Abstract
Since the enactment of Environmental Protection Act in 1989 and Department of Biotechnology (DBT) guidelines to deal with genetically modified organisms, India has embarked on establishing various levels of biosafety laboratories to deal with highly infectious and pathogenic organisms. Occurrence of outbreaks due to rapidly spreading respiratory and haemorrhagic fever causing viruses has caused an urgency to create a safe laboratory environment. This has thus become a mandate, not only to protect laboratory workers, but also to protect the environment and community. In India, technology and science are progressing rapidly. Several BSL-3 [=high containment] laboratories are in the planning or execution phase, to tackle biosafety issues involved in handling highly infectious disease agents required for basic research and diagnosis. In most of the developing countries, the awareness about biocontainment has increased but planning, designing, constructing and operating BSL-3 laboratories need regular updates about the design and construction of facilities and clear definition of risk groups and their handling which should be in harmony with the latest international practices.
This article describes the major steps involved in the process of construction of a BSL-3 laboratory in Indian settings, from freezing the concept of proposal to operationalization phase. The key to success of this kind of project is strong institutional commitment to biosafety norms, adequate fund availability, careful planning and designing, hiring good construction agency, monitoring by experienced consultancy agency and involvement of scientific and engineering personnel with biocontainment experience in the process.
Keywords: Biosafety, biosecurity, BSL-3, construction, containment, laboratory, operation, validation
Introduction
Biosafety is a major concern in every biomedical and medical setting across the world. The need of instituting biosafety and biosecurity norms/measures is increasing due to the possible dual use of microbial pathogens and increasing awareness. Laboratory associated infections (LAI) have caused diseases and fatal outcome to laboratory workers due to absence or failure of any one of the four basic controls (Engineering, Personal Protective Equipment, Standard Operating Procedure and Administrative Controls) of biosafety1,2,3. In India, apex bodies like Indian Council of Medical Research (ICMR), Council of Scientific and Industrial Research (CSIR), Department of Biotechnology (DBT), Indian Council of Agricultural Research (ICAR), and Department of Science and Technology (DST) are playing important role in biomedical research and public health. These agencies have played a leading role in evolving codes of conduct, ethics and biosafety practices.
Under the Indian Environment (Protection) Act of 1986, the Government has the power to take all the safety measures as it deems necessary for the purpose of protecting and improving the quality of the environment and preventing, controlling and abating damage to the environment, including laying down procedures and safeguards for handling hazardous substances, and carrying out and sponsoring investigations and research relating to problems of environmental pollution4. The Government enacted in 1986 rules for the manufacture, use, import, export and storage of hazardous microorganisms, genetically engineered organisms or cells. Under these rules, competent authorities have been identified to ensure implementation of the provisions of the Act and to provide guidelines on ethical and social responsibilities of scientists, institutions, industries, who conduct research, and of those who conduct, fund, administer and regulate work in the area of biological sciences5. Under this some of very important bodies such as Recombinant DNA Advisory Committee (RDAC), Institute Biosafety Committee (IBSC), Review Committee on Genetic Manipulation (RCGM), Genetic Engineering Approval Committee (GEAC), under the Ministry of Environment, Forest and Wildlife have been constituted, and some important guidelines such as Safety guidelines, by the Department of Biotechnology (1990)6,7, the Drug Policy of 20028, and National Seeds Policy, 20029 have been formulated which ensure that all biomedical research and genetically engineered crops/varieties are tested for environment safety and bio-safety before their commercial release. India has been exercising control over the export of Special Chemicals, Organisms, Materials, Equipment and Technologies (SCOMET), which also includes microorganisms/toxins, including bacteria, fungi, parasites, viruses, plant pathogens and genetically modified organisms. Conditions have been specified for export of SCOMET items, including requirement of a license10. In 2002, the DBT has developed Ethical Policies on the Human Genome, Genetic Research and Services11. The Indian Council of Medical Research under the Ministry of Health and Family Welfare, has developed code of conduct for scientists engaged in biomedical research. The Ethical Guidelines developed in 2000 for the biomedical researchers are consistent with the Declaration of Helsinki, adopted by the World Medical Assembly in 1964, and amended in October 2006 based on principles of autonomy, privacy, justice and equity12.
ICMR laboratories have a major role in supporting public health and hospital care settings in India by establishing better diagnostic facilities for detection of emerging diseases. ICMR has a mandate to focus on early detection and research on emerging and re-emerging and newly emerging highly pathogenic infectious diseases. Its regional laboratories are also involved in combating endemic diseases, outbreak situations, and national emergencies of many highly infectious and zoonotic diseases of public health importance.
The Department of Health Research (DHR) has launched a programme of establishing a network of 160 virology laboratories throughout the country in 12th Five Year Plan. ICMR network and the institutes supported by the DHR have already established sixteen BSL-2/BSL-3 laboratories to deal with many pathogenic agents of public health importance. To achieve this goal, ICMR and a number of other leading institutes are in the process of establishing several biosafety level-3 (BSL-3) laboratories at strategic locations as per XII Plan document (2012-2017), DHR, Government of India1. In the upcoming laboratories, trained scientific, technical and engineering staff with proper knowledge of biosafety principles and practices will be required in large numbers.
This article summarizes the core concept for the establishment of a BSL-3 laboartory, in various phases up to validation and functionality of the facility. Such facilities will not only lead to a reduction in the occupational exposures to pathogenic material but will also ensure safe environment by facilitating early detection of high risk groups of emerging infectious diseases.
