Abstract
The existence and identity of multipotent stem cells in the adult lung is currently highly debated. At present, it remains unclear whether candidate stem/progenitor cells are located in the airways, alveoli, or throughout the epithelial lining of the lung. Here, we introduce a method of airway microdissection, which enabled us to study the progenitor behavior of pulmonary epithelial cells in region-specific contexts. The progenitor characteristics of epithelial cells isolated from the trachea, proximal and distal airways, and lung parenchyme were evaluated in vitro and in vivo. We identified a population of airway-derived basal-like epithelial cells with the potential to self-renew and differentiate into airway and alveolar lineages in culture and in vivo after subcutaneous transplantation. The multipotent candidate progenitors originated from a minor fraction of the airway epithelial cell population characterized by high expression of α6 integrin. Results of the current study provide new insights into the regenerative potential of region-specific integrin α6-positive pulmonary epithelial cells.
Introduction
Lack of definitive growth zones and slow cellular turnover in the postnatal organism suggest that lung epithelium does not conform to “classical” stem/progenitor cell hierarchy [1]. Based on in vivo lineage analysis, it was hypothesized that the adult lung epithelium is maintained by abundant lineage-restricted progenitors that function as secretory cells at steady state but can proliferate in response to injury and account for rapid compensatory growth [2,3]. An alternative view that emerged from ex vivo studies suggests that pulmonary epithelium, similar to continuously renewing tissues, is organized in a hierarchical manner with multi-potential stem cells at the top of the hierarchy [4,5]. Recent development of powerful genetic tools, novel lung injury models, and cell separation strategies have demonstrated the remarkable plasticity and context-dependent behavior of lung epithelial cells, thus calling for integration of the two seemingly contradictory hypotheses [1,6].
Several research groups have provided evidence in support of the hypothesis that multi-potential epithelial stem cells exist in the adult lung. In a pioneering report, bronchio-alveolar stem cells (BASCs) were described as dual-positive (CCSPpos pro-SPCpos) cells capable of generating proximal and distal lung-specific epithelium in culture [7]. Clonogenic cells isolated based upon α6β4 integrin expression also exhibited multi-potential characteristics in vitro and in vivo when transplanted under the kidney capsule [5,8]. While the multi-potential stem cell hypothesis needs further experimental testing in vivo, it remains unclear whether the hierarchical model, de-differentiation model, or both are involved in lung epithelial regeneration. Using a novel murine adapted H1N1 influenza infection model, Kumar et al. showed that previously unrecognized keratin-5pos p63pos distal airway stem cells (DASCs) restored integrity of airway and alveolar epithelium within days after virus-induced lung injury [9]. Based on these findings, the authors proposed that rare multi-potential stem cells exist in the lung in a quiescent state and become activated in response to severe injury [9]. Another study recently demonstrated that following basal cell ablation, a subset of tracheal Clara cells can undergo de-differentiation enabling regeneration of the pool of basal stem cells in vivo [10], thus indicating that in the respiratory system, differentiated cells can give rise to multipotent tissue-specific stem/progenitor cells.
The precise location of candidate stem cell populations in the pulmonary system also remains controversial. It has been proposed that cells with multi-potential characteristics are distributed throughout the airways, at bronchio-alveolar junctions (BADJs), or in the alveolar compartment [4,5,8,9]. Due to the complex three-dimensional (3D) architecture of the lung, isolation of epithelial cells from its specific regions has been technically challenging, thus obscuring the identity and location of candidate progenitors. Recently, Chen et al., using the SFTPC-GFP transgenic model, described the isolation of region-specific epithelial progenitors [11]. In the present study, we introduce an alternative microdissection-based approach to isolate epithelial cell populations from different regions of the adult mouse lung. Using modifications of conventional in vitro clonogenic assays, we show that adult airway epithelium can give rise to a population of proliferative basal-like cells during in vitro cultivation and after heterotopic transplantation in vivo. These lung-derived basal-like cells self-renewed in culture and undergo multi-potential differentiation in vitro and in subcutaneous Matrigel implants. The cells of origin of the described multi-potential p63-expressing population appeared to be restricted to intralobular airways and were not found in the epithelium isolated from trachea or lung parenchyme, including BADJ regions. The described subset of candidate multipotent progenitors was isolated from other lung epithelial cells based on high expression of integrin α6 subunit. Our results suggest that the regenerative capacity of integrin α6high cells of the airway epithelial lining is broader than previously thought.
Materials and Methods
Animals
Wild-type B6:129SF2/J, transgenic C57BL/6-Tg(CAG-EGFP)1Osb/J and B6.Cg-Tg(CAG-DrRed*MST)1Nagy/J, and immunodeficient NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ mouse strains were purchased from the Jackson Laboratory (Bar Harbor, ME). All animal studies were conducted in accordance with the University of Illinois Animal Care and Use Committee and the Guide for the Care and Use of Laboratory Animals.
Microdissection of conducting airways and epithelial cell enrichment
For epithelial cell isolation, adult (6–12 week old) mouse lungs were cleared of blood and incubated in 0.5 mg/mL collagenase (Sigma-Aldrich, St. Louis, MO) and 0.125% trypsin (Sigma-Aldrich) in PBS at 4°C for 16 h. Next, the trachea and mainstem bronchi were removed and the airways were gently pulled out of lung lobes (Fig. 1A). All procedures were performed under Zeiss Discovery.V12 binocular microscope (Carl Zeiss Microscopy, Thornwood, NY). Proximal airways identified by high content of surrounding connective tissue and vessels (Fig. 1Av) were dissected from the distal airways (Fig. 1Avi, vii), and combined with the corresponding segments isolated from other lung lobes. Following airway tree removal, parenchyme was visually examined for presence of remaining fragments of the distal airways, which were extracted using ophthalmic forcepts (Fig. 1Avii), and pooled with the distal airways isolated during previous steps. Following separation, the airways and the remaining lung parenchyma were minced and incubated in 2.5 U/mL dispase (STEMCELL Technologies, Vancouver, Canada) and 1 mg/mL collagenase (Sigma-Aldrich) in PBS for 45–60 min at 37°C. Cell suspensions were filtered through 100 μm and 40 μm cell strainers (BD Biosciences, San Diego, CA), resuspended in PBS, incubated with PE-conjugated EpCAM antibody (clone G8.8; eBioscience, San Diego, CA), and separated using anti-PE magnetic microbeads (Miltenyi Biotech, Cambridge, MA). Cells were sorted using autoMACS™ separator (Miltenyi Biotech). Sort purity of EpCAMpos cells was 75%–92% as judged by PE fluorescence in sorted and control groups (Supplementary Fig. S2; Supplementary Data are available online at www.liebertpub.com/scd).
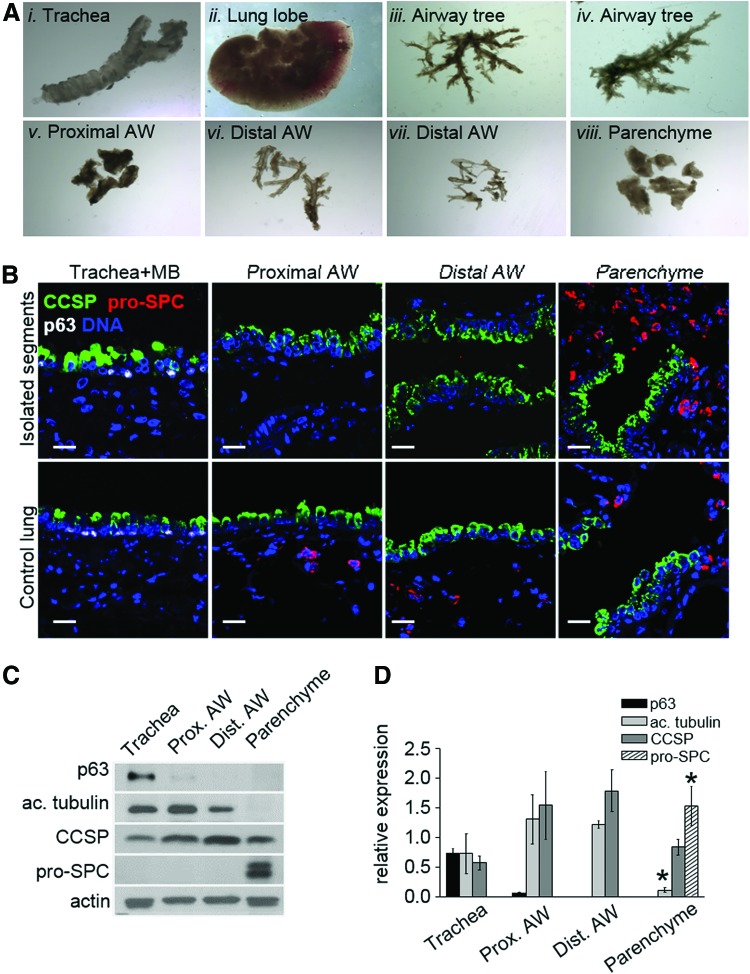
FIG. 1.
