Abstract
Objectives: To evaluate the correlation between multiparametric prostate MRI (MP-MRI) suspicion for seminal vesicle invasion (SVI) by prostate cancer (PCa) and pathology on MRI/ultrasound (US) fusion-guided biopsy.
Patients and Methods: From March 2007 to June 2013, 822 patients underwent MP-MRI at 3 Tesla and MRI/US fusion-guided biopsy. Of these, 25 patients underwent targeted biopsy of the seminal vesicles (SVs). In six patients, bilateral SVI was suspected, resulting in 31 samples. MP-MRI findings that triggered these SV biopsies were scored as low, moderate, or high suspicion for SVI based on the degree of involvement on MRI. Correlative prostate biopsy and radical prostatectomy (RP) pathology were reviewed by a single genitourinary pathologist.
Results: At the time of MP-MRI, the median age was 64 years with a median prostate-specific antigen of 10.74 ng/mL. Of the 31 SV lesions identified, MP-MRI suspicion scores of low, moderate, and high were assigned to 3, 19, and 9 lesions, respectively. MRI/US fusion-guided biopsy detected SVI in 20/31 (65%) of cases. For the four patients who underwent RP after a preoperative assessment of SVI, biopsy pathology and RP pathology were concordant in all cases.
Conclusions: As this technology becomes more available, MP-MRI and MRI/US fusion-guided biopsy may play a role in the preoperative staging for PCa. Future work will determine if improved preoperative staging leads to better surgical outcomes.
Introduction
Prostate cancer (PCa) is the most common nonskin cancer and the second most common cause of cancer-related death in American men.1 Seminal vesicle invasion (SVI) is the hallmark of stage T3b PCa and is associated with poor outcomes, including early biochemical recurrence.2,3 This is particularly true in patients with SVI and positive surgical margins after radical prostatectomy (RP). SVI is also relatively common, with recent large surgical series reporting rates of 4.5% to 12.7%.4–6 For all of these reasons, early knowledge of SVI is critical for appropriate patient counseling, treatment selection, and planning.
Historically, the preoperative assessment of SVI relied on various surrogates for definitive histologic staging. These techniques include the digital rectal examination (DRE), transrectal ultrasound (TRUS) and TRUS-guided biopsy, predictive nomograms, and MRI. However, each of these techniques suffers from significant limitations. In contemporary practice, most urologists utilize predictive models to counsel patients on the likelihood of SVI. However, these models do not incorporate advanced imaging, such as multiparametric MRI (MP-MRI), which is becoming an increasingly important part of PCa diagnosis. Moreover, predictive models provide population-based probabilities, rather than personalized results. A superior standard for preoperative staging would involve histologic confirmation in every patient; however, seminal vesicle (SV) biopsy is not routinely performed.
MRI/ultrasound (US) fusion-guided biopsy, utilizing software-based registration of diagnostic MRI and TRUS images, has been shown to accurately represent the tumor burden of PCa in a variety of settings.7,8 This technique combines the best features of two modalities: the high sensitivity and specificity of MRI for PCa and the real-time features of US, enabling accurate placement of biopsy needles for tissue diagnosis.9 Although targeted prostate biopsy has been largely used for identifying primary prostate tumors, the potential for its use as a PCa staging modality, particularly with respect to the SVs, is under active investigation. At our institution, all patients with suspected PCa undergo MP-MRI followed by MRI/US fusion-guided biopsy. When a lesion that appears to invade an SV is identified on MP-MRI, its likelihood of representing PCa is recorded, and it is included as a target on subsequent MRI/US fusion-guided biopsy. In this study, we investigate the correlation between MP-MRI suspicion for SVI and pathology on MRI/US fusion-guided biopsy, as well as final pathology when available.
