Abstract
Purpose of review
In this review, we discuss the recent advances with regard to the mTOR signaling pathway and focus on how this pathway modulates immune responses. Overall, these insights provide important clues in terms of strategically integrating mTOR and metabolic inhibitors into transplantation rejection protocols.
Recent findings
mTOR is regulated by environmental cues and activates diverse downstream pathways to guide cell growth and fate. What has emerged from recent studies is that mechanistically mTOR directs T cell differentiation and function in part by regulating metabolic programs. Such findings not only inform us with regard to the metabolic demands of effector and memory T cells but also elucidate metabolic pathways that might be targeted to selectively regulate immune responses.
Summary
Initial studies focused on the ability of the mTOR inhibitor rapamycin to suppress immune responses by inhibiting T cell proliferation. Since then, both pharmacologic and genetic studies have revealed a central role for mTOR in regulating T cell activation, differentiation and function independent of proliferation. Specifically, it has become clear that mTOR plays an important role in regulating the metabolic machinery necessary for effector, regulatory and memory T cell generation. As such, direct inhibition of metabolism may emerge as a potent and selective means of preventing graft rejection. This review will discuss new insights regarding the ability of downstream signaling pathways, including mTOR-dependent metabolic pathways in regulating T cell responses. Finally, we will discuss these new insights in the context of developing novel immunoregulatory regimens for transplantation.
Keywords: mTOR, metabolism, immunosuppression, T cells, transplantation
INTRODUCTION
Rapamycin, extracted from Streptomyces hygroscopicus, was identified as an anti-fungal agent found in soil from Easter Island in the 1970s [1]. Initially proposed as an antibiotic in yeast, FKBP12, TOR1, and TOR2 were identified as the targets of rapamycin [2]. In yeast, the proteins encoded by these genes interact as subunits of a protein complex that mediates signaling essential for cell cycle and promotes cellular proliferation in response to growth factors and nutrients [3, 4]. The Drosophila TOR homolog dTOR is required for normal growth and proliferation during larval development [5]. The mammalian target of rapamycin (mTOR) is a 289-kDa serine/threonine kinase that was identified as the target of rapamycin, which was initially found as an inhibitor of T cell proliferation [6]. Subsequent studies revealed that rapamycin can also inhibit the proliferation of tumors [7]. More recently however, it has become appreciated that mTOR acts as a central regulator of immune responses, coordinating immunologic and metabolic programs [8]. mTOR senses nutrient availability, growth factors, oxygen and energy status to regulate both innate and adaptive immune responses. Thus, mTOR signaling serves as a vital link between metabolic demand and cellular function and controls metabolic reprogramming during cell activation, proliferation, and differentiation [9-11]. This review summarizes mTOR signaling and focuses on how modulation of mTOR activity can regulate T cell responses.
Overview of mTOR Signaling pathway
In mammalian cells, mTOR exists as one gene but forms two structurally distinct complexes, mTOR Complex 1 (mTORC1) and mTORC2 [12]. In a generic sense, in mammalian cells, mTORC1 is responsible for regulating cell growth and metabolism, while mTORC2 regulates cellular functions such as actin reorganization and survival [13]. Importantly, these generalized distinctions mostly reflect the biologic systems in which mTOR as has been studied. For example, in T cells alone, our group and others have defined multiple specific functions for mTORC1 and mTORC2 [14-18].
mTORC1
mTORC1 consists of regulatory-associated protein of mTOR (Raptor), mammalian lethal with Sec13 protein 8 (mLST8), and the proline-rich Akt substrate 40 kDa (PRAS40) and DEP domain–containing mTOR-interacting protein (DEPTOR) [19-21]. Upon activation of mTORC1, mTOR phosphorylates ribosomal S6 kinase (S6K1), leading to the phosphorylation of ribosomal S6 protein, which is required for protein translation [22]. 4E-BP1, a translational repressor, is also deactivated by mTOR-mediated phosphorylation further promoting translation [22]. Along with increasing protein synthesis, mTORC1 activity also upregulates gene expression programs necessary for glucose and lipid metabolism, mitochondrial biogenesis, and inhibition of autophagy [19, 23].
mTORC2
mTORC2 is composed of mLST8 in addition to rapamycin-independent companion of mTOR (RICTOR), mammalian stress-activated protein kinase interacting protein (mSIN1), DEPTOR, and the protein observed with RICTOR (PROTOR) [19, 21]. Activation of mTORC2 leads to phosphorylation and activation of Akt, protein kinase C (PKC), and serum glucocorticoid-regulated kinase 1 (SGK1) [24-26]. For T cells, one important substrate of mTORC2 is the transcription factor Forkhead box protein O 1 (FOXO1). Phosphorylation of FOXO1 inhibits its activation by its nuclear export [27]. FOXO1 in turn regulates the expression of the transcription factor Krüppel-like factor 2 (KLF2), which promotes the expression of CD62L, and CCR7, and S1P1 [28-30]. As those are key molecules important for T cell homing to secondary lymphoid organs, recently FOXO1 has been shown to play a critical role in promoting the expression of genes important for the development of CD8+ memory T cells [31, 32].
