Abstract
Ex vivo human immunodeficiency virus type 1 (HIV-1) infection of human lymphoid tissue recapitulates some aspects of in vivo HIV-1 infection, including a severe depletion of CD4+ T cells and suppression of humoral immune responses to recall antigens or to polyclonal stimuli. These effects are induced by infection with X4 HIV-1 variants, whereas infection with R5 variants results in only mild depletion of CD4+ T cells and no suppression of immune responses. To study the mechanisms of suppression of immune responses in this ex vivo system, we used aldrithiol-2 (AT-2)-inactivated virions that have functional envelope glycoproteins but are not infectious and do not deplete CD4+ T cells in human lymphoid tissues ex vivo. Nevertheless, AT-2-inactivated X4 (but not R5) HIV-1 virions, even with only a brief exposure, inhibit antibody responses in human lymphoid tissue ex vivo, similarly to infectious virus. This phenomenon is mediated by soluble immunosuppressive factor(s) secreted by tissue exposed to virus.
The hallmark of human immunodeficiency virus type 1 (HIV-1) infection is the development of immunodeficiencies that lead to AIDS. Both cellular and humoral immunity are impaired in HIV-infected individuals at the advanced stages of disease (32, 46). CCR5-tropic (R5) (5) viruses generally transmit infection, while variants that use CXCR4 exclusively (X4 according to the suggested nomenclature) (5) or in addition to CCR5 (R5X4) (5) often emerge at later stages of the disease. The emergence of X4 or R5X4 HIV-1 variants frequently coincides with accelerated progression to AIDS (15, 51, 54, 55).
To study HIV-1 tissue immunopathogenesis, we have developed a culture system that permits ex vivo HIV-1 infection and analysis of human lymphoid tissues (18-20). Several in vivo aspects of HIV-1 infection are recapitulated in this system. In particular, ex vivo inoculation of blocks of human lymphoid tissue with X4 HIV-1 variants results in productive infection (without a requirement for exogenous activating stimuli), severe depletion of CD4+ T cells, and suppression of humoral immune responses, including the production of antibodies in response to recall antigens or polyclonal stimuli. R5 HIV-1 variants also productively infect lymphoid tissues ex vivo but deplete CD4+ T cells only mildly and do not suppress humoral immune responses (18-20).
The mechanisms of immunosuppression observed both in vivo and ex vivo and the contribution of various factors to disease progression are not fully understood (14). HIV-1 infection and the subsequent death of infected CD4+ T helper cells contribute to immunodeficiency, although abnormalities in T-cell-independent B-cell responses have been reported as well (3, 32, 38). Furthermore, it has been hypothesized that HIV-1 virions per se (without infection) (1, 25, 26, 28, 31) or their components (7-9, 11, 23, 24, 37, 45, 56, 57) can be harmful for lymphocytes. However, the contribution of such “indirect” mechanisms to overall viral pathogenesis remains unclear.
Here, we used our ex vivo system to study whether noninfectious virions impair immune responses in human lymphoid tissue, where critical events of HIV-1 disease occur. We demonstrate that chemically inactivated noninfectious X4, but not R5, HIV-1 virions with functional envelope glycoproteins inhibit humoral immune responses in human lymphoid tissue ex vivo. The efficiency of this inhibition was comparable to that mediated by infectious virus, but in contrast to the effects of infectious virus was not associated with depletion of CD4+ T cells. We establish that this phenomenon is mediated by soluble immunosuppressive factor(s) (ISF) secreted by tissue cells.
MATERIALS AND METHODS
Ex vivo cultures of human lymphoid tissue.
Human tonsils obtained from routine therapeutic tonsillectomy and not required for clinical purposes were delivered in phosphate-buffered saline within 6 h of excision. The specimens were trimmed of cauterized tissue, dissected into 2-mm blocks, and then cultured in RPMI 1640 medium supplemented with 15% fetal bovine serum, sodium pyruvate (1 mM; Invitrogen Life Technologies, Carlsbad, Calif.), minimal essential medium with nonessential amino acids (0.1 mM; Invitrogen) and a mixture of antibiotics atop collagen gel (Pharmacia & Upjohn Co., Kalamazoo, Mich.) at the medium-air interface (18-20).
Virus stocks and gp120.
Stocks of laboratory-adapted R5 HIV-1 variant SF162 (R5SF162) and X4 variant LAV.04 (X4LAV.04) were obtained from the NIH AIDS Research and Reference Reagent Program (ARRRP). A stock of X4 IIIB (LAI) (X4IIIB) was obtained from the AIDS Vaccine Program (AVP; National Cancer Institute, Frederick, Md.). Baculovirus-expressed glycosylated envelope gp120 from X4 strain LAV.04 that exhibits high affinity to immobilized CD4 (41) was obtained from ARRRP (catalog no. 2966).
Preparation of inactivated HIV.
Inactivated HIV-1 particles were prepared by methods described earlier (29, 33). Briefly, fresh (0.2 M) inactivation reagent was prepared by dissolving 2,2′-dithiodipyridine (aldrithiol-2 [AT-2]; Sigma Chemical Co., St. Louis, Mo.) in 100% ethanol. Freshly thawed virus suspension was mixed with AT-2 reagent (final concentration, 1 mM) and incubated at 37C for 1 h with gentle mixing every 15 min. Treated viral suspensions were ultrafiltered (Centriprep-500; Amicon, Beverly, Mass.), resulting in a 27,000-fold dilution of solutes smaller than 500 kDa and a 100-fold concentration of virions. Experiments with virus-free culture medium spiked with AT-2 showed that any residual AT-2 after dialysis and concentration did not mediate detectable effects on histoculture cell viability, HIV replication, or total IgG production (as assayed by means of trypan blue exclusion, HIV p24gag enzyme-linked immunosorbent assay [ELISA], or total IgG ELISA, respectively). AT-2 treatment eliminated detectable HIV-1 infectivity (50, 53; data not shown).
Tissue stimulation and culture maintenance.
We have previously shown that ex vivo human lymphoid tissue cultures retain the ability to respond to recall antigens by production of specific antibodies and to respond to polyclonal stimulators by upregulation of Ig production (20). For the present studies, on day 1 of culture, a mixture of the recall antigen, tetanus toxoid (TT; 0.1 μg/ml; Calbiochem, San Diego, Calif.), and the polyclonal stimulator pokeweed mitogen (PWM; 2.5 μg/ml; Sigma) was added to the culture medium for 3 days, after which the medium was changed every 3 days. Matched control tissues were not exposed to TT and PWM. For analysis of the effect of HIV-1 on the anti-TT immune response, we chose those tissues that upon challenge produced more than 150-μU/ml anti-TT IgG. Such an immune response was registered in tissues from 20 out of 43 (47%) donors, a fraction slightly lower than that reported for in vivo challenge in an immunized population (4). Tissues from 31 out of 43 total donors were subjected to polyclonal stimulation with PWM. Tissues that showed increased IgG production between days 9 and 18 of culture were selected for further analysis. This occurred in tissues from 20 out of 31 donors tested (65%). Although the majority of tissues from these 20 donors gave positive responses to both the recall antigen TT (increased tetanus-specific IgG) and to polyclonal stimulation (increased total IgG) according to the above mentioned criteria, a few of them responded only to one of the treatments.
Tissue exposure to AT-2-inactivated HIV-1 or gp120.