Microdissection of distinct anatomical regions of adult mouse lung. (A) Representative images of trachea, lung lobe, and airway tree following microdissection, original magnification 8×: (Ai) trachea and mainstem bronchi; (Aii) lung lobe, airways removed; (Aiii) airway tree immediately after isolation from the corresponding lobe; (Aiv) airway tree isolated from a different lobe; (Av) proximal airways; (Avi) distal airways dissected from the airway tree; (Avii) distal airway fragments recovered from parenchyme; (Aviii) lung parenchyme. (B) Representative western blot and (C) quantification of expression of p63, acetylated tubulin, CCSP, and pro-SPC in the isolated regions (n=3 independent experiments). Integrated optical density of protein bands was normalized to that of loading control (actin). (D) Representative images of the isolated segments (top) and corresponding regions of freshly fixed mouse lung (bottom) stained with antibodies against p63 (white), CCSP (green), and pro-SPC (red). Scale bar: D, 20 μm. Color images available online at www.liebertpub.com/scd
Two-dimensional cell culture
To study colony-forming efficiency (CFE), EpCAMpos cells were plated on tissue culture-treated plastic at a concentration of 1–1.8×103 cells/cm2 and fed with custom growth medium supplemented with 50% fibroblast-conditioned media. Growth media was prepared as described with modifications [12]. Briefly, Ca2+-free Eagle's minimum essential medium (EMEM) media (Lonza, Walkersville, MD) was supplemented with 8% FBS (Thermo Scientific, Rockford, IL) treated with Chelex-100 resin (BioRad Laboratories, Hercules, CA), and the following growth factors: 10 ng/mL human recombinant EGF (RnD Systems, Minneapolis, MN), 0.4 μg/mL hydrocortisone (Sigma-Aldrich), 1×10−9 M cholera toxin (Sigma-Aldrich), 2×10−9 M 3,3′,5-triiodo-L-thyronine (Sigma-Aldrich), 5 μg/mL bovine insulin (Sigma-Aldrich), 100 IU/mL penicillin (Invitrogen, Carlsbad, CA), 100 μg/mL streptomycin (Invitrogen), and 0.1 M CaCl2 to bring the final Ca2+ concentration to 0.06 mM. Contamination by nonepithelial cells in cultures derived from the AutoMACS sorted EpCAMpos fraction was <0.5% by day 10 after plating due to partial inhibition of growth of stromal cells in the low Ca2+ media. To prepare lung fibroblast-conditioned media, juvenile (P5-P10) mouse lungs were minced, treated with 2.5 U/mL dispase (STEMCELL Technologies) and 1 mg/mL collagenase (Sigma-Aldrich) in PBS for 1 h at 37°C, and cells were then plated into in 15% FBS containing high glucose Dulbecco's modified Eagle's medium (DMEM) (Invitrogen) until 80% confluent. Cultures were rinsed with Ca2+ - free PBS and then media with supplements were added for 48 h. Conditioned media was collected, sterile filtered, and stored at −20°C. To subculture epithelial cells, the plate was gently shaken to collect dividing cells, which were plated into new tissue culture vessels.
Three-dimensional cell culture
Epithelial cells, subcultured from large colonies or primary isolates from mouse lungs were combined with wild-type mouse lung fibroblasts (passage 1–2) at a concentration of 2×106 cells/mL. The cell suspensions were embedded in growth factor-reduced Matrigel (BD Biosciences) prediluted 1:1 (Vol/Vol) with DMEM/F12 growth media (Cellgro, Manassas, VA) supplemented with 10% FBS (Thermo Scientific), insulin/transferring/sodium selenite (Sigma-Aldrich), 100 IU/mL penicillin, and 100 μg/mL streptomycin (Invitrogen). One hundred and twenty-five micro liters of cell suspension in Matrigel was placed in 0.4 μm Millicell cell culture inserts (EMD Millipore Corporation, Billerica, MA) and cultured in 24-well plates in DMEM/F12 medium with supplements. Media was changed every 48 h and supplemented, where indicated, with the following growth factors: 100 ng/mL FGF-7, 100 ng/mL FGF-10, 100 ng/mL BMP-4, and 500 ng/mL R-Spondin2 (RnD Systems).
Flow cytometry
Cell suspensions were isolated from whole lung or from the airways and parenchymal regions by dispase/collagenase tissue digestion (see Microdissection of conducting airways), and prepared for flow cytometry as described [13] with modifications. Briefly, cells were resuspended in PBS supplemented with 2% FBS on ice. PE-conjugated EpCAM antibody (clone G8.8; eBioscience) and Alexa Fluor® 647-conjugated CD49f/integrin α6 antibody (BioLegend, San Diego, CA) was added for 30 min at 4°C. Dead cells were discriminated by 4′, 6-diamidino-2-phenylindole staining. Cells were sorted using a MoFlo Beckman Coulter cell sorter.
Naphthalene and bromodeoxyuridine administration
Adult (6–8 week old) B6:129SF2/J mice were injected i.p. with 300 mg/kg naphthalene in corn oil (Mazola), age-matching control animals were injected with corn oil only. The dose was adjusted to B6:129SF2/J strain resulting in a 95% decrease in CCSP mRNA in total lung RNA at 72 h after injection (data not shown). For proliferation studies, bromodeoxyuridine (Sigma-Aldrich) at concentration 50 mg/kg body weight injected i.p. 5 h before sacrifice. Proliferation and differentiation marker expression were studied at 24, 48, 72, 96 h, and 7 days after injection of naphthalene.
Subcutaneous transplantations
Epithelial cells isolated from EGFP transgenic mouse lungs were embedded in growth factor reduced Matrigel on ice (BD Biosciences). Male and female NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ mice, 4–12 weeks of age, were used as recipients. Mice anesthetized with isoflurane received injections of 150–250 μL of Matrigel containing cells under the skin of each flank and interscapular region. Animals were sacrificed at indicated times after transplantation.
Assay of self-renewal potential
EpCAMpos integrin α6pos lung cells isolated from EGFP transgenic animals were cultured in 3D Matrigel as described. At 21 days after plating, cultures were rinsed with PBS and incubated in 5 mg/mL dispase (STEMCELL Technologies) at 37°C for 30–40 min. Individual GFPpos cysts were picked out with a pipette, rinsed, and subjected to 0.25% trypsin digestion for 30 min at 37°C with agitation to obtain single cells. Cell suspensions were filtered through 40 μm cell strainers, combined with wild-type lung fibroblasts, and cultured as described for the primary isolated cells.
Immunostaining
Cells grown in two dimensional (2D) culture were fixed in 4% paraformaldehyde, permeabilized with 0.1% Triton X-100 in PBS, and incubated with antibodies after blocking nonspecific binding with 10% normal donkey serum (Jackson Immuno Research, West Grove, PA). For staining with anti-BrdU antibody, cells were fixed with Carnoy's fixative on ice, treated with 2 M HCl, and incubated with anti-BrdU mouse monoclonal antibody (BU-33) (Sigma-Aldrich).
For immunohistochemical staining of epithelial cysts in Matrigel, cultures were fixed in cell culture inserts with 2% paraformaldehyde, washed in PBS, and embedded in 1.5% agarose [20]. Samples were dehydrated using a gradient of alcohols, embedded in paraffin, and sectioned at 5 μm thickness. After rehydration, sections were subjected to antigen retrieval in Trilogy™ buffer (Cell Marque, Rocklin, CA). After cooling, slides were blocked in 10% donkey serum in TBS and incubated with primary antibodies overnight at 4°C. Primary antibodies were as follows: pro-SPC, pro-SPB, CCSP, (1:1,000 to 1:2,000, kind gifts of Dr. Whitsett, Cincinnati Children's Memorial Hospital), pan-cytokeratin (1:500; Sigma-Aldrich), Aquaporin-5 C-19, (1:200 to 1:500; Santa Cruz Biotechnology, Santa Cruz, CA), p63 mouse monoclonal 4A4 (1:100; Abcam, Cambridge, MA), ABCA3 (1:300; Seven Hills Bioreagents, Cincinnati, OH), GFP (1:2,000; Aves Labs, Tigard, OR), acetylated tubulin (1:2,000; Sigma-Aldrich), mouse monoclonal E-cadherin (1:1,000; BD Transduction Laboratories, San Diego, CA), and sheep polyclonal anti-BrdU (1:1,000; Novus Biologicals, Littleton, CO). Next, sections were incubated for 30 min at 25°C with secondary fluorophore-conjugated F(ab')2 fragments of affinity-purified antibodies raised in donkey (Jackson Immuno Research) and diluted 1:300 to 1:2,000 in TBS.