Materials and Methods
Patient selection
Patients were enrolled under an Institutional Review Board (IRB)-approved prospective trial of MP-MRI and MRI/US fusion-guided biopsy at the National Cancer Institute (NCI) of the National Institutes of Health (NIH). From March 2007 to June 2013, 822 patients underwent MP-MRI at 3 Tesla and MRI/US fusion-guided biopsy. Of these, 25 patients had lesions suspicious for SVI on MRI and underwent targeted biopsy of the SVs. Six patients had MRI findings of bilateral SVI, so that 31 targets were identified and biopsied.
Imaging and biopsy protocol
Each patient initially underwent a diagnostic MP-MRI of the prostate at 3 Tesla (Achieva; Philips Healthcare, Best, The Netherlands) with a 16-channel cardiac surface coil (SENSE; Philips Healthcare) over the pelvis and an endorectal coil (BPX-30; Medrad, Pittsburgh, PA), as previously described.10 MP-MRI sequences included tri-planar T2-weighted, dynamic contrast-enhanced (DCE), diffusion-weighted imaging (DWI), and magnetic resonance spectroscopy (MRS). MP-MRI data sets were prospectively evaluated by two radiologists (Peter L. Choyke, Baris Turkbey), who assigned a PCa suspicion level (low, moderate, or high) to each lesion using a system that has been previously validated (Fig. 1).11
FIG. 1.
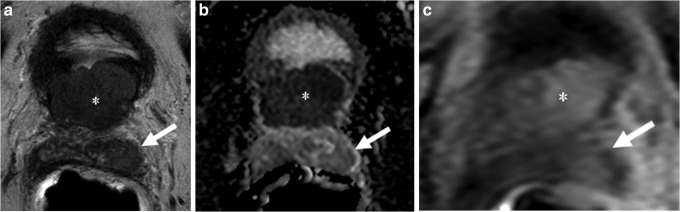
Seventy-four-year-old male with a prostate-specific antigen of 20.7 ng/mL and four prior negative biopsies. (a) Axial T2-weighted MRI demonstrates lesions in the base transitional zone (asterisk), with the latter demonstrating hypointense signal within the left seminal vesicles (SVs) (arrow). (b) ADC maps of DW MRI show restricted diffusion corresponding to hypointense signal intensity within the primary (asterisk) and the left SV (arrow) lesions. (c) Dynamic contrast-enhanced MRIs also localize both lesions (asterisk, arrow).
All patients in this study had lesions suspicious for PCa with SVI on MP-MRI and thus underwent prostate biopsy after MRI. This was done according to a protocol that has been previously described.8 In short, systematic extended sextant 12-core biopsies as well as targeted biopsies of MRI-visible lesions were performed in the same biopsy session with a system utilizing an end-fire TRUS probe. At least two cores were obtained from each MRI visible lesion, from the axial and sagittal planes (Fig. 2).
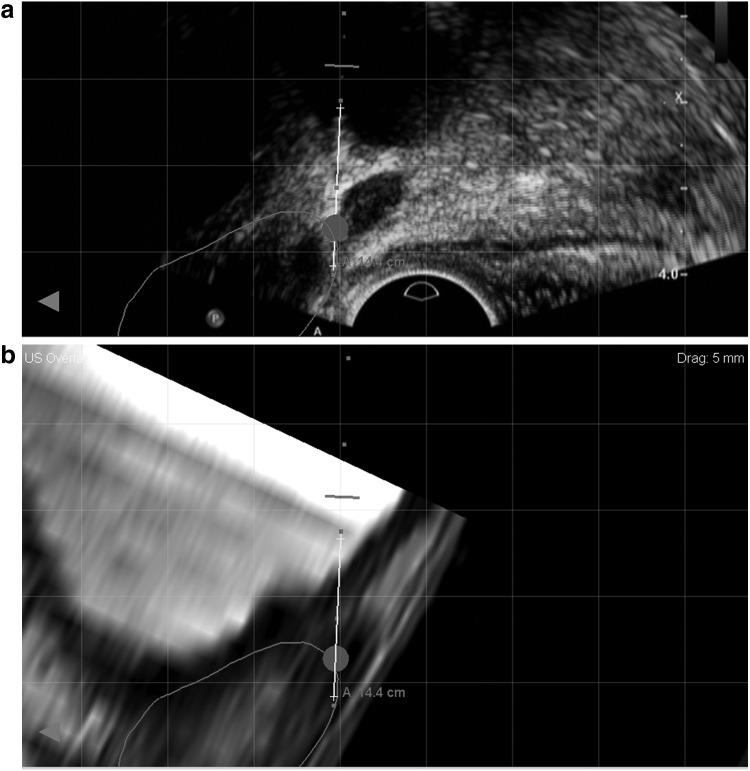
FIG. 2.
