Abstract
Background & objectives:
Dengue is an arboviral disease of public health importance in many parts of India and recently many cases have been reported from northeastern India. Aedes mosquitoes, which are the vectors of dengue, are widely prevalent in the region. A study was initiated in Sonitpur district of Assam to understand the spatiotemporal distribution and seasonal prevalence of dengue vectors and to identify the high risk zones.
Methods:
Ovitrap surveys were conducted in three randomly selected villages under each of the eight public health centres (PHC) in district Sonitpur of Assam, northeastern India during March 2011 - February 2012. Three risk zones (high, medium and low) were identified on the basis of per trap density of Aedes mosquitoes. Meteorological data were collected to study the temporal distribution of dengue vectors.
Results:
Aedes albopictus (99.3%) was the predominant dengue vector followed by Ae. aegypti (0.7%) recorded in the ovitraps. The highest vector density was observed during the post-monsoon (60.1 ± 18 per trap) while the lowest during the winter (7.6 ± 4.9 per trap) and the season-wise differences in the vector density were significant (P=0.005). Maximum temperature (correlation coefficient, r = 0.45) and minimum temperature (r = 0.408) showed the highest positive correlation with the vector density, whereas the number of rainy days showed high positive correlation (r = 0.185) than the total rainfall (r = 0.117). The high risk zone (Dekhiajuli, Behali, Bihaguri and Gohpur PHC) as indicated by the high larval densities of dengue vectors, 45.3 ± 18, 42.1 ± 22.3, 36.9 ± 29.1, 35.3 ± 22.6 per trap, respectively, was validated by dengue epidemiological data collected during 2012.
Interpretation & conclusions:
Yearlong monitoring of dengue vectors was done for the first time in this region. Monthly maximum temperature and the number of rainy days could be used for the prediction of larval density of Aedes mosquitoes. The identification high dengue risk zones would help in adopting targeted interventions for disease management in future.
Keywords: Dengue, mosquito vector, northeastern India, ovitrap surveillance, risk zone
Dengue has emerged as one of the most important arboviral diseases affecting mankind and currently about half of the world's population is at the risk of infection. It is estimated that dengue occurs in more than 100 countries affecting 50-100 million people each year1. The disease has a major socio-economic and public health impact on the countries from where the epidemics are reported2. Dengue is transmitted mainly by Aedes aegypti and also by Ae. albopictus mosquitoes3. The control of Aedes mosquitoes is the only available strategy to prevent dengue transmission and to decrease the disease burden4. Ae. aegypti, the principal vector of dengue is highly adapted to all manmade and natural environments and is closely associated with human dwellings5. Ae. albopictus, is an invasive species, which is able to transmit a large number of arboviral diseases including dengue and chikungunya. The geographical spread of this mosquito is being monitored worldwide with great concern6.
Unplanned urbanization, the lack of proper waste management and inadequate vector control measures have created favourable conditions for dengue virus and its mosquito vectors in many of the tropical developing countries including India2. Initially restricted to urban areas, the occurrence of dengue infections has now spread to the rural areas in India7. Surveillance of dengue vectors in northeastern India is important as many cases of dengue are being reported the region8. Ovitrap surveillance is a preferred method for monitoring Aedes mosquitoes due to low material costs, high sensitivity and the ease of management9. The studies on the potential vectors of dengue in Assam have indicated that Ae. albopictus is the dominant species in the semi-urban and rural areas7. However, the seasonal prevalence of Aedes mosquitoes in this part of the country and its relationship with the meteorological parameters are not understood properly.
The armed forces personnel deployed in northeastern India are more vulnerable to the incidence of vector borne diseases such as dengue, malaria, etc. due to their patrolling activities and increased exposure to the environment10. Sonitpur district of Assam, northeastern India has many military and paramilitary stations including the 4 Corps headquarters and the Air Force station, Tezpur. Hence the studies on dengue vectors and the identification of high risk zones were initiated in Sonitpur district. Yearlong ovitrap surveillance was carried out in the district to estimate the spatiotemporal distribution of the potential dengue vectors, Ae. aegypti and Ae. albopictus. The spatial distribution of the vectors as indicated by the ovitrap surveys was used for the identification of high risk zones whereas the temporal distribution was studied in relation to the meteorological parameters. The high risk zone identified in the district was validated with the data on dengue incidence during the following year.