RT-PCR and qRT-PCR
Total cell RNA was extracted using Qiagen RNeasy kit (Qiagen, Valencia, CA) and reverse transcribed. One hundred nanogram of cDNA template was used in each reaction. Real-time PCR was performed using TaqMan gene expression assays: Sftpc (Mm00488144_m1), Scgb1a1 (Mm00442046_m1), Tbp (m00446974_m1), Muc5ac (Mm01276718_m1), Aqp-5 (Mm00437578_m1), Ager (Mm00545815_m1), Pdpn (Mm00494716_m1), Cdh1 (Mm01247357_m1), Itga6 (Mm00434375_m1), Itgb4 (Mm01266840_m1), Trp63 (Mm00495788_m1), Krt5 (Mm00503549_m1), Sox2 (Mm03053810_s1), Foxm1 (Mm01266840_m1), Ly6a/Sca1 (Mm00726565_s1), Kdr (Mm01222421_m1), Kit (Mm00445212_m1), Lgr6 (Mm01291336_m1), and Id2 (Applied Biosystems, Carlsbad, CA). Expression of each gene was normalized to the housekeeping gene TATA-binding protein (Tbp). Quantitation of relative gene expression was determined using the 7500HT Fast System. Expression of each gene was analyzed within and across the studied regions. The results of analysis are shown in Supplementary Table S1. Genes that are differentially expressed at 10% FDR are highlighted in bold.
Data analysis
Results were analyzed using Origin software (OriginLab, Northampton, MA). Unless specified, data are expressed as mean±SD; P values<0.05 are indicated in figures by (*) and (#) symbols.
Results
Microdissection of conducting airways and lung parenchyme of the adult mouse lung
To study the behavior of epithelial cells from distinct regions of the pulmonary system, we developed a method to isolate proximal (up to third generation) and distal (up to sixth generation) airways from adult mouse lungs (see Materials and Methods section). Briefly, lungs were cleared of blood and incubated in collagenase/trypsin solution in cold PBS for 16 h. After removing the trachea and mainstem bronchi, the airway tree and blood vessels running alongside were gently pulled out of the lung lobes (Fig. 1A). Next, the airways were separated into proximal and distal segments based on their proximity to the mainstem bronchi. Western blot analysis showed that expression of acetylated tubulin was restricted to airway preparations, pro-SPC was detected exclusively in the parenchyme, while CCSP expression was observed in all isolated regions (Fig. 1B–D, n=3 independent experiments). Expression of p63 was detected in the trachea and to a lesser extent in the proximal airway segments (Fig. 1B–D). Consistent with the results of western blot analysis (Fig. 1C, D), a minor subset (<0.5%) of cells with nuclear p63 expression was detected by immunohistochemistry in the proximal, but not the distal freshly isolated airway segments (Supplementary Fig. S1). This procedure allowed for removal of the majority of parenchyme surrounding conducting airways as judged by staining of the isolated segments with an antibody against pro-SPC (Fig. 1B). The terminal bronchioles and BADJ regions could not be separated from gas-exchange regions using this method (Fig. 1B), thus, remaining lung tissue containing terminal bronchioles, BADJs, and the alveoli will be referred to here on as lung parenchyme. Immunohistochemical analysis showed that expression of CCSP, pro-SPC, and p63 in the isolated airways and parenchyme was similar to that in corresponding areas of whole lung tissue (Fig. 1B, lower panels).
A minor subset of airway epithelial cells gives rise to large colonies and demonstrates self-renewal in 2D culture
To evaluate growth potential of region-specific pulmonary epithelium in culture, EpCAMpos cells were isolated from trachea, proximal and distal airways, and parenchyme and plated on plastic at densities of 1.0–1.8×103 cells/cm2. Growth medium contained 0.06 mM Ca2+ and was supplemented with growth factors and lung stromal cell-conditioned medium (Fig. 2A, see Materials and Methods section for full description). It has been established that the low calcium (0.06 mM Ca2+) medium supports growth of epithelial cells derived from skin, prostate, and cornea at clonal seeding densities [12,14,15]. Cultivation of mouse keratinocytes under these conditions has been shown to maintain cells in a proliferative state enabling serial subculture [12]. Unlike co-cultures, this method requires no direct contact between epithelial and stromal (feeder layer) cells while promoting survival and growth of single cell-derived clones of epithelial progenitors.
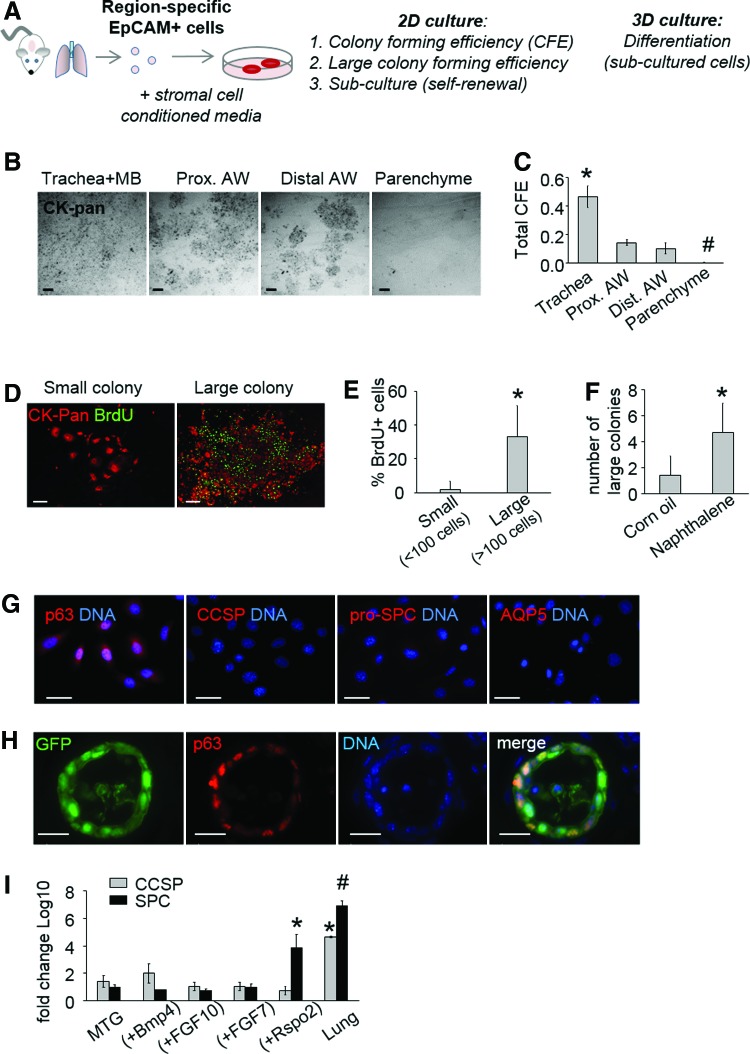
FIG. 2.
A minor subset of airway epithelial cells gives rise to large colonies and demonstrates self-renewal in 2D culture. (A) Scheme: Trachea and mainstem bronchi (MB), proximal (Prox.) and distal (Dist.) airways (AW), and lung parenchyme were dissociated into a single cell suspension, incubated with anti-EpCAM antibodies conjugated with PE, and enriched for EpCAMpos cells using magnetic-activated cell sorting (MACS). EpCAMpos cells from each region were plated at clonal seeding densities on plastic in growth medium with 0.06 mM Ca2+ and supplemented with EGF and lung stromal cell-conditioned medium. (B) Low-resolution representative images of cultures fixed and stained with anti-pan cytokeratin antibody at day 10 after plating. (C) Colony-forming efficiency (CFE) of EpCAMpos cells isolated from trachea, proximal and distal airways, and the parenchyme (mean±SD, n=3 independent experiments). (D) Representative images of small (<100 cells, left) and large (>100 cells, right) colonies with cells that incorporated BrdU over 24 h. Colonies were fixed at day 10 after plating and stained with antibodies against BrdU (green) and pan-cytokeratin (red). (E) Percentage of BrdUpos cells in small (<100 cells) and large (>100 cells) colonies at day 10 in culture; n=50 colonies. (F) Airway EpCAMpos cells were isolated 4 days after IP injection of corn oil or 300 mg/kg naphthalene and plated in culture. The graph represents the number of large colonies (>100 cells, red bars) in cultures generated by control and naphthalene challenged epithelium. Cellular input: 3×104 EpCAMpos cells (n=4, 191 colonies from control lungs and 226 colonies from naphthalene-treated lungs). (G) Representative images of the large colony-derived cells grown in low Ca2+ medium, fixed and stained with antibodies against p63, Aquaporin-5, CCSP, and pro-SPC. (H) Airway EpCAMpos cells were isolated from transgenic EGFP mice and plated in 2D culture in low Ca2+ medium. Cells derived from large colonies were subcultured and plated inside three-dimensional (3D) Matrigel. Twenty-one days after plating, the resulting cysts were fixed, sectioned, and stained with anti-p63 antibody (red). (I) Relative quantification of CCSP and SPC mRNA expression in cysts during 40 cycle real-time RT-PCR. Ct values were normalized to TBP Ct values (endogenous control). To determine fold change, CCSP and SPC mRNA expression in each sample was compared to the level of CCSP and SPC expression in cells grown on plastic in low Ca2+ medium. Results are plotted on Log10 scale and represent mean±SD of three independent experiments. Scale bars: B, 1 mm; D, 500 μm; G, 40 μm; H, 20 μm. Symbols *, # indicate P<0.05. Color images available online at www.liebertpub.com/scd
When cultivated as single cells, pulmonary EpCAMpos cells formed colonies by day 7–10 in culture (Fig. 2B and Supplementary Fig. S2B). The CFE of EpCAMpos cells from the trachea and airways was significantly higher than that of EpCAMpos cells isolated from the parenchyme (Fig. 2B, C, P<0.05, n=3). By day 10, over 80% of all colonies generated by the airway epithelium contained less than 100 cells and were designated as “small” colonies, whereas a few colonies contained 100–600 cells, which we designated as “large” colonies (Supplementary Fig. S2B–D). Importantly, the latter. were never observed in cultures derived from the parenchyme, indicating that cells capable of large colony formation are located in the trachea and conducting airways, but not in terminal bronchioles, the BADJ region, or alveoli (Fig. 2B).