Upstream Regulation of mTOR activity
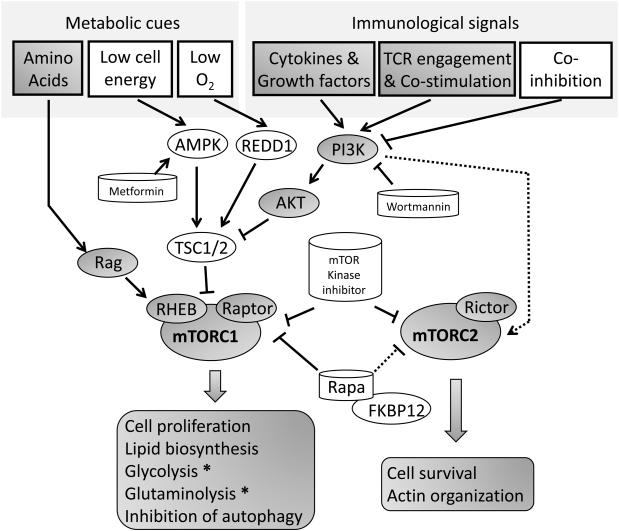
Many immunologic and non-immunologic inputs regulate mTORC1 activity (Fig. 1). TCR engagement + costimulation (for example CD28 signaling) [9, 33], growth factors, insulin and cytokine signaling lead to the activation of phosphatidylinositol 3-kinase (PI3K) activity [34, 35]. Through the production of phosphatidylinositol (3,4,5)-trisphosphate (PIP3), PI3K activates 3-phosphoinositide-dependent protein kinase-1 (PDK1), which in turn activates Akt. When Akt phosphorylates a complex consisting of tuberous sclerosis complex 1 (TSC1) and TSC2, its GTPase-activating protein (GAP) activity is inhibited, allowing for the activation of Ras homolog enriched in brain (RHEB). RHEB is a small GTPase immediately upstream of mTORC1. Through a yet unknown mechanism, active RHEB leads to the activation of mTORC1 and thus RHEB is a crucial regulator of mTORC1 signaling [36-38]. Interestingly, a recent report suggests that in T cells this activation may not be dependent on Akt [39]. mTOR can also integrate negative signals from the immune microenvironment. When the co-inhibitor PD-1 ligand 1 (PD-L1) on antigen presenting cells engages PD-1 on the surface of T cells, this leads to inhibition of PI3K and subsequent inhibition mTOR signaling [40]. The upstream regulation of mTORC2 is less understood. Physical association of mTORC2 with ribosomes in a PI3K dependent manner promotes mTORC2 kinase activity. In addition, endoplasmic reticulum (ER) stress can inhibit mTORC2 activity via GSK3β [41, 42].
Figure 1. mTOR senses metabolic cues and immunological signals to regulate T cell activation, differentiation and function.
In addition to environmental cues such as amino acid availability, energy availability, oxygen and growth factors it is clear that in T cells mTOR signaling is also regulated by TCR engagement, costimulation, co-inhibition (such as PD-1) and cytokines. As discussed in the text pharmacologic agents targeting PI-3K, AMPK, mTOR kinase and the association of mTOR and Raptor all effect mTOR activation. Arrows show activating signals, blocked lines show inhibitory signals and dashed lines indicate that the exact mechanism is unknown. Molecules or conditions that negatively impact mTOR activity are shown on a white background while those that have a positive affect are shown in grey.
Pharmacological Suppression of mTOR
Similar to FK506, the function of rapamycin is dependent on its ability to bind to the immunophilin, FK506 binding protein 12 (FKBP12) [43]. However, unlike FK506 and cyclosporin A, rapamycin does not inhibit calcineurin and hence does not block TCR-induced NF-AT activation [44]. Mechanistically, rapamycin and other rapalogs bind to FKBP12 and are believed to prevent the ability of Raptor to bind to mTOR, thus allosterically inhibiting mTORC1 [45]. Notably, prolonged treatment with rapamycin can also lead to the inhibition of mTORC2 signaling [46]. Alternatively, mTOR kinase inhibitors function as ATP-competitive inhibitors at the mTOR catalytic domain and thus inhibit both mTORC1 and mTORC2 signaling simultaneously [47-49]. Because PI3K regulates mTOR activity, PI3K inhibition can also affect mTOR activation [47](Fig. 1).
Role of mTOR in Cell-mediated Immunity
mTOR integrates diverse environmental signals and metabolic demands to coordinate T cell activation, proliferation, differentiation and trafficking.
CD4+ T cell lineage differentiation
mTORC1 has been shown to be essential for Th1 and Th17 differentiation [16]. TSC1 deletion, leading to enhanced mTORC1 activity in T cells, results in augmented Th1 and Th17 differentiation [50]. Alternatively, T cells lacking RHEB and hence decreased mTORC1 activity fail to differentiate to Th1 or Th17 cells under appropriate skewing conditions [16, 50]. On the other hand, mTORC2 is critical for Th2 differentiation as demonstrated by the observation that RICTOR−/− T cells fail to differentiate to Th2 cells under appropriate polarizing conditions [16, 18]. Interestingly, Raptor−/− T cells have mitigated mTORC1 activity like RHEB−/− T cells, but demonstrate decreased generation of Th2 cells (unlike RHEB−/− T cells) [51]. Notably, Raptor−/− T cells (unlike RHEB−/− T cells) have decreased levels of GATA-3 [51].
While mTORC1 and mTORC2 are important for Th1, Th17 and Th2 differentiation respectively, T cells specific loss of mTOR (and hence both mTORC1 and mTORC2 signaling) not only inhibits the generation of Th1, Th2 and Th17 cells but instead promotes the generation of regulatory T cells [15]. These genetic data are supported by the ability of mTOR kinase inhibitors, which simultaneously inhibit mTORC1 and mTORC2, to promote the generation of Foxp3+ Tregs [16]. Interestingly, there are numerous studies that show that rapamycin can also promote the generation of regulatory T cells [52-54]. Ostensibly, this does not seem to fit with the genetic data since rapamycin inhibits mTORC1. However, consistent with findings using cancer cell lines, rapamycin can also inhibit mTORC2 [46]. Indeed, we have found that T cells (particularly naïve T cells) are exquisitely sensitive to mTORC2 inhibition by rapamycin [16]. In addition to promoting the generation of regulatory T cells, rapamycin appears to preferentially select for the expansion of Foxp3+ T cells [55, 56]. In the presence of rapamycin, Tregs are more resistant to apoptosis, due to the upregulation of Pim-2 kinase [55, 57]. Consistent with this finding, Tregs have been noted to have less mTOR activation upon stimulation, partly because of an increase in the PI3K inhibitor phosphatase and tensin homolog (PTEN)[56]. Interestingly, regulatory T cells can promote the induction of regulatory T cells in part by promoting the depletion of leucine which in turn leads to decreased mTOR activation in the suppressed cells [58]. Additionally, progesterone has been shown to have potent Treg induction activity via suppression of the mTOR pathway [59].
