Abstract
Background:
Milky urine can be due to chyluria or lipiduria due to nephrotic syndrome. Filarial chyluria usually responds to medical management while non-filarial cases may require surgical intervention.
Aim:
To perform a prospective observational study in patients presenting with milky urine in our centre over a period of one year from July 2011 to June 2012, a complete biochemical work up and imaging to find out the site of leakage of lymph if it is a case of chyluria, its response to medical management and the requirement of surgical intervention.
Materials and Methods:
Routine blood and urine investigations, 24 hour urine protein excretion, USG abdomen, serum lipid profile and rapid filarial antigen test were done in all. MRI abdomen was done in affordable patients. Renal biopsy was done in some chyluria patients for academic purpose and in milky urine with negative urine ether test. Sclerotherapy was done with 50% dextrose and 0.2% povidone iodine. Patients were followed up with 24 hour urine protein and triglyceride estimation.
Results:
18 cases of milky urine were encountered. 8 were filarial chyluria, 9 non- filarial and 1 MCD. Mean urine TG level and median 24 hour urinary protein excretion were 37.2 ± 24.6 mg% and 4.96 g respectively. The mean age for filariasis (22.9 ± 4.5 years) was significantly different from that of non-filarial etiology (31.5 ± 4.8 years) (P = 0.005). The mean 24 hour urinary protein for normal MRI cases (4.64 ± 0.70 g) was significantly different from those with dilated lymphatics (8.15 ± 2.55 g) (P = 0.02). All the non- filarial and 4 filarial cases required sclerotherapy. One patient required a second sitting.
Conclusion:
Milky urine is most commonly due to chyluria and occasionally due to nephrotic syndrome. Nephrotic syndrome is managed in its own way while chyluria not amenable to pharmacological intervention is managed with sclerotherapy.
Keywords: Chyluria, milky urine, sclerotherapy
INTRODUCTION
Milky urine has always remained as a subject of enigma, not only in the context of a nephrologist but for any of the Urologists and practitioners who deal with it. Chyluria or leakage of lymph in urine is the most common cause for milky urine which can be non-parasitic or parasitic. Filariasis is the most common cause of parasitic chyluria in those living in endemic areas between latitude of 40° norths and 30° south including India, China, Southern Japan and South East Asia.[1] Nephrotic syndrome due to lipiduria can also cause milky urine.[2] Other causes of milky urine include urinary tract infection and crystalluria due to precipitation of phosphate.
MATERIALS AND METHODS
A meticulous history was obtained from all patients who presented with milky urine to our outpatient department (OPD) including the evolution of symptoms, past history of abdominal surgery, abdominal trauma, prior pregnancy etc., A thorough physical examination was conducted in all.
Routine basic investigations were done for all the patients including complete hemogram, urine routine and microscopy, random blood sugar, blood urea, serum creatinine, fasting serum lipid profile, electrocardiography, X-ray chest, 24 h urinary protein excretion, ultrasonogram abdomen, absolute eosinophil count, urine for triglycerides (TGs), urine ether dissolution test and rapid filarial antigen card test. Renal biopsy was done in 11 patients. Magnetic resonance imagin (MRI) plain abdomen was done in 10 patients and could not be done in the remaining, owing to the question of affordability. Filariasis was proved by rapid filarial antigen card test.
In all those in whom rapid filarial antigen card test was negative, attempts were made to delineate the cause of chyluria including malarial antigen test and mantoux test. Patients who did not respond to conservative measures underwent cystourethroscopy examination for the purpose of finding out the side of the chylous efflux facilitating the sclerotherapy. Patients in whom the initial ether test was negative were advised to take full fatty meal the previous night and also on the morning of the day of the repeat urine ether dissolution test and the test was repeated again. Urine TG was estimated after a full fatty meal the previous night.
