Abstract
Background & objectives:
Information related to nasopharyngeal carriage of Streptococcus pneumoniae among healthy children is scanty in India. This prospective study was undertaken to determine the presence of asymptomatic nasopharyngeal colonization, assess serogroups/types (SGT) and drug resistance of S. pneumoniae in children below five years of age.
Methods:
A total of 109 male and 81 female children in the age group of three months to five years belonging to different socio-economic classes were enrolled. They were recruited across all age groups from those attending paediatric OPD of a tertiary care and research centre for immunization program. Fifty three isolates identified as pneumococci were tested for their antimicrobial susceptibility pattern by Kirby-Bauer's disc diffusion and E-Test methods. Serotyping was performed by detection of the quelling reaction with specific antiserum.
Result:
The pneumococcal carriage rate in the study population was 27.9 per cent. The isolation rate was associated with age being higher (49.2%) in smaller children (3-12 months) and among male (62.2%). The most prevalent SGTs were 19 followed by 10, 14 and 7; 21 per cent of isolates belonging to serotype 10 (n=7) were 11 (n=4) were not covered in any of the conjugate vaccines currently available in Indian market. Resistance to co-trimoxazole, tetracycline, penicillin and erythromycin was observed in 91 per cent (n=48), 36 per cent (n=19), 17 per cent (n=9) and 9 per cent (n=5) isolates, respectively. All the penicillin resistant isolates were found to be intermediately resistant by E-Test. Multidrug resistance was observed in 19 per cent (n=10) isolates.
Interpretation & conclusions:
High level of antibiotic resistance was present in S. pneumoniae isolated from healthy children below age five. A pneumococcal conjugate vaccine with the prevailing SGTs would help to reduce the pool of antibiotic resistant pneumococci. Continued surveillance of serotypes and tracking susceptibility pattern of S. pneumoniae will help to introduce appropriate vaccination protocols.
Keywords: Antibiotic resistance, nasopharyngeal carriage, serotypes, Streptococcus pneumoniae
Streptococcus pneumoniae is a leading cause of life threatening infections in children in developing countries. It kills at least one million children under the age of five every year, >70 per cent of these deaths are in developing countries. The burden of the disease in youngest and oldest sections of the population is more, in both developed and less developed countries1. S. pneumonia is part of the commensal flora of the upper respiratory tract, together with Moraxella cattarrhalis, Haemophilus influenzae, Neisseria meningitis, Staphylococcus aureus and various haemolytic streptococci it colonizes the nasopharyngeal (NP) niche1. Though colonization with pneumococci is mostly asymptomatic, it remains the common cause of community-acquired pneumonia (CAP), bacterial meningitis, bacteraemia, and otitis media1. In addition, pneumococcal carriage is believed to be an important source of horizontal spread of this pathogen within community.
Serotype distribution and resistance to antibiotics among nasopharyngeal carriage isolates vary by country, age group, type of cohort, vaccine policies and change over time1. Studies have documented that pneumococcal diseases originate from nasopharyngeal colonization with homologous serotypes. Acquisition of a new serotype may lead to an invasive disease, with a risk of 15 per cent in the first month after new acquisition, and the children may act as a reservoir for the dissemination of new serotypes to others1. The pneumococcal conjugate vaccines have greatly reduced frequency of invasive disease, and has also resulted in reduction of drug resistance among the vaccine serotypes2. But several non-vaccine type strains have mutated to become more successful colonizers and are re-emerging as pathogens. Surveillance of serotype distribution of colonizing isolates in children will serve as an indicator of invasive disease, antibiotic resistance trends and usefulness of vaccine.
Initially pneumococcal strains were universally susceptible to penicillin which remained the drug of choice. Strains resistant to penicillin and multi drugs emerged during 1970s and 1980s and spread globally3. This study was carried out to determine nasopharyngeal carriage rate, serotype prevalence and in vitro antimicrobial resistance of S. pneumoniae in children during their first five years of life.
Material & Methods
A total of 190 children between the age group of 3 months and <5 yr were enrolled prospectively during December 2010-December 2011 from those attending outpatients of the department of Paediatrics, Kempegowde Institute of Medical Sciences Hospital and Research Centre, Bangalore, Karnataka, India for immunisation programme. The children belonging to different socio-economic classes were randomly recruited from the paediatric immunization centre. For the study one child per family was included.
