Abstract
Cancer antigen 125 (CA-125) is the most widely used tumor marker for ovarian cancer. Thus, monoclonal antibodies (MAbs) against CA-125 are valuable reagents for the development of diagnostic tests and immunotherapy. We describe here the generation and characterization of three novel hybridoma cell lines producing MAbs against CA-125. CA-125 purified from culture supernatant of ovarian carcinoma cell line OVCAR-3 by affinity chromatography, was used for immunization of BALB/c mice. Three stable cell lines (3C8, 2B6, and 5A12) were selected for production of antibodies against CA-125 and were expanded in mass culture. All three antibodies were shown to recognize linear epitopes. Antibodies 2B6 and 5A12 were determined to recognize epitope cluster B (M 11-like); MAb 3C8 was classified as group A-epitope binders (OC 125-like). The antibodies produced may be used for the development and improvement of CA-125 immunoassays.
Introduction
Ovarian carcinoma is an issue of major health concern worldwide. In 2013, 22,240 cases of ovarian cancer were reported in the USA; 14,030 deaths were caused solely by this type of cancer. The high death rate is mainly caused by a lack of pronounced symptoms at the early stages of the disease. In most cases ovarian cancer is diagnosed only at stages III–IV.(1) For this reason diagnostics at the presymptomatic stages are crucial for successful treatment. Cancer antigen 125 (CA-125) is the most well-established marker for epithelial ovarian tumors. Measurement of serum levels of CA-125 is routinely used for primary diagnostics of ovarian cancer, as well as for treatment response monitoring and recurrence prediction.(2–5)
CA-125 is a mucine-like transmembrane glycoprotein. Its molecular weight range is 200–1000 kDa. Such heterogeneity is considered to be a result of proteolysis. Extracellular domain of CA-125 includes numerous (>60) highly conserved tandem repeats.(6) Tandem repeats are composed of 157 amino acids, and are surrounded by highly glycosylated motifs. Antibodies against CA-125 were shown to recognize two main epitope regions, OC 125 and M 11, both being localized inside tandem repeats.(7,8) First-generation immunoassays used antibodies specific to the OC 125 region (group A antibodies) as a capture MAb and as a tracer. Second-generation assays utilized antibodies against both epitopes: antibodies specific to M 11 epitope (group B antibodies) are used as a capture antibody, whereas OC 125-related antibodies are used as a tracer.(9)
Currently marketed CA-125 immunoassays show acceptable performance, however for some samples discrepancies between assay results were observed.(10) These may be due to different antibodies included in the assays. Most commercially available anti-CA-125 reagents are characterized poorly. Introduction of novel well-characterized antibodies onto the market may help to improve existing assays. Additionally utilization of locally produced antibodies may improve cost savings for cancer diagnostics in Russia.
In the present study, we describe the production and characterization of three monoclonal antibodies with two CA-125 epitope binding specificities: one antibody is specific to OC 125 epitope cluster and two antibodies have specificity to M 11 region.
Materials and Methods
Preparation of native CA-125
CA-125 was purified from supernatants of ovarian carcinoma cell line NIH:OVCAR-3 (ATCC). OVCAR-3 cells were maintained in RPMI-1640 medium (Sigma-Aldrich, Moscow, Russia) supplemented with 10% fetal bovine serum (FBS) (HyClone, GE Healthcare, Logan, UT) at 37°C in a humidified atmosphere containing 6% CO2. To collect supernatants culture medium was centrifuged at 400 g, and cells were discarded. CA-125 was purified by gel filtration on Sephacryl S-400 (GE Healthcare, Moscow, Russia) and subsequent affinity chromatography on Sepharose coupled with anti-CA-125 antibody M86306M (Meridian Life Science, Memphis, TN).
CA-125 concentration was determined using onco-IFA CA-125 assay (Alkor Bio, St.-Petersburg, Russia). Antigen purity and molecular weight were estimated using Western blotting with MAb X306 (HyTest, Turku, Finland).