All filarial chyluria cases were given diethyl carbamazine (DEC) in the dose of 6 mg/kg/day for 3 weeks and were considered for cystoscopic examination if they did not respond to the above mentioned therapy. Sclerotherapy was done under local anesthesia and aseptic precautions by injecting a mixture of 50% of dextrose and 0.2% of povidone iodine with the patient lying head down at a tilt of 15-20°. Each time a total of 10 ml (5 ml of 50% dextrose and 5 ml of 0.2% povidone iodine) of sclerosant was injected thrice-a-day at 8 hourly intervals for 3 days. The procedure was enabled by positioning a 5F open ended ureteric axis catheter in situ which is 50 cm long, with its tip inserted into the concerned ureteric orifice for a depth of 5 cm. It's position is secured by attaching it to a 16F size Foley's catheter using a sutopack suture. Foley's was connected to urobag that was kept in position until the 3 day sclerotherapy was over. Both the ureteric axis catheter and the Foley's were removed after the 3 day therapy.
Patients were followed-up in our OPD at regular and periodic intervals after the designated therapy. They were questioned about the recurrence of symptoms. Urine TG was estimated in the early morning after overnight fast, once in 2 weeks for at least 2 months. Patient was said to have attained complete remission if early morning urine TG level was <10 mg% in two different settings done 2 weeks apart after completion of either medical or surgical therapy or both, complete disappearance of proteinuria and return of clear urine.
The distribution of continuous data was tested for normality using Shapiro-Wilk test. Continuous, normally distributed variables were represented as mean ± standard deviation. Median and inter-quartile range (IQR) was used to describe continuous non-parametric data. Parametric data were compared using Student t-test. Statistical analyses were performed using Stata version 11.0 (Stata Corp, College Station, TX) statistical software. A P ≤ 0.05 was considered statistically significant.
OBSERVATIONS AND RESULTS
We encountered 18 cases of frank milky urine over a period of 1 year. The mean age of the patients was 28.9 ± 8.9 years (range: 18-50 years). 16 cases were females (88.9%) and 2 were males (11.1%). Out of these, 17 were proven to be due to chyluria and in the remaining one, the milky urine was due to minimal change disease (MCD) (5.6%).
Among the 17 chyluria cases, 8 were due to filariasis (44.4%), 3 due to post-surgical causes (16.7%) and in the remaining 6 (33.3%), the cause could not be ascertained. The mean age for filarial causes (22.9 ± 4.5 years) was significantly different from that of nonfilarial etiology (31.5 ± 4.8 years) (P = 0.005, t-test) [Figure 1]. Three post-surgical chyluria cases were females, one occurring 2 weeks following a cesarean section and the other 2 occurring approximately 3 weeks following total abdominal hysterectomy.
Figure 1.
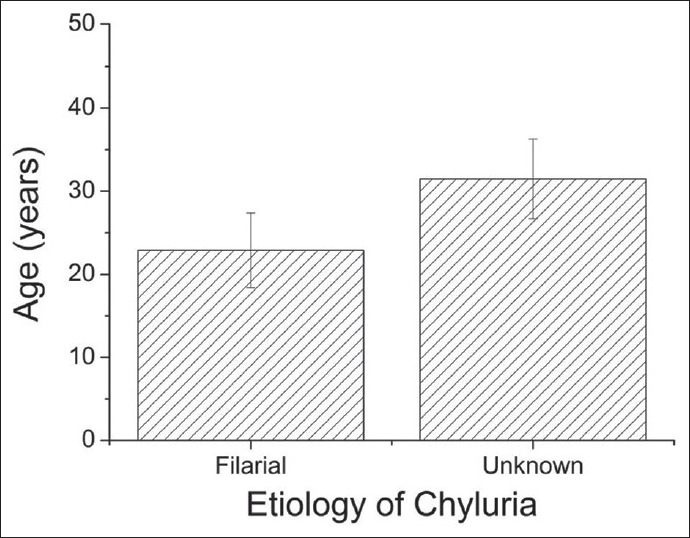
Comparison of mean ages of the filarial and unknown causes. The error bars represent standard deviation
In 2 patients in whom the random urine TG level is <10 mg%, the test was repeated after a full fatty meal the previous night and found to be >15 mg%. The mean urine TG level was 37.2 ± 24.6 mg% (range: 3-102 mg %). The median 24 h urinary protein excretion was 4.96 g (inter-quartile range, 4-6 g) with a largest value obtained being 11.3gm in a patient with filarial chyluria [Figure 2]. The ether dissolution test was positive in all except MCD patient. All patients had normal renal function before and after DEC therapy as well as after sclerotherapy.
