcl-associated and Fas-independent apoptosis in human renal cancer cells in vitro and significantly reduced in vivo tumor growth in mouse xenograft. Anticancer Res. 2007;27(1A):75–88. [PubMed] [Google Scholar]
- 87.Shen Y, Cai M, Xia W, et al. FTY720, a synthetic compound from Isaria sinclairii, inhibits proliferation and induces apoptosis in pancreatic cancer cells. Cancer Lett. 2007;254(2):288–297. doi: 10.1016/j.canlet.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 88.Nagaoka Y, Otsuki K, Fujita T, Uesato S. Effects of phosphorylation of immunomodulatory agent FTY720 (fingolimod) on antiproliferative activity against breast and colon cancer cells. Biol Pharm Bull. 2008;31(6):1177–1181. doi: 10.1248/bpb.31.1177. [DOI] [PubMed] [Google Scholar]
- 89.Estrada-Bernal A, Palanichamy K, Ray Chaudhury A, Van Brocklyn JR. Induction of brain tumor stem cell apoptosis by FTY720: a potential therapeutic agent for glioblastoma. Neuro Oncol. 2012;14(4):405–415. doi: 10.1093/neuonc/nos005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Azuma H, Takahara S, Ichimaru N, et al. Marked prevention of tumor growth and metastasis by a novel immunosuppressive agent, FTY720, in mouse breast cancer models. Cancer Res. 2002;62(5):1410–1419. [PubMed] [Google Scholar]
- 91.Ho JW, Man K, Sun CK, Lee TK, Poon RT, Fan ST. Effects of a novel immunomodulating agent, FTY720, on tumor growth and angiogenesis in hepatocellular carcinoma. Mol Cancer Ther. 2005;4(9):1430–1438. doi: 10.1158/1535-7163.MCT-05-0021. [DOI] [PubMed] [Google Scholar]
- 92.Matsuoka Y, Nagahara Y, Ikekita M, Shinomiya T. A novel immunosuppressive agent FTY720 induced Akt dephosphorylation in leukemia cells. Br J Pharmacol. 2003;138(7):1303–1312. doi: 10.1038/sj.bjp.0705182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vessey DA, Kelley M, Zhang J, Li L, Tao R, Karliner JS. Dimethylsphingosine and FTY720 inhibit the SK1 form but activate the SK2 form of sphingosine kinase from rat heart. J Biochem Mol Toxicol. 2007;21(5):273–279. doi: 10.1002/jbt.20193. [DOI] [PubMed] [Google Scholar]
- 94.Kharel Y, Lee S, Snyder AH, et al. Sphingosine kinase 2 is required for modulation of lymphocyte traffic by FTY720. J Biol Chem. 2005;280(44):36865–36872. doi: 10.1074/jbc.M506293200. [DOI] [PubMed] [Google Scholar]
- 95.Zemann B, Kinzel B, Müller M, et al. Sphingosine kinase type 2 is essential for lymphopenia induced by the immunomodulatory drug FTY720. Blood. 2006;107(4):1454–1458. doi: 10.1182/blood-2005-07-2628. [DOI] [PubMed] [Google Scholar]
- 96.Brinkmann V, Davis MD, Heise CE, et al. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem. 2002;277(24):21453–21457. doi: 10.1074/jbc.C200176200. [DOI] [PubMed] [Google Scholar]
- 97.Gräler MH, Goetzl EJ. The immunosuppressant FTY720 down-regulates sphingosine 1-phosphate G-protein-coupled receptors. FASEB J. 2004;18(3):551–553. doi: 10.1096/fj.03-0910fje. [DOI] [PubMed] [Google Scholar]
- 98.Hamada M, Nakamura M, Kiuchi M, et al. Removal of sphingosine 1-phosphate receptor-3 (S1P(3)) agonism is essential, but inadequate to obtain immunomodulating 2-aminopropane-1,3-diol S1P(1) agonists with reduced effect on heart rate. J Med Chem. 2010;53(8):3154–3168. doi: 10.1021/jm901776q. [DOI] [PubMed] [Google Scholar]