Unlike the small colonies where proliferation largely subsided by day 6–7 postplating, the growth of large colonies was continuous with up to 60% of cells incorporating BrdU over 24 h on day 10 (Fig. 2D, E). Dividing cells collected from the large colonies by mitotic shake off were subcultured at clonal seeding densities (<1×103 cells/cm2). Approximately 1%–5% of recovered dividing cells generated secondary colonies (Supplementary Fig. S2E), a fraction of which contained BrdU-incorporating cells and could be serially subcultured. Under the described conditions, we observed large colony-derived cells capable of serial propagation without a decrease in proliferative potential for over 10 consecutive passages (Supplementary Fig. S3A). The subcultured cells expressed epithelial markers, remained growth factor- and substrate-dependent, and were not tumorigenic (Supplementary Fig. S3B–E).
To evaluate the biological significance of large colony-forming cells, we analyzed cultures derived from the epithelium of control and naphthalene-challenged mouse lungs. Equal numbers of EpCAMpos cells were isolated from animals that received naphthalene or corn oil (control) 4 days after i.p. injection, which corresponded to the proliferative phase of airway reepithelialization (Supplementary Fig. S4). Colony formation was analyzed at day 10 after plating in low Ca2+ medium. The frequency of large colonies significantly increased in cultures derived from naphthalene-challenged lung epithelial cells (Fig. 2F; P<0.05 n=4 independent experiments) suggesting in vivo expansion of the large colony-forming population during reepithelialization of the airways.
When maintained and propagated in low Ca2+ medium, cells of the large colonies demonstrated no expression of major proximal or distal lung-specific differentiation markers, whereas approximately 20% of large colony-forming cells were positive for p63, a specific marker of basal epithelial cells (Fig. 2G). To assess the differentiation potential of cells subcultured in low Ca2+ medium, EpCAMpos cells were isolated from EGFP transgenic mice and expanded in 2D culture to allow for selection of large colonies. Next, cells subcultured from large colonies were combined with wild-type lung fibroblasts and cultured in 3D Matrigel in medium containing physiological Ca2+ concentration (1.2 mM) known to promote differentiation of mouse keratinocytes ([12], see Materials and Methods section). Under these conditions, the large colony-derived cells gave rise to hollow cysts (Supplementary Fig. S3F). Immunohistochemical staining showed that a fraction of cells retained nuclear p63 expression, however, differentiation markers characteristic of Clara cells or alveolocytes were not detected (Fig. 2H and Supplementary Fig. S3G). When studied at the mRNA level, expression of CCSP and SPC were increased in the large colony-derived cysts as compared with cells maintained in the low Ca2+ medium (Fig. 2I). Interestingly, while exogenously added FGF-7, Bmp-4, and FGF-10 did not affect the expression of mRNA of either of the differentiation markers, addition of Wnt signaling modulator R-Spondin-2 significantly increased SPC mRNA expression in the large colony-derived cysts (Fig. 2I, P<0.05, n=3 independent experiments).
In summary, large colony-forming cells demonstrated extensive growth in culture, nuclear p63 expression, and expansion in response to naphthalene-induced lung injury. Interestingly, when treated with R-Spondin-2, these basal-like progenitors showed increased expression of SPC mRNA suggesting they maintained the potential to differentiate into distal lung-specific cell types. Our results also indicate that large colony-forming cells reside in the trachea and intralobular air-conducting epithelium, rather than the BADJ or alveolar compartment.
A fraction of airway-derived epithelial cells gives rise to p63pos CCSPpos pro-SPCpos cysts in 3D culture and subcutaneous Matrigel implants
While cultivation in low Ca2+ medium demonstrated that a fraction of airway cells could be clonally expanded and serially propagated in the basal cell-like state, we were unable to assess differentiation potential of the described candidate progenitors. This limitation of the low Ca2+-based culture system is consistent with previously published work on mouse keratinocytes [16]. To assess the differentiation potential of airway-derived progenitors, primary EpCAMpos cells isolated from the specified regions were cultured in 3D Matrigel in the presence of stromal cells as previously described [5,11], or subcutaneously introduced into NOD-Scid (NSG) mice (Fig. 3A). Tracheal and parenchymal EpCAMpos cells were included in all experiments as controls.
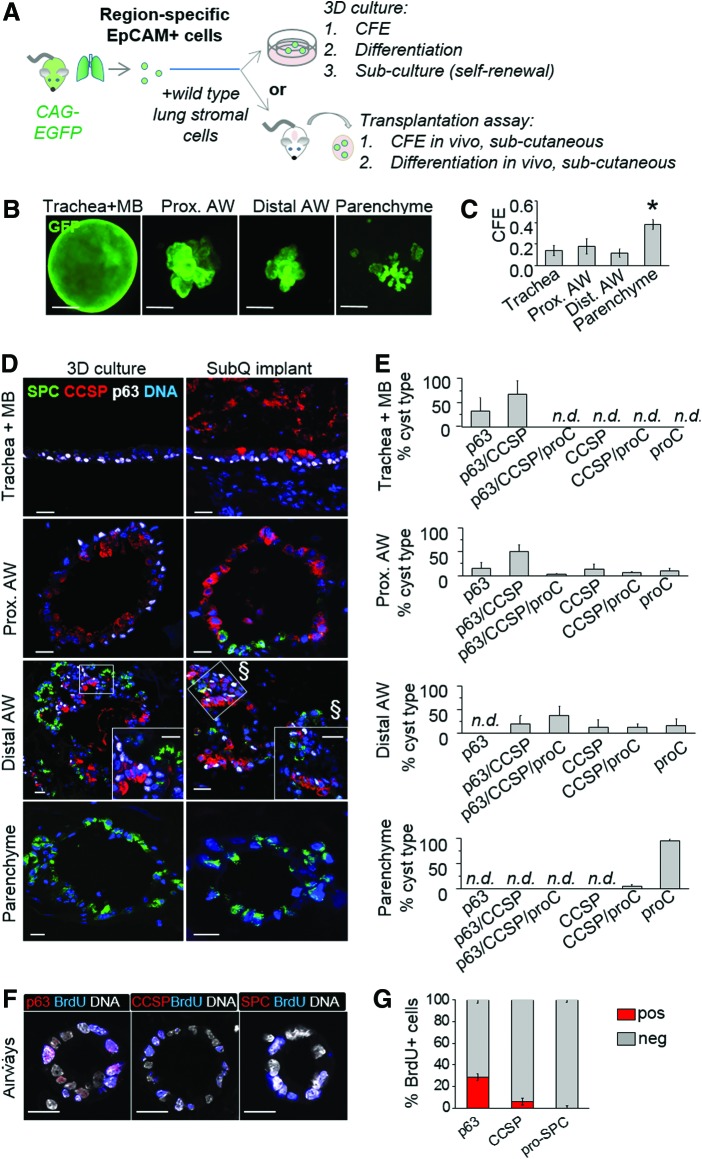
FIG. 3.
A fraction of airway-derived epithelial cells gives rise to p63pos CCSPpos pro-SPCpos cysts in 3D culture and subcutaneous Matrigel implants. (A) Scheme: EpCAMpos cells were isolated from distinct anatomical regions of EGFP mouse lungs, mixed with wild-type fibroblasts, and embedded in Matrigel. Cells were either cultured in insert plates (top) or injected under the skin of NSG mice (bottom). (B) Representative images of GFPpos cysts at day 18 in culture. (C) Cyst-forming efficiency (CFE) by EpCAMpos cells from trachea, airways, and lung parenchyme of EGFP mice; mean±SD; n=3 experiments. (D) Representative immunohistochemistry images of cysts in culture (left panels) or in subcutaneous implants (right panels) stained with antibodies against p63 (white), pro-SPC (green), and CCSP (red). The labels on the right side of the images indicate the source of epithelial cells. (E) Percentage of cyst types based on expression of differentiation markers; bars represent mean+SD, n=3 experiments, 4–25 cysts per culture. (F) At day 5 postplating, airway epithelial cells were labeled with BrdU for 5 h, fixed, and stained with antibodies against p63, CCSP, or pro-SPC (red) and BrdU (blue). (G) Quantification of p63pos, CCSPpos, and pro-SPCpos cells (red bars) in the pool of BrdU-incorporating cells at day 5 in culture. Scale bars: B, 100 μm; D, 20 μm; § indicates image acquired from the same region at a different optical plane, F, 20 μm. *, P<0.05 by ANOVA; n.d., not detectable. Color images available online at www.liebertpub.com/scd
As expected, when cultured in 3D Matrigel, EpCAMpos cells generated epithelial cysts by day 5–7 in culture, while EpCAMneg cells did not give rise to epithelial structures (Supplementary Fig. S5). In contrast to 2D cultures, growth in 3D Matrigel was observed for EpCAMpos cell populations isolated from all anatomical regions, with clonogenic efficiency (CFE) being the highest for parenchymal epithelium (Fig. 3B, C, P<0.05, n=3 independent experiments). Consistent with the study by Chen et al. [11], epithelial cysts generated by airway and parenchymal cells were morphologically distinct and contained region-specific cell types (Fig. 3B, D).