CD8+ T cell effector/memory differentiation and Trafficking
Naïve CD8+ T cells activate mTOR and down-regulate CD62L and CCR7, resulting in trafficking of the activated effector cells out of the lymph nodes and into the tissues [60]. Alternatively, memory CD8+ T cells possess low mTOR activity [61]. This is associated with re-expression of CD62L and CCR7 and homing of memory T cells to secondary lymphoid tissues [62]. Consistent with these findings regarding T cell trafficking, it has been shown that low doses of rapamycin during LCMV infection promotes the generation of memory CD8+ T cells [63]. Mechanistically rapamycin promotes the expression of eomesodermin, a transcription factor that has been associated with memory T cells, and decreases the expression of T-bet which is associated with CD8 effector cells [64]. Recently, a study employing 4-1BB aptamer-raptor siRNA, demonstrated that targeting Raptor siRNA to T cells leads to an increase in the generation of memory CD8+ T cells [65]. Furthermore, culturing LCMV-specific T cells with rapamycin generates long-lived CD8+ memory cells as demonstrated when these cells are adoptively transferred into mice [66].
mTOR coordinates T cell function and metabolism
Evolutionarily from yeast to mammalian cells, TOR acts as an integrator of nutrient-sensing pathways coordinating metabolism with nutrient availability and function [67] (Fig. 1). mTOR signaling regulates glucose, lipid, amino acid and nucleic acid metabolism for cells [68-72]. Recently, it has become clear that effector versus memory CD8+ T cells and different subsets of CD4+ T cells have different metabolic demands. Most mammalian cells rely on the tricarboxylic (TCA) cycle and oxidative phosphorylation to produce adenosine triphosphate (ATP) in the presence of oxygen. However, upon activation, effector T cells utilize glycolysis for their biosynthetic needs, similar to cancer cells [73, 74]. Although aerobic glycolysis produces less ATP, this pathway can provide more of the building blocks necessary for DNA replication, protein synthesis and lipid synthesis [75]. mTORC1 in part regulates the activity of two transcription factors, Myc and hypoxia-inducible factor 1 (HIF-1), responsible for coordinating glycolysis [76-79]. The Myc oncogene is also critical for T cell activation-induced glutaminolysis [76]. The process of glutaminolysis generates α-ketoglutarate for the TCA cycle and metabolic intermediates for biosynthesis. Inhibition of glutaminolysis significantly decreases T cell proliferation and cytokine production [80, 81]. A recent study demonstrated that mTORC1 promotes glutamine anaplerosis by activating glutamate dehydrogenase (GDH) [82]. Rapamycin treatment or acute deletion of Myc impairs the upregulation of multiple glycolytic and glutaminolytic enzymes [76]. Consequently, growth and proliferation of effector T cells are suppressed.
HIF-1 is important in the maintenance of glycolytic metabolism in specific CD4+ and CD8+ T cell subsets and its induction is dependent upon mTOR [39, 83, 84]. mTORC1 activity enhances HIF-1α expression at both the transcriptional and translational level leading to enhanced glucose transport and glycolysis [85]. siRNA mediated inhibition of HIF-1α in TSC2 null cells abrogates expression of critical enzymes for glycolysis [85]. Along these lines, HIF-1 has been shown to be necessary in order to sustain glucose metabolism and glycolysis in effector CTLs [39]. Also, HIF-1 helps to control the expression of cytolytic effector molecules and essential chemokines and adhesion receptors that regulate T cell trafficking [39]. Thus, mTOR-induced HIF-1 expression controls effector CD8+ T cell metabolism, differentiation, and migration [39]. For CD4+ T cells, HIF-1α-deficient T cells have diminished Th17 but increased Treg differentiation [83, 84]. This finding is consistent with the observation that CD4+ effector cells such as Th17 cells utilize glycolysis while Tregs employ oxidative phosphorylation and fatty acid oxidation for their metabolic needs. Indeed by regulating glycolysis the relative generation of effector versus regulatory T cells can be manipulated in vivo [86].
Lipid and cholesterol biosynthesis-SREBP1
Another important metabolic change in activated T cells is to rapidly increase lipid and cholesterol biosynthesis. This process is also in part under the control of mTORC1 via activation of the sterol regulatory element-binding protein 1 (SREBP1) [71]. mTORC1 controls SREBP via the ribosomal S6 Kinase (S6K) [72]. SREBP activation leads to upregulation of genes necessary for de novo lipid biosynthesis [69]. A recent study has demonstrated that SREBPs are essential for CD8+ T cells to undergo metabolic reprogramming in response to mitogenic signaling. Loss of SREBP function in CD8+ T cells renders them unable to efficiently blast, which results in attenuated clonal expansion during viral infection [70].
mTOR activity is regulated by nutrient, energy and oxygen availability
Activated T cells up-regulate glucose and amino acid transporters [87, 88]. Indeed, a critical aspect of CD28 mediated costimulation is to increase the expression of the glucose transporter Glut1 [89]. This increased surface expression is dependent upon PI3K-dependent Akt signaling [89]. Glucose deficiency or blocking Glut1 expression inhibits T cell function [89]. Likewise, amino acid deficiency can also inhibit T cell function. In part this is because mTORC1 activation is dependent upon the presence of branch chain amino acids such as leucine [90]. The lack of plasma membrane transporters of leucine can completely block mTORC1 activity, which can be reversed by cytoplasmic injection of leucine [91]. Recently, it has been shown that the upregulation of the leucine transporter Slc7a5 is induced by TCR stimulation in a NF-AT dependent fashion [92]. T cells lacking Slc7a5 fail to become effector cells upon stimulation [92]. Furthermore, the influx of leucine occurs via a glutamine antiporter. When intracellular glutamine is lacking, mTORC1 activity is also mitigated [93]. N-acetyl-leucine amide (NALA), a derivative of L-leucine, is capable of inhibiting mTOR signaling by acting as a competitive antagonist [11]. NALA causes cell cycle arrest at the G1 stage and blocks cell proliferation in Jurkat cells [94]. Furthermore, activating Th1 cells in the presence of NALA can promote the induction of T cell anergy [11].
