Abstract
Background& objectives:
Metabolic syndrome (MS) is a common but serious public health problem in developed countries. Chronic inflammation plays a key role in MS. Interleukins (IL)-7 and 8 are considered to have proinflammatory effects and may be involved in the pathogenesis of MS. Therefore, the aim of this study was to determine gene expression level of IL-7 and IL-8 in peripheral blood mononuclear cells (PBMCs) of patients with MS compared to healthy control subjects.
Methods:
Using real-time RT-PCR, the relative amounts of IL-7 and IL-8 mRNA were determined in PBMCs from 20 female patients with MS and compared with those of 20 healthy control subjects. Biochemical and anthropometric parameters of MS were also assessed.
Results:
Total cholesterol, triglyceride, and fasting blood sugar were significantly higher in MS patients compared to healthy subjects. There were no significant differences in HDLc and LDLc between the two groups. IL-8 expression in PBMC was significantly decreased in MS versus control subjects (fold of change was 0.395 ± 0.1824), while no difference in the IL-7 expression was detected between them. IL-8 expression had negative correlation with MS components especially with triglyceride and total cholesterol (r=0.5, P<0.001).
Interpretation & conclusions:
In this preliminary study, no detectable differences were found in IL-7 expression and decreased expression of IL-8 in PBMCs of MS patients as compared to those of control subjects. Study on a larger population and investigating the mechanisms involved can reveal more details.
Keywords: Gene expression, IL-7, IL-8, metabolic syndrome, PBMC, real-time PCR
Metabolic syndrome (MS) is one of the major public health challenges worldwide that is characterized by clustering of waist circumference, blood triglycerides (TG), high density lipoprotein (HDL) cholesterol, fasting glucose and blood pressure with different cut-off values1,2,3. MS affects approximately 25 per cent of the adult population in western countries and also is quickly increasing in young populations4. The aetiology is complex, genetic and environmental factors both have important roles5. Metabolic syndrome and its components are risk factors of cardiovascular disease and type 2 diabetes mellitus3,5,6. MS can cause dyslipidaemia, pro-thrombotic and pro-inflammatory states, fatty liver disease and finally increased cardiovascular disease (CVD) related to and all-cause mortality1,7,8. It is believed that visceral obesity can lead to development of insulin resistance, impaired glucose tolerance, hyperglycaemia, and type 2 diabetes mellitus9,10,11.
Inflammation is now thought to play a key role in the pathophysiology of MS12,13,14. Moreover, inflammation is closely related to obesity2. Adipose tissue releases a variety of molecules referred to adipocytokines11. Adipose tissue enlargement leads to an increase in the immune cells in this tissue. The process plays a role in inflammation, frequently through cytokines derived from macrophages infiltrated in the tissue15,16. Monocytes are shown to play an important role in the pathogenesis of MS, like inflammation of adipose tissue17.
Interleukin (IL)-7, known as B cell precursor growth factor, has a key role in lymphocyte homeostasis; especially in basal metabolism of glucose18. It maintains high glucose uptake and expression of GLUT1 which results in adequate glycolytic flux19,20. There are evidences to show that IL-7 contributes to inflammation in several chronic inflammatory processes21. Another key role of IL-7 is its effect on immune mediators which modulate metabolic functions22. Therefore, it may contribute to local pro-inflammatory process observed in MS.
IL-8 is known as a cytokine that involves in inflammation via neutrophil chemoattraction. Basal production of IL-8 is normally low but it can be quickly induced by different stimuli and is secreted from a variety of cells23. It is responsible for macrophage infiltration into adipose tissue in obesity and also associated with some disorders which are related to obesity24. IL-8 is secreted from adipose tissue in vitro and is shown to be involved in the pathogenesis of type 2 diabetes25,26. Plasma levels of IL-8 may be higher in patients with heart failure suffering from MS compared with non-MS group2. This study was undertaken with the aim to assess the IL-7 and IL-8 expression in PBMC of patients with MS. Detection of possible correlation between their expression and the components of disease was also studied.
Material & Methods
This study was conducted in the Research Center for Molecular Medicine, Hamadan University of Medical Sciences, Hamadan, Iran during January-March, 2011.