Material & Methods
Study areas: Sonitpur district of Assam, India, is situated on the north bank of the river Brahmaputra. This study was carried out in three randomly selected villages under each of the eight public health centres (PHC) in the district during March 2011 - February 2012. The 24 study villages were namely, Chariduar, Goroimari, Bongaon (Balipara PHC), Borgang, Behali, Helem (Behali PHC), Puthimari, Bihaguri, Baligaon (Bihaguri PHC), Biswanath Chariali, Sootea, Biswanath Ghat (Biswanath Chariali PHC), Belsiri, Sirajuli, Dekhiajuli (Dekhiajuli PHC), Kauri Pathar, Itapara, Gohpur (Gohpur PHC), Jamugurighat, Rangasukua, North Jamuguri (North Jamuguri PHC) Borjuli, Nahoroni and Rangapara (Rangapara PHC).
Ovitrap surveillance: Black polyethylene containers (350 ml) with blotting paper taped on the inner walls as the oviposition substrate and filled with 300 ml of water were used as the ovitraps. Ten ovitraps were placed nearby households in each of the selected villages and the larvae were collected twice during each month. The larval density per trap for each PHC was recorded as larval density = number of larvae / number of ovitraps. Season-wise larval density was estimated for pre-monsoon (March-May), monsoon (June-August), post-monsoon (September-November) and winter (December-February). The collected larvae were identified based on standard keys11. The district was divided into three risk zones comprising of PHC with high (>30), medium (15-30) and low (<15) per trap density of Ae. albopictus and Ae. aegypti larvae.
Meteorological data: The data on maximum temperature (Tmax), minimum temperature (Tmin), morning relative humidity (0830 h; RH1), evening relative humidity (1730 h; RH2), total rainfall (RF) and number of rainy days (RD) during March 2011 - February 2012 were obtained from the North Eastern Regional Institute of Water and Land Management (NERIWALM), Tezpur, Assam (India). Mean temperature was derived as Tmean = (Tmax + Tmin) / 2 whereas mean RH was derived as RHmean = (RH1 + RH2) / 2.
Epidemiological data: The data on dengue incidence during 2012 were obtained from the Office of the District Malaria Officer, Sonitpur, India.
Data analysis: The mean larval density for each PHC was subjected to square root transformation before statistical analyses. Analysis of variance (ANOVA) was used for season-wise analysis of larval densities. Step-wise regression analysis and Pearson correlation were used to derive the relationships between the larval density and meteorological parameters. IBM SPSS Statistics 19 (IBM, USA) software was used for statistical analysis of the data.