AT-2-inactivated HIV-1 was applied to cultures by either of two protocols. In one protocol, the tissues were continuously treated with AT-2-inactivated HIV-1, which was maintained from day 1 of culture at a constant concentration of 15-ng/ml HIV-1 p24gag equivalent (1×). This concentration is the average peak-of-replication p24gag concentration in productively infected cultures and corresponds to the very upper end of concentrations described in the plasma of HIV-1-infected patients (29). In the second protocol, tissue blocks were pulse-treated by incubation in tubes with AT-2-inactivated HIV-1 or with gp120 for 3 h. The tissue and the bathing medium of each tube were then distributed among three wells, resulting in a 12-fold dilution of inactivated virus. In several experiments, the tissues were thoroughly rinsed after incubation with virus suspension, so that no p24gag was detected in the last rinse. Control tissues were treated similarly, but were incubated in sham-treated medium instead of inactivated virus. Some of these control tissues were subsequently infected with infectious HIV-1 at 400 50% tissue culture infectious doses per block as described previously (18). Inoculation with either AT-2 or live virus was performed immediately after addition of TT and PWM to culture medium.
Analysis of IgG production and p24gag in tissue culture medium.
We assayed anti-TT IgG and total IgG in the collected medium samples by ELISA. Human anti-tetanus IgG (USP-Hyper-Tet; Miles, Inc., Elkhart, Ind.) was used as a standard, calibrated against the U.S. Control tetanus toxin, and expressed in international units. Human IgG (ICN, Costa Mesa, Calif.) was used as a standard for IgG quantitation. IgG production was expressed as a percentage of that of the control in matched tissue. HIV-1 p24gag secreted into the media of cultures productively infected with HIV-1 or exposed to AT-2-inactivated HIV-1 was measured by the p24gag antigen capture immunoassay kit (AVP, National Cancer Institute, Frederick, Md.).
Proliferation assay.
Cells were mechanically isolated from control, HIV-1-infected, or AT-2-inactivated HIV-1-exposed tissue blocks on day 3 of culture. Live cells (5 × 104), as determined by means of trypan blue exclusion, were added to each of 16 wells of a 96-well round-bottom plate (20). Cells were stimulated for 3 days with phytohemagglutinin (PHA; Sigma Chemical) or PWM and pulsed with 0.5 μCi of [3H]thymidine per well for the last 12 h of culture. Cell proliferation was measured as [3H]thymidine incorporation by means of liquid scintillation (18), and the results were expressed as a stimulation index (SI) equal to Cst/Cnst, where Cst and Cnst are the values for [3H]thymidine incorporation in cells from stimulated tissue blocks and nonstimulated tissue blocks, respectively.
Testing of virion-free conditioned medium on fresh tissues.
Freshly dissected tonsillar tissue blocks were incubated with AT-2-inactivated X4LAV.04 for 3 h or were sham treated, rinsed, and then set up in culture. The medium was collected and changed on days 3, 6, 9, and 12 after exposure to inactivated virus. Conditioned medium was filtered through a 0.22-μm-pore-size filter and then applied at a 1:3 dilution with fresh medium to new tonsil cultures challenged with TT and PWM. As a result of extensive washing, these supernatants were free of detectable viral protein or RNA (see Results). The medium was collected and changed every 3 days to assess antibody production.
Size fractionation of conditioned medium.
The conditioned media from AT-2-inactivated HIV-1 or sham-treated cultures were collected as described above and were size fractionated by serial passage through three centrifugal concentrators (Amicon) with nominal molecular weight cutoffs of 100,000, 50,000, and 30,000. Each fraction was brought back to its original volume by addition of fresh medium. The fractions as well as the original conditioned media were then applied to fresh tonsil cultures at a dilution of 1:3 with fresh medium and challenged with TT and PWM.
Isolation and culture of CD4+ T cells.
Tonsil cell suspensions were obtained by mechanical dissociation and sieving through 40-μm-pore-size filters. CD4+ T cells were isolated by magnetic bead separation with T-cell isolation kits followed by CD8+ selection (allowing CD4+ to pass through unlabeled) on AUTOMACS (Miltenyi Biotec, Auburn, Calif.). The CD4+ T-cell fraction was collected, and all other cell types were pooled together into a CD4+ T-cell-depleted fraction. CD4+ T-cell fractions were 91.8% ± 1.0% pure according to flow cytometry, a typical degree of purity for negatively immunoselected T-cell subpopulations isolated from suspensions from lymphoid tissues. The CD4+ T cells and the CD4 T-depleted cells were set up at 3 × 106 cells/ml, treated with AT-2-inactivated HIV-1 for 2 days, rinsed, and cultured. Conditioned medium from each cell fraction was collected 4 days later.
Isolation and culture of B cells.
B cells were isolated by means of magnetic bead separation using B-cell isolation kits on AUTOMACS (Miltenyi Biotec); the purity was 96.2% ± 0.6% according to flow cytometry. The B cells and the whole-cell suspensions were set up in culture at 3 × 106 cells/ml and in conditioned medium collected from tonsils treated with AT-2-inactivated LAV.04, or they were set up in control cultures at a dilution of 1:3 and activated either with Staphylococcus aureus Cowan I strain (Calbiochem) used at 0.01% (vol/vol) with 20-U/ml interleukin-2 (IL-2; Invitrogen) for B cells or with PWM for whole cells. The medium was collected and changed after 2 days of exposure to the conditioned medium and every 4 days thereafter to assess antibody production.
RESULTS
(i) AT-2-inactivated X4 but not R5 HIV-1 virions inhibit antibody production of human lymphoid tissue ex vivo.
Freshly dissected tonsillar tissue blocks were challenged with TT and/or PWM and inoculated with infectious or inactivated viruses as described in Materials and Methods. We have previously demonstrated that productive infection of lymphoid tissues by X4LAV.04 results in inhibition of antibody responses to TT and/or PWM (20). These results were confirmed here: on average, in X4LAV.04-infected tissues, anti-TT responses constituted 15% ± 6% and IgG responses to PWM were 28% ± 3% of the control (n = 10; P < 0.001). Surprisingly, exposure of the cultures to AT-2-inactivated X4LAV.04 resulted in a similar level of suppression. In tissues from 13 donors, exposure to inactivated X4LAV.04 resulted in an anti-TT response reaching on average only 17% ± 3% (P < 0.001) of that of untreated control tissue, whereas total IgG production in 17 PWM-challenged tissues was 42% ± 6% (P < 0.001) of that of the control (Fig. 1a and b). The inhibition of antibody responses by AT-2-inactivated X4LAV.04 occurs in a dose-dependent manner (Fig. 1c and d) in the range of 2 orders of magnitude. Similar levels of inhibition were observed in tissues continuously treated with inactivated virus and in tissues to which inactivated X4LAV.04 was applied only for the first 3 h of culture and removed by means of extensive washing (pulse-treated tissues) (Fig. 1 a and b). For all subsequent experiments, we pulse-treated tissues with inactivated virus.
FIG. 1.