Twenty female patients with recent diagnosis of metabolic syndrome (MS), referred to the endocrinology department of Shahid Beheshti Hospital of Hamadan University of Medical Sciences (Iran), and 20 age matched healthy women who were referred for check up to the hospital laboratory in the same period were included in this study. All patients having the inclusion criteria were selected consecutively. MS diagnosis was carried out according to the modified criteria for MS from the National Cholesterol Education Program-Adult Treatment Panel III (NCEP-ATP III)27. Based on these criteria, the patients had three or more of the following conditions: (i) Central obesity (waist circumference > 95 cm); (ii) High blood pressure ≥ 130/86 mmHg or documented use of antihypertensive therapy; (iii) High fasting glucose level (≥ 100 mg/dl); (iv) Hypertriglyceridaemia (≥ 150 mg/dl); and (v) Decreased high density lipoprotein cholesterol (HDLc) (< 50 mg/dl).
The diabetic and hypertensive patients received routine medicine of diabetes and hypertension. Participants having the following conditions were excluded from the study: estro-progesterone or testosterone treatment including oral contraceptives; smoking; pregnancy; amenorrhoea; polycystic ovarian syndrome. The subjects were not affected by chronic illness, and not taking drugs or supplements known to modify the immune system. Past medical and habitual history of participants were negative for chronic diseases (hepatic, renal, thyroid, cardiac), smoking, alcohol consumption, or taking drugs. There was no family history of early onset cardiovascular disease.
Informed written consent was obtained from all participants. Study protocol was approved by the Hamadan University of Medical Sciences ethics committee. Each participant underwent a baseline visit after an overnight fasting. Height, weight, waist circumference and blood pressure were measured prior to sample collection.
Following an overnight fasting (≥10 h) venous blood sample (6 ml) was collected. Samples for CBC (cell blood count) and gene expression analysis were collected in tubes containing 1 g EDTA/l. Serum was separated within an hour after obtaining the blood sample.
Analytical methods: Height and weight of all of participants were measured by a stadiometer (Seca, USA) and digital scale (Escali, USA), respectively. Blood pressure was measured in the sitting position after five minutes rest using a sphygmomanometer (Welch Allyn, USA). FBS (fasting blood glucose), TC (total cholesterol), TG, LDLc (low density lipoprotein cholesterol) and HDLc were measured using an autoanalyzer (Hitachi 911 sunrise Corporate company Kobe, Japan) and a colorimetric method kit (ParsAzmun - Iran). The intra- and inter-assay precisions for these biochemical parameters were 2.3-3.1 and 5.8-6.4 per cent, respectively. CBC analysis was carried out by haematology analyzer Sysmex Kx-21N (Sysmex Corporation, Japan).
Peripheral blood mononuclear cell (PBMC) isolation: Peripheral blood (4 ml) was diluted 1:1 (v/v) in BSS (Balanced salt solution); and layered on 3 ml of Ficoll-Hypaque solution (Amerhsam Biosciences, USA). Density gradient centrifugation was carried out at 400 × g for 35 min. PBMCs were harvested from the interface layer and washed twice with BSS28. Harvested PBMCs were used for RNA extraction.
RNA extraction and cDNA synthesis: Total RNA was extracted from PBMCs using RNeasy Mini Kit (Qiagen, Hilden, Germany). Quantity and purity of the extract were measured by Nanodrop spectrophotometer (Epoch, BioTek) and the ratio of A260/A280 nm of all the samples was about 2. RNA integrity was assessed using 1 per cent agarose gel. RNA was considered suitable for the next step if intact bands corresponding to 18S and 28S ribosomal RNAs were detected on electrophoresis.
Equal amounts of total RNA, approximately 0.5 micrograms, was reverse transcribed into single-stranded cDNA using QuantiTect Reverse Transcription kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. Briefly, after one step gDNA wipeout at 42○ C, cDNA synthesis was performed at 42○ C for 20 min, followed by RT inactivation at 95○ C for 3 min.
Quantitative real-time PCR: PCR analyses were performed using C1000 Thermocycler and CFX96 real time system (BioRad) and QuantiFast SYBR Green PCR Kit (Qiagen, Hilden, Germany) in a final volume of 25 μl with 10 picomol of each primer. Each reaction was performed on 1 μl of 1:9 (v/v) dilution of the first cDNA strand. Positive and negative controls were used for quality control of process.