Results
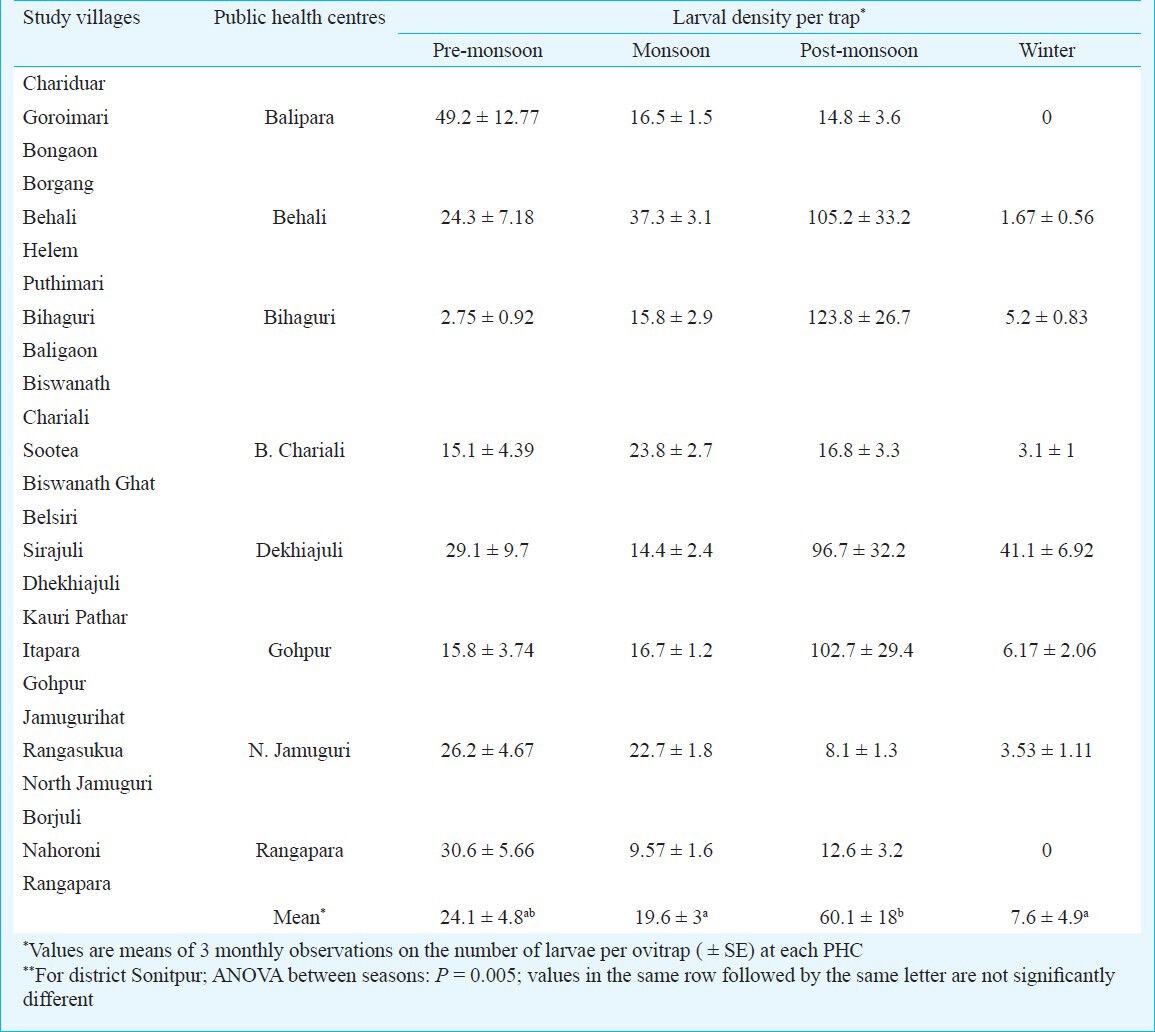
The ovitrap collections of Aedes mosquitoes were almost entirely made up of Ae. albopictus (99.3%) whereas Ae. aegypti was rarely recorded. The latter was observed in very low densities from the study areas under Balipara PHC of the district. Analysis of the month-wise density of Aedes larvae in the study areas indicated that the peak densities (mean number of larvae per trap ± SE) were recorded during September (28 ± 14.4), November (26.2 ± 15.5) and May (15 ± 4.7). There were no larvae observed in the ovitraps across the district during January (Figure). The larval density during pre-monsoon was the highest in areas under Balipara PHC (49.2) and the lowest in areas under Bihaguri PHC (2.75). The density during monsoon ranged from 9.57 (Rangapara) to 37.3 (Behali) whereas the density during post-monsoon ranged from 8.1 (North Jamuguri) to 123.8 (Bihaguri). In winter, the highest larval density was recorded in Dekhiajuli (41.1) while no larvae were collected from Rangapara and Balipara. The peak density of Aedes larvae recorded in the study was in Bihaguri PHC during the post-monsoon season. The overall larval density in the district during the pre-monsoon, monsoon, post-monsoon and winter seasons were 24.1 ± 4.8, 19.6 ± 3, 60.1 ± 18 and 7.6 ± 4.9, respectively. The seasonal variations in larval density in the district were significantly different (P = 0.005) (Table I).