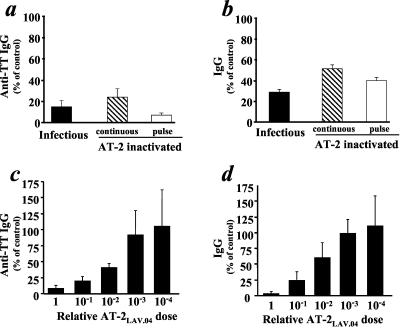
Infectious or AT-2-inactivated X4 HIV-1 variants inhibit B-cell responses in human lymphoid tissue ex vivo in a dose-dependent manner. Tissue blocks challenged with TT and PWM were inoculated with AT-2-inactivated or infectious virus X4LAV.04. Inactivated virus was applied either for the entire culture period of 15 to 18 days (continuous) or for 3 h (pulse). The antibody response was evaluated as anti-TT or total IgG (mean ± standard error) released by 27 to 36 identically treated tissue blocks from each donor and expressed as percent relative to similarly challenged matched sham-treated tissue. Concentrations of inactivated virus are indicated: 1× = 15-ng/ml p24gag, the peak concentration in the medium of productively infected tissue cultures. (a) Production of anti-TT IgG in tissues challenged with TT and inoculated with infectious X4LAV.04 or AT-2-inactivated X4LAV.04, either continuously or as a pulse (n = 10, 5, and 9 donor tissues, respectively). (b) Production of IgG in tissues challenged with PWM and inoculated with infectious X4LAV.04 or AT-2-inactivated X4 LAV.04, either continuously or as a pulse (n = 10, 10, and 5 donor tissues, respectively). (c) Production of anti-TT IgG in TT-challenged tissues inoculated with one dose (1×) of AT-2-inactivated X4LAV.04 or dilutions thereof (n = 3). (d) Production of IgG in PWM-challenged tissues inoculated with one dose (1×) of AT-2-inactivated X4LAV.04 or dilutions thereof (n = 3).
Similar to other X4 HIV-1 variants tested (18), X4IIIB infection also inhibited Ig production in TT- and PWM-challenged tissues to the level of 42 and 58% of control, respectively. AT-2-inactivated X4IIIB virions also inhibited Ig production to the levels of 38 and 55% of that of the control in pulse-treated tissues challenged with TT and PWM, respectively.
In contrast to results obtained with AT-2-inactivated X4 HIV-1, no inhibition of anti-TT IgG or total IgG was observed when stimulated tissues were exposed to comparable or larger amounts of AT-2-inactivated R5SF162 virions (Fig. 2). Inactivated R5SF162 thus behaved similarly to its infectious counterpart, which, as reported earlier (20) and confirmed in the present study, does not inhibit antibody responses in ex vivo human lymphoid tissues.
FIG. 2.
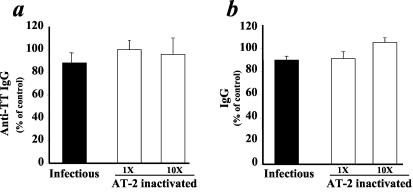
AT-2-inactivated or infectious R5 HIV-1 does not inhibit B-cell responses in human lymphoid tissue ex vivo. Tissue blocks challenged with TT and PWM were inoculated with AT-2-inactivated or infectious R5SF162 virus. The antibody response was evaluated as anti-TT or total IgG (mean ± standard error) released by 27 to 36 identically treated tissue blocks from each donor and expressed as percent relative to similarly challenged matched sham-treated tissue. Concentrations of inactivated virus are indicated: 1× = 15-ng/ml p24gag, the peak concentration in the medium of productively infected tissue cultures. (a) Production of anti-TT IgG in tissues challenged with TT and inoculated with infectious R5SF162 or AT-2-inactivated R5SF162 at 1× or 10× (n = 6, 6, and 7 donor tissues, respectively). (b) Production of IgG in tissues challenged with PWM and inoculated with infectious R5SF162 or AT-2-inactivated R5SF162 at 1× or 10× (n = 9, 9, and 7 donor tissues, respectively).
(ii) Neither denatured virions nor soluble, monomeric gp120 induces suppression of B-cell responses in human lymphoid tissue ex vivo.
To understand whether the presence of native, functional envelope glycoproteins on the virion surface, the preservation of which is a hallmark of AT-2-inactivation (2, 50), was necessary for suppression of B-cell responses from ex vivo lymphoid tissues, we denatured AT-2-inactivated virions by incubation at 100°C for 5 min. As shown in Table 1, denatured viral particles lost their ability to inhibit antibody responses in lymphoid tissues. Responses to TT and PWM stimulation in these tissues were comparable to those in sham-treated controls. Soluble monomeric gp120 also did not affect ex vivo antibody responses. We applied 1-ng/ml X4LAV.04-derived gp120 (to approximate the estimated concentration of gp120 in the intact virus preparation used in control experiments) to tissues challenged with TT and PWM (Table 1). The IgG production in gp120-treated tissues from three donors was not different from that of untreated tissue: on average, these tissues produced 89% ± 14% of anti-TT IgG and 98% ± 4% of total IgG relative to matched untreated controls. Even exposure to a 100-fold-higher concentration of gp120 did not inhibit antibody responses (102% ± 7% of control; Table 1).
TABLE 1.
Evaluation of immunosuppressive activitya
| Agent | % IgG vs control
|
|
|---|---|---|
| Anti-TT IgG | IgG | |
| Medium conditioned by AT-2 X4LAV.04-treated tissueb | 27 ± 9 | 39 ± 7 |
| Denatured medium conditioned by AT-2 X4LAV.04-treated tissuec | 100 ± 22 | 114 ± 19 |
| Denatured AT-2 X4LAV.04-virionsd | 116 ± 28 | 101 ± 4 |
| 1-ng/ml gp120e | 89 ± 14 | 98 ± 4 |
| 100-ng/ml gp120e | 102 ± 7 | |
Agents (ISF-containing conditioned medium, heat-denatured AT-2 virions, or gp120) were applied to tonsillar tissues (27 blocks from each donor tissue) challenged with TT or PWM. The immunosuppressive activity was evaluated by the level of inhibition of the immune response to TT or PWM challenge, and the results are expressed as mean percentage (± standard error) of the level of anti-TT IgG or total IgG, respectively, relative to control.
Medium (virion free) was conditioned by 27 tonsillar tissue blocks from days 6 to 9 postexposure to AT-2-inactivated X4LAV.04 or by control sham-treated blocks (n = 16 for TT and 20 for PWM).
Medium was conditioned by 27 tonsillar tissue blocks from days 6 to 9 postexposure to AT-2-inactivated X4LAV.04 tissue blocks or by control sham-treated blocks and heat-denaturated at 60°C for 30 min (n = 4).
Suspension of AT-2 inactivated X4LAV.04, heat denatured at 100°C for 5 min, was added to the tissue blocks.
LAV.04-encoded gp120 was added to the culture medium at the concentration corresponding to that in the medium of productively infected cultures at the peak of virus replication (∼1 ng/ml) or at a 100-fold-higher concentration.
(iii) In tissues with suppressed antibody responses, lymphocytes remain responsive to mitogenic stimulation.
To test whether the inhibition of Ig production described above is caused by a general impairment of lymphocytes, we evaluated their responsiveness to mitogenic stimuli. Tissue blocks pulse-exposed to AT-2-inactivated X4LAV.04 and control blocks were cultured for 3 days, and lymphocytes isolated from these blocks were then stimulated with PWM or PHA. The results shown in Fig. 3 demonstrate that there was no significant difference in mitogen-induced proliferation between lymphocytes isolated from control tissue blocks and those isolated from blocks exposed to inactivated X4LAV.04. On average, the SIs in PHA-treated lymphocytes were 47 ± 2 and 45 ± 2 for control and inactivated X4LAV.04-exposed tissue blocks, respectively. For PWM-treated lymphocytes, these measurements were 12 ± 2 and 13 ± 2, respectively.
FIG. 3.

AT-2-inactivated X4LAV.04 does not inhibit mitogenic responses of tissue lymphocytes. Cells were mechanically isolated from control or AT-2-inactivated X4LAV.04-exposed tissue blocks after 3 days and incubated for another 3 days with PWM or PHA. Cell proliferation was measured at 72 h in cultures pulsed with [3H]thymidine for the last 12 h. The results are expressed as SI (mean ± standard error of 16 replicates). Results are representative of three experiments.
(iv) Virion-free conditioned medium from immunosuppressed tissues is immunosuppressive.