The reaction mixture was incubated at 95°C for 5 min, followed by 40 cycles of 15 sec at 95°C, 30 sec at annealing temperature, 30 sec at 72°C and then fluorescence was measured. Primers, designed by software AlleleID7.6, were: IL7-forward (5’-GGCAAACAATATGAGAGT -3’); IL7-reverse (5’-CCTTATTAGCATCACAGATA -3’); IL8- forward (5’-AGACATACTCCAAACCTTT -3’); IL8- reverse (5’-GCTCTCTTCCATCAGAAA -3’); β-actin- forward (5’-AAGATCAAGATCATTGCT -3’); and β-actin- reverse (5’-TAACGCAACTAAGTCATA -3’). Gene numbers of different variants of IL-7 were NM_001199886, NM_001199887, NM_001199888, NM_000880 and the number for IL-8 was NM_000584.
Annealing temperature and size of PCR products were 48.5°C and 130, 49.5°C and 114 and 47.5°C and 177 for IL7, IL8 and β-actin, respectively. Specificity of PCR amplifications was verified by melting curve programme (70-95°C with a heating rate of 0.5°C/sec and a continuous fluorescence measurement) and analyzed by electrophoresis on a 1 per cent agarose gel, 1× TBE. Expression values were obtained as relative expression of the target gene versus the constitutively expressed β-actin gene as reference gene (ΔCT = Target gene CT - Reference gene CT). Fold change for each of studied genes was calculated by means of ΔΔCT formula (ΔΔCT of each gene = ΔCT in MS group - ΔCT in non-MS group; Fold change = 2−ΔΔCT)29.
Statistical analysis: The values were expressed as means ± SD and gene expression level was reported as means ± SEM of three independent experiments. P<0.05 was considered significant. Results were analyzed using Mann-Whitney U test for comparison between normal and MS subjects. Spearman correlation coefficient was used for the determination of relations among variables. All statistical analyses were conducted using SPSS software (Version10) (SPSS Inc., USA).
Results
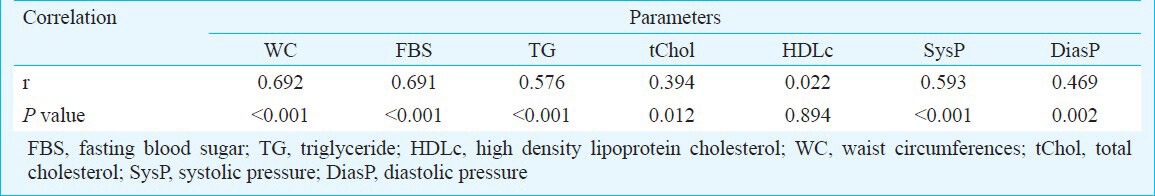
The biochemical and anthropometric parameters of patients and controls are shown in Table I. All patients in MS group and 40 per cent of the subjects of non-MS had waist circumferences more than 95 cm. Both groups had approximately similar rates of positive family history of disease and no remarkable difference was reported (55 and 60% in non-MS, and MS, respectively). All subjects with MS had waist circumferences more than 95 cm as a diagnostic factor of MS. Frequency of other components in MS group were high FBS: 75 per cent, hypertension: 65 per cent, hypertriglyceridaemia: 60 per cent, low HDLc: 45 per cent. Correlation between MS and its components is shown in Table II. Results showed significant correlations between MS and WC, FBS, TG, total cholesterol and hypertension.
Table I.
Demographic data of the studied population
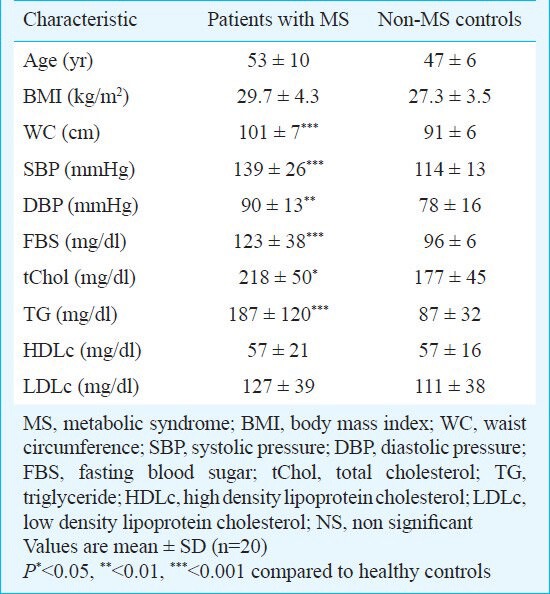
Table II.