Nasopharyngeal specimen collection: Nasopharyngeal specimens were obtained at the time of enrolment as per the technique recommended by World Health Organization4. Samples were collected using sterile miniature polyester tipped swabs on flexible aluminium shaft (Hi-Media Laboratories, Mumbai, India).
Children < 3 months of age, with a history of antibiotics in recent past (4 weeks) and recent hospitalization were excluded. Children from household with adult member suffering from acute lower respiratory tract infection or with a history of taking pneumococcal vaccines were also excluded.
The study protocol was approved by the hospital ethical committee. Informed written consent from parents or guardians was obtained at the time of enrolment.
Microbiological methods: The swabs were streaked immediately on to a 5 per cent sheep blood agar plate. The plates were incubated overnight at 35°C in a carbon dioxide incubator. Isolation of S. pneumoniae was confirmed by Gram staining, optochin sensitivity and bile solubility tests4. Serogrouping and serotyping by the Quelling reaction was done from the pure culture directly after isolation, using the pneumotest plus kit. (Statens Serum Institute, Copenhagen, Denmark).
Antimicrobial susceptibility testing: Susceptibility testing of S. pneumoniae was done on Muller-Hinton agar with 5 per cent sheep blood. The antibiotics tested were penicillin, erythromycin (15μg), Co-trimoxazole (25 μg), cefotaxime (30 μg), ciprofloxacin (5 μg) and tetracycline (30 μg). The 1μg oxacillin disc was used to screen for penicillin susceptibility. Multi drug resistant S. pneumoniae defined as resistant to penicillin and two or more non β-lactam agents such as macrolides, co-trimoxazole or tetracycline were analysed in the study.
Minimum inhibitory concentrations (MIC) for penicillin were determined using E-strips (Hi- media, Mumbai, India) S. pneumoniae quality control strain ATCC 49619 was included along with the tests.
Statistical analysis: One proportion Z-test was used to find the significance of percentage of isolation of pneumococcal carriage. The statistical softwares SPSS 15.0 and Stata 8.0 were used for the analysis of data.
Result
Pneumococci were isolated from 53 of the 190 children tested (109 male, 81 female), with a carriage rate of 27.9 per cent. The carriage of pneumococci was found to be higher in male children (34/109, 30.3%) than in female (20/81, 24.7%). Carriage rate of pneumococci (Table I) was highest in 3-12 months group, followed by 1-3 year group, and the lowest in the 3-5years group.
Table I.
Age distribution and percentage of pneumococcal isolation
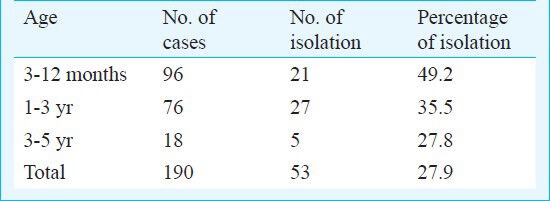
The SGT isolates were 1 (n=4), 3 (n=6), 6 (n=2), 7 (n=6), 9 (n=2), 10 (n=7), 11 (n=4), 14 (n=6), 18 (n=4) and 19 (n=11), the most common being SGT 19. Two isolates were non-vaccine type (21, 24). Six SGT (19, 10, 6, 7, 3, 14) comprised 79.2 per cent of pneumococcal isolates.
Nine (16.98%) of the 53 isolates were resistant to oxacillin. The MIC of penicillin was in the range of 0.125-0.5μg/ml indicating that all were intermediately resistant to penicillin. All the oxacillin resistant isolates were also resistant to co-trimoxazole.
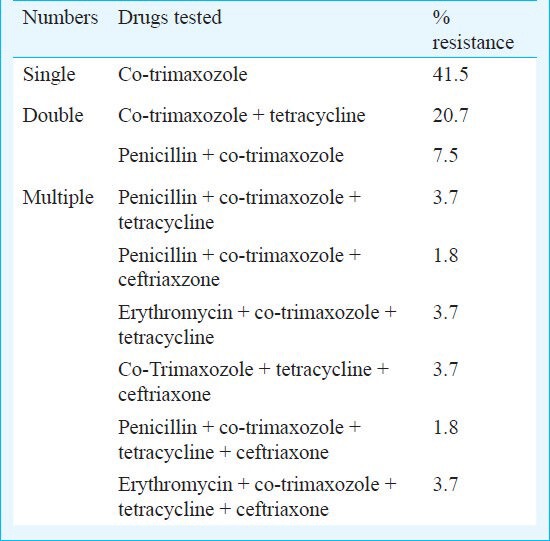
Highest degree of resistance among the six drugs was seen for co-trimoxazole, (n=48, 90.6%) (Table II). Number of resistant isolates to tetracycline, erythromycin, ciprofloxacin and cefotaxime were 19, 5, 5 and 2, respectively; 41.5 per cent of isolates were resistant to one antibiotic, 28.3 per cent to two antibiotics and 18.8 per cent to three or more antibiotics. Two among the multi drug resistant (MDR) isolates were resistant to all the six drugs tested.