Classification of laboratories on biosafety levels
The concept of developing such laboratories resides within the principles of biosafety and biosecurity. Biosafety is achieved by implementing various degrees of laboratory control and containment, through laboratory design and access restrictions, professional expertise and training, use of containment equipment, and safe methods of managing infectious materials in a laboratory setting. On the other hand, biosecurity involves ‘securing” or limiting access to the facilities, research materials and information.
The lowest level, biosafety level 1 (BSL-1) laboratory is essentially a teaching laboratory that may include research involving well-characterized agents not known to consistently cause any disease in immunocompetent adult humans, and pose minimal potential hazard to laboratory personnel and the environment. Work can be performed on open-bench with good laboratory practices, aseptic techniques, and proper waste disposal; no containment facility is required.
Biosafety level 2 (BSL-2) laboratory involves working with agents that pose moderate hazards to personnel and the environment. Basic laboratory with restricted access and containment during certain processes (i.e. aerosols, large volumes, etc.) is required. Use of autoclaves and biological safety cabinets is desired. Use of good laboratory practices, safe waste disposal measures, and aseptic techniques are mandatory. Usually non-respiratory, non-lethal agents are handled in BSL-2 laboratory.
Biosafety level 3 (BSL-3) is applicable to clinical, diagnostic, teaching, research, or production facilities where work is performed with agents that may cause serious or potentially lethal disease through inhalation, to the personnel, and may contaminate the environment. It requires that laboratory personnel receives specific training in handling pathogenic and potentially lethal agents, and be supervised by scientists competent in handling infectious agents and associated procedures. All work is performed in biocontained environments using appropriate engineering controls.
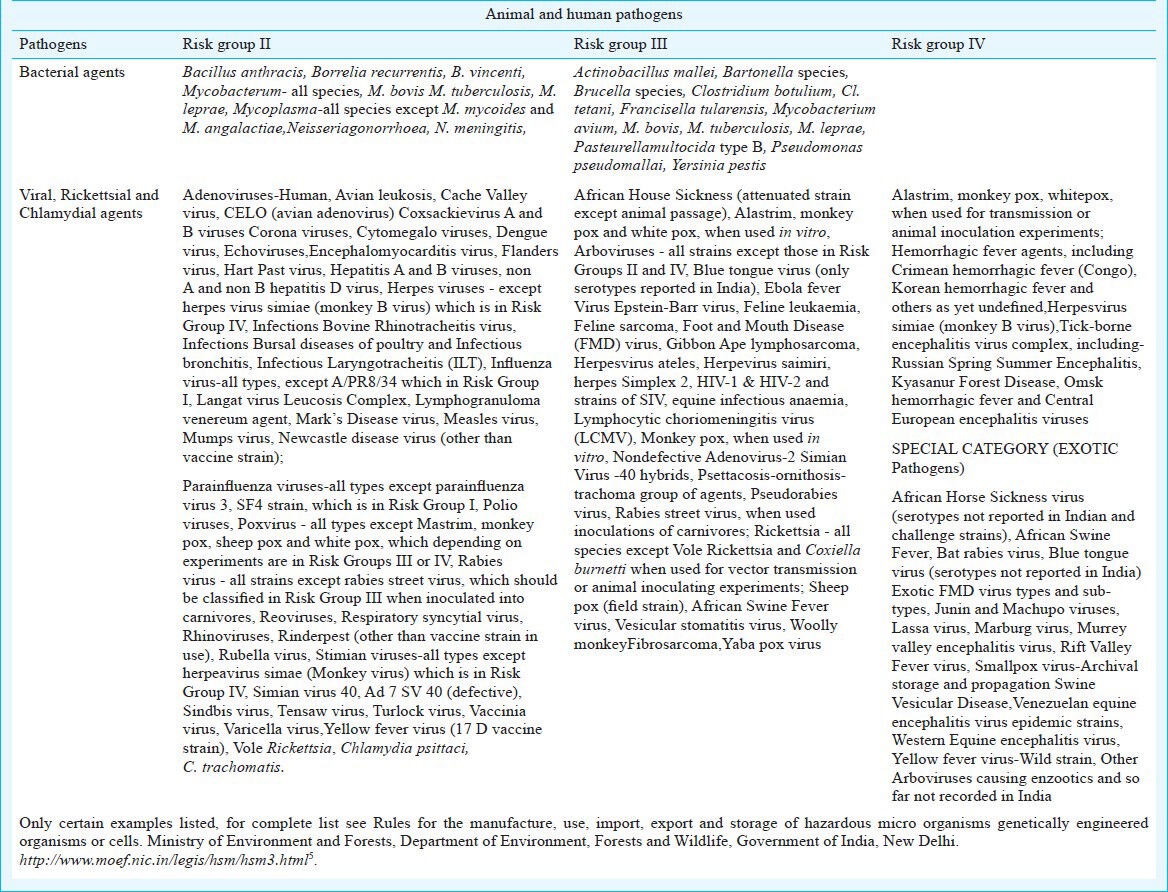
Biosafety level 4 (BSL-4), the highest level, is required for working with dangerous and exotic infectious agents that pose a high individual as well as environment risk of life-threatening disease, aerosol transmission, or a related agent with unknown risk of transmission. Laboratory personnel receive specific training in handling pathogenic and potentially lethal agents, and have to mandatorily work wearing positive pressure BSL-4 suits. As per the guidelines of the Ministry of Environment & Forests, India, various animal pathogens and plant pests are classified and defined in G.S.R. 1037(E) conferred by sections 6, 8 and 25 of the Environment (Protection) Act, 1986 (29 of 1986) with a view to protect the environment, nature and health, in connection with the application of gene-technology and microorganisms4. Some of the high risk group pathogens are listed in the Table5.
