Abstract
The mechanisms behind the resistance to human immunodeficiency virus type 2 (HIV-2) infection are still not fully understood. In the present study, we explored the HIV-2-specific humoral serum immunoglobulin A (IgA) immune response in HIV-2-exposed IgG-seronegative (EGSN) individuals. Serum samples from heterosexual EGSN individuals and their known HIV-2-infected partners, as well as controls originating from Guinea-Bissau in Africa, were studied. Antibody reactivity to native and recombinant envelope glycoproteins was investigated, and the capacity of purified serum IgA to neutralize HIV-2SBL6669 was tested. Our results showed that 16 of 25 EGSN samples exhibited reactivity against whole HIV-2 antigen, 6 of 25 samples reacted with recombinant gp36 (rgp36), and 3 of 25 samples were positive against HIV-2 rgp105; no reactivity to native HIV-2 gp125 was detected. Purified serum IgA antibodies from both EGSN and HIV-2-positive individuals, but not that from the negative controls, exhibited neutralization of HIV-2SBL6669. The most potent neutralization activity was exhibited by IgA purified from EGSN compared to infected individuals' IgA. The antigenic pattern of the HIV-2-positive partners showed that all serum IgA samples were reactive to whole HIV-2 antigen, and 14 of 15 reacted with rgp36. For rgp105 and gp125, 5 of 15 and 4 of 15 samples exhibited binding, respectively. The serum of the EGSN group had a higher mean IgA concentration than that of the negative controls (P < 0.05). Thus, we describe HIV-2-specific serum IgA antigen reactivity and show a more potent serum IgA-mediated HIV-2-neutralizing activity in EGSN individuals than in HIV-2-infected patients.
Human immunodeficiency virus type 2 (HIV-2), like HIV-1, is associated with terminal AIDS and is mainly transmitted heterosexually (1, 16, 31). It is largely confined to West Africa, with the highest prevalence rates reported in Guinea-Bissau, but a high number of cases has also been reported in Portugal and India (38, 46). Epidemiologic observations indicate a lower transmission rate for HIV-2, as well as a lower pathogenicity, than for HIV-1. The generally high CD4 T-cell count and lower circulating viral load in HIV-2-infected individuals compared to those in HIV-1-infected persons have been hypothesized to contribute to the differences seen (11).
A more vigorous immune response may also play a role in the lack of disease progression seen in HIV-2 infection. HIV-specific cell-mediated immune responses seem to be induced in a larger proportion of HIV-2 carriers than among HIV-1-infected persons (reviewed in reference 2). In addition, it has been reported that autologous neutralizing antibodies prevail in HIV-2 but not in HIV-1 infection (10). Later reports have shown that the neutralizing anti-HIV-2 immunoglobulin G (IgG) antibody response is strain specific and directed against the third variable region (V3) (9, 41).
It is generally thought that multiple factors contribute to resistance to HIV-1 infection. These factors includes inherited and acquired host factors, such as a homozygous 32-bp deletion in the gene encoding the HIV-1 coreceptor CCR5 (30), genetic HLA polymorphisms (37), HIV-specific helper and cytotoxic T cells (5, 18, 25, 28, 33, 45, 47), and mucosal and systemic anti-HIV IgA (4, 22, 26, 32, 40).
Humoral immune responses in highly exposed, persistently seronegative individuals have recently drawn greater interest in research. It is becoming more evident that both specific humoral and cellular immune responses play a role in the resistance of such individuals to HIV-1 infection (19, 24, 44). Investigations of HIV-specific IgA in several African cohorts and in female sex workers from Thailand who have been repeatedly exposed to HIV but not infected suggest that HIV-1-specific IgA antibody may act as an important component in the systemic and local mucosal compartments (6, 21, 22, 26, 39, 40, 42). The role of serum IgA immune responses in protection from HIV-2 is still not fully known. We have recently shown that HIV-2-specific serum IgA has the capacity to neutralize a well-documented HIV-2 strain, SBL6669. The serum IgA primarily bound a region within the HIV-2 transmembrane gp36 (amino acids 644 to 658) (35). Taken together, the results of HIV-1 and HIV-2 studies indicate that HIV-specific IgA immune responses directed against envelope proteins with neutralizing capability may be important in host-pathogen interactions.
To further explore and elucidate the role of humoral immune responses in resistance to HIV-2 infection, we studied serum IgA derived from highly HIV-2-exposed but IgG-seronegative (EGSN) individuals originating from Guinea-Bissau. These EGSN individuals were identified by a well-established diagnostic procedure that discriminates infected individuals from noninfected individuals. Thus, we consider these EGSN individuals to be uninfected. The HIV-2-specific IgA immune response to envelope proteins (recombinant gp36 [rgp36], rgp105, and native gp125), as well as whole-virus lysate (HIV-26669), was investigated. Furthermore, the capacity to neutralize HIV-2SBL6669 was tested. The results showed that HIV-2-specific IgA in EGSN samples preferentially bind to rgp36 rather than gp125. A high rgp36 binding capacity was also found in the HIV-2-positive partners. Furthermore, purified serum IgA exhibited neutralizing activity, with more potent neutralization elicited by EGSN IgA than by their positive partners' samples. This is the first report that describes HIV-2-specific serum IgA antigen reactivity and potent HIV-2-neutralizing activity in EGSN individuals.
MATERIALS AND METHODS
Study groups.