In the setting of decreased ATP:AMP ratio, AMPK senses the paucity of energy and in turn inactivates RHEB by phosphorylating TSC, thereby inhibiting mTORC1 activity. The diabetes drug metformin and another AMP-kinase agonist 5-aminoimidazole-4-carboxamideribonucleoside (AICAR) imitate energy deprivation and inhibit mTORC1 [11, 95]. However, AMPK is also activated upon TCR-engagement when mTORC1 activation is robust [96]. Thus, the role of AMPK in T cells is complex and its activation in the setting of antigen recognition may represent a means by which lymphocytes anticipate potential ATP exhaustion [96]. For example, while AMPK deficient CD8+ T cells display normal activation [97, 98] in the setting of glucose deprivation such cells have increased cell death [98]. Likewise, the relationship between low oxygen tension, mTOR and T cell activation is complex. In response to low oxygen tension, the hypoxia-induced factor (HIF)-responsive protein regulated in the development of DNA damage response 1 (REDD1) can also inhibit mTOR by promoting the assembly and activation of TSC [99, 100]. Alternatively, as mentioned above, mTORC1 signaling promotes the expression of HIF which in turn promotes glycolysis [83]. Indeed, in T cells, HIF1α can be induced by activating cytokine or stimuli that trigger the PI3K–AKT pathways, even under normoxic conditions [83, 84, 101].
Regulating immune responses by targeting mTOR and metabolism
Advances in long-term immunosuppressive regimens have driven the field of solid organ transplantation [102]. However, even today with the introduction of antibody therapy and costimulatory blockade, steroids and calcineurin inhibitors remain stalwarts in most post-transplantation regimens. Steroids have multiple adverse effects such as risk of infection, hyperglycemia, accelerated atherosclerosis, and gastrointestinal bleeding [103, 104]. Likewise, calcineurin inhibitors are associated with neuro- and nephrotoxicity as well as risk of infection and an increased risk of cancer [103, 105, 106]. Perhaps more importantly, such agents inhibit negative regulatory and tolerance inducing responses. Indeed, the calcineurin inhibitors are truly immunosuppressive in that they inhibit both activating and inhibitory signaling pathways [43].
In contrast, rapamycin promotes tolerance by facilitating T cell anergy, promoting the generation of regulatory T cells and blocking effector T cell differentiation. All of these properties make inhibitors of mTOR attractive agents for preventing transplantation rejection. However, we believe that until recently, the lack of the strategic integration of mTOR inhibitors into immunosuppressive protocols has limited their efficacy. For example, mechanistically, the simultaneous use of calcineurin inhibitors and mTOR inhibitors potentially mitigates the effects of blocking mTOR [107]. Calcineurin inhibitors block the generation of Tregs as well as the induction of T cell anergy [107]. Alternatively, mTOR inhibitors are not potent inhibitors of acute inflammation. Thus, by themselves, mTOR inhibitors are not effective at arresting acute rejection. To this end, in the setting of non-myeloablative Hematopoeitic Stem Cell Transplantation (HSCT) for sickle cell disease, we have employed a strategy of “Frequency depletion therapy” followed by lymphocyte recovery under the cover of mTOR inhibition [108]. That is, patients were initially treated with CAMPATH that depleted T cells and thus helped to prevent graft rejection in this setting. Subsequently, the patients were maintained on rapamycin for an extended period of time with a slow taper. Unlike previous trials of non-myeloablative HSCT for sickle cell disease which employed more conventional immunosuppression (calcineurin inhibitors) and resulted in graft rejection, our protocol resulted in a robust long term graft acceptance rate [108]. Furthermore, in as much as this procedure results in bone marrow chimerism, the patients that have been successfully completely weaned off of rapamycin represent true transplantation tolerance in humans.
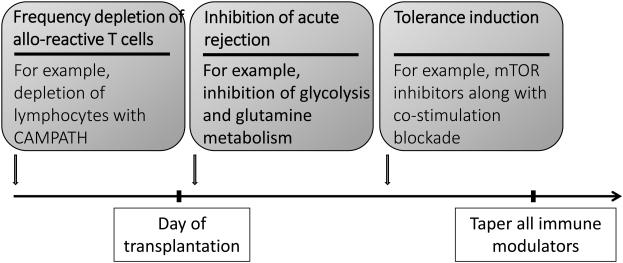
Based in part on these results, we propose that this protocol might provide a template for devising strategies to inhibit graft rejection and promote tolerance in the setting of islet cell, composite tissue graft and solid organ transplantation (Fig. 2). Such a strategy would employ initially, antibody mediated T cell depletion in order to reduce the frequency of allo-reactive cells. The immediate peri-transplant period would entail inhibition of acute effector function. Such a therapy would avoid calcinuerin inhibitors. Instead, we propose the integration of metabolic inhibitors. As mentioned above, effector T cells employ aerobic glycolysis for energy while regulatory T cells employ oxidative phosphorylation and fatty acid oxidation [86]. As such, inhibiting glycolysis can block effector function while leaving T regulatory cells generation and function intact [83, 86]. Likewise, inhibition of glutamine metabolism can also block effector T cell function [81]. Along these lines, our own lab has devised a regimen employing the hexokinase inhibitor 2-DG, the AMPK activator metformin and the glutamine analogue (6-Diazo-5-oxo-L-norleucine, DON) in a mouse model of mismatched skin transplantation (Powell et al. manuscript in preparation).