Medium conditioned by tissues exposed to AT-2-inactivated X4LAV.04 inhibited anti-TT IgG and total IgG responses of fresh cultures (Table 1): they produced anti-TT IgG at levels of 27% ± 9% (P = 0.02, n = 16) and total IgG at levels of 39% ± 7% (P = 0.009, n = 20) of matched cultures treated with control conditioned medium, respectively. This conditioned medium was virion free, as evaluated from the lack of detectable p24gag or viral RNA (the detection threshold for p24gag was 3 pg/ml, and that for HIV-1 RNA was 10 copies/ml). At this level, or even at a 10-fold-higher concentration, inactivated LAV.04 does not affect the immune response to PWM and TT (data not shown). Thus, treatment of lymphoid tissue with AT-2-inactivated X4LAV.04 virus induced the secretion of ISF. This factor(s) was inactivated by heat: when conditioned medium from tissues exposed to AT-2-inactivated X4LAV.04 was heated to 60°C for 30 min, the ability to inhibit antibody production of fresh PWM-challenged tonsil cultures was lost. Levels of anti-TT IgG and total IgG production by tissues incubated with heat-denaturated control medium were similar (100% ± 22% and 114% ± 19%, respectively; n = 6) to those of tissues incubated with heat-inactivated medium conditioned by matched tissues exposed to AT-2-inactivated virus (Table 1). Before heating, these samples of conditioned medium inhibited anti-TT IgG and total IgG production to levels of 40% ± 20% and 38% ± 11%, respectively, of that of control. In contrast, freezing and thawing did not affect the immunosuppressive activity of the factor. Thus, the ISF produced by AT-2-inactivated HIV-1-exposed tissues is heat labile but freeze/thaw resistant.
Next, we evaluated the time course of ISF production. At different time points, we collected conditioned medium from tissues exposed to AT-2-inactivated X4LAV.04 and measured their immunosuppressive activity on fresh tonsil tissues challenged with TT and PWM. Immunosuppressive activity was present in all collected samples of conditioned medium (Fig. 4).
FIG. 4.
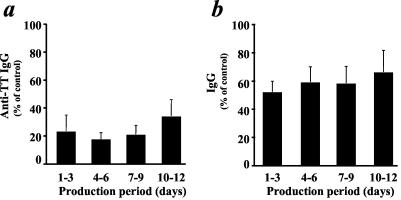
Production of ISF by tissue exposed to AT-2-inactivated X4LAV.04. Medium was conditioned by tissue blocks exposed to AT-2-inactivated X4LAV.04 for the indicated periods (days 1 to 3, 4 to 6, 7 to 9, and 10 to 12 postexposure). The conditioned media were tested for immunosuppressive activity on fresh tonsil cultures challenged with TT and PWM. Anti-TT IgG (a) and total IgG (b) are expressed as percent relative to similarly challenged matched tissue treated with conditioned medium samples (mean ± standard error, n = 3).
To evaluate the size of this factor, or factor-containing complex, virion-free conditioned medium was size fractionated and each fraction was applied separately to TT- and PWM-challenged tissue. As expected, unfractionated medium conditioned by tissues treated with AT-2-inactivated LAV.04 was immunosuppressive: it inhibited anti-TT IgG and total IgG production of TT- and PWM-challenged tissues to levels of 15% ± 9% and 23% ± 7%, respectively, relative to control conditioned medium (n = 4) (Fig. 5). Fractions containing molecules with molecular masses of >100 kDa and between 100 and 50 kDa were also immunosuppressive: relative to similar fractions isolated from control conditioned medium, they inhibited anti-TT IgG production to levels of 19% ± 10% and 47% ± 24%, respectively, and IgG production to levels of 45% ± 17% and 42% ± 6%, respectively. In contrast, responses by cultures treated with fractions containing molecules of 50 to 30 kDa or <30 kDa were not inhibited: they produced anti-TT IgG (77% ± 34% and 83% ± 16%, respectively) and total IgG (109% ± 39% and 115% ± 16%, respectively) relative to similar fractions isolated from control conditioned medium. Thus, the inhibitory activity from the media conditioned by tissue exposed to AT-2-inactivated LAV.04 is associated with the fraction containing molecules with a molecular mass of >50 kDa.
FIG. 5.
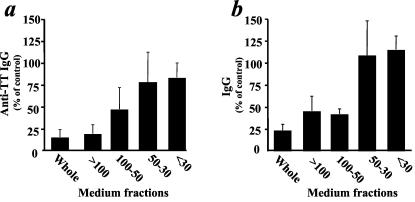
Immunosuppressive activity of size-fractionated medium conditioned by AT-2-inactivated X4LAV.04-exposed tissue. Medium conditioned by tissue blocks exposed to AT-2-inactivated X4LAV.04 was collected between days 6 and 9 postexposure and size fractionated with centrifugal concentrators. Immunosuppressive activity of each fraction was evaluated on fresh tonsil cultures challenged with TT and PWM. Anti-TT IgG(a) and total IgG (b) are expressed as percents relative to those of similarly challenged matched tissue treated with correspondent fractions of control conditioned medium (mean ± standard error of the mean; n = 4).
(v) Soluble immunosuppressive factor is produced by CD4+ T cells.
Tonsillar cells were separated in fractions enriched for or depleted of CD4+ T cells by immunoaffinity methods. Each of these fractions was divided into two portions: one of each was used as a matched control, and the other was treated with AT-2-inactivated X4LAV.04 for 2 days. Then, virion-free medium conditioned by these fractionated cell populations was collected between days 2 and 6 and tested for suppression of IgG production in PWM-challenged tissues. Medium conditioned by the CD4+ T-cell-enriched fraction incubated with AT-2-inactivated X4LAV.04 suppressed IgG production of PWM-challenged tissue to the level of 73% ± 8% (n = 7, P = 0.03) relative to the matched control medium. The supernatants of cultures of the CD4+ T-cell-depleted fraction exposed to AT-2-inactivated X4LAV.04 did not inhibit responses: the tissues treated with medium conditioned by this cell fraction produced IgG at a level similar to that of tissues treated with matched control medium (116% ± 5%; n = 7; P = 0.81).
(vi) B cells are directly affected by the immunosuppressive factor(s).
Finally, we asked whether the ISF secreted by tissues treated with AT-2-inactivated X4LAV.04 would affect B cells isolated from the tissue. To address this question, we incubated total tissue cell suspensions or a B-cell-enriched fraction with medium conditioned by tissue treated with AT-2-inactivated X4LAV.04. Medium conditioned with inactivated X4LAV.04-treated cultures mildly but significantly suppressed production of IgG in isolated cells: unfractionated cells produced IgG at the level of 56% ± 11% (P = 0.001; n = 13), and B cells produced IgG at the level of 71% ± 9% (P = 0.006; n = 13) relative to matched control medium (medium conditioned by sham-treated tonsillar blocks).
DISCUSSION
Despite more than two decades of research, the mechanisms underlying the cellular and humoral immunodeficiencies that characterize AIDS remain unclear. Both direct and indirect mechanisms contributing to viral pathogenesis have been proposed. Experimental data on the postulated mechanisms of HIV-induced immunodeficiency come largely from in vitro experiments with isolated cells, whereas the in vivo milieu is very different. In an attempt to replicate it, at least in part, we have developed a system for studying HIV biology in human lymphoid tissues cultured ex vivo. Such cultures of lymphoid tissue retain the multiple cell populations and cytoarchitectural microenvironment of in vivo lymphoid tissues and are fully permissive for HIV replication by both X4 and R5 HIV-1, without a requirement for exogenous stimulation (18-20). In the experiments that we report here, we used this ex vivo system to study potential mechanisms of HIV-induced humoral immunodeficiency.