A group of HIV-2-discordant married couples, i.e., in which one of the individuals in each couple was HIV-2 infected and the other was not, were identified through a population-based prospective cohort study in Guinea-Bissau (34). Of the 25 EGSN persons enrolled in our study, 6 were men and 19 were women, with a median age of 39 years (25th and 75th percentiles, 29 and 51 years). The median number of children was 6 per EGSN individual (25th and 75th percentiles, 4 and 8; range, 0 to 17). Fifteen HIV-2-positive partners were also included. Fifteen control samples were collected from a group of healthy, HIV-negative individuals from Bissau, and 14 samples were used as controls in the analysis of enzyme-linked immunosorbent assay (ELISA) data. Blood samples were drawn from the subjects, whole blood was centrifuged at 400 × g, and serum was separated and stored immediately at −20°C until analyzed. The serum samples were inactivated by heating at 56°C for 30 min before use and run blindly in all of the assays. To determine serological status, the samples from the population-based cohort (EGSN persons and HIV-2-positive persons) were screened with the Murex ICE HIV-1.O.2 ELISA (Murex, Dartford, England). Screening-reactive samples were confirmed by Pepti-LAV 1-2 (Sanofi Diagnostics Pasteur, Marnes-la-Coquette, France) as previously described (20). The group of healthy controls was tested for HIV antibodies in accordance with a similar alternative testing strategy described elsewhere (3).
Purification of IgA and IgG antibodies.
IgA was purified from serum on Artocarpus integrifolia agglutinin (Jacalin; Vector, Burlingame, Calif.), and IgG was purified on protein G-Sepharose (Pharmacia, Uppsala, Sweden) as previously described in detail (35). Briefly, a 200-μl volume of Jacalin-agarose beads was added to 20 μl of serum diluted 1:10 in dilution buffer (2% [vol/vol] Triton X-100, 150 mM NaCl, 600 mM KCl, 5 mM disodium EDTA, pH 7.8). The mixture was incubated on an end-to-end rotator at room temperature for 2 h. After binding, the mixture was centrifuged for 3 min (10,000 × g), washed three times with extraction buffer (2% [vol/vol] Triton X-100, 150 mM NaCl, 600 mM KCl, 5 mM disodium EDTA, 3 mM phenylmethylsulfonyl fluoride, 1 μg of aprotinin/ml in 10 mM Tris-HCl, pH 7.8), and then eluted overnight at 4°C with 50 μl of 200 mM methyl-α-d-galactopyranoside. On the following day, the supernatant was harvested after centrifugation for 3 min (3,000 × g). An equal volume of protein G-Sepharose (Pharmacia, Uppsala, Sweden) was added, and the mixture was incubated on an end-to-end rotator for 2 h to exclude remaining IgG from the first purification step. After centrifugation for 3 min (3,000 × g), the supernatant containing IgA was harvested. The protein G-Sepharose was washed with start buffer (20 mM Na2HPO4, pH 7.0), and IgG was eluted with elution buffer (0.1 M glycine-HCl, pH 2.2) for 10 min, followed by centrifugation for 1 min (10,000 × g). After harvesting, the eluted IgG was immediately adjusted to pH 7 with neutralization buffer (2.5 M Tris-base, pH 9.0).
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
Ready gels were commercially obtained from Bio-Rad Laboratories. Purified IgA and IgG samples (5 μl) were mixed with 15 μl of sample buffer in Eppendorf tubes and heated to 100°C for 3 min; thereafter, the samples (20 μl) were loaded onto the gel. A 5-μl volume of molecular weight markers was included in each run as a reference (Amersham Laboratories, Amersham, Buckinghamshire, Great Britain). The electrophoresis procedure was performed as detailed before (35).
Quantification of immunoglobulins.
An ELISA was used for quantification of total (and purified) IgA and IgG and also as a control for residual serum IgA and IgG, together with sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Human IgA (Jackson Laboratories, West Grove, Pa.) and IgG (Calbiochem, San Diego, Calif.) were used to generate standards. Optical density (OD) values were analyzed with DS3-1.46B software (DeltaSoft 3; Biometallic Inc.) to calculate the concentrations of IgA and IgG.
Whole HIV-2 antigen, native gp125, and recombinant glycoproteins used in ELISA.
Whole killed HIV-2SBL6669 virions purified from cultured U937:2 cells were used as whole HIV-2 antigen and were kindly provided by G. Biberfeld at the Swedish Institute for Infectious Disease Control (8). rgp105, derived from HIV-2ROD, was produced in a baculovirus expression system. rgp105 and rgp36 were commercially obtained from Bartels, Trinity Biotech Plc., Wicklow, Ireland, and ViroGen Corp., Watertown, Mass., respectively. Galanthus nivalis agglutinin-purified native gp125 derived from HIV-2SBL6669 was kindly provided by the Department of Virology at the Swedish Institute for Infectious Disease Control (27).
ELISA.
Whole HIV-2 antigen, rgp36, rgp105, and native gp125 ELISAs were performed on purified immunoglobulin fractions and whole serum samples as described elsewhere (35). Briefly, microtiter plates (Nunc, Roskilde, Denmark) were coated overnight with whole HIV-2 antigen (0.1 μg/well), rgp36 (0.1 μg/well), gp105 (0.1 μg/well), or gp125 (0.1 μg/well). Blocking was performed with 10% bovine serum albumin at 37°C for 2 h. After washing, purified IgA or IgG (diluted 1:20) and serum (diluted 1:100) were added and the mixture was incubated for 1 h at 37°C. After another wash, for IgG detection, alkaline phosphatase-conjugated goat anti-human IgG (Sigma, St. Louis, Mo.) diluted 1:2,000 in buffer (20% goat serum, 20% fetal calf serum, 0.5% bovine serum albumin, 0.05% Tween 20) was added. p-Nitrophenylphosphate (Sigma) was used as the substrate. After a 30-min reaction, OD values were read at 405 nm. To detect IgA, biotinylated goat anti-human IgA (Southern Biotechnology, Birmingham, Ala.) was used at a 1:10,000 dilution, followed by streptavidin-horseradish peroxidase (Southern Biotechnology). For visualization of the horseradish peroxidase conjugates, o-phenylenediamine dihydrochloride substrate (Sigma) was added. The reaction was allowed to continue for 30 min and was stopped by adding 50 μl of 2.5 M H2SO4 per well. OD values were read at 490 and 630 nm. Samples were tested in duplicate, repeated at least twice, and regarded as positive when the mean OD values of duplicates exceeded the mean OD obtained with negative control serum plus three standard deviations.