Representative images obtained during MRI/ultrasound (US) fusion-guided biopsy at sagittal plane, demonstrating the SV target (circle) on (a) real-time transrectal ultrasound and (b) fused diagnostic MRI. Biopsy pathology revealed Gleason 8 prostate cancer in the SV core.
Prostate biopsy results were then combined with clinical variables to determine treatment plans based on individual patient preferences in the setting of a multidisciplinary PCa clinic. When indicated, patients underwent robot-assisted radical prostatectomy (RARP) by a single surgeon (Peter A. Pinto). All biopsy and RP pathology were reviewed by a single genitourinary pathologist (Maria J. Merino).
Data analysis
Logistic regression models for univariate analysis as well as descriptive statistics were calculated using JMP Pro 10.0 (SAS Institute, Cary, NC).
Results
Demographic information for the 25 patients is presented in Table 1. The 25 patients had a median age of 64 years (interquartile range [IQR]: 57–68) and median prostate-specific antigen (PSA) of 10.74 ng/mL (IQR: 6.9–20.9). Fourteen of 25 (56%) had not been previously diagnosed with PCa. MP-MRI suspicion levels of low, moderate, and high for PCa were assigned to 3, 19, and 9 lesions, respectively. For those patients with MP-MRIs that were considered moderately or highly suspicious for PCa, extracapsular extension (ECE) at the site of the primary cancer was also seen in 42% and 67% of cases, respectively (Table 2).
Table 1.
Patient Demographics
| Total No. of patients (%) | 25 (100) |
| Age median (IQR), years | 64 (57–68) |
| Race, n (%) | |
| White | 14 (56.0) |
| Black | 8 (32.0) |
| Other | 3 (12.0) |
| PSA median (IQR), ng/mL | 10.74 (6.9–20.9) |
| Pre-MRI clinical stage | |
| No history of PCa, n (%) | 14 (56) |
| Prior history of PCa, n (%) | 11 (44) |
| cT1c, n | 9 |
| cT2a, n | 2 |
| Past biopsies per patient, mean (range) | 1.28 (0–5) |
IQR=interquartile range; PCa=prostate cancer; PSA=prostate-specific antigen.
Table 2.
MP-MRI Findings of T3 Disease
| SV lesions, n (%) | MRI ECE+, n (%) | |
|---|---|---|
| Total lesions | 31 (100) | 14 (100) |
| MRI suspicion level | ||
| Low | 3 (10) | 0/3 (0) |
| Moderate | 19 (61) | 8/19 (42) |
| High | 9 (29) | 6/9 (67) |
ECE=extracapsular extension; MP-MRI=multiparametric MRI; SV=seminal vesicle.
Biopsy results are presented in Table 3. SV biopsies were classified as unknown when the SV cores contained fibroadipose tissue rather than SV tissue. Complete biopsy results are also presented, as both cores obtained by means of 12-core biopsy and MRI/US fusion-guided biopsy of visualized lesions. The overall SVI detection rate by MRI/US fusion-guided biopsy for the 31 lesions was 20/31 (65%). Age, race, PSA, clinical stage, number of past biopsies, and MRI suspicion levels were not significantly associated with SVI on targeted biopsy.
Table 3.