Table II.
Drug resistance pattern
Discussion
As the highest frequency of pneumococcal colonization and highest crowding index are found in young children, this group is the most important reservoir for horizontal dissemination of the pathogen within the community3. Therefore, strategies to prevent pneumococcal disease need to focus on prevention of nasopharyngeal colonization especially in children. Industrialized countries like Israel, Finland have reported nasopharyngeal carriage rates ranging from 43-90 per cent5,6. Studies conducted in various regions of India have found similar carriage of 6.5-83 per cent7,8,9. There are many factors that are independent determinants for nasopharyngeal colonization like ethnicity, age, environmental features, season and antibiotic usage practices. Some of the identified specific risk factors for carriage establishment are the presence of children in the household, day-care attendance, overcrowded living conditions, upper respiratory tract infections and rural residence10,11. The carriage rate in our study was higher than the results reported from Lucknow (20.2%)7. There was a progressive decrease of carriage rate with increasing age of children similar to other reports12,13,14.
Treatment of infections due to S. pneumoniae has become a complicated global problem due to antibiotic resistance. Antibiotic resistance patterns found among carriage specimens provide a rough estimate of those found among invasive isolates15 which indicates that pneumococcal carriage studies may be used for monitoring resistance patterns16. The risk factors for development of antibiotic resistance in pneumococci are use of antibiotic particularly at low levels for prolonged periods, easy availability over the counter and wider, indiscriminate use in the hospital as well as in the community. The emergence of resistance to penicillin in 1980's let to the increased use of macrolides, flouroquinolones and other drugs17. While these antibiotics provided effective alternate therapy for S. pneumoniae infections, selective pressure associated with the widespread use of these antibiotics, has resulted in resistance to various classes of antimicrobial agents.
In our study, the penicillin resistance rate was found to be 16.9 per cent and all isolates were intermediately resistant. Penicillin resistant pneumococci in various studies from India have been reported to be from 0-15.4 per cent7,11,17,18. In these studies, high level penicillin resistant strains were not detected. Studies in urban areas of Southeast Asia have found a much higher proportion of penicillin resistance, sometimes up to 60 per cent19. Increasing emergence of intermediate resistance of S. pneumoniae as observed in the present study is of great concern as it can cause spread of resistant strains in the community. This may lead to higher rate of treatment failure, additional cost of therapy and extra days of hospitalization. Moreover, it is also known that strain showing penicillin resistance can have genes responsible for resistance to other antibiotics15.
Excessive consumption, inappropriate and overuse of co-trimoxazole are possibly the contributory factors for high resistance in our study. Hence the empirical use of co-trimoxazole is not justified in this region. The nasopharyngeal isolates also demonstrated resistance to other antimicrobial agents like tetracycline, erythromycin, ciprofloxacin and cefotaxime.
Multi drug resistant S. pneumoniae is increasingly being reported from many parts of the globe19. The first MDR strain of S. pneumoniae was reported in 1977 from Johannesburg14. Penicillin susceptibility is an important marker for the presence or absence of a multi drug resistant phenotype. Strains with reduced susceptibility to penicillin are usually cross-resistant to other antibiotics also14. Prolonged carriage and rapid re-acquisition provide an increased chance of exposure to antibiotics and thus may be important selective factor in predisposing to antibiotic resistance21. In our study, 19 per cent isolates belonged to multi drug resistance group. Majority of our multi resistant isolates showed profile of resistance to penicillin, co-trimoxazole and tetracycline, and were found to be associated with four serotypes 6, 9, 14 and 19.