Table.
Hazardous microorganisms rules published in the Gazette No. 6215
The milestone steps in construction of a BSL-3 laboratory are pre-design assessment of risk involved in proposed research or clinical activity, designing, construction, commissioning, validation, operation and maintenance as shown in Box-1. This is also applicable to BSL-2 laboratories.
Box 1.
Action plan for the establishment of BSL-3 laboratory
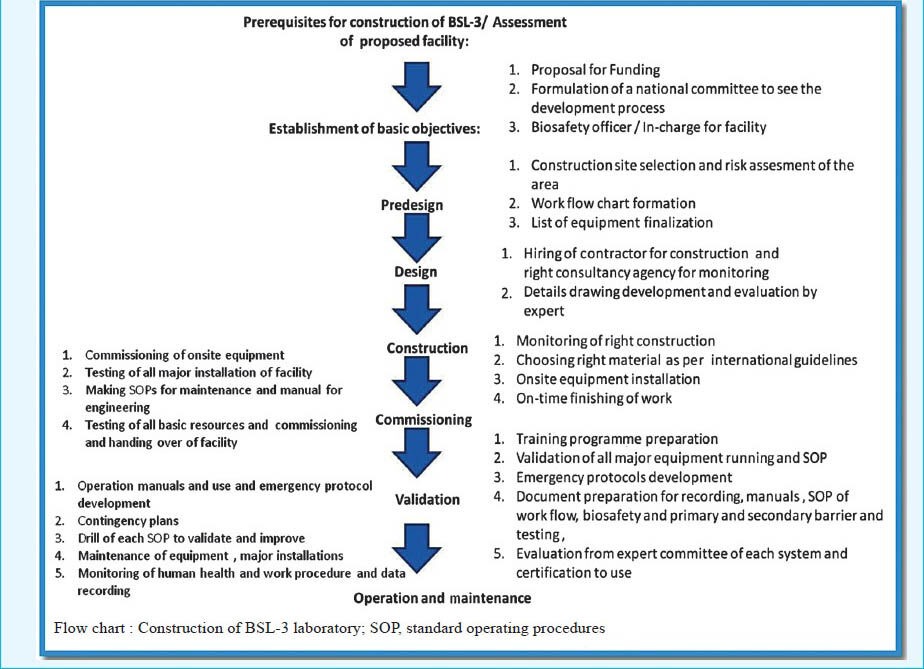
I. Pre-requisites for construction
A clear and detailed outline of scientific framework, objectives, pre-requisites (manpower, space and funding), plan for proper utilization of the facilities should be made before preparing conceptual proposal. An assessment of scientific strength including available BSL-2 laboratories and biosafety strength of existing working group is required for establishing goals and dimensions of the work. Proposed preliminary flowchart of laboratory work should be defined to accomplish objectives of the laboratory. One of the important prerequisite is specifying the need of engineering controls to accomplish the desired task. Laboratory protocols should be developed to identify the areas where biosafety can be breached or compromised using existing standard operating procedures (SOP), administrative and personal protective equipments (PPE) control, or engineering control of existing laboratory. This will provide information as to which type of laboratory, modular construction or reinforced cement concrete (RCC) type construction would be suitable for the proposed work. While deciding this, both advantages and disadvantages should be carefully evaluated in terms of funding, sustainability and man- power for managing such a facility. In both the cases selection of site for establishing BSL-3 is important and the preference should be given to the work practices encompassing engineering controls, which will be sustainable and long lasting.
If the proposed work can be performed in the enhanced BSL-2 laboratory, following BSL-3 work practices, the proposal should be for modifying the existing BSL-2 laboratory.
As per the scientific tasks and biosafety requirements, the BSL-3 laboratories can be categorized as standard level or BSL-3 plus level. However, the common essential features of BSL-3 laboratory include unidirectional air flow using room pressure gradients of negative pressure, exhaust air being HEPA (high efficiency particulate air) filtered and proper procedures for disposal of biomedical waste. Measures of waste disposal and effluent decontamination should be followed as per the guidelines of biosafety of containment laboratories. The air discharged from the containment laboratories is passed through exhaust HEPA filters which are capable of filtering 0.3 micron air-borne particles with an efficiency of 99.97 per cent. These measures are common for all types of BSL-3 containment laboratories irrespective of their designs14.
(i) Personnel decontamination: As a primary practice, the first step should be protective clothing (outer scrubs/apron removal) followed by washing of hands and other exposed areas with 0.5 per cent solution or intermediate detergent solution. Any kind of splashes on body of personnel should be immediately treated with sufficient amount of water from a nearby eye-wash station. For large quantities of spill/splashes on skin or clothing, an emergency body shower with disinfectant soap and water is to be utilized immediately.
(ii) Solid waste disposal: Solid biomedical waste generated from laboratories which includes gloves, soiled gauze pads, cotton, etc. should be soaked in 2.5 per cent solution of sodium hypochlorite and 0.25N NaOH for 16 h or more. Also if the laboratory has an autoclave facility, the biohazardous laboratory waste should be autoclaved at 121°C at 15 psi pressure for 20 min for complete decontamination; it can then be disposed off in accordance with the State/local pollution control bodies requirements.
(iii) Liquid effluents: Toxic liquid effluents generated from the BSL-3 laboratories should be decontaminated with a 1:1 (v/v) mixture of 2.5 per cent sodium hypochlorite and 0.25N NaOH, mixed well and kept for 8 h. Also, if the laboratory has an autoclave facility, the liquid effluent can be autoclaved at 121°C at 15 psi pressure for 20 min through a specific liquid cycle in the autoclave. This decontaminated effluent can then be disposed off in accordance with the State/local pollution control bodies requirements.