Fig.
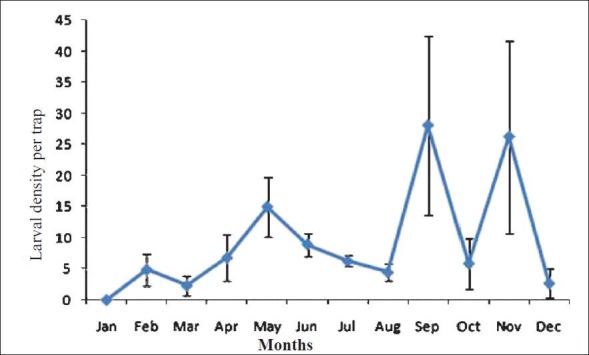
Month-wise pattern of abundance of dengue vectors in district Sonitpur of Assam, India.
Table I.
Seasonal prevalence of dengue vectors in district Sonitpur of Assam, India
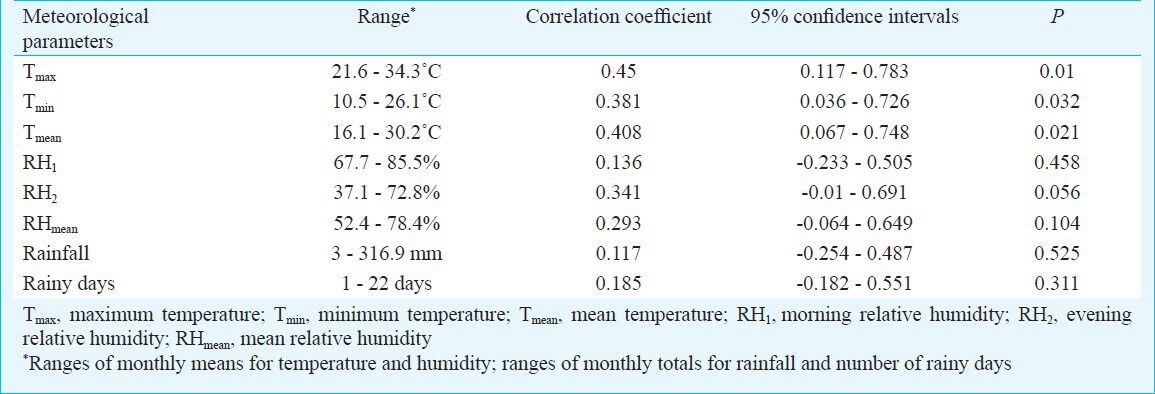
The ranges of the monthly means of maximum temperature, minimum temperature, mean temperature, morning relative humidity, evening relative humidity and mean relative humidity were 21.6 - 34.3˚C, 10.5 - 26.1˚C, 16.1 - 30.2˚C, 67.7 - 85.5 per cent, 37.1 - 72.8 per cent and 52.4 - 78.4 per cent, respectively. The monthly total rainfall and the number of rainy days ranged from 3 to 316.9 mm and 1 to 22, respectively. Maximum temperature was having the highest positive correlation with the vector density (r = 0.45; P = 0.01) followed by mean temperature (r = 0.408; P = 0.021) and minimum temperature (r = 0.381; P = 0.032). Positive but not significant correlations were observed between the larval density, morning relative humidity (r = 0.136), evening relative humidity (r = 0.341), mean relative humidity (r = 0.293), rainfall (r = 0.117) and number of rainy days (r = 0.185) (Table II). Step-wise regression analysis between the weather parameters and dengue vector density indicated that the monthly maximum temperature (Tmax) along with the number of rainy days (RD) have relatively high predictive value on the larval density (LD). The expression of larval density in terms of these weather parameters was derived as LD = 1.068 Tmax - 0.15 RD - 23.359 (R2 = 0.385; P = 0.001).
Table II.