Neutralization assay.
The capacity of purified IgA and IgG, as well as whole serum, to neutralize HIV-2SBL6669 was tested in an assay with phytohemagglutinin-stimulated peripheral blood mononuclear cells (35). Briefly, antibodies or sera were incubated for 1 h at 37°C with diluted tissue culture supernatant of virus-infected peripheral blood mononuclear cells (40 to 100 50% tissue culture infective doses, 100 μl). Peripheral blood mononuclear cells (105 in 50 μl) were added to the virus-antibody reaction mixture, and the mixture was incubated overnight. All dilutions were performed with RPMI 1640 medium (GIBCO, Life Technologies Ltd., Paisley, Scotland) supplemented with 10% fetal calf serum, 3 mM glutamine, 20 IU of interleukin-2, and antibiotics. Medium changes were performed on days 1 and 4. Seven days after infection, supernatants were collected and analyzed for HIV-2 antigen by a capture ELISA (48). The neutralization titer was defined as the reciprocal of the last dilution step that showed an 80% or greater reduction in the OD at 490 nm of the culture supernatant compared to that of HIV antibody-negative serum. Titers equal to or greater than 20 were considered to represent positive neutralization. A previously defined HIV-2-neutralizing serum sample with a known neutralization titer was used as a positive control in the neutralization assays. The purified immunoglobulin samples were not concentrated after purification. The purified immunoglobulin samples used in the neutralization assay constituted a one-fifth dilution since 20 μl of serum was used initially and the final volume after elution was 100 μl. The neutralization assay was performed in six steps at twofold dilutions on at least two occasions. Results are reported as the reciprocal dilution in the neutralization assay and not as the final serum dilution.
Statistical analysis.
The unpaired t test was used for comparison of IgA concentrations in serum samples. The Wilcoxon signed rank sum test was used for comparison of the HIV-2-neutralizing capacities of purified EGSN and HIV-2-positive IgA, which are given as the titer per milligram. Analysis was performed with StatView (Abacus Concepts, Inc., Berkeley, Calif.).
RESULTS
Purification of IgA.
An average efficiency of IgA1 purification from all whole serum samples of 60% was achieved, with yields ranging from 0.32 to 1.19 (average, 0.58) mg/ml.
IgA purity and possible residual IgG contamination were assessed with Coomassie-stained electrophoresis gels. No high-molecular-weight proteins or residual IgG in the serum samples could be detected. To confirm these results, an ELISA was performed with the same samples to determine that the amount of residual IgG did not exceed 0.1 μg/ml. A similar procedure was performed for purified IgG to eliminate IgA contamination. The IgG purification yields ranged from 0.16 to 2.31 (average, 0.69) mg/ml.
Quantification of total serum IgA.
The ranges of total IgA concentrations detected in serum samples from the EGSN group, HIV-2-positive individuals, and healthy controls were 2.05 to 8.46, 3.61 to 7.09, and 2.33 to 6.23 mg/ml, respectively. Owing to lack of material, HIV-2-positive sample 458 could not be quantified. The mean serum IgA concentrations in the three groups were as follows: EGSN group, 4.94 mg/ml; HIV-2-infected group, 4,77 mg/ml; healthy controls, 3.81 mg/ml. The mean total serum IgA concentration derived from EGSN persons did not differ from that of the HIV-2-positive group. However, serum IgA levels in EGSN persons were significantly higher than those of the HIV-2-negative controls (P < 0.05, unpaired t test; Table 1).
TABLE 1.
HIV-2-specific serum IgA reactivity to whole HIV-2 antigen, rgp36, rgp105, and gp125 in EGSN individuals, HIV-2-positive patients, and HIV-2-seronegative controls
| Antigen or parameter | No. of samples reactive/total (%)
|
||
|---|---|---|---|
| EGSN individuals | HIV-2-positive patients | Controls | |
| Whole HIV-2 | 16/25 (64) | 15/15 (100) | 0/15 |
| rgp36 | 6/25 (24) | 14/15 (93) | 0/15 |
| rgp105 | 3/25 (12) | 5/15 (33) | 0/15 |
| gp125 | 0/25 (0) | 4/15 (27) | 0/15 |
| Serum IgA (mg/ml)a | 4.94b | 4.77 | 3.81 |
Serum IgA concentrations are mean values.
P < 0.05 (unpaired t test) compared to negative controls.
Anti-HIV-2 antibody reactivity in EGSN individuals.
To determine the HIV-2-specific antibody profiles of EGSN persons, HIV-2-specific IgA and IgG ELISAs were used to examine antibody reactivity against whole HIV-2 (strain SBL6669) lysate, rgp36, rgp105, and native gp125. For IgA antibody, 16 (64%) and 6 (24%) out of 25 EGSN serum samples showed reactivity against whole HIV-2 antigen and rgp36, respectively. Three samples exhibited IgA directed against rgp105. Only one sample (no. 323; OD = 0.502, cutoff = 0.497) had borderline IgA reactivity to gp125 (Table 1 and Fig. 1A to D). The purified IgA fractions elicited a similar binding pattern (data not shown). For IgG, no reactivity to HIV-2 proteins was seen in the EGSN individuals, thus confirming their HIV-2-seronegative status (data not shown).
FIG. 1.