Correlation between metabolic syndrome (MS) and some biochemical parameters
For each person and gene, ΔCT was computed and compared between the two groups. There was a significant difference in the IL-8 expression between the two groups (ΔCT3.48 ± 1.45 in MS group versus 2.14 ± 1.12 in Non-MS subjects, P=0.002), but IL-7 expression was not significantly different between the two groups (12.79 ± 1.1 versus 12.77 ± 0.7 in MS and non-MS groups P=0.94, respectively). Fold changes of gene expression in MS group compared to non-MS subjects was calculated by 2-ΔΔCT formula. MS patients expressed 1.01 ± 0.4 (mean ± SEM) fold IL-7 expression compared to non-MS subjects. IL-8 expression in PBMCs was significantly decreased in MS versus control subjects (fold change was 0.395±0.182).
Correlation study of both genes in the two groups showed that IL-7 was only associated with WBC count. A strong relationship was observed between IL-8 expression (ΔCT) and MS components (waist circumferences, systolic and diastolic blood pressure, TG, total cholesterol and LDLc). This association for TG and tChol was stronger than other factors (P=0.001, r = 0.511 and r = 0.500, respectively), while there was no association with HDLc and FBS. In MS group, IL-8 ΔCT showed a direct association with TG (r =0.470, P=0.036) but this relation was not observed in non-MS subjects.
Discussion
In the present study, we quantified the relative gene expression of IL-7 and IL-8 in PBMCs in MS subject and investigated correlation between these gene expression and other biochemical parameters. A study on mice showed that IL-7 had a regulatory effect on adipose tissue mass through a lymphocyte-independent mechanism but protective role of this cytokine on glucose homeostasis would be mediated by immune cells22. Relationship between this cytokine and insulin sensitivity and also adipose tissue mass indicated that the gene expression of IL-7 might be altered in process of metabolic syndrome22. Regulatory function of IL-7 in glucose utilization by lymphocytes supports this theory30. However, we did not find any change in gene expression of the cytokine.
IL-8 is hypothesized to involve in macrophage infiltration into adipose tissue in obesity and is reported to be associated with the development of obesity related disorders24. It is also reported that circulating IL-8 is increased in MS2,25,27. Thus, an increase in IL-8 expression in PBMC of MS subjects was expected compared to normal subjects, but our results showed vice versa. Therefore, it can be deduced that most of the IL-8 secreted in blood is from other sources including adipose tissue. It has been recently reported that IL-8 is released from adipose tissue in vitro26; our result also displayed that all MS subject had central obesity. It is possible that the increase in production from other sites induce negative feedback control in PBMC IL-8 expression.
It has been shown that calcium is a second messenger in many cell types and plays a key role in different cellular processes, additionally it has been previously reported that Ca++ is required for IL-8 production in neutrophils and mast cells. Increase in intracellular Ca++ may induce IL-8 gene expression and protein secretion through transcriptional and posttranscriptional regulation31,32. Further, results of another study from our laboratory with a large sample size (n=400) showed that Ca++ was diminished in MS subjects (Our unpublished data). Therefore, reduction in IL-8 expression may be considered as one of the Ca++ downfall consequences.
The major limitation of our study was small sample size that can could lead to type II errors. Another limitation was the fact that we intended the control group not to have any parameter of MS but finding age-matched women without any MS component was very difficult.
In summary, our preliminary findings showed an absence of detectable differences in IL-7 expression and decreased expression of IL-8 in PBMCs of patients with MS compared to control subjects. Study on a larger population and detecting synthesis of these proteins can reveal more details.
Acknowledgment
The authors thank the Hamadan University of Medical Sciences for financial support of this study. This study is a part of Ph.D thesis, of the second author (AST).