To evaluate the differentiation potential of airway epithelial cells, we characterized the expression of p63, CCSP, and pro-SPC at day 18–21 after culture initiation. A cyst was considered positive for a marker if at least one cell on cross section expressed it (Fig. 3D, E). Cysts derived from trachea and mainstem bronchi contained p63pos cells and lacked CCSPpos or pro-SPCpos cell types. In the parenchyme-derived cultures, no p63pos cysts were observed, whereas 95%±4% of cysts were pro-SPC-only positive and 4.5%±4.1% contained both CCSPpos and pro-SPCpos cells (Fig. 3D, E). When compared to trachea and parenchyme epithelium-derived, cysts generated by airway cells displayed the maximum variety of cell types (Fig. 3D). Strikingly, the airway cultures contained cysts comprised of p63pos, CCSPpos, and pro-SPCpos cell types. This phenotype was not observed in the cysts generated by either tracheal or parenchymal epithelial cells (Fig. 3D, E). The alveolocyte-like differentiation in the airway epithelium-derived cysts with multi-potenital characteristics was confirmed by immunostaining of sections with an antibody against ABCA3, a member of the ATP-binding cassette (ABC) protein family required for synthesis and storage of pulmonary surfactant in mature type II alveolocytes (Supplementary Fig. S6) [17]. To exclude the possibility that the p63pos CCSPpos pro-SPC/ABCA3pos phenotype resulted from cell aggregation in culture, we cultivated mixtures of epithelial cells isolated from lungs of EGFP and RFP transgenic animals and demonstrated that the cysts were single cell-derived (Supplementary Fig. S7A).
To assess the functional significance of p63pos cells in airway epithelial morphogenesis, we evaluated the phenotype of cells surviving and proliferating in 3D Matrigel on day 0–7 after culture initiation (Supplementary Fig. S7B). Analysis of TUNEL and nuclear PCNA staining showed that the peak of cell death in 3D Matrigel occurred at day 1 postplating and was followed by proliferation of surviving epithelial cells from day 4–7 (Supplementary Fig. S7B). When cultures generated by the airway epithelial cells were labeled with BrdU during the proliferative phase of cyst growth at day 5 in culture, 28%±3% of label-incorporating cells were p63pos (Fig. 3F). In comparison, only 5.5%±3% of BrdU-incorporating cells demonstrated CCSP expression at this time point and expression of pro-SPC was not detected by immunohistochemistry (Fig. 3F, G). The predominance of p63pos over CCSPpos and pro-SPCpos cells in the proliferative pool suggests that the p63pos cells behaved as progenitors of the other cell types at least in this subset of airway epithelium-derived cysts.
The results of these experiments suggest that airway-derived epithelial progenitors represent a heterogeneous population, a fraction of which are capable of airway (CCSPpos) and alveolar (pro-SPCpos/ABCA3pos) cell-specific differentiation. Importantly, the same multi-potential characteristics associated with p63pos cysts were observed in 3D Matrigel cultures and subcutaneous implants (Fig. 3D, E). Thus, under these conditions, only airway epithelial progenitors demonstrate the potential to generate p63pos, CCSPpos, and pro-SPCpos cell types.
Prospective isolation of multi-potential airway epithelium-derived progenitors based on expression of α6 integrin
In the epithelium of skin and mammary gland, stem/progenitor populations can be discriminated from terminally differentiated cells by their integrin profile [18,19]. In the pulmonary system, tracheal basal stem cells are characterized by high expression of α6 integrin subunit [20]. Likewise, integrin α6 and β4 were associated with multi-potential epithelial stem/progenitor cells in the adult mouse lung [5,8]. Here, we asked whether the airway epithelial cell subset characterized by high integrin α6 expression is enriched in candidate progenitors that demonstrate basal-like characteristics and multi-potentiality ex vivo.
Immunohistochemical analysis of α6 integrin expression showed that in the adult mouse lung, this subunit is expressed by the majority of airway epithelial cells and a number of epithelial cells in the parenchyme (Supplementary Fig. S8). Despite the ubiquitous pattern of expression, integrin α6high and integrin α6low epithelial subsets could be clearly discriminated when freshly isolated lung cells were analyzed by flow cytometry (Fig. 4A). Single live EpCAMpos integrin α6high and EpCAMpos integrin α6low cells were isolated by FACS from airways and compared to corresponding cell populations derived from trachea and parenchyme (Fig. 4Ai–vi). The percentage of integrin α6high cells in the EpCAMpos population decreased in the proximal-to-distal direction from 44%±11% in trachea to 17%±6.2% in airways and 5.3%±4% in parenchyme (Fig. 4Avii, mean±SD, n=3 experiments).
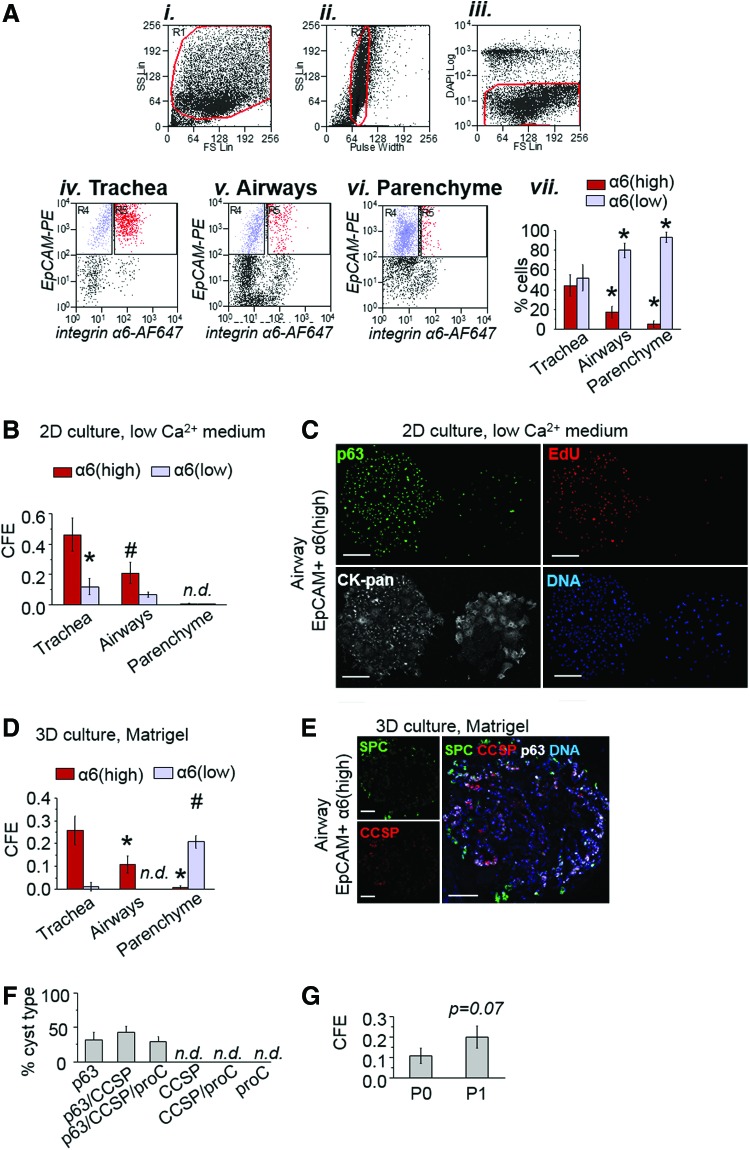
FIG. 4.