Figure 2.

A filarial chyluria patient with frank milky urine. This patient had heavy proteinuria of 11.3 g/d, urine triglyceride of 100 mg% and recurrence after first sclerotherapy
MRI plain abdomen could be done only in 10 patients and in MCD patient it was normal. Three filarial and 2 non filarial cases (50%) showed dilated clusters of lymphatic channels as hyper intense linear tubular structures in T2 FS images in retropelvic, retroperitoneal and paraaortic regions [Figure 3]. The MRI was normal in the remaining 5 cases (50%). The mean 24 h urinary protein for normal MRI cases (4.64 ± 0.70 g) was significantly different from those with dilated lymphatics (8.15 ± 2.55 g) (P = 0.02, t-test) [Figure 4].
Figure 3.
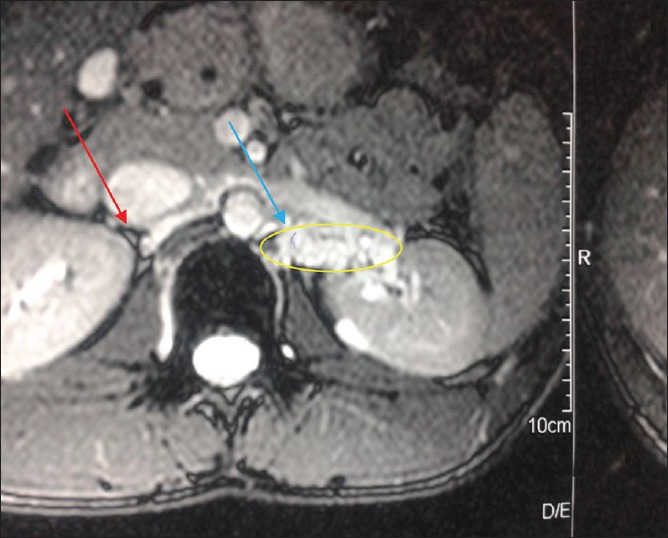
Magnetic resonance imaging showing dilated lymphatic clusters on left side in a patient near the renal hilum, marked by the blue arrow. Note the normal hilum indicated by the red arrow on the right side
Figure 4.
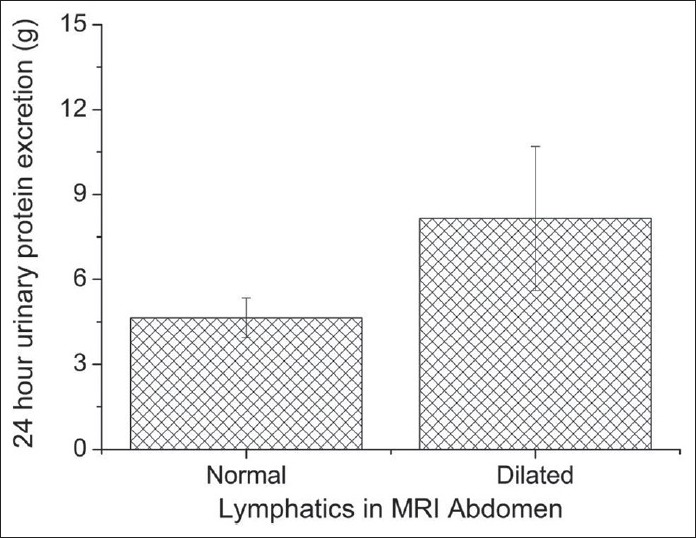
Comparison of mean 24 h urinary protein excretion of the patients who had magnetic resonance imaging abdomen done. The error bars represent standard deviation
Eight cases required DEC therapy. Complete remission was noted in 4 cases (50%), recurrence was observed in 3 (37.5%) and one showed no response (12.5%). Among the cases which had cystoscopy done, left sided leak was observed in 8 patients (57.2%), right sided leak in 3 (21.4%), leak from both sides in 2 (14.3%) and no leak in 1 (7.1%). Renal biopsy was done in 11 patients with one showing MCD, 3 out of 6 (50%) filarial cases showing filarial parasite within the glomerulus [Figure 5]. Biopsies of non-filarial cases were essentially normal.