Preparation of recombinant CA-125
In order to study MAb binding with isolated CA-125 epitope we prepared recombinant CA-125 repeat 11 (rCA-125, 156 amino acids). DNA fragment encoding R11 was synthesized by GenScript and subcloned in-frame with a His-tag into pQE30 vector (Qiagen, Valencia, CA) as described elsewhere.(8) Recombinant protein was expressed in E. coli M15 strain (Qiagen) and purified from lysates using His-Trap columns (GE Healthcare) under denaturing conditions.
Immunization
Four-week-old BALB/c mice were immunized with affinity purified CA-125. Antigen (20 μg) emulsified in an equal volume of complete Freund's adjuvant (Sigma-Aldrich) was injected subcutaneously in footpads. 1 month later mice were injected with 20 μg of CA-125 in incomplete Freund's adjuvant. Booster injections with 20 μg of CA-125 in normal saline were given intraperitoneally at 1-month intervals for at least 3 months. Blood was collected from the retro-orbital sinus, and antisera titers were determined by indirect ELISA.
Hybridoma production and purification of MAbs
Splenocytes collected from the mouse with the highest antisera titer were fused with Sp2/0 myeloma cells at a ratio of 1:2 in the presence of 41% PEG-1500 (Fluka) using standard protocol. Cells were then plated into 96-well plates on a feeder layer of mouse peritoneal macrophages and maintained in selective media (RPMI medium supplemented with HAT (Sigma-Aldrich), 10% FBS, 50 U/mL penicillin, 50 μg/mL streptomycin) for at least 10 days. After 10–15 days supernatants were screened for the presence of anti-CA-125 antibodies using ELISA. Cells from the positive wells were sub-cloned by limiting dilution. Established cell lines were designated as 2B6, 3C8, and 5A12.
For production of ascites BALB/c and (BALB/cxDBA/2)F1 mice were injected intraperitoneally with 0.4 mL pristane (Sigma-Aldrich) and 7–10 days later with 1–2×106 of hybridoma cells. Ascites were collected 10–14 days after injection. MAbs from ascitic fluids were purified by affinity chromatography on protein-G Sepharose (GE Healthcare) and concentration was measured by spectrophotometry at 280 nm.
Labeling of antigen and monoclonal antibodies
Affinity-purified CA-125 was biotinylated using biotin N-hydroxysuccinimide ester (Sigma-Aldrich) according to the manufacturer's instruction. Antibodies were conjugated with HRP by periodate method.(11)
ELISA
Determination of antibody titers in sera and screening of culture supernatant were performed by indirect ELISA. Maxisorp plates (Nunc, Thermo Scientific) were coated with goat anti-mouse IgG antibodies (GAM). Sera were titrated in serial five-fold dilutions; supernatants were diluted 1:2. After washing, plates were blocked with PBS containing 0.1% Nonidet P-40 and 2% non-fat dry milk. Then biotinylated CA-125 (600 IU/mL) was added. After washing, plates were incubated with streptavidin-HRP conjugate (Str-HRP) (Sigma-Aldrich) and developed with TMB substrate solution (Seramun Diagnostica GmbH, Heidesee, Germany). Reaction was stopped with HCl. Optical density was measured at 450 nm. In all ELISA variant plates were developed in the same manner.
Isotypes of MAbs were determined using Immuno-Type mouse monoclonal antibody isotyping kit (Sigma-Aldrich), according to the manufacturer's instruction.
Epitope mapping was carried out using two approaches: single- and two-step competitive ELISA.
Reference antibodies X325, X52, and X75 (M 11-like) and X306 (OC 125-like) were purchased from HyTest. In a single-step competitive assay reference MAbs or MAbs under test were immobilized at a concentration of 5 μg/mL on a microplate. After washing, biotinylated CA-125 and MAbs to compete with adsorbed antibodies for labeled antigen binding were added. All MAb combinations were tested. No antibodies were added into control wells. Plates were developed using Str-HRP reagent. Results were expressed as percent inhibition value relative to the control (biotinylated antigen binding in the absence of competitor).
In a two-step competition assay plates were coated with MAbs X52, X306, and 2B6. After washing, unlabeled CA-125 was added. After 1 h incubation, plates were washed and incubated with HRP-conjugated MAbs X306, X52, and 3C8 and unlabeled competing MAbs. Signal was developed as above. All assays were performed in triplicate.