(iv) Equipment/Work surfaces: For most toxins and chemicals, 0.5 per cent sodium hypochlorite solution is an effective decontaminant. Additionally, one should read carefully the Material Safety Data Sheet (MSDS) for the appropriate decontaminant for a particular toxin/chemical used.
(v) Glassware: All the contaminated glasswares should be soaked in a mixture of 2.5 per cent sodium hypochlorite and 0.25N NaOH solution for 8 h. Alternatively, glasswares can also be soaked in 5 per cent sodium hypochlorite solution for 8 h.
Different types of BSL-3 have been described and some important features are summarized below, which can be considered while planning for that type of BSL-3 laboratory:
BSL-3 laboratory with anteroom or workroom as an access zone: A simplest BSL-3 facility includes a two-space facility with an entry door from an access internal laboratory corridor into an anteroom, which can serve as an access zone for the BSL-3 laboratory of the facility. This anteroom can serve for clothes changing/donning PPE, supply storage and other functions that support the work in the BSL-3 laboratory. This model of a BSL-3 facility generally does not have a dedicated autoclave within it. The discarded infectious biomedical waste generated in this kind of BSL-3 laboratories is surface decontaminated/sprayed with an intermediate-level liquid disinfectant (e.g. lyzol spray), or sealed in biohazard plastic bags and transported to the nearest autoclave in the facility by trained personnel. However, storage of frozen agents in the anteroom has been found to be acceptable by many organizations. This model is popular due to the low cost of construction and the ease of downgrading it for a BSL-2 tissue culture work when BSL-3 controls are not required. This type of simple BSL-3 laboratory execution is generally found where small-scale sporadic BSL-3 work is anticipated.
BSL-3 laboratory with restricted corridor as an access zone: This model provides access to BSL-3 spaces directly from the laboratory corridor that has been closed to other traffic. The first door is encountered after entering the corridor and the BSL-3 space begins when entering the rooms. This model is often used when there are large number of small separate BSL-3 scientific programmes in execution. This model should be considered when upgrading existing laboratory space to BSL-3 level and to reduce the cost of construction involved.
BSL-3 laboratory with BSL-2 laboratory as access zone: A modification to the above model is to enlarge the anteroom into a working BSL-2 laboratory and provide small BSL-3 modules as an extension. This model works well for isolation rooms and small functional laboratories in clinical laboratory settings.
BSL-3 laboratory suite: The larger suite concept works well for more complex multi-functional BSL-3 laboratories, used for large volume testing, identification and large research programmes dedicated to studying BSL-3 agents. An anteroom leads into the suite with a BSL-3 workroom. This workroom functions as a preparation area for dedicated purpose such as tissue culture, serology and molecular biology laboratory. Additional rooms can be dedicated for equipment. This segregation can increase safety and effectiveness by creating good workflow in the BSL-3 laboratory. This type of facility often includes dunk tank-cum-pass box and double door autoclave to allow decontamination to take place prior to hazardous material leaving the facility.
Enhanced BSL-3 laboratories: In certain instances, it may be necessary to add some special features which are not specified in the international guidelines. Based on the requirement, this can increase environmental protection using HEPA filtration of exhaust air, effluent decontamination or chemical kill tank, personnel shower in the changing area, etc. Inner and outer change rooms for showers, if provided, allow easy entry and exit protocols for the laboratory personnel. Considerations should include lockers for personnel effects, shelving for gloves, gowns, masks and booties or jump suits and positive air pressure respirators.
Biosafety cabinets (BSC) and decontamination autoclaves are essential part of such a containment facility, although autoclaves are not required to be located within BSL-3 laboratories, but the laboratories with significant amounts of hazardous waste should consider providing pass through autoclaves to decontaminate materials leaving the facility. Exhaust ventilation should be provided above the exterior door of the autoclave to remove the heat and steam when the door is opened. The types of BSCs needed in the facility should be decided based on the risk assessment performed on the work protocols and agents to be handled. Similarly, rodent proofing of laboratory and installing air curtains particularly in the laboratories which would be dealing with arthropod-borne diseases, are essential features.
Animal Biosafety Level-3 Laboratory (ABSL-3)
Animal holding space may be incorporated in BSL-3 facilities. Whenever animals are handled in such facilities, some specific needs as per the animal usages have to be incorporated. Generally, the requirement of small laboratory animals (rabbit, mouse, guinea pigs) and birds (chickens) can be handled easily by placing individual ventilated cages (IVCs) to prevent aerosols generated by animal care from entering the general room environment. Also, requirement of large animals may be restricted to lower primates. In such a scenario, the most important parameters to be maintained are sound, vibrations, temperature and humidity, and air ventilation rates. Besides, the outlets or exit air ducts have to be provided with double washable pre-filter to block skin flaxes, feather and hair which quickly block the HEPA filters. Depending on the agents used, the large animal handling facility requires many more modifications. Animal facility often contains separate personal showers and change room2,15,19.
Establishment of basic objectives, workflow, SOPs and evaluation for need of a BSL-3 laboratory
The basic objectives for establishment of containment laboratories for handling Risk Group-III agents normally include: handling of clinical samples (seasonal or during outbreaks), which may be caused by high-risk pathogenic organisms; creating safe environment for detection, identification, propagation and manipulation of such organisms in the laboratory; and maintaining safety of the community and the environment. Besides these, there are other national and international criteria like procedures involving propagation of Risk Group-II agents in larger volumes, procedures involving potential aerosol infection risks and DNA recombination technology, which might lead to higher virulence of Risk Group-II agents, suggests use of BSL-3 laboratory and work practices. Once goals and objectives are set, the flowcharts of proposed work practices can be established. This exercise would help in planning the physical facility and engineering controls for the risk assessed for the proposed work practices.