Prospective isolation of multi-potential airway epithelium-derived progenitors based on expression of α6 integrin. To test the hypothesis that expression of integrin α6 marks progenitor cells in the adult mouse lung, EpCAMpos integrin α6high and EpCAMpos integrin α6low populations were sorted and plated in 3D Matrigel. (A) FACS strategy: (Ai) total cell population, (Aii) single cell selection, (Aiii) live cell selection. Single live EpCAMpos integrin α6high (red) and single live EpCAMpos integrin α6low (blue) cells were sorted from trachea (Aiv), airways (Av), and the lung parenchyme (Avi); (Avii) percentage of single live integrin α6high (red) and α6low (blue) cells in the EpCAMpos population sorted from distinct lung regions; mean±SD, n=3 experiments; *P<0.05 versus trachea. (B) Total epithelial colony-forming efficiency (CFE) of EpCAMpos integrin α6high (red) and EpCAMpos integrin α6low (blue) cells isolated from distinct regions and plated on plastic in low Ca2+ medium; mean±SD, n=3 experiments. (C) Representative images of large (left) and small (right) colonies formed by airway EpCAMpos integrin α6high cells at day 10 in culture. Cells were fixed and stained with anti-p63 (green) and anti-pan-cytokeratin (white) antibodies following incubation with EdU (red) for 12 h. (D) Cyst-forming efficiency (CFE) in 3D Matrigel was evaluated for GFPpos EpCAMpos integrin α6high (red) and GFPpos EpCAMpos integrin α6low (blue) cells isolated from distinct regions; mean±SD, n=3 experiments; *P<0.05 versus trachea. (D) Representative images of a cyst generated by EpCAMpos integrin α6high cells isolated from the airways. Cultures were stained with antibodies against p63 (white), pro-SPC (green), and CCSP (red) at day 18 after plating in 3D Matrigel. (E) Quantification of types of cysts generated by the airway EpCAMpos integrin α6high cells based on constituent cell type; bars represent mean±SD, n=3 experiments, 9–12 cysts per experiment. (F) To study the self-renewal potential of airway EpCAMpos integrin α6high cells, single cells were recovered from cysts after 18–21 days in culture, mixed with lung fibroblasts, and embedded in Matrigel. (G) The graph shows efficiency of cyst formation (CFE) in primary (P0) and secondary (P1) cultures; results represent mean±SD, n=3 cultures from one of two independent experiments. Scale bars: C, 200 μm; E, 100 μm. n.d., not detectable; *, #=P<0.05 by ANOVA for α6(high) and α6(low), respectively. Color images available online at www.liebertpub.com/scd
CFE and proliferative potential of EpCAMpos integrin α6high cells isolated from the specified regions were analyzed under 2D and 3D culture conditions. When cultivated in low Ca2+ medium, integrin α6high cells isolated from tracheal and airway epithelium demonstrated significantly higher CFE, as compared with the integrin α6low population from the same region (Fig. 4B). A fraction of tracheal and airway EpCAMpos integrin α6high cells gave rise to large colonies containing p63pos cells that incorporated proliferative label at day 10 in culture (Fig. 4C). Consistent with results of CFE assessment in the total EpCAMpos cell population (Fig. 2C and Supplementary Fig. S2C, D), large colonies represented a minor fraction of all colonies generated by integrin α6high epithelial cells. Large colonies were not observed in cultures derived from epithelial cells with low integrin α6 expression (Supplementary Fig. S9). Epithelial cells isolated from parenchyme demonstrated low CFE and produced transient small-sized colonies (<100 cells) irrespective of their integrin α6 status (Fig. 4B and Supplementary Fig. S9).
When co-cultured with fibroblasts in 3D Matrigel, airway-derived EpCAMpos integrin α6high but not integrin α6low cells gave rise to cysts (Fig. 4D). In contrast, EpCAMpos integrin α6high cells isolated from parenchyme demonstrated low CFE under the same conditions. Unlike airway-derived epithelial cells, parenchyme-derived integrin α6low cells generated cysts with high efficiency. These results demonstrate that the CFE of EpCAMpos integrin α6high cells are not the same throughout the pulmonary epithelium (Fig. 4D). Rather, the progenitor characteristics of this population are defined by their location in the lung.
To assess the differentiation potential of airway EpCAMpos integrin α6high cells, on day 18–21, cultures were fixed and stained with antibodies against p63 (white), CCSP (blue), and pro-SPC (green) (Fig. 4E). A fraction of cysts (29%±8%, n=3) contained p63pos, CCSPpos, and pro-SPCpos cells suggesting that one-third of airway progenitor cells isolated based on integrin α6 expression were multi-potential basal cell-like progenitors. The remaining cysts in cultures from integrin α6high cells contained p63pos and CCSPpos cell types (Fig. 4F), whereas CCSPpos/pro-SPCpos, and pro-SPCpos-only cysts characteristic of parenchymal cultures were not observed.
To evaluate the in vitro self-renewal potential of airway integrin α6high progenitors, Matrigel cultures were dissociated by dispase on day 21 and individual epithelial cysts were picked out with a pipette. To obtain a single cell suspension, the cysts were treated with trypsin and the recovered single cells were combined with lung fibroblasts before being placed into secondary culture. After 18–21 days, a fraction of subcultured cells gave rise to secondary epithelial cysts, thus demonstrating self-renewal potential of airway integrin α6high epithelial cells under these conditions (Fig. 4G).
Based on these results, airway EpCAMpos integrin α6high cells represent a heterogeneous population of cells, which include cyst-forming progenitors with the potential to generate cell types with basal-like, airway- and alveolar-specific features.
Airway α6 integrinhigh cells demonstrate increased expression of p63, Sca-1, and KDR, and decreased expression of differentiation markers
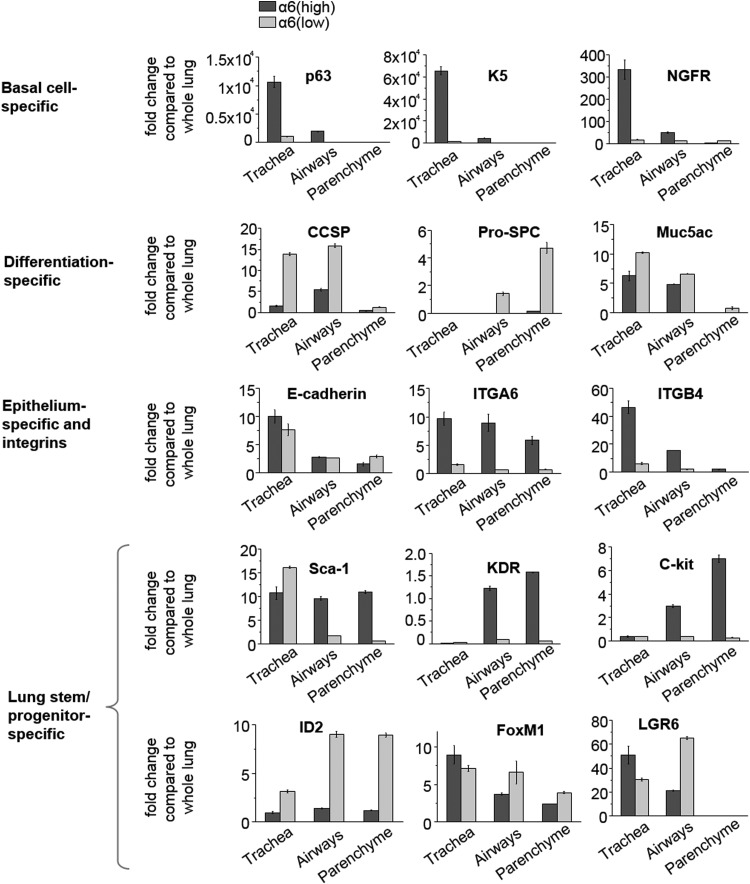
Expression of selected stem/progenitor and differentiated cell-specific markers in the airway integrin α6high fraction was studied by real-time PCR, and compared to that in the integrin α6low cells within and across indicated regions (Fig. 5 and Supplementary Table S1). Similar to tracheal basal cells [20,21], airway integrin α6high cells demonstrated increased p63, keratin-5 (K5), and NGFR expression as compared with the integrin α6low population. However, the level of expression of these genes in airway integrin α6high cells was significantly lower than in those from the trachea, indicating that tracheal and airway integrin α6high cell fractions were not identical (Fig. 5).
FIG. 5.
Airway α6 integrinhigh cells demonstrate increased expression of p63, Sca-1, and KDR, and decreased expression of differentiation markers. To study expression of differentiated and progenitor cell-specific markers, mRNA was collected from FACS isolated EpCAMpos integrin α6high and EpCAMpos integrin α6low cells. Relative quantification of mRNA expression was performed by real-time RT-PCR. Ct values of each sample were normalized to TBP Ct values (endogenous control). To determine fold change, mRNA expression in each sample was compared to the level of expression of corresponding gene in the whole lung. Results are plotted as fold change in expression compared to that in whole lung mRNA extract and represent mean±SD of triplicate samples from one of two independent experiments. In the graphs, gene expression in EpCAMpos integrin α6high cells is reflected by the dark gray bars and that in the EpCAMpos integrin α6low cells by light gray bars.