Numerous B-cell abnormalities have been described in HIV-infected patients since 1983 (32, 46). Infection of CD4+ T cells and their depletion compromises T-cell help to B cells (3, 32, 38). Also, in patients, HIV infection perturbs B-cell responsiveness to CD4+ T-cell help (39, 40). Both T-cell-dependent and -independent abnormalities in B-cell function have been attributed to ongoing viral replication (39, 42, 44). Here, we evaluated the possible contribution of noninfectious HIV virions to the impairment of B-cell responses observed in human lymphoid tissues infected ex vivo with HIV-1. The vast majority of HIV-1 virions circulating in HIV-1-infected patients are not detectably infectious (12, 48), making it more likely for cells to interact with noninfectious particles than with their infectious counterparts. For this study, we used HIV-1 virions rendered noninfectious by treatment with AT-2. These AT-2-treated virions retain both the structure and function of the viral envelope glycoproteins, allowing authentic interactions with a variety of target cells, but are not infectious (6, 16, 17, 33, 36, 50). Consistent with prior observations in vitro and in vivo (34, 50; J. Lifson, J. L. Rossio, M. Piatak, J. Bess, E. Chertova, D. K. Schneider, V. J. Coalter, B. Poore, R. F. Kiser, R. J. Imming, A. J. Scarzello, L. E. Henderson, V. M. Hirsch, R. C. Desrosiers, R. E. Benveniste, and L. O. Arthur, submitted for publication), AT-2-inactivated HIV-1 was not detectably infectious when inoculated in ex vivo cultures of human lymphoid tissues. Also, in human tissues ex vivo, in contrast to suspension cultures of peripheral blood mononuclear cells (13), treatment with AT-2-inactivated HIV-1 did not induce CD4+ T-cell loss (53). However, here, we report that despite the absence of appreciable depletion of CD4+ T cells, these inactivated HIV-1 virions impair the ability of ex vivo human lymphoid tissue to produce IgG in response to stimulation.
Infectious HIV-1 variants differentially affect immune responses in human lymphoid tissue ex vivo depending on whether they are of the X4 or R5 phenotype. Infection with X4 but not R5 HIV-1 inhibits production of antibodies by tissues challenged with recall antigens (diphtheria toxoid or TT) as well as by those challenged with polyclonal stimulators (18-20). Infection of ex vivo human lymphoid tissues by X4 HIV-1 variants severely depletes the total CD4+ T-cell population, whereas R5 HIV-1 isolates deplete CD4+ T cells only mildly, reflecting the relative abundance of their respective target cells (21). Our present results with AT-2-inactivated virions recapitulate the effect on B-cell responses seen with the corresponding infectious viruses. AT-2-inactivated X4 HIV-1 virions potently suppressed antibody responses to both recall antigen and to polyclonal stimulation, albeit in contrast to infectious X4 virus, without CD4+ T-cell depletion. Similarly to infectious R5 HIV-1, AT-2-inactivated R5 virions did not inhibit antibody responses even when applied continuously or at a concentration 1,000-fold-greater than that used for inactivated X4 virus. Since inactivation of both R5 and X4 was done in parallel, these results clearly demonstrate that the inhibitory effect is not caused by the inactivation procedure. While the concentrations of AT-2-inactivated virus used for these studies are relatively high in comparison to plasma virus levels found in infected patients, they are comparable to levels observed with productive infection of lymphoid tissues ex vivo in our culture system, suggesting that these concentrations may be in the range that is obtained in infected lymphoid tissues in vivo.
Experiments performed with various concentrations of inactivated virus indicate that immunosuppression is dose dependent, which may suggest that the more targets the virus accesses, the greater the inhibition. Since the inoculated AT-2-treated virus fuses with cells (50) but does not replicate and is applied only for the first 3 h, the initial interaction of virus with cells seems to be sufficient to trigger the secretion of the ISF.
Thus, neither productive viral infection nor CD4+ T-cell depletion seems to be necessary to mediate HIV-induced inhibition of antibody production in human lymphoid tissue ex vivo. The inhibition of B-cell responses demonstrated here seemed to be dependent on the maintenance of native X4 virion structure, since neither baculovirus-expressed gp120 nor heat-denatured AT-2-inactivated X4 virus inhibited immune responses of ex vivo human lymphoid tissue. Moreover, the virus itself is only required to trigger suppression of B-cell responses; its suppressive activity is maintained without its continuous presence and can be transferred by (virion-free) conditioned medium. (Control experiments demonstrated that even if trace amounts of residual inactivated virus were still present in this conditioned medium, the levels present were insufficient to mediate the inhibitory effects we observed.)
We conclude that medium conditioned by tissue incubated with AT-2-inactivated X4 HIV-1 contains soluble factors that are responsible for suppression of B-cell responses. These factors are heat labile but tolerate freezing and thawing. Several cytokines are known to play a role in B-cell immune responses (47), and their depletion may suppress these responses. However, such an effect should not be transferred by the medium conditioned by HIV-infected tissue. Blocks of ex vivo tissues secrete large amounts of IL-6 and IL-8 and detectable amounts of IL-1β, IL-16, gamma interferon-inducible protein 10, gamma interferon, and tumor necrosis factor alpha (22, 27), and HIV-1 infection does not alter the spectrum of these cytokines. Recently we have shown that X4 but not R5 HIV-1 infection of human lymphoid tissue ex vivo upregulates CC chemokines and SDF-1 (27). These chemokines do not affect B-cell responses (data not shown) and are of small molecular weight, whereas, our preliminary results on size fractionation of supernatants containing suppressive activity indicate that this putative factor is larger than 50 kDa and is thus not compatible with chemokines and cytokines affecting B-cell immune response. However, ISF may be of smaller size but associated with carriers. Only the ultimate isolation and definitive identification of this factor, objectives beyond the scope of the present experiments, will answer the main questions regarding the mechanism of its action.
The study reported here was restricted to the demonstration that noninfectious HIV-1 can suppress B-cell responses in human lymphoid tissues ex vivo and that this effect is mediated by a (soluble) factor secreted by HIV-treated tissues. Nevertheless, some basic aspects of ISF action can be studied prior to its isolation. In particular, we attempted to determine which cells are sensitive to and which cells are the main producers of ISF. Our experiments showed that ISF contained in the conditioned medium from HIV-1-infected tissue blocks directly affects Ig production by B cells, without affecting their proliferative capacity. However, the much less potent inhibition of pure B cells relative to the unfractionated cell populations likely indicates that other cells enhance the effect of ISF. Fractionation studies also demonstrated that cells enriched for CD4+ T cells are the main producers of ISF, while tissue cells depleted of CD4+ T lymphocytes do not produce ISF. However, also in this case, the medium conditioned by the intact lymphoid tissue showed greater suppressive activity than medium conditioned by suspension cultures of cells isolated from this tissue, once again suggesting that cell-cell interactions in the context of the intact tissue architecture are important for optimal ISF production. Thus, tissue integrity seems to be important both for production of ISF and for its efficiency in suppressing B-cell responses.