Institutional site to be selected for construction of a new stand-alone facility should have adequate and consistent utilities like electricity and water, good connectivity to airport and three-tier security protection. The risk analysis based on geographical data of the selected site should be performed to ensure that it is out of high risk zone for earthquakes. Similarly, the possibility of damage to the site during the occurrence of landslides and heavy rains should also be assessed. Two meters of excavation of the proposed construction plot and examination of “Borehole logs” and soil analysis can provide information on suitability of the construction.
II. Milestone steps to proceed for constructing a BSL-3 laboratory
The milestone steps in early construction phase include drawing development phase, preparation of construction document, final specifications, finding the right construction agency/contractor and awarding the tender to agency, hiring a consultant agency for supervising the quality of construction as per the requirement, and commissioning. The most important part of the project is to constitute a Project Implementary Committee (PIC). Project-team members should be selected carefully so that accountability and authority resides with this committee. It is important that project-team recognizes the amount of work required before the laboratory design is begun. The most Important member of the PIC should be the person who will have overall responsibility for the functioning and safety of the laboratory. When the establishment of BSL-3 is decided in any region or part of the country, availability of persons with knowledge and experience should be assessed. It is advisable to place a few people from scientific and engineering background for a few months in any working BSL-3 laboratory, so that they get exposure and experience in a functional laboratory. However, all the project team members should familiarize themselves with the available information applicable to their project. Team members should meet frequently at least once every month. This will help the committee to evolve criteria for the proposed BSL-3 laboratory, based on the specific laboratory priorities and requirements.
Drawing development phase, drawing evaluation and channelization of documents for funding
Complete action plan for constructing a BSL-3 laboratory should be crystallized during the planning of the proposed laboratory. In this phase, a detailed flowchart of the construction work must be chalked out and a schematic drawing be prepared to enable detailed planning. This should be followed by a detailed drawing of the development of the entire facility which will help in monitoring the progress of work. Hiring qualified and experienced architects and engineers to prepare design and construction leads to a successful and timely completion of the project. An important aspect in the construction of the BSL-3 laboratory is putting forward the conceptual proposal with detailed drawings and plans, justifying the objectives for the construction as per the requirements and also abiding with the guidelines of biosafety.
An evaluation of the prepared drawings should be done by an expert committee, which should include biosafety experts, engineers and architects to review whether the proposed design is as per the requirements and also complies with the “General Guidelines of Biosafety and Biosecurity”2,19 for the BSL-3 laboratories in State/country.
At most places, qualified and experienced engineers suitable for such projects are not available, hence team members need to meet routinely and advise in concert with the hired consulting agency/engineer and the contractor. All the detailed drawing of the proposed containment laboratory must be developed as a mandate. These detailed drawings of the facility must reflect the layouts of all the laboratory areas along with the room numbers to facilitate the placement of essential on-site as well as stand-alone equipment in the facility. The placement and installation of all on-site equipment like autoclaves, Biological liquid effluent decontamination (BLED) plant/chemical kill tank, air handling units, exhaust filters within the facility must be identified. Placement of all safety equipment like fire extinguishers, water sprinklers, etc. within the facility should also be incorporated in the detailed drawing. Approvals should also be taken at this stage from the local fire departments, so that at later stages facility can pass the fire norms. Complete Heating Ventilation Air Condition (HVAC) design calculations for maintenance of unidirectional airflow and negative pressure as compared to the ambient within the facility and air flow diagrams must be prepared.
Placement of microscopes and all such equipment in the facility, which are sensitive to vibration, are crucial. Normally placing them near structural columns or over the slab-on-grade is not advised. Adequate considerations must be given to the ability of the laboratory facility engineering staff to start learning to operate modern mechanical systems as soon as these are installed in the laboratory. Specialized skills are required to operate and maintain these, that include knowledge of traditional skills of pneumatic controls and logic principles of mechanical engineering in handling of complex building management systems (BMS). During planning with various laboratory work flowcharts, and on-site and stand-alone equipment and the requirement of electrical supply of transient or low voltage fluctuations with low harmonics along with adequate voltage requirement of uninterrupted power supply (UPS), should be taken care of. At this stage, staff that will be required for working in the facility must be identified and relocated or appointed for carrying out specific responsibilities. A preliminary finish schedule and required material selection for the hardware, and other construction requirements should be finalized. Developing an “Engineering Manual” for the operation of the facility is recommended.
Requirements during construction of BSL-3
Preparation of construction document with final specifications is essential at this stage. The final proposal with drawings and all other details including specifications of the essential and stand-alone equipment for release of funds should also be put forward so that tenders can be advertised. Tender must be customized to select a professional organization, having experience of constructing this kind of laboratory. Some of the construction documents setting basic criteria and requirements for this facility should be part of the tender document. Major documents to be included are general conditions of contract, description and scope of work, qualification criteria, instructions to bidders and evaluation of bids, and notice inviting tender.
Finding the right construction agency/contractor and awarding the tender to agency
Finding and hiring the right construction agency for the facility is the key to success of the project. The construction agency with adequate qualification and expertise helps in making the containment laboratory functional and achieve standards of biosafety practices for safer working environments.