Biopsy Results by MRI Suspicion Level for Each of the 31 Lesions Suspicious for SVI on MP-MRI
| Seminal vesicles | Prostate | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MRI/US fusion biopsies | Random biopsies | MRI/US fusion biopsies | |||||||||||||
| Gleason sum, n | Gleason sum, n | Gleason sum, n | |||||||||||||
| Total Lesions | Benign | 6 | 7 | 8+ | Unknown | SVI+ n (%) | Benign | 6 | 7 | 8+ | Benign | 6 | 7 | 8+ | |
| MRI suspicion level | |||||||||||||||
| Low | 3 | — | — | — | — | 3 | 0 (0) | 2 | — | 1 | — | 0 | 1 | 2 | 0 |
| Moderate | 19 | 3 | — | 3 | 12 | 1 | 15 (79) | 4 | 1 | 5 | 9 | 4 | 1 | 2 | 12 |
| High | 9 | 3 | — | — | 5 | 1 | 5 (56) | — | — | — | 9 | — | — | — | 9 |
| Total | 31 | 6 | — | 3 | 17 | 5 | 20 (65)a | 6 | 1 | 6 | 18 | 4 | 2 | 4 | 21 |
SV biopsies were classified as unknown when the SV cores contained fibroadipose tissue rather than SV tissue.
Moderate or high suspicion led to histologic confirmation of SVI in 20/28 (71%) of cases.
The gray shaded areas signify the important parts of the respective tables (i.e., “SVI+” and “total” sections).
SVI=seminal vesicle invasion; US, ultrasound.
Eight of the 25 patients (32%) underwent RP with curative intent (Table 4). Of these, four patients had SV biopsy tissue that allowed for a preoperative determination of SVI. SV biopsy and final pathology were concordant in 4/4 (100%) of these cases. In addition, the highest biopsy Gleason grade was a significant predictor of SVI on final pathology (p=0.018).
Table 4.
Biopsy and RARP Pathology for Those Patients Who Underwent Surgery
| Prostate biopsy | RARP | |||||
|---|---|---|---|---|---|---|
| Patient | MRI suspicion | SVI | Gleason sum | SV | Gleason sum | SV biopsy–RARP concordance |
| 1 | Low | Unknown | 3+4=7 | No | 6 | — |
| 2 | Low | Unknown | 3+4=7 | No | 3+4=7 | — |
| 3 | Low | Unknown | 3+4=7 | No | 3+4=7 | — |
| 4 | Moderate | Unknown | 6 | No | 6 | — |
| 5 | Moderate | Yes | 10 | Yes | 4+5=9 | Yes |
| 6 | Moderate | Yes | 10 | Yes | 4+5=9 | Yes |
| 7 | Moderate | Yes | 4+5=9 | Yes | 10 | Yes |
| 8 | Moderate | No | 3+4=7 | No | 3+4=7 | Yes |
| Total | 4/4 (100%) | |||||
The gray shaded area highlights the SV biopsy–RARP pathology concordance, which is the most important part of the table.
RARP=robot-assisted radical prostatectomy.
MRI/US fusion-guided biopsy significantly decreased the preoperative staging of one patient. This patient was a 51-year-old African American male with a PSA of 8.18 ng/mL and no prior prostate biopsy. On MP-MRI, bilateral lesions within the prostate gland were visualized with a moderate likelihood for PCa but with no ECE. The left SV appeared to be involved by PCa. Extended sextant biopsy revealed PCa bilaterally in 6 of 12 cores with a highest Gleason grade of 3+4=7. This was confirmed on targeted biopsy of the intraprostatic lesions, but targeted biopsy of the SV revealed benign SV tissue. Thus, this patient's preoperative stage was reduced from T3b to T2c. This patient opted for surgical treatment with RARP. Final pathology confirmed pT2c PCa with a highest Gleason grade of 3+4=7 and no SVI or ECE.