In tissues in vivo, HIV-1 infection can modulate immune functions in multiple ways. In HIV-infected individuals, B cells typically show signs of both hyperactivity and depression. Hyperactivation has been reported in the form of hypergammaglobulinemia (32, 46), spontaneous secretion of Igs in culture (30), and increased expression of activation markers (35, 49), while B-cell depression includes decreased antibody production following immunizations or in vitro stimulation of B cells with antigens or mitogens (32, 43, 46). In our ex vivo tissue system, productive infection with R5 viruses recapitulates some aspects of hyperactivation, whereas productive infection with X4 viruses results in the suppression of B-cell responses (20). Thus, both ex vivo and in vivo studies suggest that HIV-1 replication is associated with some aspects of B-cell dysfunction and there is a clear link between high levels of viremia and B-cell dysfunction (10, 39, 42). Our current experiments show that noninfectious virions may also contribute to impairment of B-cell function and that this effect is associated with virions of the X4 phenotype. The impairment of B-cell function by X4 HIV-1 may be mediated by CD4+ T cells, which upon interactions with the virions produce soluble factors. This immunosuppression becomes evident before HIV kills CD4+ T cells, as shown earlier (20), and does not require actual infection, as shown here. Moreover, short interaction with inactivated virions seems to be sufficient to trigger ISF production. Since these virions generated immunosuppressive activity in a dose-dependent manner, it is consistent with the idea that the more target cells virions interact with the larger the amount of secreted ISF. This may also explain why neither infectious R5 HIV-1 nor inactivated R5 virions trigger detectable immunosuppression. The number of targets for R5 HIV-1 in tonsillar tissue is approximately 10-fold less than the number of X4 HIV-1 targets (21), and their amount in tissues might not be sufficient to produce detectable levels of ISF upon R5 HIV-1 inoculation. Alternatively virion interactions with CCR5 may not trigger production of ISF at all. The above hypotheses can be directly tested when ISF is identified. Whichever the mechanisms of ISF induction, our work indicates that noninfectious HIV-1 virions are immunosuppressive.
Since the majority of the virions circulating in vivo are not detectably infectious, one can speculate that some of these defective HIV-1 virions may suppress immune responses, similar to what we have shown for AT-2-inactivated virus. This may contribute to various defective B-cell functions, such as loss of anti-Gag responses and decreased responsiveness to vaccination typically observed at the late stages of HIV-1 infection when X4 variants may emerge (10, 32, 52).
In summary, our results demonstrate conclusively that HIV-1 can inhibit immune responses by human lymphoid tissue ex vivo, in the absence of viral replication and CD4+ T-cell death. This inhibition is mediated by a (soluble) factor(s) produced by HIV-challenged tissue. If such a mechanism operates in HIV-infected individuals, identification and isolation of this factor(s) and elucidation of the exact mechanism(s) by which it inhibits humoral immune responses may help to understand the immunopathogenesis of HIV-1 infection and AIDS and may suggest avenues for therapeutic intervention.
Acknowledgments
The work of J.D.L. is supported in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract N01-CO-12400.
REFERENCES
- 1.Amadori, A., G. P. Faulkner-Valle, A. De Rossi, P. Zanovello, D. Collavo, and L. Chieco-Bianchi. 1988. HIV-mediated immunodepression: in vitro inhibition of T-lymphocyte proliferative response by ultraviolet-inactivated virus. Clin. Immunol. Immunopathol. 46:37-54. [DOI] [PubMed] [Google Scholar]
- 2.Arthur, L. O., J. W. Bess, Jr., E. N. Chertova, J. L. Rossio, M. T. Esser, R. E. Benveniste, L. E. Henderson, and J. D. Lifson. 1998. Chemical inactivation of retroviral infectivity by targeting nucleocapsid protein zinc fingers: a candidate SIV vaccine. AIDS Res. Hum. Retrovir. 14(Suppl. 3):S311-S319. [PubMed] [Google Scholar]
- 3.Ballet, J. J., G. Sulcebe, L. J. Couderc, F. Danon, C. Rabian, M. Lathrop, J. P. Clauvel, and M. Seligmann. 1987. Impaired anti-pneumococcal antibody response in patients with AIDS-related persistent generalized lymphadenopathy. Clin. Exp. Immunol. 68:479-487. [PMC free article] [PubMed] [Google Scholar]
- 4.Barbi, M., M. R. Biffi, S. Binda, M. Clerici-Schoeller, G. Ferraris, C. Luraschi, P. Masella, P. Mazzoni, A. Pozzi, F. Pregliasco, et al. 1992. Immunization in children with HIV seropositivity at birth: antibody response to polio vaccine and tetanus toxoid. AIDS 6:1465-1469. [PubMed] [Google Scholar]
- 5.Berger, E. A., R. W. Doms, E. M. Fenyo, B. T. Korber, D. R. Littman, J. P. Moore, Q. J. Sattentau, H. Schuitemaker, J. Sodroski, and R. A. Weiss. 1998. A new classification for HIV-1. Nature 391:240. [DOI] [PubMed] [Google Scholar]
- 6.Buseyne, F., S. Le Gall, C. Boccaccio, J. P. Abastado, J. D. Lifson, L. O. Arthur, Y. Riviere, J. M. Heard, and O. Schwartz. 2001. MHC-I-restricted presentation of HIV-1 virion antigens without viral replication. Nat. Med. 7:344-349. [DOI] [PubMed] [Google Scholar]
- 7.Capobianchi, M. R. 1996. Induction of lymphomonocyte activation by HIV-1 glycoprotein gp120. Possible role in AIDS pathogenesis. J. Biol. Regul. Homeost. Agents 10:83-91. [PubMed] [Google Scholar]
- 8.Chen, Y. H., A. Christiansen, and M. P. Dierich. 1995. HIV-1 gp41 selectively inhibits spontaneous cell proliferation of human cell lines and mitogen- and recall antigen-induced lymphocyte proliferation. Immunol. Lett. 48:39-44. [DOI] [PubMed] [Google Scholar]
- 9.Chirmule, N., and S. Pahwa. 1996. Envelope glycoproteins of human immunodeficiency virus type 1: profound influences on immune functions. Microbiol. Rev. 60:386-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conge, A. M., K. Tarte, J. Reynes, M. Segondy, J. Gerfaux, M. Zembala, and J. P. Vendrell. 1998. Impairment of B-lymphocyte differentiation induced by dual triggering of the B-cell antigen receptor and CD40 in advanced HIV-1-disease. AIDS 12:1437-1449. [DOI] [PubMed] [Google Scholar]
- 11.Denner, J., C. Persin, T. Vogel, D. Haustein, S. Norley, and R. Kurth. 1996. The immunosuppressive peptide of HIV-1 inhibits T and B lymphocyte stimulation. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 12:442-450. [DOI] [PubMed] [Google Scholar]