In the current study the predominant capsular serotype encountered was 19. A study conducted at Delhi also identified serotype 19 as the most common9. Other centres in India have identified serotypes 6 and 33 as the commonest highlighting the need to conduct prevalence studies in various regions17,22. In a study recently conducted in our centre it was observed that most prevalent serotypes in invasive pneumococcal disease in children below five years were 6, 14, 18, 5, 1, 3, 19, 9, 4, 10 and 15 in descending order; 81 per cent of SGTs found in nasopharyngeal carriage were also present in invasive pneumococcal group. Therefore, it can be concluded that most types of invasive S.pneumoniae are those found in nasopharynx. This finding is compatible with the concept that the types of S.pneumoniae that cause disease in man are usually derived from normal human flora21,23.
With the emergence of resistant pneumococcal strains specifically MDR and their isolation from healthy carriers is a reason for consideration of active immunization. Immunization with a conjugate pneumococcal vaccine has been reported to result in reduced carriage rate of pneumococcal serotypes included in the vaccine24. Further studies and analysis of the serotypes of nasopharyngeal isolates of different regions will provide useful information on the applicability of these conjugate pneumococcal vaccines.
The limitation of the study was its short duration and small number of children included from those attending paediatric OPD for immunization program.
In conclusion, a high rate of drug resistance was found in colonized S. pneumoniae in healthy children. A diversity was seen among the serotypes compared to other regions. Data obtained emphasize need to control the use of antibiotics and introduction of vaccines. The increasing penicillin and multidrug resistance has important clinical implications.
Acknowledgment
Authors thank Drs Nandan and Anjana Gopi for laboratory assistance.
References
- 1.Bogaert D, De Groot R, Hermans PW. Streptococcus pneumoniae colonization: the key to pneumococcal disease. Lancet Infect Dis. 2004;4:144–54. doi: 10.1016/S1473-3099(04)00938-7. [DOI] [PubMed] [Google Scholar]
- 2.Pilishvili T, Noggle B, Moore MR. Roush SW, McIntyre L, Baldy LM, editors. Pneumococcal disease. VPD surveillance manual. 5th ed. 2012. pp. 1–11. Available from: www.cdc.gov/vaccines/pubs/surv-manual/chpt11-pneumo.pdf .
- 3.Bokaeian M, Khazaei HA, Javadimehr M. Nasopharyngeal carriage, antibiotic resistance and serotype distribution of Streptococcus pneumoniae among healthy adolescents in Zahedan. Iran Red Crescent Med J. 2011;13:328–33. [PMC free article] [PubMed] [Google Scholar]
- 4.O’Brien KL, Nohynek H the WHO pneumococcal vaccine trials carriage working group. Report from a WHO working Group. Standard method for detecting upper respiratory carriage of Streptococcus pneumoniae. Paediatr Infect Dis J. 2003;22:e1–11. doi: 10.1097/01.inf.0000049347.42983.77. [DOI] [PubMed] [Google Scholar]
- 5.Hill PC, Akisanya A, Sankareh K, Cheung YB, Saaka M, Lahai G, et al. Nasopharyngeal carriage of Streptococcus pneumoniae in Gambian villagers. Clin infect Dis. 2006;43:673–9. doi: 10.1086/506941. [DOI] [PubMed] [Google Scholar]
- 6.Syrjänen RK, Kilpi TM, Kaijalainen TH, Herva EE, Takala AK. Nasopharyngeal carriage of Streptococcus pneumoniae in Finnish children younger than 2 years old. J infect Dis. 2001;184:451–9. doi: 10.1086/322048. [DOI] [PubMed] [Google Scholar]
- 7.Jain A, Kumar P, Awasthi S. High nasopharyngeal carriage of drug resistant Streptococcus pneumoniae and Haemophilus influenzae in North Indian schoolchildren. Trop Med Int Health. 2005;10:234–9. doi: 10.1111/j.1365-3156.2004.01379.x. [DOI] [PubMed] [Google Scholar]
- 8.Goyal R, Singh NP, Kaur M, Talwar V. Antimicrobial resistance in invasive and colonising Streptococcus pneumoniae in North India. Indian J Med Microbiol. 2007;25:256–9. doi: 10.4103/0255-0857.34770. [DOI] [PubMed] [Google Scholar]
- 9.Wattal C, Oberoi JK, Pruthi PK, Gupta S. Nasopharyngeal carriage of Streptococcus pneumonia. Indian J Pediatr. 2007;74:905–7. doi: 10.1007/s12098-007-0166-z. [DOI] [PubMed] [Google Scholar]