In a separate analysis, we combined biopsy and final pathology findings to assess the performance of MP-MRI for detecting SVI. Overall, 0/3 (0%) patients with low suspicion, 15/19 (79%) patients with moderate suspicion, and 5/9 (56%) patients with high suspicion on MP-MRI proved to have documented SVI. Thus, moderate or high suspicion led to histologic confirmation of SVI in 20/28 (71%) of cases versus 0/3 (0%) for low suspicion. This difference was statistically significant (p=0.04).
Discussion
For patients with PCa, the diagnosis of SVI has significant prognostic implications.3,12 T3b disease is considered “very high risk” and can significantly alter treatment pathways, often resulting in multimodal therapy that exposes patients to associated risks and consequences that can affect quality of life. Furthermore, the presence of a very high-risk disease may steer more patients toward radiation therapy, if there is doubt regarding the ability to obtain surgical cure or a need for wide surgical margins that could affect postoperative potency and continence.13
Despite all of these factors, the accurate preoperative assessment of SVI has remained a significant challenge. Historically, SVI was only detectable by DRE in cases of high-stage palpable diseases. However, DRE suffered from poor accuracy, leading to a shift toward imaging-based diagnosis. A number of studies have evaluated the performance of TRUS biopsy in detecting SVI. However, these studies are composed of a heterogeneous group of patients, limiting their generalizability. Nonetheless, in these carefully selected study populations, the sensitivity and specificity of TRUS biopsy for SVI range from 47.4% to 66.7% and 98.4% to 100%, respectively.14,15
This low sensitivity, in turn, led to the development of models used to predict SVI based on various clinical variables.16,17 Such models combine PSA, clinical stage, and biopsy findings, but may be further improved by the use of imaging. For example, Wang et al. recently demonstrated the incremental value of adding MRI to the Kattan nomogram for the prediction of SVI.18 Utilizing a 1.5-Tesla scanner and relying solely on T1- and T2-weighted images, the authors found that combining the imaging findings with results from the Kattan nomogram produced the highest area under the curve for the prediction of SVI on final pathology (p<0.05). The authors identified SV biopsy as a particularly appealing additional tool, but felt that enthusiasm for the idea has been limited because of poor results with the TRUS-guided techniques.
In part, because MRI also provides anatomical information that may help guide surgery, it offers a useful new alternative to the existing methods of identifying T3 disease.19 MP-MRI has become the de facto standard for how MRI should be performed and is generally based on a T2-weighted scan, a diffusion-weighted scan, and a DCE MRI. In a recent study, Soylu et al. retrospectively evaluated the performance of MP-MRI for the detection of SVI in patients who had undergone RP.20 This group utilized a 1.5-Tesla magnet and endorectal coil to perform MP-MRI. For the 23 patients with SVI on final pathology, MP-MRI had a specificity of 93.1% to 93.6% and sensitivity of 52% to 59%, depending on the radiologist's experience. The addition of DW imaging significantly improved detection of SVI, while the addition of DCE did not. In a separate study, Kim et al. assessed 30 patients with PCa and SVI using T2 and DW imaging on a 3-Tesla magnet.21 The addition of sequenced imaging significantly improved SVI detection, particularly for the less experienced radiologist. However, this group did not utilize DCE, and neither study validated the findings with preoperative biopsy. This highlights the need for delineating the most accurate MP-MRI protocol for PCa diagnosis and staging. Most importantly, neither predictive models nor imaging alone is able to provide reliable SV tissue samples that can allow for appropriate data-driven patient counseling, treatment selection, and planning.
In this study, we demonstrate the feasibility of the preoperative detection of SVI with MRI/US fusion-guided biopsy. For the 31 lesions that were suspicious for SVI on MP-MRI, the overall SVI detection rate on MRI/US fusion-guided biopsy was 20/31 (65%) and 71% if low-suspicion lesions are not included. Targeted SV biopsies provide an improvement over the current standard of care. Patients with targeted biopsy-proven SVI can be definitively counseled as to the presence of T3b disease. This finding has a much greater utility to individual patients than would a population-based probability or the results of imaging alone without guided biopsy. In addition, a negative targeted SV biopsy may be helpful in the setting of an equivocal MRI suggesting SVI. The biopsy could impact the patient's choice of definitive therapy, particularly when surgical extirpation is being considered.