ELISA with recombinant CA-125 was used to further compare binding properties of antibodies generated here with reference MAbs. Antibodies were incubated in rCA-125-coated plates and plates were developed with GAM-HRP.
Western blotting
To elucidate whether epitopes recognized by established MAbs are linear or conformation-dependent, Western blotting was used. Binding of MAbs with rCA 125 was confirmed using blotting as well. Samples were reduced by boiling in the presence of 2-mercaptoethanol and separated by SDS-PAGE using 7.5% gels for cell culture-derived CA-125 and 12.5% gels for recombinant protein. Proteins were transferred onto nitrocellulose membranes and blocked with PBS containing 5% BSA. Unlabeled antibody 5A12 (10 μg/mL) and antibodies X52, X306, X75, X325, 3C8, and 2B6 conjugated with HRP (1:10,000) were diluted in blocking solution. After washing, blot with MAb 5A12 was incubated with GAM-HRP. Membranes were developed with TMB staining solution (Sigma-Aldrich).
Results
Antigen purification
Native CA-125 protein was purified from supernatants of OVCAR-3 cells by subsequent gel-filtration and affinity chromatography. The resulting preparation runs on Western blotting stained with MAb X306 as a diffuse band far above 200 kDa, which indicates the removal of the majority of impurities (Fig. 1). Purified antigen was used for immunization.
FIG. 1.
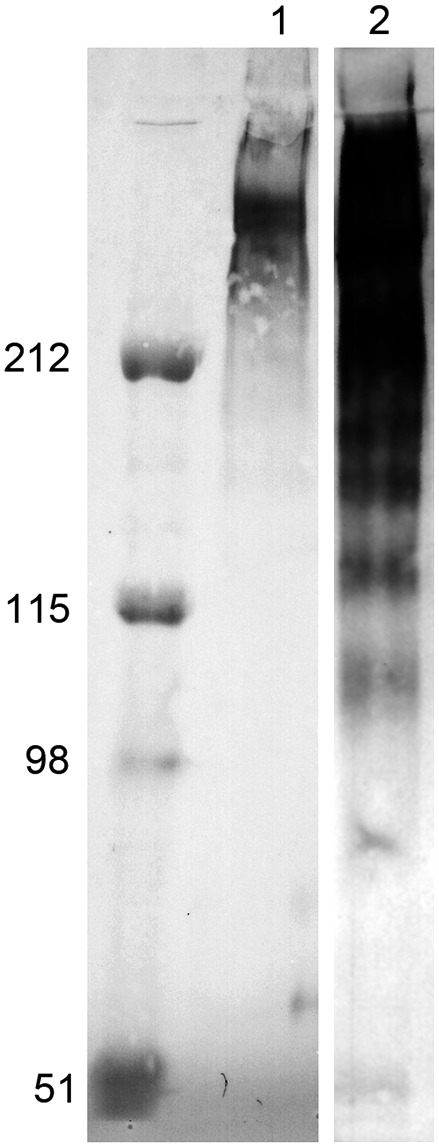
Purification of CA-125 from supernatant of OVCAR-3 cells. Supernatant was purified solely by gel filtration (lane 2) or by gel filtration and subsequent immunoaffinity chromatography (lane 1). Samples were stained by Western blot using MAb X306.
Hybridoma production
Splenocytes were derived from the animal with the highest anti-CA-125 antibodies titer (1:200,000). After fusion, screening and cloning three stable cell lines—3C8, 2B6, and 5A12—were selected for production of antibodies against CA-125. The same codes were further used for MAbs produced by the corresponding cell lines. Hybridomas 3C8 and 2B6 were shown to produce antibodies of IgG1 subtype—5A12 of IgG2a.
Epitope mapping
Epitopes recognized by established MAbs were mapped by means of competitive ELISA using four reference antibodies: X306, specific to OC 125 epitope; and X52, X325, and X72, specific to M 11 epitope. The results of single-step competitive ELISAs are summarized in Table 1.
Table 1.