- 10.Sung RY, Ling JM, Fung SM, Oppenheimer SJ, Crook DW, Lau JT, et al. Carriage of Haemophilus influenzae and Streptococcus pneumoniae in healthy Chinese and Vietnamese children in Hong Kong. Acta Paediatr. 1995;84:1262–7. doi: 10.1111/j.1651-2227.1995.tb13545.x. [DOI] [PubMed] [Google Scholar]
- 11.Ussery XT, Gessner BD, Lipman H, Elliott JA, Crain MJ, Tien PC, et al. Risk factors for nasopharyngeal carriage of resistant Streptococcus pneumoniae and detection of a multiply resistant clone among children living in the Yukon-Kuskokwim Delta region of Alaska. Pediatr Infect Dis J. 1996;15:986–92. doi: 10.1097/00006454-199611000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Hansman D, Morris S, Gregory M, McDonald B. Pneumococcal carriage amongst Australian aborigines in Alice Springs, Northern Territory. J Hyg (Lond) 1985;95:677–84. doi: 10.1017/s0022172400060782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leino T, Auranen K, Jokinen J, Leinonen M, Tervonen P, Takala AK. Pneumococcal carriage in children during their first two years: important role of family exposure. Pediatr Infect Dis J. 2001;20:1022–7. doi: 10.1097/00006454-200111000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Loda FA, Collier AM, Glezen WP, Strangert K, Clyde WA, Jr, Denny FW. Occurrence of Diplococcus pneumoniae in the upper respiratory tract of children. J Pediatr. 1975;87:1087–93. doi: 10.1016/s0022-3476(75)80120-x. [DOI] [PubMed] [Google Scholar]
- 15.Lehmann D, Gratten M, Montgomery J. Susceptibility of pneumococcal carriage isolates to penicillin provides a conservative estimate of susceptibility of invasive pneumococci. Pediatr Infect Dis J. 1997;16:297–305. doi: 10.1097/00006454-199703000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Cizman M. The use and resistance to antibiotics in the community. Int J Antimicrob Agents. 2003;21:297–307. doi: 10.1016/s0924-8579(02)00394-1. [DOI] [PubMed] [Google Scholar]
- 17.Jebaraj R, Cherian T, Raghupathy P, Brahmadathan KN, Lalitha MK, Thomas K, et al. Nasopharyngeal colonization of infants in southern India with Streptococcus pneumoniae. Epidemiol Infect. 1999;123:383–8. doi: 10.1017/s0950268899003131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coles CL, Rahmathullah L, Kanungo R, Thulasiraj RD, Katz J, Santosham M, et al. Nasopharyngeal carriage of resistant pneumococci in young South Indian infants. Epidemiol Infect. 2002;129:491–7. doi: 10.1017/s0950268802007586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soewignjo S, Gessner BD, Sutanto A, Steinhoff M, Prijanto M, Nelson C, et al. Streptococcus pneumoniae nasopharyngeal carriage prevalence, serotype distribution, and resistance patterns among children on Lombok Island, Indonesia. Clin Infect Dis. 2001;32:1039–43. doi: 10.1086/319605. [DOI] [PubMed] [Google Scholar]
- 20.Chawla K, Gurung B, Mukhopadhyay C, Bairy I. Reporting emerging resistance of Streptococcus pneumoniae from India. J Glob Infect Dis. 2010;2:10–4. doi: 10.4103/0974-777X.59245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ciftci E, Dogru U, Aysev D, Ince E, Guriz H. Nasopharyngeal colonization with penicillin-resistant Streptococcus pneumoniae in Turkish children. PediatrInt. 2000;42:552–6. doi: 10.1046/j.1442-200x.2000.01269.x. [DOI] [PubMed] [Google Scholar]
- 22.Devi U, Ayyagari A, Rekha Devi K, Narain K, Dilip Kumar P, Sharma A. Serotype distribution & sensitivity pattern of nasopharyngeal colonizing Streptococcus pneumoniae among rural children of eastern India. Indian J Med Res. 2012;136:495–8. [PMC free article] [PubMed] [Google Scholar]
- 23.Boken DJ, Chartrand SA, Moland ES, Goering RV. Colonization with penicillin - non susceptible Streptococcus pneumoniae in urban and rural child-care centers. Pediatr Infect Dis J. 1996;15:667–72. doi: 10.1097/00006454-199608000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Obaro SK. The new pneumococcal vaccine. Clin Microbiol Infect. 2002;8:623–33. doi: 10.1046/j.1469-0691.2002.00424.x. [DOI] [PubMed] [Google Scholar]