This study is the first description, to our knowledge, of the use of MRI/US fusion-guided biopsy to detect SVI. In select cases, these biopsies significantly altered treatment plans. In all cases, the biopsies allowed for increased confidence in preoperative patient counseling. Given the large number of recent studies that have demonstrated the utility of targeted biopsy for intraprostatic lesions, this technique is likely to become increasingly widespread.8,10,22–24 The present study supports targeted biopsy of the SVs when indicated by imaging. It should be noted that the targeted biopsies performed in this study were based on diagnostic MRIs obtained on a 3-Tesla magnet with an endorectal coil and MP-MRI sequences, including T2-weighted, DCE, DWI, and MRS. This is a particularly comprehensive approach to prostate imaging that has been validated in other studies.10,25 However, we and others do not believe that MRS contributes to the diagnosis of SVI because of the naturally occurring increase in choline and lowering of citrate in the SVs. Finally, this study provides a unique assessment of the positive predictive value (PPV) of MRI for SVI. While this PPV is thought to be high, the studies that established this relied on just the subset of patients who underwent prostate MRI and subsequent RP. In the present study, targeted biopsy allowed for the procurement of SV tissue preoperatively. In this overall cohort, 6/31 (19%) of the MRI lesions suspicious for SVI were benign on targeted biopsy. This suggests the possibility that MRI may, in fact, be less specific for SVI than previously thought. Given this possibility, it becomes even more critical for patients to undergo targeted biopsy of the SVs, when indicated.
This study has certain limitations. First, it is not possible to calculate a sensitivity or NPV of MP-MRI and MRI/US fusion biopsy for SVI, as we do not routinely biopsy the SVs of those patients with negative MRIs. We were not able to target the SVs with both positive and negative MRI findings, since SV biopsy is not routinely performed and can cause protracted hematospermia. Second, the majority of the patients in our study received systemic therapy and/or radiation rather than RP due to advanced disease. This limits our ability to verify SVI on final pathology. However, MRI/US fusion-guided biopsy appears to offer a useful surrogate for RP pathology.26 This is supported by the 100% concordance between biopsy and final pathology in the subset of patients who underwent surgery after a conclusive biopsy. Finally, in five cases, MRI/US fusion-guided biopsy failed to yield SV or PCa tissue. This may be a result of SV anatomy itself being distorted by the prostate nerve block, which is administered at the level of the junction between the prostate and SVs bilaterally. Because the resulting anatomic deformation was not present during the diagnostic MRI, accurate image registration on MRI/US fusion may be challenging. Finally, targeted biopsy of the SVs may be especially sensitive to motion artifact. Even in cases where the prostatic nerve blocks do not distort the anatomy, the SVs remain relatively mobile. The resulting inaccuracy may justify performing a repeat image registration immediately before SV biopsy.
Conclusions
MP-MRI and MRI/US fusion-guided biopsy offer a useful novel tool with which to detect SVI by PCa. Importantly, it provides a preoperative targeted tissue confirmation and accurate staging, thus allowing for appropriate data-driven patient counseling, treatment selection, and planning. As this technology becomes more available to physicians, MP-MRI and MRI/US fusion-guided biopsy may play a role in the preoperative staging for PCa. Future work will determine if improved preoperative staging leads to better surgical outcomes.