- 99.Watson DG, Tonelli F, Al Osaimi M, et al. The roles of sphingosine kinases 1 and 2 in regulating the Warburg effect in prostate cancer cells. Cell Signal. 2013 doi: 10.1016/j.cellsig.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shimizu H, Takahashi M, Kaneko T, et al. KRP-203, a novel synthetic immunosuppressant, prolongs graft survival and attenuates chronic rejection in rat skin and heart allografts. Circulation. 2005;111(2):222–229. doi: 10.1161/01.CIR.0000152101.41037.AB. [DOI] [PubMed] [Google Scholar]
- 101.Fujishiro J, Kudou S, Iwai S, et al. Use of sphingosine-1-phosphate 1 receptor agonist, KRP-203, in combination with a subtherapeutic dose of cyclosporine A for rat renal transplantation. Transplantation. 2006;82(6):804–812. doi: 10.1097/01.tp.0000232687.78242.cd. [DOI] [PubMed] [Google Scholar]
- 102.Bieberich E, Kawaguchi T, Yu RK. N-acylated serinol is a novel ceramide mimic inducing apoptosis in neuroblastoma cells. J Biol Chem. 2000;275(1):177–181. doi: 10.1074/jbc.275.1.177. [DOI] [PubMed] [Google Scholar]
- 103.Loveridge C, Tonelli F, Leclercq T, et al. The sphingosine kinase 1 inhibitor 2-(p-hydroxyanilino)-4-(p-chlorophenyl)thiazole induces proteasomal degradation of sphingosine kinase 1 in mammalian cells. J Biol Chem. 2010;285(50):38841–38852. doi: 10.1074/jbc.M110.127993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kono K, Tanaka M, Mizuno T, Kodama K, Ogita T, Kohama T. B-535a, b and c, new sphingosine kinase inhibitors, produced by a marine bacterium; taxonomy, fermentation, isolation, physico-chemical properties and structure determination. J Antibiot (Tokyo) 2000;53(8):753–758. doi: 10.7164/antibiotics.53.753. [DOI] [PubMed] [Google Scholar]
- 105**.Struckhoff AP, Bittman R, Burow ME, et al. Novel ceramide analogs as potential chemotherapeutic agents in breast cancer. J Pharmacol Exp Ther. 2004;309(2):523–532. doi: 10.1124/jpet.103.062760. Introducing conjugated system into sphingosine backbone increasing apoptotic activity. [DOI] [PubMed] [Google Scholar]
- 106.De Jonghe S, Van Overmeire I, Gunst J, et al. Synthesis and apoptogenic activity of fluorinated ceramide and dihydroceramide analogues. Bioorg Med Chem Lett. 1999;9(21):3159–3164. doi: 10.1016/s0960-894x(99)00553-3. [DOI] [PubMed] [Google Scholar]
- 107.Shikata K, Niiro H, Azuma H, Ogino K, Tachibana T. Apoptotic activities of C2-ceramide and C2-dihydroceramide homologues against HL-60 cells. Bioorg Med Chem. 2003;11(13):2723–2728. doi: 10.1016/s0968-0896(03)00228-1. [DOI] [PubMed] [Google Scholar]
- 108.Shikata K, Niiro H, Azuma H, Tachibana T, Ogino K. Synthesis of non-natural C2-homo-ceramide and its apoptotic activity against HL-60 cells. Bioorg Med Chem Lett. 2003;13(4):613–616. doi: 10.1016/s0960-894x(02)01026-0. [DOI] [PubMed] [Google Scholar]
- 109.Niiro H, Azuma H, Tanago S, et al. (3Z)-2-Acetylamino-3-octadecen-1-ol as a potent apoptotic agent against HL-60 cells. Bioorg Med Chem. 2004;12(1):45–51. doi: 10.1016/j.bmc.2003.10.040. [DOI] [PubMed] [Google Scholar]