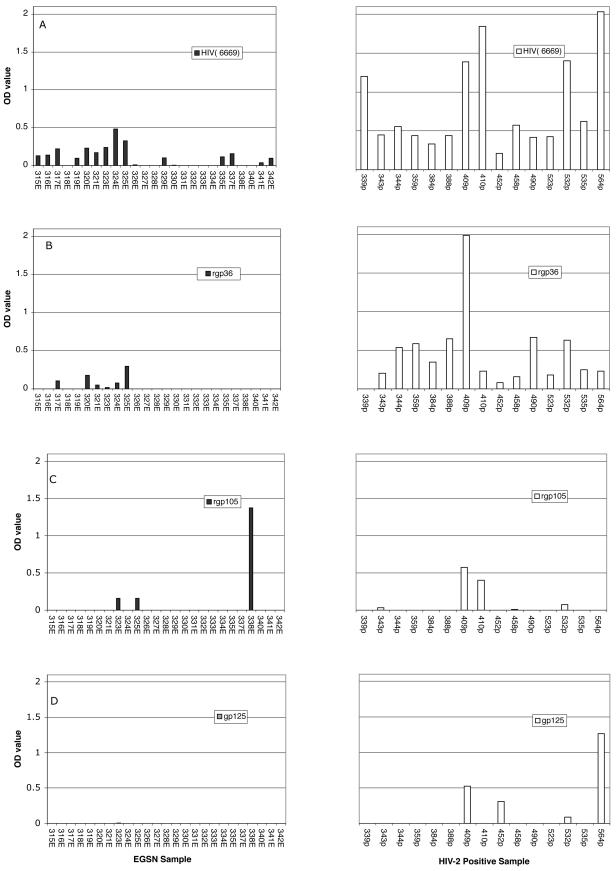
Reactivity to HIV-2 proteins of purified serum IgA as determined by ELISA and given as OD values with correction for cutoff values. Antibody responses to HIV-2 whole antigen (A), rgp36 (B), rgp105(C), and gp125 (D) of EGSN and HIV-2-positive individuals are shown. The cutoff values, set as described in Materials and Methods, for the assays were as follows: HIV-2 antigen IgA, 0.322; rgp36 IgA, 0.434; rgp105 IgA, 0.45; gp125 IgA, 0.497; HIV-2 antigen IgG, 0.454; rgp36 IgG, 0.363; rgp105 IgG, 0.34; gp125 IgG, 0.269.
Anti-HIV-2 antibody reactivity in HIV-2-positive patients and HIV-negative controls.
All serum samples from HIV-2-positive patients (15 of 15) showed strong IgA reactivity against whole HIV-2 antigen, and 93% (14 of 15) displayed rgp36 reactivity. However, only 33% (5 of 15) and 27% (4 of 15) of the serum samples showed rgp105 and gp125 reactivity, respectively (Table 1 and Fig. 1A to D). A similar pattern of reactivity was detected when IgA fractions were analyzed (data not shown). All serum samples (15 of 15) showed strong HIV-2 IgG antibody reactivity to whole HIV-2 antigen and rgp36, while 86% (13 of 15) showed IgG reactivity to gp125 antigen and only a few samples (3 of 15) showed gp105 reactivity (data not shown).
None of the negative controls showed any antigen binding reactivity to any of the proteins tested (Table 1).
Neutralizing activity against HIV-2SBL6669.
All of the IgA purified from the serum of EGSN individuals exhibited neutralizing activity against HIV-2SBL6669, with titers ranging from 40 to 80. Similarly, 13 out of 15 serum IgA samples from HIV-2-positive partners elicited HIV-2 neutralization. No neutralizing activity was detected in IgA from the seronegative control group (Table 2).
TABLE 2.
Serum IgA-mediated HIV-2 neutralization in EGSN individuals, HIV-2-positive patients, and HIV-2-seronegative controls
| Sample source and/or IDa no. | IgA neutralizing antibody titer |
|---|---|
| EGSN individuals | |
| 315 | 40 |
| 316 | 80 |
| 317 | 40 |
| 318 | 40 |
| 319 | 40 |
| 320 | 40 |
| 321 | 40 |
| 323 | 40 |
| 324 | 80 |
| 325 | 40 |
| 326 | 40 |
| 327 | 40 |
| 328 | 80 |
| 329 | 40 |
| 330 | 40 |
| 331 | 40 |
| 332 | 40 |
| 333 | 40 |
| 334 | 40 |
| 335 | 40 |
| 337 | 40 |
| 338 | 40 |
| 340 | 40 |
| 341 | 40 |
| 342 | 40 |
| HIV-2-positive patients | |
| 339 | 40 |
| 343 | 40 |
| 344 | 40 |
| 359 | 40 |
| 384 | 40 |
| 388 | 40 |
| 409 | 40 |
| 410 | 40 |
| 452 | 40 |
| 458 | 40 |
| 490 | 40 |
| 523 | 80 |
| 532 | 40 |
| 535 | ≤20 |
| 564 | ≤20 |
| HIV-2-negative controls (n = 15) | ≤20 |
| Methyl-α-d-galactopyranoside | ≤20 |
ID, identification.
To adjust for the amount of IgA present in the purifications, neutralizing activity was also reported as the neutralizing titer per milligram of serum IgA. (Fig. 2). The ratios (neutralizing titer per milligram) in the EGSN individuals ranged between 1,200 and 370, and those in the HIV-2-infected individuals ranged between 1,480 and 300. The mean ratio was higher for the samples from the EGSN individuals (580) than for those from the HIV-2-positive patients (480, P < 0.05), indicating a qualitative difference between the serum IgAs of the two groups.
FIG. 2.
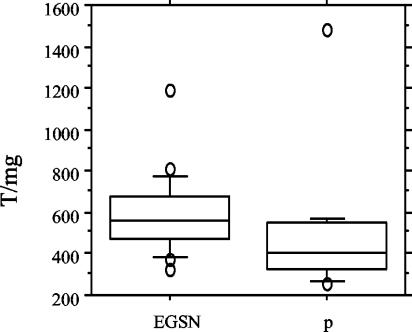
Box plot illustrating the anti-HIV-2 IgA neutralizing reactivity expressed as the titer per milligram (T/mg) of the serum IgA preparation. Shown are the 10th, 25th, 50th, 75th, and 90th percentiles of the reactivity titer after calculation of the IgA amount (milligrams) in purifications from EGSN (n = 23, 2 were not included owing to a lack of samples) and HIV-2-infected individuals (p) (n = 15), respectively (P < 0.05).