Abbreviations Used
- DCE
dynamic contrast enhanced
- DRE
digital rectal examination
- DWI
diffusion-weighted imaging
- ECE
extracapsular extension
- IQR
interquartile range
- IRB
Institutional Review Board
- MP-MRI
multiparametric magnetic resonance imaging
- MRI
magnetic resonance imaging
- MRI/US
magnetic resonance imaging/ultrasound
- MRS
magnetic resonance spectroscopy
- NCI
National Cancer Institute
- NIH
National Institutes of Health
- PCa
prostate cancer
- PPV
positive predictive value
- PSA
prostate-specific antigen
- RARP
robot-assisted radical prostatectomy
- RP
radical prostatectomy
- SV
seminal vesicle
- SVI
seminal vesicle invasion
- TRUS
transrectal ultrasound
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research, and the Center for Interventional Oncology. NIH and Philips Healthcare have a cooperative research and development agreement. NIH and Philips share intellectual property in the field. This research was also made possible through the National Institutes of Health Medical Research Scholars Program, a public–private partnership supported jointly by the NIH and generous contributions to the Foundation for the NIH from Pfizer, Inc., the Doris Duke Charitable Foundation, the Alexandria Real Estate Equities, Inc., Mr. and Mrs. Joel S. Marcus, and the Howard Hughes Medical Institute, as well as other private donors. For a complete list, please visit the Foundation website at http://fnih.org/work/education-training-0/medical-research-scholars-program.
Disclosure Statement
No competing financial interests exist.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11–30 [DOI] [PubMed] [Google Scholar]
- 2.NCCN. NCCN Clinical Practice Guidelines in Oncology 2014. [March1, 2014]. Available from: www.nccn.org/professionals/physician_gls/pdf/prostate.pdf (Accessed March1, 2014)
- 3.Hull GW, Rabbani F, Abbas F, Wheeler TM, Kattan MW, Scardino PT. Cancer control with radical prostatectomy alone in 1,000 consecutive patients. J Urol 2002;167(Pt 1):528–534 [DOI] [PubMed] [Google Scholar]
- 4.Gofrit ON, Zorn KC, Shikanov SA, Zagaja GP, Shalhav AL. Is seminal vesiculectomy necessary in all patients with biopsy Gleason score 6? J Endourol 2009;23:709–713 [DOI] [PubMed] [Google Scholar]
- 5.Jeong IG, Lim JH, You D, et al. . Incremental value of magnetic resonance imaging for clinically high risk prostate cancer in 922 radical prostatectomies. J Urol 2013;190:2054–2060 [DOI] [PubMed] [Google Scholar]
- 6.Somford DM, Hamoen EH, Futterer JJ, et al. . The predictive value of endorectal 3 Tesla multiparametric magnetic resonance imaging for extraprostatic extension in patients with low, intermediate and high risk prostate cancer. J Urol 2013;190:1728–1734 [DOI] [PubMed] [Google Scholar]
- 7.Logan JK, Rais-Bahrami S, Turkbey B, et al. . Current status of MRI and ultrasound fusion software platforms for guidance of prostate biopsies. BJU Int 2013 [Epub ahead of print]; DOI: 10.1111/bju.12593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pinto PA, Chung PH, Rastinehad AR, et al. . Magnetic resonance imaging/ultrasound fusion guided prostate biopsy improves cancer detection following transrectal ultrasound biopsy and correlates with multiparametric magnetic resonance imaging. J Urol 2011;186:1281–1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rais-Bahrami S, Siddiqui MM, Vourganti S, et al. . Diagnostic value of biparametric MRI as an adjunct to PSA-based Detection of prostate cancer in men without prior biopsies. BJU Int 2014. DOI: 10.1111/bju.12639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turkbey B, Pinto PA, Mani H, et al. . Prostate cancer: Value of multiparametric MR imaging at 3 T for detection—histopathologic correlation. Radiology 2010;255:89–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rais-Bahrami S, Siddiqui MM, Turkbey B, et al. . Utility of multiparametric magnetic resonance imaging suspicion levels for detecting prostate cancer. J Urol 2013;190:1721–1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramsden AR, Chodak G. An analysis of risk factors for biochemical progression in patients with seminal vesicle invasion: Validation of Kattan's nomogram in a pathological subgroup. BJU Int 2004;93:961–964 [DOI] [PubMed] [Google Scholar]