One-step Competitive ELISA
| Competitor | OC 125 | M 11 | |||||
|---|---|---|---|---|---|---|---|
| Solid phase | X306 | 3C8 | 2B6 | 5A12 | X52 | X75 | X325 |
| X306 | 71±1 | 71±1 | 14±0 | −82±0 | −13±2 | −1±2 | −8±2 |
| 3C8 | 40±0 | 87±0 | 40±1 | 23±2 | 17±0 | 30±0 | 20±0 |
| 2B6 | 13±7 | 34±6 | 66±1 | 35±5 | 27±6 | 67±2 | 44±6 |
| 5A12 | −10±2 | 51±12 | 85±1 | 85±1 | 84±1 | 87±0 | 84±0 |
| X52 | 12±4 | 30±6 | 87±0 | 93±0 | 82±2 | 94±0 | 77±1 |
| X75 | 12±4 | 38±3 | 84±0 | 52±1 | 43±3 | 93±0 | 80±0 |
| X325 | 1±6 | 30±3 | 79±1 | 38±6 | 31±4 | 91±0 | 78±0 |
Inhibition of biotinylated CA-125 binding to MAb-coated solid phase by corresponding antibodies expressed as percentage relative to control (no competitor added)±standard error. Inhibition of binding >50% was assumed significant (grey cells).
Inhibition of biotinylated antigen binding for more than 50% relative to the control was assumed as a strong competition between antibodies. Antibody producing such inhibition pattern was considered to share the epitope group with the reference antibody.
MAb 3C8 strongly competed with immobilized X306 antibody (binding decreased by 71%). However, 3C8 when used as a coating antibody, did not compete with X306 significantly. 3C8 poorly inhibited binding of other MAbs with CA-125, whether on solid phase or in solution. Therefore 3C8 belongs to the OC 125-like group.
Antibody 2B6 significantly competed with all M 11-like antibodies (X52, X75, X325) adsorbed onto solid phase. Immobilized 2B6 showed low to moderate competition with M 11-like antibodies, and negligible competition with OC 125-related antibody X306. Summarizing the data, we concluded that antibody 2B6 is M 11-like.
Antibody 5A12 strongly inhibited binding of biotinylated CA-125 to X52 and, to a lesser extent, to X75 and X325 absorbed on the wells. When plates were coated with 5A12, all M 11-like reference antibodies decreased signals significantly. 5A12 did not inhibit but rather markedly increased binding of antigen with X306 in both experimental arrangements. Summarizing the data, we considered 5A12 to belong to the M 11-like antibody group.
Antibodies under test exhibited moderate competition with each other, except 5A12 on solid phase and 2B6 in solution (86%). This further suggests that 5A12 and 2B6 bind to the same epitope cluster, while 3C8 to the other.
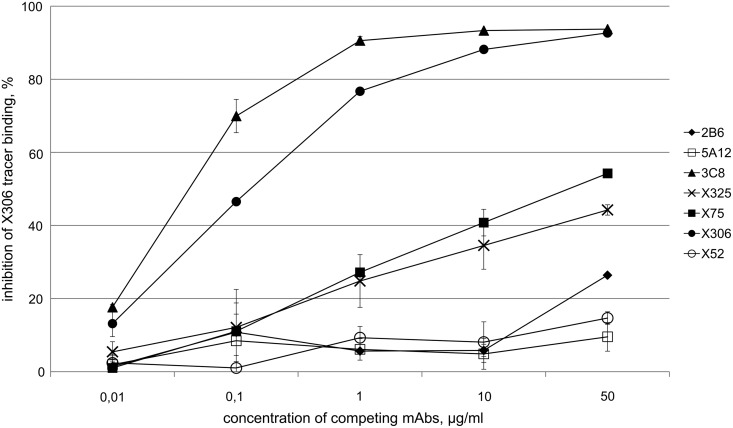
To confirm our epitope mapping results we performed a two-step competitive ELISA using three antibodies: solid phase antibody, HRP-labeled tracer antibody and competing unlabeled MAb. Competing antibodies were added in a range of concentrations.