Figure 5.
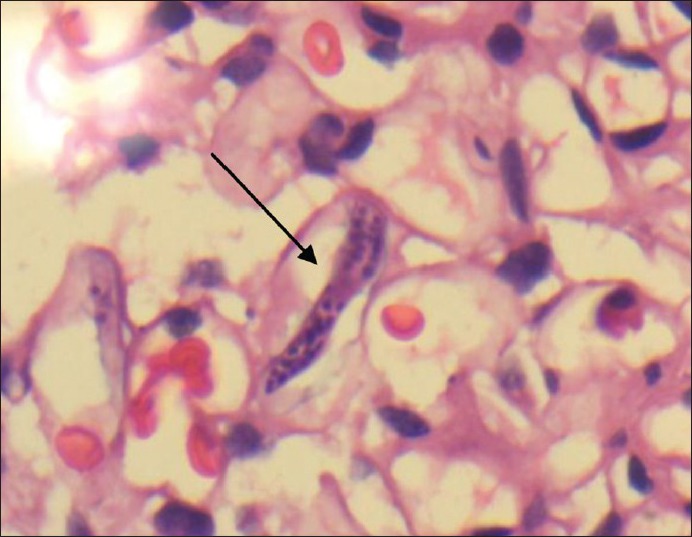
Arrow in the picture pointing the sheathed microfilaria inside the glomerlus in a filarial chyluria patient
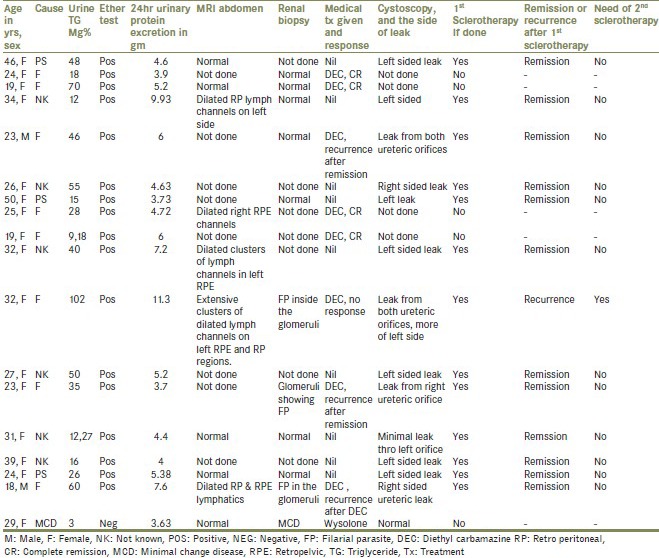
A total of 4 filarial cases [Figure 5] required sclerotherapy as they showed either no response to DEC therapy, partial remission or remission followed by recurrence. All the non-filarial cases required sclerotherapy. Only one among 13 required sclerotherapy in the second sitting who initially had very high urine TG levels secondary to filarial chyluria. The details and management strategies of the patients in the study are summarized in Table 1.
Table 1.
Details and management strategies of the patients
DISCUSSION
Chyluria, passage of lymph per urine has got a multitude of etiologies. Filariasis appears to be a common cause especially in endemic areas. 90-95% of filarial causes are due to wuchereria bancrofti. Other rare causes include trichinella spiralis, echinococcus and ankylostoma duodenale infections. Cases of chyluria have rarely been reported in malaria too.
Among the non-filarial causes, congenital, trauma to the thoracic duct,[3] pregnancy, abdominal surgery, obstruction of the thoracic duct due to tumors are the common causes.