- 13.Holmes JA, Wang AZ, Hoffman KE, et al. . Is primary prostate cancer treatment influenced by likelihood of extraprostatic disease? A surveillance, epidemiology and end results patterns of care study. Int J Radiat Oncol Biol Phys 2012;84:88–94 [DOI] [PubMed] [Google Scholar]
- 14.Terris MK, McNeal JE, Freiha FS, Stamey TA. Efficacy of transrectal ultrasound-guided seminal vesicle biopsies in the detection of seminal vesicle invasion by prostate cancer. J Urol 1993;149:1035–1039 [DOI] [PubMed] [Google Scholar]
- 15.Vallancien G, Prapotnich D, Veillon B, Brisset JM, Andre-Bougaran J. Seminal vesicle biopsies in the preoperative staging of prostatic cancer. Eur Urol 1991;19:196–200 [DOI] [PubMed] [Google Scholar]
- 16.Partin AW, Mangold LA, Lamm DM, Walsh PC, Epstein JI, Pearson JD. Contemporary update of prostate cancer staging nomograms (Partin Tables) for the new millennium. Urology 2001;58:843–848 [DOI] [PubMed] [Google Scholar]
- 17.Koh H, Kattan MW, Scardino PT, et al. . A nomogram to predict seminal vesicle invasion by the extent and location of cancer in systematic biopsy results. J Urol 2003;170(Pt 1):1203–1208 [DOI] [PubMed] [Google Scholar]
- 18.Wang L, Hricak H, Kattan MW, et al. . Prediction of seminal vesicle invasion in prostate cancer: Incremental value of adding endorectal MR imaging to the Kattan nomogram. Radiology 2007;242:182–188 [DOI] [PubMed] [Google Scholar]
- 19.D'Amico AV, Schnall M, Whittington R, et al. . Endorectal coil magnetic resonance imaging identifies locally advanced prostate cancer in select patients with clinically localized disease. Urology 1998;51:449–454 [DOI] [PubMed] [Google Scholar]
- 20.Soylu FN, Peng Y, Jiang Y, et al. . Seminal vesicle invasion in prostate cancer: Evaluation by using multiparametric endorectal MR imaging. Radiology 2013;267:797–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim CK, Choi D, Park BK, Kwon GY, Lim HK. Diffusion-weighted MR imaging for the evaluation of seminal vesicle invasion in prostate cancer: Initial results. J Magn Reson Imaging 2008;28:963–969 [DOI] [PubMed] [Google Scholar]
- 22.Rastinehad AR, Turkbey B, Salami SS, et al. . Improving detection of clinically significant prostate cancer: MRI/TRUS fusion-guided prostate biopsy. J Urol 2014;19:1749–1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stamatakis L, Siddiqui MM, Nix JW, et al. . Accuracy of multiparametric magnetic resonance imaging in confirming eligibility for active surveillance for men with prostate cancer. Cancer 2013;119:3359–3366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siddiqui MM, Rais-Bahrami S, Truong H, et al. . Magnetic resonance imaging/ultrasound-fusion biopsy significantly upgrades prostate cancer versus systematic 12-core transrectal ultrasound biopsy. Eur Urol 2013;64:713–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turkbey B, Merino MJ, Gallardo EC, et al. . Comparison of endorectal coil and nonendorectal coil T2W and diffusion-weighted MRI at 3 Tesla for localizing prostate cancer: Correlation with whole-mount histopathology. J Magn Reson Imaging 2014;39:1443–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turkbey B, Mani H, Shah V, et al. . Multiparametric 3T prostate magnetic resonance imaging to detect cancer: Histopathological correlation using prostatectomy specimens processed in customized magnetic resonance imaging based molds. J Urol 2011;186:1818–1824 [DOI] [PMC free article] [PubMed] [Google Scholar]