When M 11-like reference antibody X52 was used for coating and OC 125-like X306 as a tracer, competing antibodies clearly fell into two groups: one group included all M 11-related reference antibodies and MAbs 2B6 and 5A12, whereas the second group contained X306 and 3C8 (Fig. 2). The same pattern of inhibition was seen when 2B6 was used as a coating MAb and 3C8 as a tracer (data not shown). Our results could not be explained by competition between solid phase antibody and unlabeled MAb, as M 11-like antibodies (X52, X75, and X325) did not significantly reduce binding of tracer on X52-coated plate. Thus unlabeled antibodies competed generally with the tracer, and data obtained confirm that MAb 3C8 belongs to the OC 125 group whereas 2B6 and 5A12 are M 11-like.
FIG. 2.
Two-step competitive ELISA. Solid phase was coated with MAb X52 and incubated with CA 125, labeled X306 tracer and competitors at different concentrations. Data represent the average of three replicates±standard error.
Experiments with reciprocal combinations of antibodies (OC 125-like antibody used for coating and M 11-like used as a tracer) produced no clear discrimination between antibodies (data not shown).
Binding of MAbs with recombinant CA-125
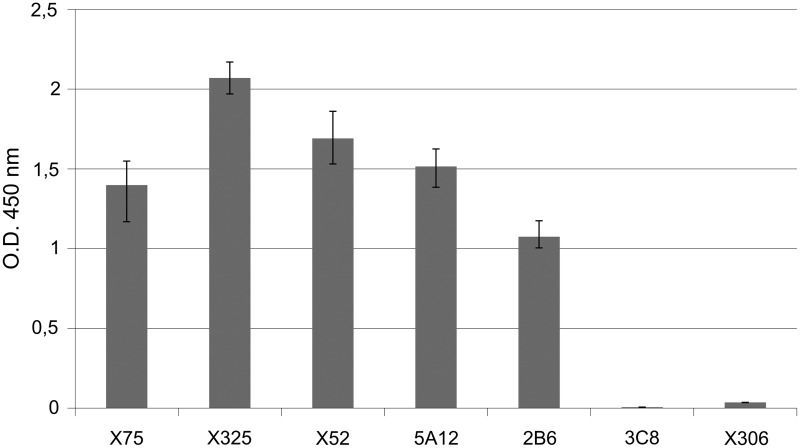
To confirm our data regarding epitope specificity of the antibodies produced, we analyzed interaction of antibodies with recombinant CA-125 tandem repeat. M 11-like reference antibodies X52, X75, and X325 were shown to bind to recombinant CA-125-coated wells. Similarly, antibodies 2B6 and 5A12, which we assigned to M 11-like group, were also shown to interact with rCA125. Reference antibody X306, as well as antibody 3C8, failed to bind to rCA-125 (Fig. 3).
FIG. 3.
Binding of HRP-conjugated MAbs with rCA-125-coated microplates (data represent optical densities measured at 450 nm (mean values±standard error).
Western blotting
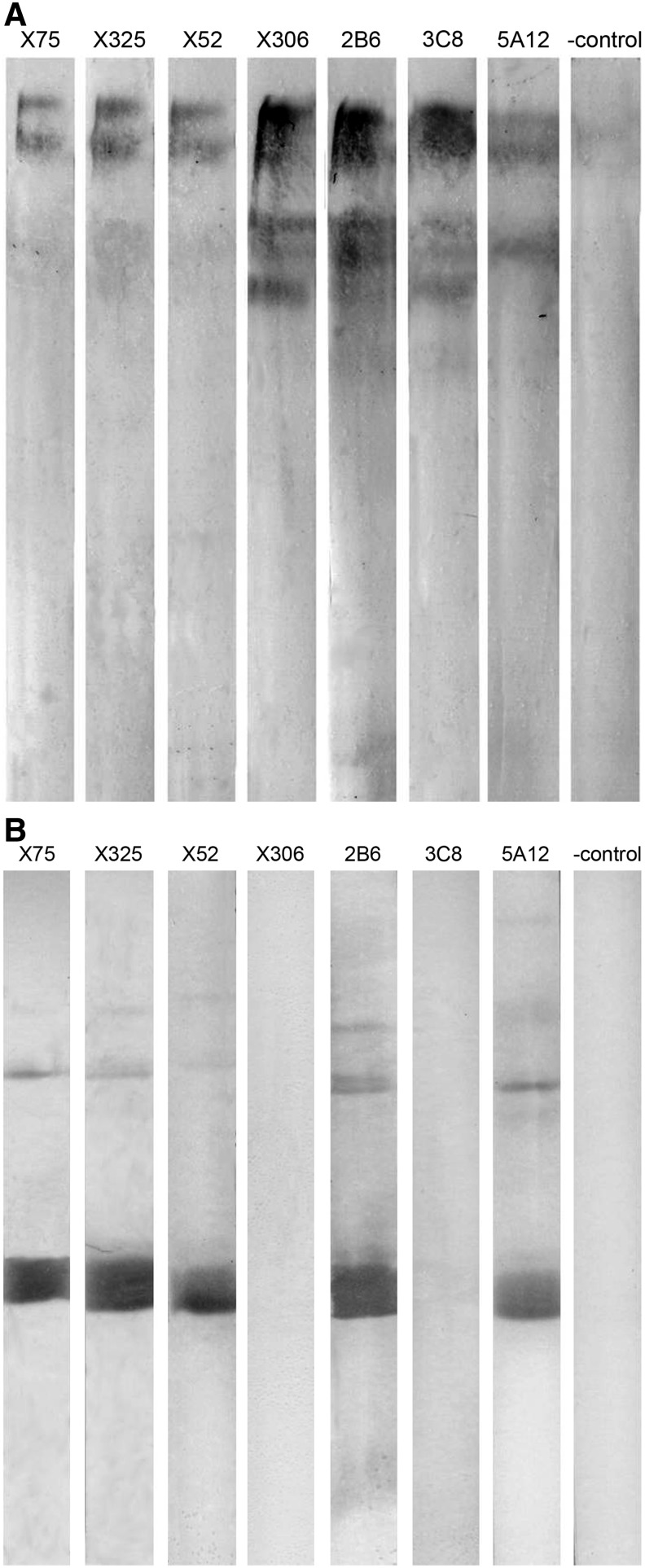