- 110.Bedia C, Canals D, Matabosch X, et al. Cytotoxicity and acid ceramidase inhibitory activity of 2-substituted aminoethanol amides. Chem Phys Lipids. 2008;156(1–2):33–40. doi: 10.1016/j.chemphyslip.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 111.Lu X, Arthur G, Bittman R. Synthesis of a novel ceramide analogue via Tebbe methylenation and evaluation of its antiproliferative activity. Org Lett. 2005;7(8):1645–1648. doi: 10.1021/ol0503440. [DOI] [PubMed] [Google Scholar]
- 112.Kang JH, Garg H, Sigano DM, Francella N, Blumenthal R, Marquez VE. Ceramides: branched alkyl chains in the sphingolipid siblings of diacylglycerol improve biological potency. Bioorg Med Chem. 2009;17(4):1498–1505. doi: 10.1016/j.bmc.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Singh A, Ha HJ, Park J, Kim JH, Lee WK. 3,4-Disubstituted oxazolidin-2-ones as constrained ceramide analogs with anticancer activities. Bioorg Med Chem. 2011;19(21):6174–6181. doi: 10.1016/j.bmc.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 114.Yasuda S, Kitagawa H, Ueno M, et al. A novel inhibitor of ceramide trafficking from the endoplasmic reticulum to the site of sphingomyelin synthesis. J Biol Chem. 2001;276(47):43994–44002. doi: 10.1074/jbc.M104884200. [DOI] [PubMed] [Google Scholar]
- 115.Ďuriš A, Wiesenganger T, Moravčíková D, et al. Expedient and practical synthesis of CERT-dependent ceramide trafficking inhibitor HPA-12 and its analogues. Org Lett. 2011;13(7):1642–1645. doi: 10.1021/ol2001057. [DOI] [PubMed] [Google Scholar]
- 116.Nakamura Y, Matsubara R, Kitagawa H, et al. Stereoselective synthesis and structure-activity relationship of novel ceramide trafficking inhibitors. (1R,3R)-N-(3-hydroxy-1-hydroxymethyl-3-phenylpropyl)dodecanamide and its analogues. J Med Chem. 2003;46(17):3688–3695. doi: 10.1021/jm0300779. [DOI] [PubMed] [Google Scholar]
- 117.Camacho L, Simbari F, Garrido M, et al. 3-Deoxy-3,4-dehydro analogs of XM462. Preparation and activity on sphingolipid metabolism and cell fate. Bioorg Med Chem. 2012;20(10):3173–3179. doi: 10.1016/j.bmc.2012.03.073. [DOI] [PubMed] [Google Scholar]
- 118.Novgorodov SA, Szulc ZM, Luberto C, et al. Positively charged ceramide is a potent inducer of mitochondrial permeabilization. J Biol Chem. 2005;280(16):16096–16105. doi: 10.1074/jbc.M411707200. [DOI] [PubMed] [Google Scholar]
- 119.Kim S, Cho M, Lee T, Lee S, Min HY, Lee SK. Design, synthesis, and preliminary biological evaluation of a novel triazole analogue of ceramide. Bioorg Med Chem Lett. 2007;17(16):4584–4587. doi: 10.1016/j.bmcl.2007.05.086. [DOI] [PubMed] [Google Scholar]
- 120.Grijalvo S, Bedia C, Triola G, et al. Design, synthesis and activity as acid ceramidase inhibitors of 2-oxooctanoyl and N-oleoylethanolamine analogues. Chem Phys Lipids. 2006;144(1):69–84. doi: 10.1016/j.chemphyslip.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 121.Senkal CE, Ponnusamy S, Rossi MJ, et al. Potent antitumor activity of a novel cationic pyridinium-ceramide alone or in combination with gemcitabine against human head and neck squamous cell carcinomas in vitro and in vivo. J Pharmacol Exp Ther. 2006;317(3):1188–1199. doi: 10.1124/jpet.106.101949. [DOI] [PubMed] [Google Scholar]