References
- 1.Sookoian S, Pirola CJ. Genetics of the cardiometabolic syndrome: new insights and therapeutic implications. Ther Adv Cardiovasc Dis. 2007;1:37–47. doi: 10.1177/1753944707082702. [DOI] [PubMed] [Google Scholar]
- 2.Shin MJ, Lee KH, Chung JH, Park YK, Choi MK, Oh J, et al. Circulating IL-8 levels in heart failure patients with and without metabolic syndrome. Clin Chim Acta. 2009;405:139–42. doi: 10.1016/j.cca.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 3.Ford ES, Li C, Cook S, Choi HK. Serum concentrations of uric acid and the metabolic syndrome among US children and adolescents. Circulation. 2007;115:2526–32. doi: 10.1161/CIRCULATIONAHA.106.657627. [DOI] [PubMed] [Google Scholar]
- 4.Warnberg J, Marcos A. Low-grade inflammation and the metabolic syndrome in children and adolescents. Curr Opin Lipidol. 2008;19:11–5. doi: 10.1097/MOL.0b013e3282f4096b. [DOI] [PubMed] [Google Scholar]
- 5.Isomaa B, Almgren P, Tuomi T, Forsén B, Lahti K, Nissén M, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24:683–9. doi: 10.2337/diacare.24.4.683. [DOI] [PubMed] [Google Scholar]
- 6.Ford ES, Giles WH, Dietz WH. Prevalence of metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–9. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 7.Bjorntorp P. Metabolic implications of body fat distribution. Diabetes Care. 1991;14:1132–43. doi: 10.2337/diacare.14.12.1132. [DOI] [PubMed] [Google Scholar]
- 8.Camargo A, Ruano J, Fernandez JM, Parnel LD, Jimenez A, Santos-Gonzalez M, et al. Gene expression changes in mononuclear cells in patients with metabolic syndrome after acute intake of phenol-rich virgin olive oil. BMC Genomics. 2010;11:253. doi: 10.1186/1471-2164-11-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bosello O, Zamboni M. Visceral obesity and metabolic syndrome. Obes Rev. 2000;1:47–56. doi: 10.1046/j.1467-789x.2000.00008.x. [DOI] [PubMed] [Google Scholar]
- 10.Pop-Busui R, Pietropaolo M. Metabolic syndrome and inflammation. In: Eisenbarth GS, editor. Immunoendocrinology: Scientific and clinical aspects. Totowa, NJ: Humana Press; 2011. pp. 69–92. [Google Scholar]
- 11.Sandhofer A, Kaser S, Ritsch A, Laimer M, Engl J, Paulweber B, et al. Cholesteryl ester transfer protein in metabolic syndrome. Obesity (Silver Spring) 2006;14:812–8. doi: 10.1038/oby.2006.94. [DOI] [PubMed] [Google Scholar]
- 12.Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288:2709–16. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 13.Pischon T, Hu FB, Rexrode KM, Girman CJ, Manson JE, Rimm EB. Inflammation, the metabolic syndrome, and the risk of coronary heart disease in women and men. Atherosclerosis. 2008;197:392–9. doi: 10.1016/j.atherosclerosis.2007.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shim WS, Kim HJ, Kang ES, Ahn CW, Lim SK, Lee HC, et al. The association of total and differential white blood cell count with metabolic syndrome in type 2 diabetic patients. Diabetes Res Clin Pract. 2006;73:284–91. doi: 10.1016/j.diabres.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Wang Z, Nakayama T. Inflammation, a link between obesity and cardiovascular disease. Mediators Inflamm. Available from: http://www.hindawi.com/journals/mi/2010/535918 . [DOI] [PMC free article] [PubMed]
- 16.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Odegaard JI, Chawla A. Mechanisms of macrophage activation in obesity induced insulin resistance. Nat Clin Pract Endocrinol Metab. 2008;4:619–26. doi: 10.1038/ncpendmet0976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacobs SR, Michalek RD, Rathmell JC. IL-7 is essential for homeostatic control of T cell metabolism in vivo. J Immunol. 2010;184:3461–9. doi: 10.4049/jimmunol.0902593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wofford JA, Wieman HL, Jacobs SR, Zhao Y, Rathmell JC. IL-7 promotes Glut1 trafficking and glucose uptake via STAT5-mediated activation of Akt to support T-cell survival. Blood. 