- 12.Dimitrov, D. S., R. L. Willey, H. Sato, L. J. Chang, R. Blumenthal, and M. A. Martin. 1993. Quantitation of human immunodeficiency virus type 1 infection kinetics. J. Virol. 67:2182-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Esser, M. T., J. W. Bess, Jr., K. Suryanarayana, E. Chertova, D. Marti, M. Carrington, L. O. Arthur, and J. D. Lifson. 2001. Partial activation and induction of apoptosis in CD4+ and CD8+ T lymphocytes by conformationally authentic noninfectious human immunodeficiency virus type 1. J. Virol. 75:1152-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fauci, A. S., G. Pantaleo, S. Stanley, and D. Weissman. 1996. Immunopathogenic mechanisms of HIV infection. Ann. Intern. Med. 124:654-663. [DOI] [PubMed] [Google Scholar]
- 15.Fenyo, E. M. 1995. HIV biological phenotype: prognosis marker for transmission, disease progression and therapy. J. Biol. Regul. Homeost. Agents 9:88-90. [PubMed] [Google Scholar]
- 16.Frank, I., M. Piatak, Jr., H. Stoessel, N. Romani, D. Bonnyay, J. D. Lifson, and M. Pope. 2002. Infectious and whole inactivated simian immunodeficiency viruses interact similarly with primate dendritic cells (DCs): differential intracellular fate of virions in mature and immature DCs. J. Virol. 76:2936-2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frank, I., J. J. Santos, E. Mehlhop, L. Villamide-Herrera, C. Santisteban, A. Gettie, R. Ignatius, J. D. Lifson, and M. Pope. 2003. Presentation of exogenous whole inactivated simian immunodeficiency virus by mature dendritic cells induces CD4+ and CD8+ T-cell responses. J. Acquired Immune Defic. Syndr. 34:7-19. [DOI] [PubMed] [Google Scholar]
- 18.Glushakova, S., B. Baibakov, L. B. Margolis, and J. Zimmerberg. 1995. Infection of human tonsil histocultures: a model for HIV pathogenesis. Nat. Med. 1:1320-1322. [DOI] [PubMed] [Google Scholar]
- 19.Glushakova, S., B. Baibakov, J. Zimmerberg, and L. B. Margolis. 1997. Experimental HIV infection of human lymphoid tissue: correlation of CD4+ T cell depletion and virus syncytium-inducing/non-syncytium-inducing phenotype in histocultures inoculated with laboratory strains and patient isolates of HIV type 1. AIDS Res. Hum. Retrovir. 13:461-471. [DOI] [PubMed] [Google Scholar]
- 20.Glushakova, S., J. C. Grivel, W. Fitzgerald, A. Sylwester, J. Zimmerberg, and L. B. Margolis. 1998. Evidence for the HIV-1 phenotype switch as a causal factor in acquired immunodeficiency. Nat. Med. 4:346-349. [DOI] [PubMed] [Google Scholar]
- 21.Grivel, J. C., and L. B. Margolis. 1999. CCR5- and CXCR4-tropic HIV-1 are equally cytopathic for their T-cell targets in human lymphoid tissue. Nat. Med. 5:344-346. [DOI] [PubMed] [Google Scholar]
- 22.Grivel, J.-C., F. Santoro, S. Chen, G. Fagá, M. S. Malnati, Y. Ito, L. Margolis, and P. Lusso. 2003. Pathogenic effects of human herpesvirus 6 in human lymphoid tissue ex vivo. J. Virol. 77:8280-8289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gutheil, W. G., M. Subramanyam, G. R. Flentke, D. G. Sanford, E. Munoz, B. T. Huber, and W. W. Bachovchin. 1994. Human immunodeficiency virus 1 Tat binds to dipeptidyl aminopeptidase IV (CD26): a possible mechanism for Tat's immunosuppressive activity. Proc. Natl. Acad. Sci. USA 91:6594-6598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haraguchi, S., R. A. Good, G. J. Cianciolo, R. W. Engelman, and N. K. Day. 1997. Immunosuppressive retroviral peptides: immunopathological implications for immunosuppressive influences of retroviral infections. J. Leukoc. Biol. 61:654-666. [DOI] [PubMed] [Google Scholar]
- 25.Herbein, G., U. Mahlknecht, F. Batliwalla, P. Gregersen, T. Pappas, J. Butler, W. A. O'Brien, and E. Verdin. 1998. Apoptosis of CD8+ T cells is mediated by macrophages through interaction of HIV gp120 with chemokine receptor CXCR4. Nature 395:189-194. [DOI] [PubMed] [Google Scholar]
- 26.Hofmann, B., E. Langhoff, B. O. Lindhardt, N. Odum, J. J. Hyldig-Nielsen, L. P. Ryder, P. Platz, B. K. Jakobsen, K. Bendtzen, N. Jacobsen, et al. 1989. Investigation of immunosuppressive properties of inactivated human immunodeficiency virus and possible neutralization of this effect by some patient sera. Cell Immunol. 121:336-348. [DOI] [PubMed] [Google Scholar]
- 27.Ito, Y., J.-C. Grivel, S. Chen, Y. Kiselyeva, P. Reichelderfer, and P. Margolis. 2004. CXCR4-tropic HIV-1 suppresses replication of CCR5-tropic HIV-1 in human lymphoid tissue by selective induction of CC-chemokines. J. Infect. Dis. 189:506-514. [DOI] [PubMed] [Google Scholar]
- 28.Kameoka, M., T. Kimura, Y.-H. Zheng, S. Suzuki, K. Fujinaga, R. B. Luftig, and K. Ikuta. 1997. Protease-defective, gp120-containing human immunodeficiency virus type 1 particles induce apoptosis more efficiently than does wild-type virus or recombinant gp120 protein in healthy donor-derived peripheral blood T cells. J. Clin. Microbiol. 35:41-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kappes, J. C., M. S. Saag, G. M. Shaw, B. H. Hahn, P. Chopra, S. Chen, E. A. Emini, R. McFarland, L. C. Yang, M. Piatak, Jr., et al. 1995. Assessment of antiretroviral therapy by plasma viral load testing: standard and ICD HIV-1 p24 antigen and viral RNA (QC-PCR) assays compared. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 10:139-149. [DOI] [PubMed] [Google Scholar]
- 30.Katz, I. R., S. E. Krown, B. Safai, H. F. Oettgen, and M. K. Hoffmann. 1986. Antigen-specific and polyclonal B-cell responses in patients with acquired immunodeficiency disease syndrome. Clin. Immunol. Immunopathol. 39:359-367. [DOI] [PubMed] [Google Scholar]
- 31.Kinter, A. L., C. A. Umscheid, J. Arthos, C. Cicala, Y. Lin, R. Jackson, E. Donoghue, L. Ehler, J. Adelsberger, R. L. Rabin, and A. S. Fauci. 2003. HIV envelope induces virus expression from resting CD4+ T cells isolated from HIV-infected individuals in the absence of markers of cellular activation or apoptosis. J. Immunol. 170:2449-2455. [DOI] [PubMed] [Google Scholar]
- 32.Lane, H. C., H. Masur, L. C. Edgar, G. Whalen, A. H. Rook, and A. S. Fauci. 1983. Abnormalities of B-cell activation and immunoregulation in patients with the acquired immunodeficiency syndrome. N. Engl. J. Med. 309:453-458. [DOI] [PubMed] [Google Scholar]
- 33.Larsson, M., J. F. Fonteneau, M. Lirvall, P. Haslett, J. D. Lifson, and N. Bhardwaj. 2002. Activation of HIV-1 specific CD4 and CD8 T cells by human dendritic cells: roles for cross-presentation and non-infectious HIV-1 virus. AIDS 16:1319-1329. [DOI] [PubMed] [Google Scholar]
- 34.Lifson, J. D., M. Piatak, Jr., J. L. Rossio, J. Bess, Jr., E. Chertova, D. Schneider, R. Kiser, V. Coalter, B. Poore, R. Imming, R. C. Desrosiers, L. E. Henderson, and L. O. Arthur. 2002. Whole inactivated SIV virion vaccines with functional envelope glycoproteins: safety, immunogenicity, and activity against intrarectal challenge. J. Med. Primatol. 31:205-216. [DOI] [PubMed] [Google Scholar]
- 35.Martinez-Maza, O., E. Crabb, R. T. Mitsuyasu, J. L. Fahey, and J. V. Giorgi. 1987. Infection with the human immunodeficiency virus (HIV) is associated with an in vivo increase in B lymphocyte activation and immaturity. J. Immunol. 138:3720-3724. [PubMed] [Google Scholar]