Western blot assay revealed that all the antibodies tested were able to bind with cell culture-derived denaturated CA-125 (Fig. 4A). They stained several bands of MW >200 kDa. Antibodies X306, 2B6, 3C8, and 5A12 stained additional bands of lower molecular mass, probably products of antigen degradation. This infers that all three antibodies produced here bind to conformation-independent epitopes of CA-125. At the same time, five of the MAbs (X52, X325, X75, 2B6, and 5A12) were able to bind recombinant CA-125. Antibodies X306 and 3C8 failed to produce any staining, which is in agreement with our ELISA data (Fig. 4B). Binding of 2B6 and 5A12 with rCA125 implicates that their antigenic determinants are non-glycosylated.
FIG. 4.
Western blot analysis. Native (A) and recombinant (B) CA-125 were probed with a panel of MAbs.
Discussion
CA-125 is an important marker for ovarian cancer, and many antibodies against it have been produced to date. However not many of them are commercially available. Introduction of novel well-characterized antibodies to the market may help in the improvement of existing assays and cost reduction.
Production of antibodies against CA-125 is hampered by low immunogenicity of the protein.(12) Antigens from various sources were used previously for production of antibodies against CA-125 (OVCAR-3 cells, supernatants of OVCAR-3 cells, ascitic fluids).(13,14) In our preliminary studies, we immunized animals with OVCAR-3-derived protein purified by gel filtration, OVCAR-3 crude cell extracts, commercially available antigens of human origin, and recombinant CA-125 fragment. Immunization with all of the listed substances did not lead to appropriate immune response against the antigen of interest. Introduction of affinity chromatography into purification protocol enabled us to isolate pure antigen that was able to elicit strong immune response. Moreover, affinity-purified CA-125 turned out to be more stable and suitable for labeling. This purification technique may be used to improve the quality of standard CA-125 preparations.
We used two settings of competitive ELISA to determine epitope specificity of the established antibodies. To further confirm our data, we assayed binding of antibodies with rCA-125.
In both arrangements of competitive ELISA antibody 3C8 showed significant competition with X306, but not with X52, X75, and X325 antibodies for binding of native CA-125. Similarly to X306, 3C8 showed almost no interaction with rCA-125 in ELISA and Western blotting. According to these data, antibody 3C8 may be described as OC 125-like.