Figure 2. Proposed novel regimen for transplantation.
The figure outlines a potential template for strategically integrating mTOR and metabolic inhibitors into regimens designed to prevent graft rejection. We propose that initially, antibody mediated therapy should be employed to markedly reduce the frequency of alloreactive T cells. In the peri-transplant setting we propose the use of inhibitors of glycolysis and glutamine metabolism that can mitigate effector function while leaving (and potentially promoting) the generation of regulatory T cells intact. Finally, we propose the use of tolerance inducing agents such as mTOR inhibitors and costimulatory blockade. After long-term tolerance is established, the immune modulators could be tapered and long-term graft survival in the absence of long-term immunosuppression might be achieved.
Finally, the third component of our regimen would involve tolerance induction therapy. For this, we envision employing mTOR inhibitors along with agents such as biologics that block costimulation or even the transfer of Tregs. While this phase of treatment would be more prolonged, the goal is that eventually this would lead to the tapering of all medications upon the establishment of tolerance.
PERSPECTIVES AND CONCLUSION
Rapamcyin was originally developed as an immunosuppressive agent [6]. Yet in the past 30-40 years its impact has paled compared to other cyclophilin binding agents such as Cyslosporin A and FK506. Ironically, the past 10 years have revealed a critical role for mTOR in regulating activation, differentiation and function of cells of the immune system [8]. We believe that this new insight will lead to the strategic and potent integration of mTOR inhibitors into pharmacologic regimens to treat autoimmune disease, inhibit transplant rejection and even enhance tumor immunotherapy [65]. Furthermore, specifically targeting molecules downstream of mTORC1 and mTORC2 will enable the selective targeting of immune function rather than “wholesale” immunosuppression [14]. Along these lines, we believe that the differential metabolic requirements of effector and regulatory T cells reveal an important therapeutic window in order to simultaneously inhibit rejection and promote tolerance. In this way, based on the insight gained by understanding the role that mTOR and metabolism play in regulating immunity we advocate the end of immunosuppression in favor of immunoregulation.
Key Points.
mTOR plays a central role in integrating environmental cues that guide the outcome of antigen recognition.
mTOR plays a critical role in regulating the metabolic machinery necessary for effector, regulatory and memory T cell generation.
Targeting glycolysis can potentially inhibit effector function while leaving the generation of T regulatory cells intact.
Selective and strategic inhibition of mTOR signaling and metabolism represents a potentially novel means to prevent acute graft rejection and promote long-term tolerance.
Acknowledgements
We thank Dr. Kristen N. Pollizzi for critical review of this manuscript. This work was supported by National Institutes of Health grants R01AI077610.
Footnotes
Conflicts of interest:
The authors of the present manuscript do not have any conflict of interest to disclose
References
- [1].Vezina C, Kudelski A, Sehgal SN. Rapamycin (AY-22,989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle. J Antibiot (Tokyo) 1975;28:721–726. doi: 10.7164/antibiotics.28.721. [DOI] [PubMed] [Google Scholar]
- [2].Heitman J, Movva NR, Hall MN. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science. 1991;253:905–909. doi: 10.1126/science.1715094. [DOI] [PubMed] [Google Scholar]
- [3].Barbet NC, Schneider U, Helliwell SB, et al. TOR controls translation initiation and early G1 progression in yeast. Mol Biol Cell. 1996;7:25–42. doi: 10.1091/mbc.7.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hardwick JS, Kuruvilla FG, Tong JK, et al. Rapamycin-modulated transcription defines the subset of nutrient-sensitive signaling pathways directly controlled by the Tor proteins. Proc Natl Acad Sci U S A. 1999;96:14866–14870. doi: 10.1073/pnas.96.26.14866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zhang H, Stallock JP, Ng JC, et al. Regulation of cellular growth by the Drosophila target of rapamycin dTOR. Genes Dev. 2000;14:2712–2724. doi: 10.1101/gad.835000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Dumont FJ, Staruch MJ, Koprak SL, et al. Distinct mechanisms of suppression of murine T cell activation by the related macrolides FK-506 and rapamycin. J Immunol. 1990;144:251–258. [PubMed] [Google Scholar]
- [7].Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer. 2006;6:729–734. doi: 10.1038/nrc1974. [DOI] [PubMed] [Google Scholar]
- [8].Powell JD, Pollizzi KN, Heikamp EB, Horton MR. Regulation of immune responses by mTOR. Annu Rev Immunol. 2012;30:39–68. doi: 10.1146/annurev-immunol-020711-075024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Powell JD, Delgoffe GM. The mammalian target of rapamycin: linking T cell differentiation, function, and metabolism. Immunity. 2010;33:301–311. doi: 10.1016/j.immuni.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zeng H, Chi HB. mTOR and lymphocyte metabolism. Current Opinion in Immunology. 2013;25:347–355. doi: 10.1016/j.coi.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zheng Y, Delgoffe GM, Meyer CF, et al. Anergic T cells are metabolically anergic. J Immunol. 2009;183:6095–6101. doi: 10.4049/jimmunol.0803510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Heikamp EB, Patel CH, Collins S, et al. The AGC kinase SGK1 regulates T1 and T2 differentiation downstream of the mTORC2 complex. Nat Immunol. 2014 doi: 10.1038/ni.2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *This study demonstrates how the selective inhibition of a downstream target of mTORC2 can specifically regulate Th1 and Th2 cell differentiation and function.