We could not detect neutralizing activity in any of the IgG purifications or whole serum samples of any of the study groups (data not shown).
DISCUSSION
Our study presents for the first time the HIV-2-specific serum IgA immune responses of EGSN individuals who have been determined to be uninfected through a well-established diagnostic procedure. Serum IgA reactivity to HIV-2, primarily directed against whole HIV-2 viral lysate, was observed in a large proportion of the EGSN individuals. Furthermore, HIV-2 neutralization was induced by all serum IgA preparations.
The majority of the HIV-2-infected patients exhibited anti-HIV-2 serum IgA with reactivity to both whole viral lysate and rgp36 and induced HIV-2 neutralization. This HIV-2 reactivity pattern was similar to that which we recently reported for another group of HIV-2-infected patients (35).
In the present study of EGSN individuals, all serum IgA samples exhibited HIV-2 neutralization of HIV-2SBL6669. However, only 7 (28%) of 25 reacted with either of the HIV-2 envelope glycoproteins tested. The fact that the remaining serum IgA samples lacked detectable binding to envelope proteins in an ELISA does not necessarily indicate that an interaction with the envelope protein did not occur. Previous studies have shown that antigen binding epitopes may be different from epitopes targeted by neutralizing antibodies (13, 29). In the majority of the EGSN cases, we found binding of IgA to whole virus lysate but not to recombinant envelope proteins, which raises the question of the target epitopes of HIV-2-specific serum IgA. In a study of six HIV-1 discordant couples, Clerici and coworkers have suggested that the serum IgA of HIV-1-exposed uninfected individuals exclusively binds to an epitope within gp41 and thereby inhibits infection (17). In our study, the overall epitope usage was not elucidated and further study is required to identify possible nonenvelope epitopes.
We could detect HIV-2 neutralization in all purified serum IgA fractions from the EGSN individuals and the majority of the HIV-2-infected patients, although no neutralization was seen when we used either purified IgG or whole serum samples. The lack of detectable neutralization in whole sera could be due to the steric blocking by other immunoglobulins, and a similar phenomenon has been discussed earlier in a report on a study concerning influenza virus neutralization in which a small amount of purified IgG was shown to be more efficient at neutralization than whole serum, owing to the axial rotation and mobility necessary for the antibody to bind and inhibit the virus (23).
The presence of HIV-2-specific serum IgA in EGSN individuals could be a result of an abortive infection. However, recent studies suggest that exogenous antigens can also be processed for presentation by professional presenting cells, such as dendritic cells (15, 43, 49), particularly in HIV infection (12). Thus, the detection of HIV-2-specific IgA in EGSN individuals might not be a consequence of an abortive infection but rather could result from a mechanism of presentation by dendritic cells or/and other, unknown, mechanisms. So far, there has been no report of aborted infections in HIV-1-exposed uninfected individuals (7, 36).
Recent studies have raised the possibility that transiently exposed conformations of proteins that are required for HIV-1 infection can elicit neutralizing antibody immune responses (14). We have observed that some of the serum IgA preparations from the EGSN subjects recognized the transmembrane region of the envelope protein, a region that is more accessible after binding of gp125 on the target cells. However, the epitope specificity of HIV-2-neutralizing serum IgA was not elucidated in this study and further investigation is therefore warranted.
When we compared the neutralizing titer per milligram of IgA in EGSN individuals to that exhibited by HIV-2-infected patients, we found that the mean of the ratios in the EGSN individuals was higher than that in the HIV-2 patients. Furthermore, when determining the total IgA concentration in serum, we found that the EGSN group had higher concentrations (mean, 4.94 mg/ml) than did the healthy controls (mean, 3.81 mg/ml). Taken together, this suggests the presence of a strong IgA-mediated immune response in EGSN individuals with a quality different from that found in HIV-2-infected patients.
In conclusion, this is the first report showing serum IgA specific for HIV-2 with neutralizing capacity in samples obtained from EGSN individuals. Importantly, we found that purified serum IgA from EGSN individuals was more potent in neutralizing HIV-2 than was that from their HIV-2-positive partners, as shown by comparison of their respective neutralizing titers per milligram of IgA. This extends our previous studies, in which we demonstrated that serum IgA derived from HIV-2-positive individuals is able to neutralize HIV-2 (35). Given that IgA had potent neutralizing capacity in vitro, it may confer protection on populations at high risk of HIV-2 exposure. It remains to be determined whether these HIV-2-specific IgA responses are induced on mucosal surfaces as well and whether primary virus isolates can be neutralized in vitro and in vivo. Enhanced understanding of the role of IgA immunity in resistance to HIV-2 infection may provide insight into correlates of protection, both systemically and mucosally, and assist in the rational design of HIV vaccines.
Acknowledgments
We thank Kerstin Andreasson and Helen Linder for expert technical assistance.
This study was supported by the Lars Hiertas Minnes Foundation, the Translational Research Project at the Research Center, South Hospital, the Swedish Society for Medical Research, and the Swedish Research Council.