The 3C8 binding profile differs greatly from that of X306. Binding of biotinylated CA-125 to X306-coated solid phase was slightly enhanced by M 11-like reference antibodies, and 5A12 augmented the binding greatly. In contrast “negative inhibition” was not shown for 3C8. A similar situation was shown by Nustad and colleagues.(15) Antibody B43.13 demonstrated greatly enhanced binding of recombinant CA-125 fragment in the presence of M 11-like antibodies. Other antibodies tested did not display negative inhibition in the presence of competitor antibodies. Authors of the TD-1 report assigned B43.13 to a separate epitope group, A3. Probably antibody X308 belongs to the same epitope group since it shares similar properties, while the 3C8 binding site lies in the other epitope subgroup. Most likely negative inhibition originates from conformational changes caused by antibody binding. Such changes may render certain epitopes more accessible for antibody binding.
Unlike the X306 antibody, 3C8 displayed moderate competition with M 11-like reference MAbs as well as with 2B6 and 5A12 (3C8 decreased tracer binding to 5A12-coated solid phase two-fold). Such results further imply that 3C8 binding site is remote from X306 and is in close proximity to M 11 epitope cluster.
Antibodies 2B6 and 5A12 displayed moderate to high competition with M 11-like reference antibodies but not with X306 in both competitive assays. Moreover similarly to M 11-like antibodies, 2B6 and 5A12 demonstrated strong binding with recombinant CA-125, as determined by ELISA and Western blotting. According to these results, we classified MAbs 2B6 and 5A12 as M 11-like. These two antibodies performed differently in single-step assay. 2B6 showed more pronounced competition with all solid phase-bound M 11-like reference MAbs. When 2B6 was adsorbed to the solid support, reference antibodies exhibited poor to moderate competition. However, 5A12 competed with solid phase-bound antibodies in a more moderate manner, and all M 11-like reference antibodies and 2B6 almost completely abrogated binding of biotinylated CA-125 to 5A12-coated solid phase. These results indicate that 2B6 likely has higher affinity to CA-125 than does 5A12.
Our two-step competitive assay has demonstrated that some combinations of solid phase-bound antibody and tracer produce clear discrimination between M 11-like and OC 125-like antibodies, while others do not. This may reflect intrinsic properties of antibodies; some of them perform better on solid phase, while others are more suitably used as a tracer. Similarly Nustad and colleagues demonstrated that antibodies have different binding capability to antigen, depending on whether the antibody is free or adsorbed.(15) Such peculiarities are to be kept in mind during development of immunoassays.
Binding of all antibodies produced with denaturated CA-125 in western blot indicates that antibodies are directed against linear epitopes. As CA-125 is prone to partial degradation, antibodies against conformation-independent determinants may be found valuable in quantitation of products of CA-125 decay with perturbed conformation.
As a result of the present study, we established three hybridomas, producing antibodies to CA-125. Our cell lines were adapted for large-scale antibody production, which is a prerequisite for their further use in diagnostic assays. Precise description of antibody properties will allow the developers of immunoassays to select the appropriate antibody pairs to optimize performance. Introduction of novel antibodies to the market may reduce costs of CA-125 assays, especially in developing countries such as Russia.
Acknowledgments
We thank A. Saenko, Dr. D. Lukyanov, and A. Krylova for excellent technical assistance in MAb and protein purification and labeling and in animal colony management; and Dr. A. Klimovich for editing the manuscript.
Author Disclosure Statement
Authors have no conflicts of interest to declare.