- [15].Delgoffe GM, Kole TP, Zheng Y, et al. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30:832–844. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Delgoffe GM, Pollizzi KN, Waickman AT, et al. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat Immunol. 2011;12:295–303. doi: 10.1038/ni.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kim JS, Sklarz T, Banks LB, et al. Natural and inducible TH17 cells are regulated differently by Akt and mTOR pathways. Nat Immunol. 2013;14:611–618. doi: 10.1038/ni.2607. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [18].Lee K, Gudapati P, Dragovic S, et al. Mammalian target of rapamycin protein complex 2 regulates differentiation of Th1 and Th2 cell subsets via distinct signaling pathways. Immunity. 2010;32:743–753. doi: 10.1016/j.immuni.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Laplante M, Sabatini DM. Regulation of mTORC1 and its impact on gene expression at a glance. J Cell Sci. 2013;126:1713–1719. doi: 10.1242/jcs.125773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **This review comprehensively describes the genetic programs regulated by mTORC1 signaling.
- [20].Wang L, Harris TE, Roth RA, Lawrence JC., Jr. PRAS40 regulates mTORC1 kinase activity by functioning as a direct inhibitor of substrate binding. J Biol Chem. 2007;282:20036–20044. doi: 10.1074/jbc.M702376200. [DOI] [PubMed] [Google Scholar]
- [21].Yecies JL, Manning BD. Transcriptional control of cellular metabolism by mTOR signaling. Cancer Res. 2011;71:2815–2820. doi: 10.1158/0008-5472.CAN-10-4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Beugnet A, Tee AR, Taylor PM, Proud CG. Regulation of targets of mTOR (mammalian target of rapamycin) signalling by intracellular amino acid availability. Biochem J. 2003;372:555–566. doi: 10.1042/BJ20021266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Roczniak-Ferguson A, Petit CS, Froehlich F, et al. The transcription factor TFEB links mTORC1 signaling to transcriptional control of lysosome homeostasis. Sci Signal. 2012;5:ra42. doi: 10.1126/scisignal.2002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Garcia-Martinez JM, Alessi DR. mTOR complex 2 (mTORC2) controls hydrophobic motif phosphorylation and activation of serum- and glucocorticoid-induced protein kinase 1 (SGK1) Biochem J. 2008;416:375–385. doi: 10.1042/BJ20081668. [DOI] [PubMed] [Google Scholar]
- [25].Guertin DA, Stevens DM, Thoreen CC, et al. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev Cell. 2006;11:859–871. doi: 10.1016/j.devcel.2006.10.007. [DOI] [PubMed] [Google Scholar]
- [26].Ikenoue T, Inoki K, Yang Q, et al. Essential function of TORC2 in PKC and Akt turn motif phosphorylation, maturation and signalling. EMBO J. 2008;27:1919–1931. doi: 10.1038/emboj.2008.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hedrick SM, Hess Michelini R, Doedens AL, et al. FOXO transcription factors throughout T cell biology. Nat Rev Immunol. 2012;12:649–661. doi: 10.1038/nri3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Carlson CM, Endrizzi BT, Wu J, et al. Kruppel-like factor 2 regulates thymocyte and T-cell migration. Nature. 2006;442:299–302. doi: 10.1038/nature04882. [DOI] [PubMed] [Google Scholar]
- [29].Fabre S, Carrette F, Chen J, et al. FOXO1 regulates L-Selectin and a network of human T cell homing molecules downstream of phosphatidylinositol 3-kinase. J Immunol. 2008;181:2980–2989. doi: 10.4049/jimmunol.181.5.2980. [DOI] [PubMed] [Google Scholar]
- [30].Kerdiles YM, Beisner DR, Tinoco R, et al. Foxo1 links homing and survival of naive T cells by regulating L-selectin, CCR7 and interleukin 7 receptor. Nat Immunol. 2009;10:176–184. doi: 10.1038/ni.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hess Michelini R, Doedens AL, Goldrath AW, Hedrick SM. Differentiation of CD8 memory T cells depends on Foxo1. J Exp Med. 2013;210:1189–1200. doi: 10.1084/jem.20130392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kim MV, Ouyang W, Liao W, et al. The transcription factor Foxo1 controls central-memory CD8+ T cell responses to infection. Immunity. 2013;39:286–297. doi: 10.1016/j.immuni.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Colombetti S, Basso V, Mueller DL, Mondino A. Prolonged TCR/CD28 engagement drives IL-2-independent T cell clonal expansion through signaling mediated by the mammalian target of rapamycin. J Immunol. 2006;176:2730–2738. doi: 10.4049/jimmunol.176.5.2730. [DOI] [PubMed] [Google Scholar]
- [34].Abraham RT. Mammalian target of rapamycin: immunosuppressive drugs uncover a novel pathway of cytokine receptor signaling. Curr Opin Immunol. 1998;10:330–336. doi: 10.1016/s0952-7915(98)80172-6. [DOI] [PubMed] [Google Scholar]
- [35].Stephenson LM, Park DS, Mora AL, et al. Sequence motifs in IL-4R alpha mediating cell-cycle progression of primary lymphocytes. J Immunol. 2005;175:5178–5185. doi: 10.4049/jimmunol.175.8.5178. [DOI] [PubMed] [Google Scholar]
- [36].Saucedo LJ, Gao X, Chiarelli DA, et al. Rheb promotes cell growth as a component of the insulin/TOR signalling network. Nat Cell Biol. 2003;5:566–571. doi: 10.1038/ncb996. [DOI] [PubMed] [Google Scholar]
- [37].Yamagata K, Sanders LK, Kaufmann WE, et al. rheb, a growth factor- and synaptic activity-regulated gene, encodes a novel Ras-related protein. J Biol Chem. 1994;269:16333–16339. [PubMed] [Google Scholar]