- 122.Separovic D, Saad ZH, Edwin EA, et al. C16-Ceramide Analog Combined with Pc 4 Photodynamic Therapy Evokes Enhanced Total Ceramide Accumulation, Promotion of DEVDase Activation in the Absence of Apoptosis, and Augmented Overall Cell Killing. J Lipids. 2011;2011:713867. doi: 10.1155/2011/713867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dindo D, Dahm F, Szulc Z, et al. Cationic long-chain ceramide LCL-30 induces cell death by mitochondrial targeting in SW403 cells. Mol Cancer Ther. 2006;5(6):1520–1529. doi: 10.1158/1535-7163.MCT-05-0513. [DOI] [PubMed] [Google Scholar]
- 124.Johnson CR, Chun J, Bittman R, Jarvis WD. Intrinsic cytotoxicity and chemomodulatory actions of novel phenethylisothiocyanate sphingoid base derivatives in HL-60 human promyelocytic leukemia cells. J Pharmacol Exp Ther. 2004;309(2):452–461. doi: 10.1124/jpet.103.060665. [DOI] [PubMed] [Google Scholar]
- 125.Antoon JW, Liu J, Ponnapakkam AP, Gestaut MM, Foroozesh M, Beckman BS. Novel D. -erythro N-octanoyl sphingosine analogs as chemo- and endocrine-resistant breast cancer therapeutics. Cancer Chemother Pharmacol. 2010;65(6):1191–1195. doi: 10.1007/s00280-009-1233-0. [DOI] [PubMed] [Google Scholar]
- 126.Kim JW, Kim YW, Inagaki Y, et al. Synthesis and evaluation of sphingoid analogs as inhibitors of sphingosine kinases. Bioorg Med Chem. 2005;13(10):3475–3485. doi: 10.1016/j.bmc.2005.02.053. [DOI] [PubMed] [Google Scholar]
- 127.Oh JE, So KS, Lim SJ, Kim MY. Induction of apoptotic cell death by a ceramide analog in PC-3 prostate cancer cells. Arch Pharm Res. 2006;29(12):1140–1146. [PubMed] [Google Scholar]
- 128.Wong L, Tan SS, Lam Y, Melendez AJ. Synthesis and evaluation of sphingosine analogues as inhibitors of sphingosine kinases. J Med Chem. 2009;52(12):3618–3626. doi: 10.1021/jm900121d. [DOI] [PubMed] [Google Scholar]
- 129.Antoon JW, Liu J, Gestaut MM, Burow ME, Beckman BS, Foroozesh M. Design, synthesis, and biological activity of a family of novel ceramide analogues in chemoresistant breast cancer cells. J Med Chem. 2009;52(18):5748–5752. doi: 10.1021/jm9009668. [DOI] [PubMed] [Google Scholar]
- 130.Liu J, Antoon JW, Ponnapakkam A, Beckman BS, Foroozesh M. Novel anti-viability ceramide analogs: design, synthesis, and structure-activity relationship studies of substituted (S)-2-(benzylideneamino)-3-hydroxy-N-tetradecylpropanamides. Bioorg Med Chem. 2010;18(14):5316–5322. doi: 10.1016/j.bmc.2010.05.044. [DOI] [PubMed] [Google Scholar]
- 131.Antoon JW, Beckman BS. Anti-proliferative effects of the novel ceramide analog (S)-2-(benzylideneamino)-3-hydroxy-N-tetrade-cylpropanamide in chemoresistant cancer. Bioorg Med Chem Lett. 2012;22(7):2624–2628. doi: 10.1016/j.bmcl.2012.01.087. [DOI] [PubMed] [Google Scholar]
- 132.Nichols CE, Lamb HK, Lockyer M, et al. Characterization of Salmonella typhimurium YegS, a putative lipid kinase homologous to eukaryotic sphingosine and diacylglycerol kinases. Proteins. 2007;68(1):13–25. doi: 10.1002/prot.21386. [DOI] [PubMed] [Google Scholar]
- 133.Cho US, Xu W. Crystal structure of a protein phosphatase 2A heterotrimeric holoenzyme. Nature. 2007;445(7123):53–57. doi: 10.1038/nature05351. [DOI] [PubMed] [Google Scholar]
- 134.Inoue T, Okino N, Kakuta Y, et al. Mechanistic insights into the hydrolysis and synthesis of ceramide by neutral ceramidase. J Biol Chem. 2009;284(14):9566–9577. doi: 10.1074/jbc.M808232200. [DOI] [PMC free article] [PubMed] [Google Scholar]