REFERENCES
- 1.Albert, J., U. Bredberg, F. Chiodi, B. Böttiger, E-M. Fenyö, E. Norrby, and G. Biberfeld. 1987. A new human retrovirus isolate of West African origin and its relationship to HTLV-IV, LAV-II and HTLV-IIIB. AIDS Res. Hum. Retrovir. 3:3-10. [DOI] [PubMed] [Google Scholar]
- 2.Andersson, S. 2001. HIV-2 and the immune response. AIDS Rev. 3:11-23. [Google Scholar]
- 3.Andersson, S., Z. da Silva, H. Norrgren, F. Dias, and G. Biberfeld. 1997. Field evaluation of alternative testing strategies for diagnosis and differentiation of HIV-1 and HIV-2 infections in an HIV-1 and HIV-2 prevalent area. AIDS 11:1815-1822. [DOI] [PubMed] [Google Scholar]
- 4.Belec, L., P. D. Ghys, H. Hocini, J. N. Nkengasong, J. Tranchot-Diallo, M. O. Diallo, V. Ettiegne-Traore, C. Maurice, P. Becquart, M. Matta, A. Si-Mohamed, N. Chomont, I. M. Coulibaly, S. Z. Wiktor, and M. D. Kazatchkine. 2001. Cervicovaginal secretory antibodies to human immunodeficiency virus type 1 (HIV-1) that block viral transcytosis through tight epithelial barriers in highly exposed HIV-1-seronegative African women. J. Infect. Dis. 184:1412-1422. [DOI] [PubMed] [Google Scholar]
- 5.Bernard, N. F., C. M. Yannakis, J. S. Lee, and C. M. Tsoukas. 1999. Human immunodeficiency virus (HIV)-specific cytotoxic T lymphocyte activity in HIV exposed seronegative persons. J. Infect. Dis. 179:538-547. [DOI] [PubMed] [Google Scholar]
- 6.Beyrer, C., A. W. Artenstein, S. Rugpao, H. Stephens, T. C. VanCott, M. L. Robb, M. Rinkaew, D. L. Birx, C. Khamboonruang, P. A. Zimmerman, K. E. Nelson, and C. Natpratan. 1999. Epidemiologic and biologic characterization of a cohort of HIV-1 highly exposed, persistently seronegative commercial sex workers in Northern Thailand. J. Infect. Dis. 179:59-67. [DOI] [PubMed] [Google Scholar]
- 7.Biasin, M., S. L. Caputo, L. Speciale, F. Colombo, L. Racioppi, A. Zagliani, C. Ble, F. Vichi, L. Cianferoni, A. M. Masci, M. L. Villa, P. Ferrante, F. Mazzotta, and M. Clerici. 2000. Mucosal and systemic immune activation is present in human immunodeficiency virus-exposed seronegative women. J Infect. Dis. 182:1365-1374. [DOI] [PubMed] [Google Scholar]
- 8.Biberfeld, G., R. Thorstensson, M. Bergström, A. Naucler, and C. Mendes Costa. 1988. Enzyme immunoassay for demonstration of the antibodies to HIV-2 SBL6669 and HTLV-IV (SIVmac). AIDS 2:195-199. [PubMed] [Google Scholar]
- 9.Björling, E., F. Chiodi, G. Utter, and E. Norrby. 1994. Two V3 associated important neutralizing domains in the envelope glycoprotein gp125 of human immunodeficiency virus type 2. J. Immunol. 152:1952-1959. [PubMed] [Google Scholar]
- 10.Björling, E., G. Scarlatti, A. von Gegerfelt, J. Albert, G. Biberfeld, F. Chiodi, E. Norrby, and E.-M. Fenyö. 1993. Autologous neutralizing antibodies prevail in HIV-2 but not in HIV-1 infection. Virology 193:528-530. [DOI] [PubMed] [Google Scholar]
- 11.Bock, P. J., and D. M. Markowitz. 2001. Infection with HIV-2. AIDS 15(Suppl. 3):S35-S45. [DOI] [PubMed] [Google Scholar]
- 12.Buseyne, F., S. Le Gall, C. Boccaccio, J. P. Abastado, J. D. Lifson, L. O. Arthur, Y. Riviere, J. M. Heard, and O. Schwartz. 2001. MHC-I-restricted presentation of HIV-1 virion antigens without viral replication. Nat. Med. 7:344-349. [DOI] [PubMed] [Google Scholar]
- 13.Cavacini, L. A., M. Duval, J. Robinson, and M. R. Posner. 2002. Interactions of human antibodies, epitope exposure, antibody binding and neutralization of primary isolate HIV-1 virions. AIDS 16:2409-2417. [DOI] [PubMed] [Google Scholar]
- 14.Chan, D. C., and P. S. Kim. 1998. HIV entry and its inhibition. Cell 93:681-684. [DOI] [PubMed] [Google Scholar]
- 15.Chefalo, P. J., and C. V. Harding. 2001. Processing of exogenous antigens for presentation by class I MHC molecules involves post-Golgi peptide exchange influenced by peptide-MHC complex stability and acidic pH. J. Immunol. 167:1274-1282. [DOI] [PubMed] [Google Scholar]
- 16.Clavel, F., D. Guetard, F. Brun-Vezinet, S. Chamaret, M. A. Rey, M. O. Santos-Ferreria, A. G. Laurent, C. Dauguet, C. Katlama, and C. Rouzioux. 1986. Isolation of a new human retrovirus from West African patients with AIDS. Science 233:343-346. [DOI] [PubMed] [Google Scholar]
- 17.Clerici, M., C. Barassi, C. Devito, C. Pastori, S. Piconi, D. Trabattoni, R. Longhi, J. Hinkula, K. Broliden, and L. Lopalco. 2002. Serum IgA of HIV-exposed uninfected individuals inhibits HIV through recognition of a region within the α-helix of gp41. AIDS 16:1731-1741. [DOI] [PubMed] [Google Scholar]