Relationships between the meteorological parameters and the density of dengue vectors in district Sonitpur of Assam, India
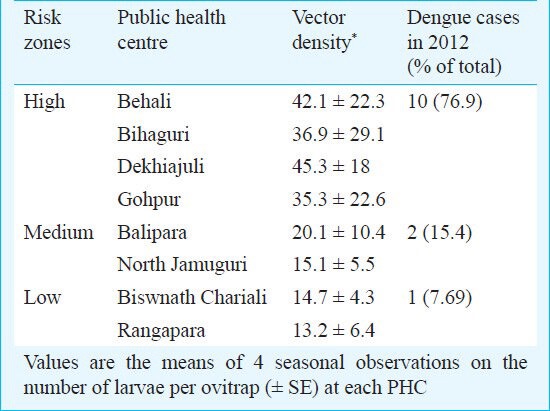
Dekhiajuli (45.3 ± 18), Behali (42.1 ± 22.3), Bihaguri (36.9 ± 29.1) and Gohpur (35.3 ± 22.6) had high larval densities of Aedes spp., whereas Balipara (20.1 ± 10.4) and North Jamuguri (15.1 ± 5.5) showed medium densities. The lowest vector densities were recorded in Biswanath Chariali (14.7 ± 4.3) and Rangapara (13.2 ± 6.4). Of the 13 dengue cases reported in 2012, 10 (76.9%) were from the high risk zone comprising Behali, Bihaguri, Dekhiajuli and Gohpur PHC. There were two dengue cases reported from the medium risk zone Balipara and North Jamuguri, whereas one case was reported from the low risk zone Biswnath Chariali and Rangapara (Table III).
Table III.
High risk zones identified and the dengue cases reported in Sonitpur district of Assam, India
Discussion
The epidemics of dengue, transmitted by Ae. aegypti and Ae. albopictus, are on the rise worldwide especially in the tropical developing countries12. The outbreak of dengue in Manipur, northeastern India during 2007 and the subsequent increase in the number of cases show the vulnerability of this region to the disease8. The control measures of this mosquito borne disease are largely dependent on the reduction of vector populations12. Ovitrap is a rapid, inexpensive and sensitive tool for the monitoring of dengue vectors and is being widely used for mosquito surveillance, spatial distribution studies and for the evaluation of the efficacy of control measures13. In the present study, ovitrap surveys throughout the year for the monitoring of dengue vectors were carried out. The PHCs of Sonitpur district were taken as the sampling units as the larval density of dengue vectors could be correlated with the dengue incidence reported from each PHC in case of future outbreaks.
The transmission of mosquito borne diseases is climate sensitive as the mosquitoes need water to breed and ambient temperature is critical to the larval development and the feeding behaviour of adults14. As per the theoretical models, the transmission patterns of dengue are influenced by temperature and precipitation15. Temperature affects the egg viability, larval development, adult longevity and dispersal, whereas rainfall affects the abundance and productivity of the breeding habitats of Aedes mosquitoes16. The temporal dynamics of Ae. aegypti populations in San Juan City, Puerto Rico was found to be positively associated with rainfall and temperature17. Ovitrap surveys in northern Sri Lanka revealed a significant positive correlation between Aedes density and rainfall18. The increase in mosquito density and the number of dengue cases in Manipur, northeastern India were attributed to the significant changes in rainfall, temperature and relative humidity between 2000-2004 and 2005-20088. A temperature range of 18-33.2°C is considered to be ideal for the transmission of dengue fever and the frequency of feeding increases with temperature19. The increase in temperature has been found to augment dengue incidence in many countries including Thailand, Indonesia and Mexico20.
The analysis of the seasonal pattern of dengue vector density in the study areas indicated that high densities occur during September-November (post-monsoon), which received 183.1 mm of total rainfall. This could be attributed to the availability of breeding habitats such as plant axils, bamboo stumps and discarded coconut shells, which are filled with water after the rains. However, the larval density was relatively low during the monsoon as heavy rains during this period (643.6 mm) were detrimental to the larval breeding in the container habitats. Hence, vector control measures should be intensified after the monsoon rains so as to prevent probable disease outbreaks. The density of dengue vectors was very low during the winter due to low temperature and drying up of the container habitats. All weather parameters studied, including temperature, relative humidity and rainfall were observed to be positively correlated with the dengue vector density. Maximum temperature had the significant positive correlation with the Aedes populations, whereas the number of rainy days rather than the total rainfall was positively correlated with the larval density. Maximum temperature and the number of rainy days accounted for 38.5 per cent variability in dengue vector density in the district.