While hiring the construction agency for the proposed facility, one should ensure that the bidder meets the following minimum essential qualification criteria: (i) the minimum average annual turnover during the last three financial years (as per their audited balance sheets) should be at adequate level for making it sure that agency would be able to complete the project. (ii) Successful and timely completion of at least one similar project, which involved construction, testing, commissioning and validation of BSL-2 /or BSL-3 laboratory including civil works, electrical works, HVAC works, BMS, door interlocks, access control system, primary barrier containment equipment, decontamination system, etc., during the previous five years. The ability of construction agency for designing and planning, correct evaluation of architectural layout plans, men and material movement plans, zoning plans, specialized systems and services schemes, services and utilities schemes, laboratory commissioning and validation protocols, laboratory security protocols and integration of laboratory and equipment should be assessed. The most important part is providing correct magnitude/valuation of the project and completion on time, customer satisfaction, cost overrun, if any and litigation, if any.
After satisfactorily meeting the qualification criteria, before awarding the contract to the construction agency, an agreement should be signed specifying all the requirements and guidelines to be followed, mentioning the time limit given for the completion of the project and the penalty clause, if not completed in time.
Hiring project management consultant for supervising the quality of construction as per the requirement
One of the major difficulties being encountered is non availability of typical regulations, codes and standards for a high standard construction which is required for such a laboratory. Also the International standards are not easy for the contractors and engineers to understand, since they do not illustrate implementation of these standards. Hence, quality of construction must be supervised by a competent agency or group of engineers. If required, the project staff should be sent for onsite training at other laboratories. Development of scientific and engineering SOPs for the operation and maintenance should be started at this stage. Construction supervision by local engineering department may be arranged. Installation/placement of equipment, which is an integral part of the laboratory, must be initiated as construction work progresses. Procurement and placement of other essential equipment required to functionalize the laboratory should also be done at this stage. While ordering equipment, care should be taken to understand their installation, calibration, requirements and essential details for its maintenance.
The staff requirement includes one maintenance in-charge, one HVAC technician, one electrical technician, one person for instrumentation and staff for general building and services maintenance. A well trained person on biosafety and biosecurity should be the in-charge of the facility with one or two well trained technical staff. Box 2 lists equipment in BSL-3, laboratory.
Box 2.
Onsite equipments in BSL-3 laboratory

III. Milestone steps to operate the facility after construction
Commissioning of the facility: The commissioning process of the laboratory includes three phases:
(i) Testing and commissioning of “on-site” equipment– this should be initially performed by the construction contractor alone. It should be repeated and demonstrated to authorized person or project management consultant for the facility.
(ii) Testing and validation of the commissioning process of equipment are performed in presence of the facility In-charge and Biosafety Officer.
(iii) Final testing and commissioning should take place in presence of committee / project team. On completion the laboratory is to be made functional, ready for take over.
Commissioning procedure for the laboratory should be well designed and implemented to verify the safe facility operation. Testing and commissioning of some of the elements are crucial to the proper functioning of the containment, such as airflow patterns and pressures within isolators and biosafety cabinets, temperature profiles in autoclaves, procedures for decontamination and sterilization, verification of light lux level (must be between 400-600 lux), operation of HVAC systems, capacity calculations of HVAC systems plant, chilled water pumps capacity, air quantities at outlet diffusers / grilles, and air compressor, testing air curtains, steam boiler, clean room garment storage cabinet, floor traps, drains, dunk tanks, checking of ceiling panels, pass box, shower cabinets/air shower, water outlets, air leak in ducts and plenums, doors and view panels and functioning of all the alarm systems. Taking over the facility includes verfying all the basic requirements as per the approved layouts, electrical connections (raw, essential and UPS), local area network (LAN) connections, servers, water connections, sewage connection, hardware fitting, telephones and intercoms, functioning of the BMS with all the desired parameters, fine setting of access control and all the inventories.
On-site essential component and importance of training of the staff in advance: An integral component in setting-up the BSL-3 laboratory is designating an experienced and qualified Biosafety Officer and the Scientist-In-charge for the overall functioning i.e. operation and maintenance of the facility. In-house staff training periodically with provision of safety drills is to be conducted every six months. As the laboratory construction is custom-made as per the requirement of the user, specific training programme should be developed to cater to the needs of that specific laboratory. Besides general biosafety training programme, laboratory specific training programme should also be prepared. Training programmes and protocols must be developed by the Biosafety Officer and In-charge of the facility. SOPs for All the equipment, and laboratory working, and clinical sample handling and processing protocols must be developed. Validation of the developed SOPs is also a mandate that needs to be taken care of by the designated person. Another issue of great concern is the development and testing of emergency protocols that should be strategically designed from the very beginning of the construction plans.
The staff working within the containment laboratory must be well-trained in the concepts and practices of the biosafety and biosecurity. Laboratory workers should be trained to understand and tackle any kind of emergency situations within the containment laboratory without panic while ensuring their own safety first and ensuring that laboratory equipment are put into safe operating mode. Filing the incident report, assessment and management must be ensured for future risk assessment and management.
Operation phase of the laboratory: After taking over, the training programmes for the staff should be implemented. The completion of training programmes follows the mock drills and validation of all the SOPs of the on-site and stand-alone equipment, followed by training from the contractor on the facility operations equipment. After obtaining approvals from the local statutory authorities like the Fire Department and municipal corporation, the laboratory can be put into operation. Once this is achieved, the laboratory can go into validation phase.
Operation of the facility, preparation of essential documents and programmes for validation: While the facility is being completed, some important documents certifying compliance with the international guidelines need to be prepared. Validation document includes commissioning reports of all major equipment, important SOPs of laboratory workflow, equipment use, and engineering controls (AHU filters changing and operating records and main engineering controls), maintenance (servicing, calibration and validation), decontamination and emergency protocols and other SOPs. The training programme should cover all the basic and specific training modules, training imparted report and list of trainees with assessment results.