- 36.Mehlhop, E., L. A. Villamide, I. Frank, A. Gettie, C. Santisteban, D. Messmer, R. Ignatius, J. D. Lifson, and M. Pope. 2002. Enhanced in vitro stimulation of rhesus macaque dendritic cells for activation of SIV-specific T cell responses. J. Immunol. Methods 260:219-234. [DOI] [PubMed] [Google Scholar]
- 37.Meyaard, L., S. A. Otto, H. Schuitemaker, and F. Miedema. 1992. Effects of HIV-1 Tat protein on human T cell proliferation. Eur. J. Immunol. 22:2729-2732. [DOI] [PubMed] [Google Scholar]
- 38.Miedema, F., A. J. Petit, F. G. Terpstra, J. K. Schattenkerk, F. de Wolf, B. J. Al, M. Roos, J. M. Lange, S. A. Danner, J. Goudsmit, et al. 1988. Immunological abnormalities in human immunodeficiency virus (HIV)-infected asymptomatic homosexual men. HIV affects the immune system before CD4+ T helper cell depletion occurs. J. Clin. Investig. 82:1908-1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moir, S., A. Malaspina, K. M. Ogwaro, E. T. Donoghue, C. W. Hallahan, L. A. Ehler, S. Liu, J. Adelsberger, R. Lapointe, P. Hwu, M. Baseler, J. M. Orenstein, T. W. Chun, J. A. Mican, and A. S. Fauci. 2001. HIV-1 induces phenotypic and functional perturbations of B cells in chronically infected individuals. Proc. Natl. Acad. Sci. USA 98:10362-10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moir, S., K. M. Ogwaro, A. Malaspina, J. Vasquez, E. T. Donoghue, C. W. Hallahan, S. Liu, L. A. Ehler, M. A. Planta, S. Kottilil, T. W. Chun, and A. S. Fauci. 2003. Perturbations in B cell responsiveness to CD4+ T cell help in HIV-infected individuals. Proc. Natl. Acad. Sci. USA 100:6057-6062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moore, J. P., J. A. McKeating, I. M. Jones, P. E. Stephens, G. Clements, S. Thomson, and R. A. Weiss. 1990. Characterization of recombinant gp120 and gp160 from HIV-1: binding to monoclonal antibodies and soluble CD4. AIDS 4:307-315. [DOI] [PubMed] [Google Scholar]
- 42.Morris, L., J. M. Binley, B. A. Clas, S. Bonhoeffer, T. P. Astill, R. Kost, A. Hurley, Y. Cao, M. Markowitz, D. D. Ho, and J. P. Moore. 1998. HIV-1 antigen-specific and -nonspecific B cell responses are sensitive to combination antiretroviral therapy. J. Exp. Med. 188:233-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nicholson, J. K., J. S. McDougal, T. J. Spira, G. D. Cross, B. M. Jones, and E. L. Reinherz. 1984. Immunoregulatory subsets of the T helper and T suppressor cell populations in homosexual men with chronic unexplained lymphadenopathy. J. Clin. Investig. 73:191-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Notermans, D. W., J. J. de Jong, J. Goudsmit, M. Bakker, M. T. Roos, L. Nijholt, J. Cremers, J. A. Hellings, S. A. Danner, and A. de Ronde. 2001. Potent antiretroviral therapy initiates normalization of hypergammaglobulinemia and a decline in HIV type 1-specific antibody responses. AIDS Res. Hum. Retrovir. 17:1003-1008. [DOI] [PubMed] [Google Scholar]
- 45.Pahwa, S., R. Pahwa, C. Saxinger, R. C. Gallo, and R. A. Good. 1985. Influence of the human T-lymphotropic virus/lymphadenopathy-associated virus on functions of human lymphocytes: evidence for immunosuppressive effects and polyclonal B-cell activation by banded viral preparations. Proc. Natl. Acad. Sci. USA 82:8198-8202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pahwa, S. G., M. T. Quilop, M. Lange, R. N. Pahwa, and M. H. Grieco. 1984. Defective B-lymphocyte function in homosexual men in relation to the acquired immunodeficiency syndrome. Ann. Intern. Med. 101:757-763. [DOI] [PubMed] [Google Scholar]
- 47.Paul, W. E., and R. A. Seder. 1994. Lymphocyte responses and cytokines. Cell 76:241-251. [DOI] [PubMed] [Google Scholar]
- 48.Piatak, M., Jr., M. S. Saag, L. C. Yang, S. J. Clark, J. C. Kappes, K. C. Luk, B. H. Hahn, G. M. Shaw, and J. D. Lifson. 1993. High levels of HIV-1 in plasma during all stages of infection determined by competitive PCR. Science 259:1749-1754. [DOI] [PubMed] [Google Scholar]
- 49.Rosenberg, Y. J., M. G. Lewis, and M. H. Kosco-Vilbois. 1998. Enhanced follicular dendritic cell-B cell interaction in HIV and SIV infections and its potential role in polyclonal B cell activation. Dev. Immunol. 6:61-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rossio, J. L., M. T. Esser, K. Suryanarayana, D. K. Schneider, J. W. Bess, Jr., G. M. Vasquez, T. A. Wiltrout, E. Chertova, M. K. Grimes, Q. Sattentau, L. O. Arthur, L. E. Henderson, and J. D. Lifson. 1998. Inactivation of human immunodeficiency virus type 1 infectivity with preservation of conformational and functional integrity of virion surface proteins. J. Virol. 72:7992-8001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schuitemaker, H., M. Koot, N. A. Kootstra, M. W. Dercksen, R. E. de Goede, R. P. van Steenwijk, J. M. A. Lange, J. K. M. E. Schattenkerk, F. Miedema, and M. Tersmette. 1992. Biological phenotype of human immunodeficiency virus type 1 clones at different stages of infection: progression of disease is associated with a shift from monocytotropic to T-cell-tropic virus populations. J. Virol. 66:1354-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Steinhoff, M. C., B. S. Auerbach, K. E. Nelson, D. Vlahov, R. L. Becker, N. M. Graham, D. H. Schwartz, A. H. Lucas, and R. E. Chaisson. 1991. Antibody responses to Haemophilus influenzae type B vaccines in men with human immunodeficiency virus infection. N. Engl. J. Med. 325:1837-1842. [DOI] [PubMed] [Google Scholar]
- 53.Sylwester, A. W., J. C. Grivel, W. Fitzgerald, J. L. Rossio, J. D. Lifson, and L. B. Margolis. 1998. CD4+ T-lymphocyte depletion in human lymphoid tissue ex vivo is not induced by noninfectious human immunodeficiency virus type 1 virions. J. Virol. 72:9345-9347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tersmette, M., J. M. Lange, R. E. de Goede, F. de Wolf, J. K. Eeftink-Schattenkerk, P. T. Schellekens, R. A. Coutinho, J. G. Huisman, J. Goudsmit, and F. Miedema. 1989. Association between biological properties of human immunodeficiency virus variants and risk for AIDS and AIDS mortality. Lancet i:983-985. [DOI] [PubMed] [Google Scholar]
- 55.Tscherning, C., A. Alaeus, R. Fredriksson, A. Bjorndal, H. Deng, D. R. Littman, E. M. Fenyo, and J. Albert. 1998. Differences in chemokine coreceptor usage between genetic subtypes of HIV-1. Virology 241:181-188. [DOI] [PubMed] [Google Scholar]
- 56.Viscidi, R. P., K. Mayur, H. M. Lederman, and A. D. Frankel. 1989. Inhibition of antigen-induced lymphocyte proliferation by Tat protein from HIV-1. Science 246:1606-1608. [DOI] [PubMed] [Google Scholar]
- 57.Wang, H., P. Nishanian, and J. L. Fahey. 1995. Characterization of immune suppression by a synthetic HIV gp41 peptide. Cell Immunol. 161:236-243. [DOI] [PubMed] [Google Scholar]