Dengue vector surveillance could be improved by Aedes larval sampling methods and by the estimation of the spatial distribution of larvae21. In the present study, the larval density of dengue vectors was used for the estimation of the risk of disease transmission in Sonitpur district. The high risk zone (Dekhiajuli, Behali, Gohpur and Bihaguri PHC) was validated by the dengue incidence data during 2012. The majority (76.9%) of the cases reported during the year were from the high risk zone. Surveillance for probable detection of dengue infections, monitoring of vector activity and initiation of vector control measures should be ensured so as to prevent disease transmission in the high risk zones. The medium risk zone (Balipara and North Jamuguri PHC) had 15.4 per cent of the dengue cases, whereas the low risk zone (Biswanath Chariali and Rangapara PHC) had 7.69 per cent of the cases. In the medium and low risk zones, the proliferation of dengue vectors could be prevented by reducing the availability of water-holding containers suitable for mosquito breeding.
The relationships established between the weather parameters and the abundance of dengue vectors in the study areas could provide valuable inputs for the development of a decision support system for dengue in northeastern India. Further studies are needed in other parts of the region to understand the seasonal prevalence of Aedes mosquitoes and the factors contributing to their distribution and abundance. The identification of dengue risk zones in Sonitpur district was based solely on the larval density of the potential dengue vectors. However, disease outbreaks depend on factors such as the source of infection, climate and a susceptible human population apart from vector density14. As dengue cases are being reported from Assam and other parts of northeastern India, there is a need to identify high risk zones so that future outbreaks could be avoided by targeted interventions.
Acknowledgment
The authors thank the District Malaria Officer and Staff of Sonitpur, Assam, India for their help during the conduct of the study.
References
- 1.Sabchareon A, Wallace D, Sirivichayakul C, Limkittikul K, Chanthavanich P, Suvannadabba S, et al. Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: a randomised, controlled phase 2b trial. Lancet. 2012;380:1559–67. doi: 10.1016/S0140-6736(12)61428-7. [DOI] [PubMed] [Google Scholar]
- 2.Gubler DJ. The economic burden of dengue. Am J Trop Med Hyg. 2012;86:743–4. doi: 10.4269/ajtmh.2012.12-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta N, Srivastava S, Jain A, Chaturvedi UC. Dengue in India. Indian J Med Res. 2012;136:373–90. [PMC free article] [PubMed] [Google Scholar]
- 4.Arunachalam A, Tyagi BK, Samuel M, Krishnamoorthi R, Manavalan R, Tewari SC, et al. Community-based control of Aedes aegypti by adoption of eco-health methods in Chennai City, India. Pathog Glob Health. 2012;106:488–96. doi: 10.1179/2047773212Y.0000000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oo TT, Storch V, Madon MB, Becker N. Factors influencing the seasonal abundance of Aedes (Stegomyia)aegypti and the control strategy of dengue and dengue haemorrhagic fever in Thanlyin Township, Yangon City, Myanmar. Trop Biomed. 2011;28:302–11. [PubMed] [Google Scholar]
- 6.Guillaumot L, Ofanoa R, Swillen L, Singh N, Bossin HC, Schaffner F. Distribution of Aedes albopictus (Diptera, Culicidae) in southwestern Pacific countries, with a first report from the Kingdom of Tonga. Parasit Vectors. 2012;5:247. doi: 10.1186/1756-3305-5-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dutta P, Mahanta J. Potential vectors of dengue and the profile of dengue in the north-eastern region of India: an epidemiological perspective. Dengue Bull. 2006;30:234–42. [Google Scholar]