- 18.Clerici, M., J. M. Levin, H. A. Kessler, A. Harris, J. A. Berzofsky, A. L. Landay, and G. M. Shearer. 1994. HIV-specific T-helper activity in seronegative health care workers exposed to contaminated blood. JAMA 271:42-46. [PubMed] [Google Scholar]
- 19.Clerici, M., J. V. Giorgi, C. C. Chou, V. K. Gudeman, J. A. Zack, P. Gupta, H. N. Ho, P. G. Nishanian, J. A. Berzofsky, and G. M. Shearer. 1992. Cell-mediated immune response to human immunodeficiency virus (HIV) type 1 in seronegative homosexual men with recent sexual exposure to HIV-1. J. Infect. Dis. 165:1012-1019. [DOI] [PubMed] [Google Scholar]
- 20.De Kock, K. M., A. Porter, J. Kouadio, M. Maran, M.-F. Lafontaine, G.-M. Gershy-Damet, W. Heyward, and R. George. 1991. Cross-reactivity on Western blots in HIV-1 and HIV-2 infections. AIDS 5:859-863. [DOI] [PubMed] [Google Scholar]
- 21.Devito, C., J. Hinkula, R. Kaul, J. Kimani, P. Kiama, L. Lopalco, C. Barass, S. Piconi, D. Trabattoni, J. J. Bwayo, F. Plummer, M. Clerici, and K. Broliden. 2002. Cross-clade HIV-1-specific neutralizing IgA in mucosal and systemic compartments of HIV-1-exposed, persistently seronegative subjects. J. Acquir. Immune Defic. Syndr. 30:413-420. [DOI] [PubMed] [Google Scholar]
- 22.Devito, C., J. Hinkula, R. Kaul, L. Lopalco, J. J. Bwayo, F. Plummer, M. Clerici, and K. Broliden. 2000. Mucosal and plasma IgA from HIV-exposed seronegative individuals neutralizes a primary HIV-1 isolate. AIDS 14:1917-1920. [DOI] [PubMed] [Google Scholar]
- 23.Dimmock, N. 1993. Neutralization of animal viruses. Curr. Top. Microbiol. Immunol. 183:32-34. [DOI] [PubMed] [Google Scholar]
- 24.Ferrantelli, F., and R. M. Ruprecht. 2002. Neutralizing antibodies against HIV—back in the major leagues? Curr. Opin. Immunol. 14:495-502. [DOI] [PubMed] [Google Scholar]
- 25.Fowke, K. R., R. Kaul, K. L. Rosenthal, J. Oyugi, J. Kimani, W. J. Rutherford, N. J. Nagelkerke, T. B. Ball, J. J. Bwayo, J. N. Simonsen, G. M. Shearer, and F. A. Plummer. 2000. HIV-1-specific cellular immune responses among HIV-1-resistant sex workers. Immunol. Cell Biol. 78:586-595. [DOI] [PubMed] [Google Scholar]
- 26.Ghys, P. D., L. Belec, M. O Diallo, V. Ettiegne-Traore, P. Becquart, C. Maurice, J. N. Nkengasong, I. M. Coulibaly, A. E. Greenberg, M. Laga, and S. Z. Wiktor. 2000. Cervicovaginal anti-HIV antibodies in HIV-seronegative female sex workers in Abidjan, Cote d'Ivoire. AIDS 14:2603-2608. [DOI] [PubMed] [Google Scholar]
- 27.Gilljam, G. 1993. Envelope glycoproteins of HIV-1, HIV-2, and SIV purified with Galanthus nivalis agglutinin induce strong immune responses. AIDS Res. Hum. Retrovir. 9:431-438. [DOI] [PubMed] [Google Scholar]
- 28.Goh, W. C., J. Markee, R. E. Akridge, M. Meldorf, L. Musey, T. Karchmer, M. Krone, A. Collier, L. Corey, M. Emerman, and M. J. McElrath. 1999. Protection against human immunodeficiency virus type 1 infection in persons with repeated exposure: evidence for T cell immunity in the absence of inherited CCR5 coreceptor defects. J. Infect. Dis. 179:548-557. [DOI] [PubMed] [Google Scholar]
- 29.Herrera. C., C. Spenlehauer, M. S. Fung, D. R. Burton, S. Beddows, and J. P. Moore. 2003. Nonneutralizing antibodies to the CD4-binding site on the gp120 subunit of human immunodeficiency virus type 1 do not interfere with the activity of a neutralizing antibody against the same site. J. Virol. 77:1084-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Just, J. J. 1995. Genetic predisposition to HIV-1 infection and acquired immunodeficiency virus syndrome: a review of the literature examining associations with HLA. Hum. Immunol. 44:156-169. [DOI] [PubMed] [Google Scholar]
- 31.Kanki, P. J., S. M′Boup, D. Ricard, F. Barin, F. Denis, C. Boye, L. Sangare, K. Travers, M. Albaum, and R. Marlink. 1987. Human T-lymphotropic virus type 4 and the human immunodeficiency virus in West Africa. Science 236:827-831. [DOI] [PubMed] [Google Scholar]
- 32.Kaul, R., D. Trabattoni, J. J. Bwayo, D. Arienti, A. Zagliani, F. M. Mwangi, C. Kariuki, E. N. Ngugi, K. S. MacDonald, T. B. Ball, M. Clerici, and F. A. Plummer. 1999. HIV-1-specific mucosal IgA in a cohort of HIV-1-resistant Kenyan sex workers. AIDS 13:23-29. [DOI] [PubMed] [Google Scholar]
- 33.Kaul, R., F. A. Plummer, J. Kimani, T. Dong, P. Kiama, T. Rostron, E. Njagi, K. S. MacDonald, J. J. Bwayo, A. J. McMichael, and S. L. Rowland-Jones. 2000. HIV-1-specific mucosal CD8+ lymphocyte responses in the cervix of HIV-1-resistant prostitutes in Nairobi. J. Immunol. 164:1602-1611. [DOI] [PubMed] [Google Scholar]