- [38].Yee WM, Worley PF. Rheb interacts with Raf-1 kinase and may function to integrate growth factor- and protein kinase A-dependent signals. Mol Cell Biol. 1997;17:921–933. doi: 10.1128/mcb.17.2.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Finlay DK, Rosenzweig E, Sinclair LV, et al. PDK1 regulation of mTOR and hypoxia-inducible factor 1 integrate metabolism and migration of CD8+ T cells. J Exp Med. 2012;209:2441–2453. doi: 10.1084/jem.20112607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Francisco LM, Salinas VH, Brown KE, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Chen CH, Shaikenov T, Peterson TR, et al. ER stress inhibits mTORC2 and Akt signaling through GSK-3beta-mediated phosphorylation of rictor. Sci Signal. 2011;4:ra10. doi: 10.1126/scisignal.2001731. [DOI] [PubMed] [Google Scholar]
- [42].Zinzalla V, Stracka D, Oppliger W, Hall MN. Activation of mTORC2 by association with the ribosome. Cell. 2011;144:757–768. doi: 10.1016/j.cell.2011.02.014. [DOI] [PubMed] [Google Scholar]
- [43].Powell JD, Zheng Y. Dissecting the mechanism of T-cell anergy with immunophilin ligands. Curr Opin Investig Drugs. 2006;7:1002–1007. [PubMed] [Google Scholar]
- [44].Henderson DJ, Naya I, Bundick RV, et al. Comparison of the effects of FK-506, cyclosporin A and rapamycin on IL-2 production. Immunology. 1991;73:316–321. [PMC free article] [PubMed] [Google Scholar]
- [45].Abraham RT, Wiederrecht GJ. Immunopharmacology of rapamycin. Annu Rev Immunol. 1996;14:483–510. doi: 10.1146/annurev.immunol.14.1.483. [DOI] [PubMed] [Google Scholar]
- [46].Sarbassov DD, Ali SM, Sengupta S, et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- [47].Ballou LM, Lin RZ. Rapamycin and mTOR kinase inhibitors. J Chem Biol. 2008;1:27–36. doi: 10.1007/s12154-008-0003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Yu K, Shi C, Toral-Barza L, et al. Beyond rapalog therapy: preclinical pharmacology and antitumor activity of WYE-125132, an ATP-competitive and specific inhibitor of mTORC1 and mTORC2. Cancer Res. 2010;70:621–631. doi: 10.1158/0008-5472.CAN-09-2340. [DOI] [PubMed] [Google Scholar]
- [49].Yu K, Toral-Barza L, Shi C, et al. Biochemical, cellular, and in vivo activity of novel ATP-competitive and selective inhibitors of the mammalian target of rapamycin. Cancer Res. 2009;69:6232–6240. doi: 10.1158/0008-5472.CAN-09-0299. [DOI] [PubMed] [Google Scholar]
- [50].Park Y, Jin HS, Lopez J, et al. TSC1 regulates the balance between effector and regulatory T cells. J Clin Invest. 2013;123:5165–5178. doi: 10.1172/JCI69751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Yang K, Shrestha S, Zeng H, et al. T cell exit from quiescence and differentiation into Th2 cells depend on Raptor-mTORC1-mediated metabolic reprogramming. Immunity. 2013;39:1043–1056. doi: 10.1016/j.immuni.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Haxhinasto S, Mathis D, Benoist C. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J Exp Med. 2008;205:565–574. doi: 10.1084/jem.20071477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Sauer S, Bruno L, Hertweck A, et al. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc Natl Acad Sci U S A. 2008 doi: 10.1073/pnas.0800928105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Kang J, Huddleston SJ, Fraser JM, Khoruts A. De novo induction of antigen-specific CD4+CD25+Foxp3+ regulatory T cells in vivo following systemic antigen administration accompanied by blockade of mTOR. J Leukoc Biol. 2008;83:1230–1239. doi: 10.1189/jlb.1207851. [DOI] [PubMed] [Google Scholar]
- [55].Strauss L, Czystowska M, Szajnik M, et al. Differential responses of human regulatory T cells (Treg) and effector T cells to rapamycin. Plos One. 2009;4:e5994. doi: 10.1371/journal.pone.0005994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Zeiser R, Leveson-Gower DB, Zambricki EA, et al. Differential impact of mammalian target of rapamycin inhibition on CD4+CD25+Foxp3+ regulatory T cells compared with conventional CD4+ T cells. Blood. 2008;111:453–462. doi: 10.1182/blood-2007-06-094482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Basu S, Golovina T, Mikheeva T, et al. Cutting edge: Foxp3-mediated induction of pim 2 allows human T regulatory cells to preferentially expand in rapamycin. J Immunol. 2008;180:5794–5798. doi: 10.4049/jimmunol.180.9.5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Cobbold SP, Adams E, Farquhar CA, et al. Infectious tolerance via the consumption of essential amino acids and mTOR signaling. Proc Natl Acad Sci U S A. 2009;106:12055–12060. doi: 10.1073/pnas.0903919106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Lee JH, Lydon JP, Kim CH. Progesterone suppresses the mTOR pathway and promotes generation of induced regulatory T cells with increased stability. Eur J Immunol. 2012;42:2683–2696. doi: 10.1002/eji.201142317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Sinclair LV, Finlay D, Feijoo C, et al. Phosphatidylinositol-3-OH kinase and nutrient-sensing mTOR pathways control T lymphocyte trafficking. Nat Immunol. 2008;9:513–521. doi: 10.1038/ni.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].van der Windt GJ, Everts B, Chang CH, et al. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity. 2012;36:68–78. doi: 10.1016/j.immuni.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Finlay D, Cantrell D. Phosphoinositide 3-kinase and the mammalian target of rapamycin pathways control T cell migration. Ann N Y Acad Sci. 2010;1183:149–157. doi: 10.1111/j.1749-6632.2009.05134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Araki K, Turner AP, Shaffer VO, et al. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Rao RR, Li Q, Odunsi K, Shrikant PA. The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T-bet and Eomesodermin. Immunity. 2010;32:67–78. doi: 10.1016/j.immuni.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Berezhnoy A, Castro I, Levay A, et al. Aptamer-targeted inhibition of mTOR in T cells enhances antitumor immunity. J Clin Invest. 2014;124:188–197. doi: 10.1172/JCI69856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].He S, Kato K, Jiang J, et al. Characterization of the metabolic phenotype of rapamycin-treated CD8+ T cells with augmented ability to generate long-lasting memory cells. PLoS One. 2011;6:e20107. doi: 10.1371/journal.pone.0020107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Peter C, Waldmann H, Cobbold SP. mTOR signalling and metabolic regulation of T cell differentiation. Current Opinion in Immunology. 2010;22:655–661. doi: 10.1016/j.coi.2010.08.010. [DOI] [PubMed] [Google Scholar]