Masayoshi Akisada and Tani observed a wide age range for filarial chyluria varying from 14 to 67 years, an average of 42 years. They also observed a female preponderance with 20 female cases of 30 total cases they encountered.[4] A long-term study in BHU, India showed a male preponderance.[5] In our study, 15 patients out of 17 chyluria patients were females.
In a study done by Peng et al., presence of lipids in postprandial urine was assessed in 116 patients with a history of filariasis and 70 normal individuals using a biochemical autoanalyzer. Urinary TGs ranging from 10 to 1955 mg/dl were detected in 13 individuals with a history of chyluria, including 3 with TG levels ranging from 233 to 1955 mg/dl and cholesterol levels of 6-35 mg/dl. Neither TGs nor cholesterol were detected in the urine of normal individuals.[6] Several other studies indicate the importance of urine TGs in evaluating chyluria patients.[7,8,9]
Most of lipids in the urine of chyluria patients are of dietary origin that is exemplified by the fact that the urine contains major portion of the fatty acids that are fed orally the previous night when measured postprandially.
Urine TGs are highly specific for chyluria and estimation of early morning urine TGs after a fatty meal appears to be the most specific way of seeing the response to therapy, medical or surgical either.
Among the imaging modalities, cystourethroscopy, lymphangiography, lymphoscintigraphy,[10] MRI abdomen and retrograde pyelography can be employed to find out the site of leakage of lymph. MRI appears to be an easily available non-invasive modality that delineates the dilated lymphatic clusters as multiple tortuous fluid filled channels. They are seen as diffuse homogenous hyperintensities in T2-weighted images.
Lymphangiography is probably the investigation of choice[11] as it demonstrates the site of leakage, the actual diameter of the dilated lymph channels. The disadvantages of requiring high technical expertise, high cost, being cumbersome and non-availability in standard centers outweigh its advantages and make it less preferred.
Cystourethroscopy is easily available, can be performed without much needed technical expertise and can be used therapeutically to inject the sclerosing agent while attempting to find the site of leakage.
Nearly 0.1-3% silver nitrate, 0.2% of povidone iodine, 15% of sodium iodide and 50% of dextrose are the common sclerotherapeutic agents employed to sclerose the lymphatics. They produce chemical lymphangitis and produce permanent relief. In the present study, 100% of non-filarial and 50% of filarial patients required cystoscopic sclerotherapy for permanent relief. Only one of these 13 patients required a second sitting of sclerotherapy. Our success rate was higher as we employed a combination of sclerosants, a mixture of 50% dextrose and 0.2% povidone iodine.
In a study done at BHU, Varanasi, India by Nandy et al., complete remission of hematochyluria was observed in 40 out of 46 persons treated with a combination of 50% dextrose and 0.2% povidone iodine as sclerosants.[12] Many of the other studies do exist to mention the efficacy of povidone iodine as an effective sclerosant.[13]
Nausea, vomiting, flank pain and hematuria are some common and self-limiting complications of sclerotherapy.[14] Other cumbersome though rare side-effects include necrotising ureteritis especially with silver nitrate.[15]
CONCLUSION
Frank milky urine is most commonly due to chyuria or lipiduria secondary to nephrotic syndrome. Chyluria is the passage of milky urine secondary to the leakage of lymph through a fistulous communication between the lymphatic and urinary tracts. Wuchereria infection, trauma to the lymph channels mainly post-surgical, retroperitoneal tumors and pregnancy are the important causes. Wastage of significant quantities of lipids and proteins in urine make it necessary to be treated promptly and precisely.
MRI abdomen can be employed as a non-invasive method to detect the dilated channels. Urine TGs are the best way of estimating the response to treatment. Cystoscopy is a cost effective, simple, easily available modality that can also be therapeutically employed to inject sclerosants. A combination of 50% dextrose and 0.2% povidone iodine can produce a higher success rate and much lesser chances of recurrence than 0.2% povidone iodine alone.