- 34.Larsen, O., Z. Silva, A. Sandström, P. K. Andersen, S. Andersson, A. G. Poulsen, M. Melbye, F. Dias, A. Nauclér, and P. Aaby. 1998. Declining HIV-2 prevalence and incidence among men in a community study from Guinea-Bissau. AIDS 12:1707-1714. [DOI] [PubMed] [Google Scholar]
- 35.Lizeng, Q., P. Skott, S. Sourial, C. Nilsson, S. Andersson, M. Ehnlund, N. Taveira, and E. Björling. 2003. Serum immunoglobulin A (IgA) mediated immunity in human immunodeficiency virus type 2 (HIV-2) infection. Virology 308:225-232. [DOI] [PubMed] [Google Scholar]
- 36.Lo Caputo, S., D. Trabattoni, F. Vichi, S. Piconi, L. Lopalco, M. L. Villa, F. Mazzotta, and M. Clerici. 2003. Mucosal and systemic HIV-1-specific immunity in HIV-1-exposed but uninfected heterosexual men. AIDS 17:531-539. [DOI] [PubMed] [Google Scholar]
- 37.MacDonald, K. S., K. R. Fowke, J. Kimani, V. A. Dunand, N. J. Nagelkerke, T. B. Ball, J. Oyugi, E. Njagi, L. K. Gaur, R. C. Brunham, J. Wade, M. A. Luscher, P. Krausa, S. Rowland-Jones, E. Ngugi, J. J. Bwayo, and F. A. Plummer. 2000. Influence of HLA supertypes on susceptibility and resistance to human immunodeficiency virus type 1 infection. J. Infect. Dis. 181:1581-1589. [DOI] [PubMed] [Google Scholar]
- 38.Marlink, R. 1996. Lessons from the second AIDS virus, HIV-2. AIDS 10:689-699. [DOI] [PubMed] [Google Scholar]
- 39.Mazzoli, S., D. Trabattoni, S. Lo Caputo, S. Piconi, C. Ble, F. Meacci, S. Ruzzante, A. Salvi, F. Semplici, R. Longhi, M. L. Fusi, N. Tofani, M. Biasin, M. L. Villa, F. Mazzotta, and M. Clerici. 1997. HIV-specific mucosal and cellular immunity in HIV-seronegative partners of HIV-seropositive individuals. Nat. Med. 3:1250-1257. [DOI] [PubMed] [Google Scholar]
- 40.Mazzoli, S., L. Lopalco, A. Salvi, D. Trabattoni, S. Lo Caputo, F. Semplici, M. Biasin, C. Bl, A. Cosma, C. Pastori, F. Meacci, F. Mazzotta, M. L. Villa, A. G. Siccardi, and M. Clerici. 1999. Human immunodeficiency virus (HIV)-specific IgA and HIV neutralizing activity in the serum of exposed seronegative partners of HIV-seropositive persons. J. Infect. Dis. 180:871-875. [DOI] [PubMed] [Google Scholar]
- 41.Mörner, A., A. Achour, M. Norin, R. Thorstensson, and E. Björling. 1999. Fine characterization of a neutralizing epitope in the third variable region (V3) of the surface glycoprotein (gp125) of human immunodeficiency virus type 2. Virus Res. 59:49-60. [DOI] [PubMed] [Google Scholar]
- 42.Pastori, C., C. Barassi, S. Piconi. R. Longhi, M. L. Villa, A. G. Siccardi, M. Clerici, and L. Lopalco. 2000. HIV neutralizing IgA in exposed seronegative subjects recognizes an epitope within the gp41 coiled-coil pocket. J. Biol. Regul. Homeost. Agents 14:15-21. [PubMed] [Google Scholar]
- 43.Reimann, J., and R. Schirmbeck. 1999. Alternative pathways for processing exogenous and endogenous antigens that can generate peptides for MHC class I-restricted presentation. Immunol. Rev. 172:131-152. [DOI] [PubMed] [Google Scholar]
- 44.Rowland-Jones, S., J. Sutton, K. Ariyoshi, K. Ariyoshi, T. Dong, F. Gotch, S. Mcadam, D. Whitby, S. Sabally, and A. Gallimore. 1995. HIV-specific cytotoxic T-cells in HIV-exposed but uninfected Gambian women. Nat. Med. 1:59-64. [DOI] [PubMed] [Google Scholar]
- 45.Rowland-Jones, S., T. Dong, K. R. Fowke, J. Kimani, P. Krausa, H. Newell, T. Blanchard, K. Ariyoshi, J. Oyugi, E. Ngugi Bwayo, K. S. MacDonald, A. J. McMichael, and F. A. Plummer. 1998. Cytotoxic T cell responses to multiple conserved HIV epitopes in HIV-resistant prostitutes in Nairobi. J. Clin. Investig. 102:1758-1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schim van der Loeff, M. F., and P. Aaby. 1999. Towards a better understanding of the epidemiology of HIV-2. AIDS 13:S69-S84. [PubMed] [Google Scholar]
- 47.Skurnick, J. H., P. Palumbo, A. DeVico, B. L. Shacklett, F. T. Valentine, M. Merges, R. Kamin-Lewis, J. Mestecky, T. Denny, G. K. Lewis, J. Lloyd, R. Praschunus, A. Baker, D. F. Nixon, S. Stranford, R. Gallo, S. H. Vermund, and D. B. Louria. 2002. Correlates of nontransmission in US women at high risk of human immunodeficiency virus type 1 infection through sexual exposure. J. Infect. Dis. 185:428-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thorstensson, R., L. Walther, P. Putkonen, J. Albert, and G. Biberfeld. 1991. A capture enzyme immunoassay for detection of HIV-2/SIV antigen. J. Acquir. Immune Defic. Syndr. 4:374-379. [PubMed] [Google Scholar]
- 49.Wick, M. J., and H. G. Ljunggren. 1999. Processing of bacterial antigens for peptide presentation on MHC class I molecules. Immunol. Rev. 172:153-162. [DOI] [PubMed] [Google Scholar]