Consistent with the study by Chapman et al. [8], expression of mRNA encoding primary lung-specific differentiation markers, such as CCSP, SPC, and Muc5AC, was lower in airway EpCAMpos integrin α6high (red bars) as compared with the integrin α6low population isolated from the same region (blue bars) (Fig. 5 and Supplementary Table S1). As expected, expression of integrin α6 and corresponding β4 subunit was significantly higher in the integrin α6high than in the integrin α6low fraction. The level of E-cadherin mRNA expression was not different between airway integrin α6high and integrin α6low cells, confirming the epithelial origin of both populations (Fig. 5 and Supplementary Table S1).
Expression of Sca-1, a marker of BASCs [7] and a subpopulation of Type 2 cells [22], was significantly higher in airway EpCAMpos integrin α6high cells consistent with previous observations that this marker is shared by several progenitor cell populations [5]. Expression of KDR mRNA in the airway EpCAMpos integrin α6high epithelial population was increased as well, supporting the notion that KDR marks airway epithelial progenitors [11]. While we observed higher expression of c-kit mRNA in primary sorted integrin α6high cells, it is unlikely that these cells are related to the previously described human c-kitpos stem cells [23] since our analysis focused only on epithelial (EpCAMpos) cells.
Expression of Id2, the fetal lung multi-potential stem cell marker [24], was found to be lower in integrin α6high than in the integrin α6low epithelial fraction from the same region (Fig. 5 and Supplementary Table S1). FoxM1, a marker associated with proliferative distal lung epithelial progenitors [23,25], and LGR6, a marker that identifies a subpopulation of integrin α6high cells with stem cell characteristics in the human lung [26], were not differentially expressed in integrin α6high and integrin α6low populations across the studied regions (Supplementary Table S1).
Thus, analysis of gene expression showed increased expression of key basal cell-specific markers (p63, keratin-5, and NGFR), a distal airway progenitor marker (K.D.R.), and a distal lung stem/progenitor marker (Sca-1) in the integrin α6high airway epithelial cells. In addition, these results suggest that integrin α6high airway progenitors are distinct from fetal lung Id2pos stem cells, FoxM1pos distal lung progenitors, and LGR6-expressing stem cells described in human lung.
Discussion
The current study provides evidence that the airway epithelium harbors a cell population capable of generating basal cell-like cells that demonstrate multi-potentiality and self-renewal in culture and in vivo after heterotopic transplantation. The described subset of progenitor cells was derived exclusively from airway epithelial cells characterized by high expression of the α6 integrin subunit (Fig. 6).
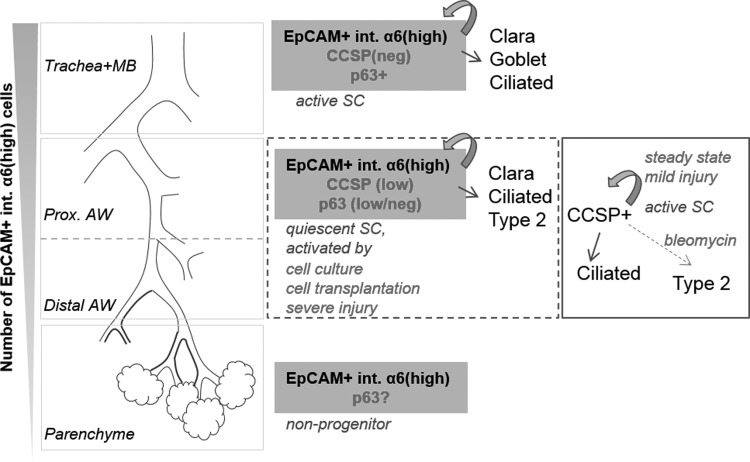
FIG. 6.
Scheme. The number of EpCAMpos integrin α6high cells located along the airway tree and in lung parenchyme decreases in the proximal-to-distal direction. Stem/progenitor characteristics of the EpCAMpos integrin α6high cells appear to be different in the air-conducting versus gas-exchanging regions. In the trachea and upper airways, EpCAMpos integrin α6high cells represent basal cells, which function as active stem cells [20]. In airways lined by simple cuboidal epithelium, EpCAMpos integrin α6high cells represent a heterogeneous population that includes a pool of quiescent candidate stem cells with low/no expression of CCSP protein. In this region, Clara cells perform stem cell functions during tissue maintenance or repair after mild injury [3]. Severe injury (eg, viral infection) or disintegration of tissue (eg, prior to transplantation or placing cells in culture) results in the induction of p63 expression and converts airway EpCAMpos integrin α6high cells into multi-potential p63pos stem cells. Our results provide evidence that airway EpCAMpos integrin α6high epithelial cells have the potential to regenerate both airway and alveolar compartments. In the uninjured lung parenchyme, which contains the fewest EpCAMpos integrin α6high cells, we were unable to detect progenitor activity of the EpCAMpos integrin α6high population.
Isolation of region-specific pulmonary epithelial cells
The precise location of candidate stem/progenitor cells in the pulmonary system remains ambiguous due to the notoriously complex architecture of the lung and lack of region-specific surface markers. Utilizing the expression pattern of the human SPC promoter in a transgenic mouse model, Chen et al. showed that CD24low populations give rise to region-specific epithelium in vitro and identified a set of prospective markers to help discriminate between cells from distinct locations [11]. Here, we introduce an airway tree microdissection technique as an alternative method to isolate region-specific pulmonary epithelial cells. Western blotting and immunohistochemical analyses showed that this approach allowed for separation of proximal (p63pos, acetylated tubulinpos) and distal (pro-SPCpos) lung-specific cell types. Although approximately 10%–15% of cysts in airway epithelial Matrigel cultures had an alveolar-like phenotype, suggesting there was incomplete removal of parenchymal cells and therefore a limitation of this method, the majority of cysts were region-specific, consistent with previously published studies [11]. Regardless, our results demonstrate significant differences in the progenitor potential of airway- and parenchyme-derived EpCAMpos integrin α6high cell populations, thus emphasizing the importance of studying progenitor behavior in region-specific contexts.
Multi-potential progenitor cells of the adult lung epithelium
Unlike skin or intestinal epithelium, epithelium of the lung is lacking definitive growth zones at steady state [1]. During regeneration after injury proliferative cells could be found in prospective niches, such as close to NEB or in BADJ, although it is thought that multiple cells not associated with a specific location can enter proliferation [1,27]. To date it remains unclear whether cells with multi-potential characteristics in vitro originate from airways, BADJ or alveolar region [4,5,8,9]. These multipotent in vitro progenitors appear to originate from airway, BADJ, or alveolar regions. In this study we used ex vivo methods to study progenitor properties of epithelial cells differentially isolated from upper and lower epithelium and parenchymal region including BADJ. Although our method does not allow us to determine location of cells with multipotent properties more precisely, it suggests that these cells are associated with airways instead of BADJ or alveoli. We would like to emphasize that it remains unclear whether candidate stem cells are present in vivo postnatally and to what extent they play a role in lung regeneration [1,27]. In vivo behavior of regenerative cells is pertinent to both type and severity of the injury [1]. Following severe injury or tissue disruption, differentiation potencies of epithelial cells can be drastically different from those documented during physiological tissue maintenance and repair after a mild injury [28]. We observed a subset of airway epithelial progenitors with a basal-like phenotype in vitro and upon heterotopic transplantation that were capable of generating airway- and alveolar-specific cell types. The multi-potential p63pos airway progenitors identified in the present study are possibly related to DASCs described in the mouse lung following H1N1 influenza [9]. Consistent with others, we observed rare p63-expressing cells in proximal airway preparations and did not detect p63pos cells in the distal airways or parenchyme [27] (Fig. 1). Interestingly, more than half of all cysts derived from the distal airway epithelium contained p63pos cells (Fig. 3). Since we excluded cell migration between proximal and distal airways, the possibility remains that the p63pos phenotype was induced from preexisting distal airway epithelial cells. Indeed, two reports using genetic lineage tracing recently demonstrated that p63-expressing cells were derived from CCSPpos cells [10,29]. An alternative possibility is that tissue disaggregation and plating of cells in culture results in expansion of rare preexisting p63pos cells and/or an increase in p63 expression in this minor subset of cells. Although neither scenario can be excluded at this point, to directly address the origin of the described basal-like multi-potential progenitors, it is essential to perform genetic lineage tracing studies of p63-expressing cells of the airway epithelial lining.
Characterization of pulmonary progenitors isolated based on integrin α6 expression
Integrin-based cell sorting results in heterogeneous cell populations in part due to the ubiquitous nature of integrin expression in pulmonary epithelium [1]. Interestingly, our results show that while surface molecule signature of the sorted cell populations was the same (EpCAMpos integrin α6high), their progenitor potential was significantly different depending on the region of the pulmonary system where they were isolated from. In our assays, approximately one-third of cysts generated by integrin α6high epithelial cells demonstrated multi-potentiality in culture indicating that additional and region-specific surface markers may be needed to increase the purity of isolation of the described progenitors.