2008;111:2101–11. doi: 10.1182/blood-2007-06-096297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barata JT, Silva A, Brandao JG, Nadler LM, Cardoso AA, Boussiotis VA. Activation of PI3K is indispensable for interleukin 7-mediated viability, proliferation, glucose use, and growth of T cell acute lymphoblastic leukemia cells. J Exp Med. 2004;200:659–69. doi: 10.1084/jem.20040789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bikker A, van Woerkom JM, Kruize AA, Wenting-van Wijk M, de Jager W, Bijlsma JW, et al. Increased expression of interleukin-7 in labial salivary glands of patients with primary Sjogren's syndrome correlates with increased inflammation. Arthritis Rheum. 2010;62:969–77. doi: 10.1002/art.27318. [DOI] [PubMed] [Google Scholar]
- 22.Lucas S, Taront S, Magnan C, Fauconnier L, Delacre M, Macia L, et al. Interleukin-7 regulates adipose tissue mass and insulin sensitivity in high-fat diet-fed mice through lymphocyte-dependent and independent mechanisms. PLoS One. 2012;7:e40351. doi: 10.1371/journal.pone.0040351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim HJ, Byun SJ, Kim TY. Differential regulation of IGF-II-induced IL-8 by extracellular signal-regulated kinase 1/2 and p38 mitogen-activated protein kinases in human keratinocytes. Biochem Biophys Res Commun. 2004;317:276–84. doi: 10.1016/j.bbrc.2004.03.042. [DOI] [PubMed] [Google Scholar]
- 24.Herder C, Haastert B, Müller-Scholze S, Koenig W, Thorand B, Holle R, et al. Association of systemic chemokine concentrations with impaired glucose tolerance and type 2 diabetes: results from the Cooperative Health Research in the Region of Augsburg Survey S4 (KORA S4) Diabetes. 2005;54(Suppl 2):S11–7. doi: 10.2337/diabetes.54.suppl_2.s11. [DOI] [PubMed] [Google Scholar]
- 25.Zozulinska D, Majchrzak A, Sobieska M, Wiktorowicz K, Wierusz-Wysocka B. Serum interleukin-8 level is increased in diabetic patients. Diabetologia. 1999;42:117–8. doi: 10.1007/s001250051124. [DOI] [PubMed] [Google Scholar]
- 26.Bruun JM, Lihn AS, Madan AK, Pedersen SB, Schiøtt KM, Fain JN, et al. Higher production of IL-8 in visceral vs subcutaneous adipose tissue. Implication of nonadipose cells in adipose tissue. Am J Physiol Endocrinol Metab. 2004;286:E8–13. doi: 10.1152/ajpendo.00269.2003. [DOI] [PubMed] [Google Scholar]
- 27.Azizi F, Hadaegh F, Khalili D, Esteghamati A, Hosseinpanah F, Delavari A, et al. Appropriate definition of metabolic syndrome among Iranian adults: Report of the Iranian National Committee of Obesity. Arch Iran Med. 2010;13:426–8. [PubMed] [Google Scholar]
- 28.Nilsson C, Aboud S, Karlén K, Hejdeman B, Urassa W, Biberfeld G. Optimal blood mononuclear cell isolation procedures for gamma interferon enzyme-linked immunospot testing of healthy Swedish and Tanzanian subjects. Clin Vaccine Immunol. 2008;154:585–9. doi: 10.1128/CVI.00161-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real time quantitative pcr and the 2-ΔΔct. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 30.Chehtane M, Khaled AR. Interleukin-7 mediates glucose utilization in lymphocytes through transcriptional regulation of the hexokinase II gene. Am J Physiol Cell Physiol. 2010;298:C1560–C71. doi: 10.1152/ajpcell.00506.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim MS, Lim WK, Park RK, Shin T, Yoo YH, Hong SH, et al. Involvement of mitogen-activated protein kinase and NF-kB activation in Ca2+↰-induced IL-8 production in human mast cells. Cytokine. 2005;32:226–33. doi: 10.1016/j.cyto.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 32.Kuhns DB, Young HA, Gallin EK, Gallin JI. Ca2+-dependent production and release of IL-8 in human neutrophils. J Immunol. 1998;161:4332–9. [PubMed] [Google Scholar]