- 8.Sankari T, Hoti SL, Singh TB, Shanmugavel J. Outbreak of dengue virus serotype-2 (DENV-2) of Cambodian origin in Manipur, India - association with meteorological factors. Indian J Med Res. 2012;136:649–55. [PMC free article] [PubMed] [Google Scholar]
- 9.Carrieri M, Albieri A, Angelini P, Baldacchini F, Venturelli C, Zeo SM, et al. Surveillance of the chikungunya vector Aedes albopictus (Skuse) in Emilia-Romagna (northern Italy): organizational and technical aspects of a large scale monitoring system. J Vector Ecol. 2011;36:108–16. doi: 10.1111/j.1948-7134.2011.00147.x. [DOI] [PubMed] [Google Scholar]
- 10.Dhiman S, Gopalakrishnan R, Goswami D, Rabha B, Baruah I, Singh L. Malaria incidence among paramilitary personnel in an endemic area of Tripura. Indian J Med Res. 2011;133:665–9. [PMC free article] [PubMed] [Google Scholar]
- 11.Barraud PJ. London, UK: Taylor and Francis; 1934. The fauna of British India, including Ceylon and Burma. Diptera, vol. V. Family Culicidae: Tribes Megarhinini and Culicini. [Google Scholar]
- 12.Chang AY, Parrales ME, Jimenez J, Sobieszczyk ME, Hammer SM, Copenhaver DJ, et al. Combining Google Earth and GIS mapping technologies in a dengue surveillance system for developing countries. Int J Health Geogr. 2009;8:49. doi: 10.1186/1476-072X-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ríos-Velásquez CM, Codeço CT, Honório NA, Sabroza PS, Moresco M, Cunha IC, et al. Distribution of dengue vectors in neighborhoods with different urbanization types of Manaus, state of Amazonas, Brazil. Mem Inst Oswaldo Cruz. 2007;102:617–23. doi: 10.1590/s0074-02762007005000076. [DOI] [PubMed] [Google Scholar]
- 14.Hales S, de Wet N, Maindonald J, Woodward A. Potential effect of population and climate changes on global distribution of dengue fever: an empirical model. Lancet. 2002;360:830–4. doi: 10.1016/S0140-6736(02)09964-6. [DOI] [PubMed] [Google Scholar]
- 15.Johansson MA, Dominici F, Glass GE. Local and global effects of climate on dengue transmission in Puerto Rico. PLoS Negl Trop Dis. 2009;3:e382. doi: 10.1371/journal.pntd.0000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Honório NA, Codeço CT, Alves FC, Magalhães MA, Lourenço-De-Oliveira R. Temporal distribution of Aedes aegypti in different districts of Rio de Janeiro, Brazil, measured by two types of traps. J Med Entomol. 2009;46:1001–14. doi: 10.1603/033.046.0505. [DOI] [PubMed] [Google Scholar]
- 17.Barrera R, Amador M, MacKay AJ. Population dynamics of Aedes aegypti and dengue as influenced by weather and human behavior in San Juan, Puerto Rico. PLoS Negl Trop Dis. 2011;5:e1378. doi: 10.1371/journal.pntd.0001378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Surendran SN, Kajatheepan A, Sanjeefkumar KF, Jude PJ. Seasonality and insecticide susceptibility of dengue vectors: an ovitrap based survey in a residential area of northern Sri Lanka. Southeast Asian J Trop Med Public Health. 2007;38:276–82. [PubMed] [Google Scholar]
- 19.Hu W, Clements A, Williams G, Tong S, Mengersen K. Spatial patterns and socioecological drivers of dengue fever transmission in Queensland, Australia. Environ Health Perspect. 2012;120:260–6. doi: 10.1289/ehp.1003270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gharbi M, Quenel P, Gustave J, Cassadou S, La Ruche G, Girdary L, et al. Time series analysis of dengue incidence in Guadeloupe, French West Indies: Forecasting models using climate variables as predictors. BMC Infect Dis. 2011;11:166. doi: 10.1186/1471-2334-11-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chansang C, Kittayapong P. Application of mosquito sampling count and geospatial methods to improve dengue vector surveillance. Am J Trop Med Hyg. 2007;77:897–902. [PubMed] [Google Scholar]