Footnotes
Source of Support: Nil
Conflict of Interest: None.
REFERENCES
- 1.Cheng JT, Mohan S, Nasr SH, D’Agati VD. Chyluria presenting as milky urine and nephrotic-range proteinuria. Kidney Int. 2006;70:1518–22. doi: 10.1038/sj.ki.5001703. [DOI] [PubMed] [Google Scholar]
- 2.Sharma S, Hemal AK. Chyluria-an overview. Int J Nephrol Urol. 2009;1:14–26. [Google Scholar]
- 3.Lazarus JA, Marks MS. Non-parasitic chyluria with special reference to traumatic chyluria. J Urol. 1946;56:246–58. doi: 10.1016/S0022-5347(17)69804-5. [DOI] [PubMed] [Google Scholar]
- 4.Akisada M, Tani S. Filarial chyluria in Japan-lymphography, etiology and treatment in 30 cases. Radiology. 1968;90:311–7. [Google Scholar]
- 5.Tandon V, Singh H, Dwivedi US, Mahmood M, Singh PB. Filarial chyluria: Long-term experience of a university hospital in India. Int J Urol. 2004;11:193–8. doi: 10.1111/j.1442-2042.2003.00761.x. [DOI] [PubMed] [Google Scholar]
- 6.Peng HW, Chou CF, Shiao MS, Lin E, Zheng HJ, Chen CC, et al. Urine lipids in patients with a history of filariasis. Urol Res. 1997;25:217–21. doi: 10.1007/BF00941986. [DOI] [PubMed] [Google Scholar]
- 7.Amin S. Letter: Simple test for chyle in the urine. Am J Clin Pathol. 1975;64:286. doi: 10.1093/ajcp/64.2.286a. [DOI] [PubMed] [Google Scholar]
- 8.Hashim SA, Roholt HB, Babayan VK, Vanitallie TB. Treatment of chyluria and chylothorax with medium-chain triglyceride. N Engl J Med. 1964;270:756–61. doi: 10.1056/NEJM196404092701502. [DOI] [PubMed] [Google Scholar]
- 9.Lock DR, Hockenberry D, Cunningham J, Hahm KS, Kantor O, Schonfeld G. Apolipoprotein B subspecies in chylomicrons isolated from a patient with chyluria. Am J Med. 1983;75:360–4. doi: 10.1016/0002-9343(83)91218-4. [DOI] [PubMed] [Google Scholar]
- 10.Liu N, Wang C, Sun M. Noncontrast three-dimensional magnetic resonance imaging vs lymphoscintigraphy in the evaluation of lymph circulation disorders: A comparative study. J Vasc Surg. 2005;41:69–75. doi: 10.1016/j.jvs.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 11.Choi JK, Wiedemer HS. Chyluria: Lymphangiographic study and review of literature. J Urol. 1964;92:723–7. doi: 10.1016/S0022-5347(17)64041-2. [DOI] [PubMed] [Google Scholar]
- 12.Nandy PR, Dwivedi US, Vyas N, Prasad M, Dutta B, Singh PB. Povidone iodine and dextrose solution combination sclerotherapy in chyluria. Urology. 2004;64:1107–9. doi: 10.1016/j.urology.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 13.Ramana Murthy KV, Jayaram Reddy S, Prasad DV, Purusotham G. Povidone iodine instillation into the renal pelvis in the management of chyluria: Our experience. Urol Int. 2010;84:305–8. doi: 10.1159/000288233. [DOI] [PubMed] [Google Scholar]
- 14.Desai R. Complications and precautions of sclerotherapy for chyluria. Indian J Urol. 2005;21:27–30. [Google Scholar]
- 15.Su CM, Lee YC, Wu WJ, Ke HL, Chou YH, Huang CH. Acute necrotizing ureteritis with obstructive uropathy following instillation of silver nitrate in chyluria: A case report. Kaohsiung J Med Sci. 2004;20:512–5. doi: 10.1016/S1607-551X(09)70251-7. [DOI] [PubMed] [Google Scholar]