References
- 1.Meyer T, and Rustin CG: Role of tumour markers in monitoring epithelial ovarian cancer. Br J Cancer 2000;82:1535–1538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maggino T, and Gadducci A: Serum markers as prognostic factors in epithelial ovarian cancer: an overview. Eur J Gynaecol Oncol 2000;21: 64–69 [PubMed] [Google Scholar]
- 3.van Dalen A, Favier J, Burges A, Hasholzner U, de Bruijn HW, Dobler-Girdziunaite D, Dombi VH, Fink D, Giai M, McGing P, Harlozinska A, Kainz C, Markowska J, Molina R, Sturgeon C, Bowman C, and Einarsson R: Prognostic significance of CA 125 and TPS levels after 3 chemotherapy courses in ovarian cancer patients. Gynecol Oncol 2000;79:444–450 [DOI] [PubMed] [Google Scholar]
- 4.van Dalen A, Favier J, Hallensleben E, Burges F, Stieber P, de Bruijn HWA, Fink D, Ferrero A, McGing P, Harlozinska A, Kainz C, Markowska J, Molina R, Sturgeon C, Bowman A, Einarsson R, and Goike H: Significance of serum CA125 and TPS antigen levels for determination of overall survival after three chemotherapy courses in ovarian cancer patients during long-term follow-up. Eur J Gynaecol Oncol 2009;30:609–615 [PubMed] [Google Scholar]
- 5.Bidart JM, Thuillier F, Augereau C, Chalas J, Daver A, Jacob N, Labrousse F, and Voitot H: Kinetics of serum tumor marker concentrations and usefulness in clinical monitoring. Clin Chem 1999;45: 1695–1707 [PubMed] [Google Scholar]
- 6.Davis HM, Zurawski VR, Bast RC, and Klug TL: Characterization of the CA 125 antigen associated with human epithelial ovarian carcinomas. Cancer Res 1986;46:6143–6148 [PubMed] [Google Scholar]
- 7.Bast RC, Feeney JM, Lazarus H, Nadler LM, Colvin RB, and Knapp RC: Reactivity of a monoclonal antibody with human ovarian carcinoma. J Clin Invest 1981;68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Brien TJ, Beard JB, Underwood LJ, Dennis RA, Santin AD, and York L: The CA 125 gene: an extracellular superstructure dominated by repeat sequences. Tumour Biol 2001;22:348–366 [DOI] [PubMed] [Google Scholar]
- 9.Kenemans P, van Kamp GJ, Oehr P, and Verstraeten RA: Heterologous double-determinant immunoradiometric assay CA 125 II: reliable second-generation immunoassay for determining CA 125 in serum. Clin Chem 1993;39:2509–2513 [PubMed] [Google Scholar]
- 10.Mongia SK, Rawlins ML, Owen WE, and Roberts WL: Performance characteristics of seven automated CA 125 assays. Am J Clin Pathol 2006;125:921–927 [DOI] [PubMed] [Google Scholar]
- 11.Wisdom GB: Horseradish peroxidase labeling of antibody using periodate oxidation. In The Protein Protocols Handbook, Walker JM. (Ed). Humana Press, Totowa, 1996, pp. 273–274 [Google Scholar]
- 12.Shojaeian S, Allameh A, Zarnani AH, Chamankhah M, Ghods R, Bayat AA, and Jeddi-Tehrani M: Production and characterization of monoclonal antibodies against the extracellular domain of CA 125. Immunol Invest 2010;39:114–131 [DOI] [PubMed] [Google Scholar]
- 13.Lloyd KO, Yin BW, and Kudryashov V: Isolation and characterization of ovarian cancer antigen CA 125 using a new monoclonal antibody (VK-8): identification as a mucin-type molecule. Intl J Cancer 1997;71:842–850 [DOI] [PubMed] [Google Scholar]
- 14.O'Brien TJ, Raymond LM, Bannon GA, Ford DH, Hardardottir H, Miller FC, and Quirk JG: New monoclonal antibodies identify the glycoprotein carrying the CA 125 epitope. Am J Obstet Gyn 1991;165:1857–1864 [DOI] [PubMed] [Google Scholar]
- 15.Nustad K, Bast J, O'Brien TJ, Nilsson O, Seguin P, Suresh MR, Saga T, Nozawa S, Børmer OP, de Bruijn HWA, Nap M, Vitali A, Gadnell M, Clark J, Shigemasa K, Karlsson B, Kreutz FT, Jette D, Sakahara H, Endo K, Paus E, Warren D, Hammarström S, Kenemans P, and Hilgers J: Specificity and affinity of 26 monoclonal antibodies against the CA 125 antigen: first report from the ISOBM TD-1 Workshop. Tum Biol 1996;17:196–219 [DOI] [PubMed] [Google Scholar]