It is currently unclear whether there are lung stem/progenitor cells characterized by an undifferentiated (primitive) phenotype. Reduced SPC expression was observed in a candidate distal lung α6β4 integrin-expressing stem cell population [6], however, a more recent study indicated that fully differentiated SPC-expressing distal lung cells can also function as stem cells in vivo [30]. Furthermore, it was demonstrated that cells of CCSP lineage were capable of generating functional stem cells via de-differentiation, wherein a subset of these cells with lower CCSP expression appeared to be more likely to become a source of the induced stem cells as compared with cells with higher CCSP expression [10]. Our results show decreased expression of CCSP mRNA in clonogenic airway-derived EpCAMpos integrin α6high cells (Fig. 5), thus supporting the idea that there is an inverse correlation between the differentiated phenotype and progenitor behavior of airway epithelial cells. It remains unclear whether the observed difference in CCSP expression is sufficient to discriminate between the progenitor and nonprogenitor airway cell populations when performing lineage tracing based on CCSP promoter activity, further emphasizing the need for additional genetic models to clarify lineage relationships in adult airway epithelium.
In conclusion, results of the current study are in agreement with the regional progenitor hypothesis. However, assessment of progenitor behavior of airway epithelium in a number of different contexts suggested hierarchical organization of epithelial cells of this region. In 2D and 3D culture systems and in vivo in subcutaneous implants, we observed a previously undescribed p63pos cell population derived from integrin α6high airway epithelial cells. These candidate progenitors demonstrated a primitive phenotype and the ability to self-renew in culture and the potential to give rise to proximal and distal lung-specific cell types. Subsequent studies of airway integrin α6high epithelial cells may provide novel insights into the regenerative potential of adult lung epithelium in vivo.
Supplementary Material
Acknowledgments
The authors acknowledge M. Castellon, L. Menhennett, and the UIC Research Resources Histology and Tissue Imaging, Confocal Microscopy, and Flow Cytometry Services staff for skilled technical assistance. We thank Dr. I. Bertoncello for insightful comments and Dr. J. Whitsett for helpful discussions and generous gifts of CCSP and pro-SPC antibodies. The work was supported by NIH P01 HL060678 (R.D.M.), NIH R01 HL071626 (R.D.M.), and UIC CCTS Grant UL1RR029879 (O.C). Results of this study were presented in part at a number of meetings, including the Keystone Symposium on Lung Development and Repair (2011) and the Stem Cells and Cell Therapies in Lung Biology and Lung Diseases Conference (2011).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Bertoncello I. and McQualter JL. (2013). Lung stem cells: do they exist?. Respirology 18:587–595 [DOI] [PubMed] [Google Scholar]
- 2.Rawlins EL, Okubo T, Xue Y, Brass DM, Auten RL, Hasegawa H, Wang F. and Hogan BL. (2009). The role of Scgb1a1+Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell 4:525–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stripp BR. (2008). Hierarchical organization of lung progenitor cells: is there an adult lung tissue stem cell?. Proc Am Thorac Soc 5:695–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim CF. (2007). Paving the road for lung stem cell biology: bronchioalveolar stem cells and other putative distal lung stem cells. Am J Physiol Lung Cell Mol Physiol 293:L1092–L1098 [DOI] [PubMed] [Google Scholar]
- 5.McQualter JL, Yuen K, Williams B. and Bertoncello I. (2010). Evidence of an epithelial stem/progenitor cell hierarchy in the adult mouse lung. Proc Natl Acad Sci U S A 107:1414–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desai TJ. and Krasnow MA. (2013). Stem cells: Differentiated cells in a back-up role. Nature 503:204–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT. and Jacks T. (2005). Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell 121:823–835 [DOI] [PubMed] [Google Scholar]
- 8.Chapman HA, Li X, Alexander JP, Brumwell A, Lorizio W, Tan K, Sonnenberg A, Wei Y. and Vu TH. (2011). Integrin alpha6beta4 identifies an adult distal lung epithelial population with regenerative potential in mice. J Clin Invest 121:2855–2862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar PA, Hu Y, Yamamoto Y, Hoe NB, Wei TS, Mu D, Sun Y, Joo LS, Dagher R, et al. (2011). Distal airway stem cells yield alveoli in vitro and during lung regeneration following H1N1 influenza infection. Cell 147:525–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tata PR, Mou H, Pardo-Saganta A, Zhao R, Prabhu M, Law BM, Vinarsky V, Cho JL, Breton S, et al. (2013). Dedifferentiation of committed epithelial cells into stem cells in vivo. Nature in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen H, Matsumoto K, Brockway BL, Rackley CR, Liang J, Lee JH, Jiang D, Noble PW, Randell SH, Kim CF. and Stripp BR. (2012). Airway epithelial progenitors are region specific and show differential responses to bleomycin-induced lung injury. Stem Cells 30:1948–1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hager B, Bickenbach JR. and Fleckman P. (1999). Long-term culture of murine epidermal keratinocytes. J Invest Dermatol 112:971–976 [DOI] [PubMed] [Google Scholar]
- 13.Bertoncello I. and McQualter J. (2011). Isolation and clonal assay of adult lung epithelial stem/progenitor cells. Curr Protoc Stem Cell Biol Chapter 2:Unit 2G.1 [DOI] [PubMed] [Google Scholar]
- 14.Litvinov IV, Vander Griend DJ, Xu Y, Antony L, Dalrymple SL. and Isaacs JT. (2006). Low-calcium serum-free defined medium selects for growth of normal prostatic epithelial stem cells. Cancer Res 66:8598–8607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma X, Shimmura S, Miyashita H, Yoshida S, Kubota M, Kawakita T. and Tsubota K. (2009). Long-term culture and growth kinetics of murine corneal epithelial cells expanded from single corneas. Invest Ophthalmol Vis Sci 50:2716–2721 [DOI] [PubMed] [Google Scholar]
- 16.Caldelari R, Suter MM, Baumann D, De Bruin A. and Müller E. (2000). Long-term culture of murine epidermal keratinocytes. J Invest Dermatol 114:1064–1065 [DOI] [PubMed] [Google Scholar]
- 17.Davé V, Childs T, Xu Y, Ikegami M, Besnard V, Maeda Y, Wert SE, Neilson JR, Crabtree GR. and Whitsett JA. (2006). Calcineurin/NFAT signaling is required for perinatal lung maturation and function. J Clin Invest 116:2597–2609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watt FM. (2002). The stem cell compartment in human interfollicular epidermis. J Dermatol Sci 28:173–180 [DOI] [PubMed] [Google Scholar]
- 19.Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML, Wu L, Lindeman GJ. and Visvader JE. (2006). Generation of a functional mammary gland from a single stem cell. Nature 439:84–88 [DOI] [PubMed] [Google Scholar]
- 20.Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, Xue Y, Randell SH. and Hogan BL. (2009). Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci U S A 106:12771–12775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hegab AE, Ha VL, Gilbert JL, Zhang KX, Malkoski SP, Chon AT, Darmawan DO, Bisht B, Ooi AT, et al. (2011). Novel stem/progenitor cell population from murine tracheal submucosal gland ducts with multipotent regenerative potential. Stem Cells 29:1283–1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Y, Sadikot RT, Adami GR, Kalinichenko VV, Pendyala S, Natarajan V, Zhao YY. and Malik AB. (2011) FoxM1 mediates the progenitor function of type II epithelial cells in repairing alveolar injury induced by Pseudomonas aeruginosa. J Exp Med 208:1473–1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kajstura J, Rota M, Hall SR, Hosoda T, D'Amario D, Sanada F, Zheng H, Ogórek B, Rondon-Clavo C, et al. (2011). Evidence for human lung stem cells. N Engl J Med 364:1795–1806 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Rawlins EL, Clark CP, Xue Y. and Hogan BL. (2009). The Id2+ distal tip lung epithelium contains individual multipotent embryonic progenitor cells. Development 136:3741–3745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalin TV, Ustiyan V. and Kalinichenko VV. (2011). Multiple faces of FoxM1 transcription factor: Lessons from transgenic mouse models. Cell Cycle 10:396–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oeztuerk-Winder F, Guinot A, Ochalek A, Ventura JJ. (2012). Regulation of human lung alveolar multipotent cells by a novel p38α MAPK/miR-17-92 axis. EMBO J 31: 3431–3441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rock JR. and Hogan BL. (2011). Epithelial progenitor cells in lung development, maintenance, repair, and disease. Annu Rev Cell Dev Biol 27:493–512 [DOI] [PubMed] [Google Scholar]
- 28.Van Keymeulen A. and Blanpain C. (2012). Tracing epithelial stem cells during development, homeostasis, and repair. J Cell Biol 197:575–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng D, Yin L. and Chen J. (2014). Evidence for Scgb1a1+cells in the generation of p63+ cells in the damaged lung parenchyma. Am J Respir Cell Mol Biol 50:595–604 [DOI] [PubMed] [Google Scholar]
- 30.Barkauskas CE, Cronce MJ, Rackley CR, Bowie EJ, Keene DR, Stripp BR, Randell SH, Noble PW. and Hogan BL. (2013). Type 2 alveolar cells are stem cells in adult lung. J Clin Invest 123:3025–3036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benjamini Y. and Hochberg Y. (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B 57:289–300 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.