Some of the features in a BSL-3 Laboratory infrastructure and environment are given in Box 3.
Box 3.
BSL-3 laboratory infrastructure
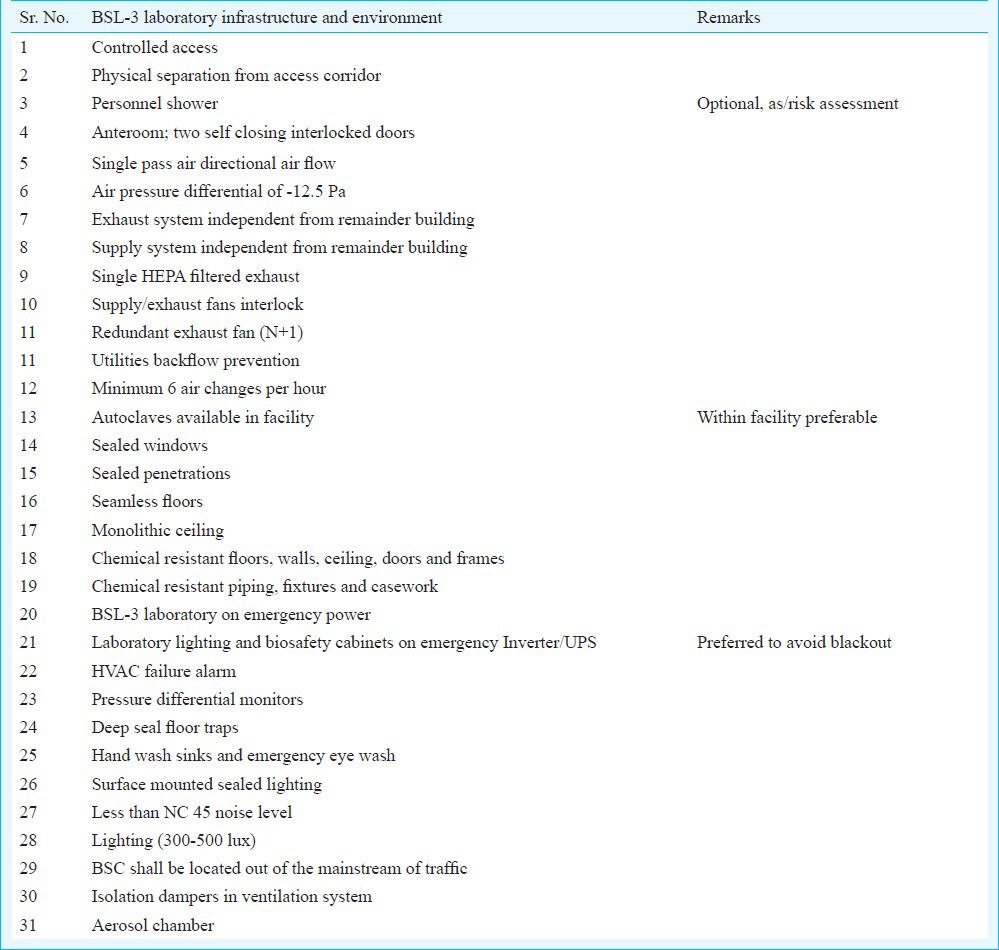
To maintain all records for all equipment, log books, certification details and maintenance report book should be available in the laboratory. Other documents should include entry and exit record sheets, entry of shower printouts from access control, records of daily checks, requisition file, and equipment calibration file. During validation procedure the methods of decontamination and their records should be presented with the details of tests done (spores strips validation of autoclaves, area fumigation records and surface swab tests). The record and performance of BMS, room pressures, temperature and humidity should also be presented to the validation committee / agency. Inventory of the samples and the agents in digitalized system and storing refrigerator [-20°C, -86°C & Liquid Nitrogen refrigerators] and the back-up plans should be defined. Facility and operation manuals should be prepared in such a way that these explain not only maintenance of engineering system but also the biosafety aspects. Documents should also describe the mandate and features of the facility. A technical manual of the facility should be prepared for good performance.
An internal expert committee as well as an external expert committee in conjunction with facility-in-charge and/or Biosafety Officer, construction agency and project management consultant agency should validate the facility and ensure that the work flow programme and all aspects of operation for balancing the safety and security of person, environment and products are achieved.
Operation and maintenance of BSL-3 laboratory: After completion of validation procedure and acquiring certificate to use the facility, it goes in safe running operation mode. The major maintenance requires re-review of the contingency plan for emergency during regular operation mode, so that any possible failure of any ongoing controls can be handled without breaching biosafety such as BMS, UPS, DG set and autoclaves. In case of non-sustainability of emergency, contingency plan for exit should be evolved. It is mandatory for all research institutions/universities/industries that are handling microorganism/genetically engineered organisms to constitute institute IBSC that prepares an up-to-date site emergency plan according to the manuals/guidelines of the Review Committee on Genetic Manipulation (RCGM)20. About 300 such committees are already functioning in various research institutions/universities/industries handling microorganism/genetically engineered organisms. This committee also looks into the biosafety aspects including experimentation and containment issues5,21. Annual Maintenance Contracts (AMC) of all important equipment should be done in advance to ensure continuity of routine maintenance. A contract should be made for maintenance of the facility by facility contractor to maintain and train the engineering personnel for future. Memorandum of understanding should be signed with the contractor and sub-contractors for providing support for at least five years for the spares and services as and when needed2,16,17,18,19.
Conclusion
The establishment of a containment laboratory is by and large a learning experience in India. This article provides some guidance on roles and responsibilities for those who are planning to establish such facilities. This will also promote awareness about the roles and responsibilities of different team members to be involved in establishment of BSL-3 facilities. There are no national agencies in India to audit and validate BSL-3 laboratories. The management of biosafety programme in such laboratories is to be accorded highest priority. It was out of the scope of this paper to touch upon such issues. Also, there are no national guidelines or standards available for operation and maintenance of BSL-3 laboratories. There is need to formulate such guidelines and establish agencies, which can help in managing biocontainment programmes in the country.
References
- 1.Callaway E. Biosafety concerns for labs in the developing world. Nature. 2012;485:425. doi: 10.1038/485425a. [DOI] [PubMed] [Google Scholar]
- 2.Biosafety in microbiological and biomedical laboratories. 5th ed. Washington. U.S: 2007. [accessed on June 23, 2013]. Department of Health and Human Services. Centers for Disease Control and Prevention and National Institutes of Health. Available from: http://www.cdc.gov/OD/OHS/biosfty/bmbl5/BMBL_5th_edition . [Google Scholar]
- 3.Kant L, Mourya DT. Managing dual use technology: it takes two to tango. Sci Eng Ethics. 2010;16:77–83. doi: 10.1007/s11948-008-9062-9. [DOI] [PubMed] [Google Scholar]
- 4.New Delhi: Ministry of environment and forests. Department of environment, forests and wildlife, Government of India; 1986. [accessed on June 23, 2013]. The environment (protection) act. Available from: www.moef.nic.in/sites/default/files/eprotect_act_1986.pdf . [Google Scholar]
- 5.New Delhi: Ministry of environment and forests, Department of environment, forests and wildlife, Government of India; 1986. [accessed on June 23, 2013]. Rules for the manufacture, use, import, export and storage of hazardous microorganisms, genetically engineered organisms or cells. Available from: http://www.moef.nic.in/legis/hsm/hsm3.html . [Google Scholar]
- 6.Department of Biotechnology, Ministry of Science and Technology, Government of India; 1990. [accessed on June 23, 2013]. Recombinant DNA safety guidelines. Available from: http://dbtbiosafety.nic.in/guideline%5Cpdf%5CAnnex-5.doc↱ . [Google Scholar]
- 7.Department of Biotechnology, Ministry of Science and Technology, Government of India; [accessed on June 23, 2013]. Revised guidelines for safety in biotechnology. Available from: http://dbtbiosafety.nic.in/guideline/pdf/guidelines_94.pdf . [Google Scholar]
- 8.Department of Biotechnology, Ministry of Science and Technology, Government of India. Drug Policy. 2002. [accessed on June 23, 2013]. Available from: http://dbtbiosafety.nic.in/Files/CD_IBSC/Files/2002.PDF .
- 9.National seed policy. Ministry of Agriculture. Govt. of India. 2002. [accessed on June 23, 2013]. Available from: http://tnau.ac.in/eagri/eagri50/GPBR112/ pdf /lec02.pdf .
- 10.Guidelines for export of SCOMET. Ministry of commerce and trades, Government of India. [accessed on June 23, 2013]. Available from: http://dgft.gov.in/ exim /2000/scomet /scomet2011.pdf .
- 11.Ethical policies on the human genome, genetic research & services. Department of Biotechnology, Ministry of Science and Technology, Government of India. [accessed on June 23, 2013]. Available from: http://dbtindia.nic.in/uniquepage.asp?id_pk=113 .
- 12.New Delhi: Indian council of Medical Research; 2006. [accessed on June 23, 2013]. Ethical guidelines for biomedical research on human participants. Available from: http://icmr.nic.in/ethical_guidelines.pdf . [Google Scholar]
- 13.New Delhi: 2012. [accessed on June 23, 2013]. Department of Health Research. XII plan document (2012-2017) Available from: http://icmr.nic.in/Publications/plan/ICMR%20XIIth%20Plan%20(2012-2017).pdf . [Google Scholar]
- 14.Richmond JY. Anthology of biosafety II. Facility design considerations. American Biological Safety Association. 2000 [Google Scholar]
- 15.Lam SK. Emerging infectious diseases - South-east Asia. Emerg Infect Dis. 1998;4:145–7. doi: 10.3201/eid0402.980201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.WHO. Biorisk management: Laboratory biosecurity guidance. 2006. [accessed on June 23, 2013]. Available from: http://www.who.int/ihr/biosafety/publications/en/index.html .
- 17.WHO. Geneva: World Health Organisation; 2012. Laboratory biorisk management strategic framework for action 2012-2016, WHO/HSE/2012.3. Enhancement of laboratory biosafety (WHA58.29) [Google Scholar]
- 18.3rd ed. Geneva: World Health Organization; 2004. World Health Organization. Laboratory biosafety manual. [Google Scholar]
- 19.Le Due JW, Anderson K, Bloom ME, Estep JE, Feldmann H, Geisbert JB, et al. Framework for leadership and training of biosafety level 4 laboratory workers. Emerg Infect Dis. 2008;14:1685–8. doi: 10.3201/eid1411.080741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Due JW, Anderson K, Bloom ME, Estep JE, Feldmann H, Geisbert JB, et al. Framework for leadership and training of biosafety level 4 laboratory workers. Emerg Infect Dis. 2008;14:1685–8. doi: 10.3201/eid1411.080741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaiser J. Biosafety breaches. Accidents spur a closer look at risks at biodefense labs. Science. 2007;317:1852–4. doi: 10.1126/science.317.5846.1852. [DOI] [PubMed] [Google Scholar]