- [68].Ben-Sahra I, Howell JJ, Asara JM, Manning BD. Stimulation of de novo pyrimidine synthesis by growth signaling through mTOR and S6K1. Science. 2013;339:1323–1328. doi: 10.1126/science.1228792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Duvel K, Yecies JL, Menon S, et al. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Molecular Cell. 2010;39:171–183. doi: 10.1016/j.molcel.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Kidani Y, Elsaesser H, Hock MB, et al. Sterol regulatory element-binding proteins are essential for the metabolic programming of effector T cells and adaptive immunity. Nat Immunol. 2013;14:489–499. doi: 10.1038/ni.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Porstmann T, Santos CR, Griffiths B, et al. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab. 2008;8:224–236. doi: 10.1016/j.cmet.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Yecies JL, Zhang HH, Menon S, et al. Akt stimulates hepatic SREBP1c and lipogenesis through parallel mTORC1-dependent and independent pathways. Cell Metab. 2011;14:21–32. doi: 10.1016/j.cmet.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Pearce EL, Poffenberger MC, Chang CH, Jones RG. Fueling immunity: insights into metabolism and lymphocyte function. Science. 2013;342:1242454. doi: 10.1126/science.1242454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **Comprehensive and up to date review concerning the role of metabolism in regulating immunity.
- [74].Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- [75].Fox CJ, Hammerman PS, Thompson CB. Fuel feeds function: energy metabolism and the T-cell response. Nat Rev Immunol. 2005;5:844–852. doi: 10.1038/nri1710. [DOI] [PubMed] [Google Scholar]
- [76].Wang R, Dillon CP, Shi LZ, et al. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity. 2011;35:871–882. doi: 10.1016/j.immuni.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Gordan JD, Thompson CB, Simon MC. HIF and c-Myc: sibling rivals for control of cancer cell metabolism and proliferation. Cancer Cell. 2007;12:108–113. doi: 10.1016/j.ccr.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Sengupta S, Peterson TR, Sabatini DM. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Molecular Cell. 2010;40:310–322. doi: 10.1016/j.molcel.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Shi Y, Sharma A, Wu H, et al. Cyclin D1 and c-myc internal ribosome entry site (IRES)-dependent translation is regulated by AKT activity and enhanced by rapamycin through a p38 MAPK- and ERK-dependent pathway. J Biol Chem. 2005;280:10964–10973. doi: 10.1074/jbc.M407874200. [DOI] [PubMed] [Google Scholar]
- [80].MacIver NJ, Michalek RD, Rathmell JC. Metabolic regulation of T lymphocytes. Annu Rev Immunol. 2013;31:259–283. doi: 10.1146/annurev-immunol-032712-095956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **This review discusses in detail cellular metabolism in T cell development, activation, differentiation, and function.
- [81].Carr EL, Kelman A, Wu GS, et al. Glutamine uptake and metabolism are coordinately regulated by ERK/MAPK during T lymphocyte activation. J Immunol. 2010;185:1037–1044. doi: 10.4049/jimmunol.0903586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Csibi A, Fendt SM, Li C, et al. The mTORC1 pathway stimulates glutamine metabolism and cell proliferation by repressing SIRT4. Cell. 2013;153:840–854. doi: 10.1016/j.cell.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Shi LZ, Wang R, Huang G, et al. HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J Exp Med. 2011;208:1367–1376. doi: 10.1084/jem.20110278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Dang EV, Barbi J, Yang HY, et al. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell. 2011;146:772–784. doi: 10.1016/j.cell.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Duvel K, Yecies JL, Menon S, et al. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol Cell. 2010;39:171–183. doi: 10.1016/j.molcel.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Michalek RD, Gerriets VA, Jacobs SR, et al. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol. 2011;186:3299–3303. doi: 10.4049/jimmunol.1003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Frauwirth KA, Riley JL, Harris MH, et al. The CD28 signaling pathway regulates glucose metabolism. Immunity. 2002;16:769–777. doi: 10.1016/s1074-7613(02)00323-0. [DOI] [PubMed] [Google Scholar]
- [88].Hayashi K, Jutabha P, Endou H, et al. LAT1 is a critical transporter of essential amino acids for immune reactions in activated human T cells. J Immunol. 2013;191:4080–4085. doi: 10.4049/jimmunol.1300923. [DOI] [PubMed] [Google Scholar]
- [89].Jacobs SR, Herman CE, Maciver NJ, et al. Glucose uptake is limiting in T cell activation and requires CD28-mediated Akt-dependent and independent pathways. J Immunol. 2008;180:4476–4486. doi: 10.4049/jimmunol.180.7.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Sancak Y, Peterson TR, Shaul YD, et al. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science. 2008;320:1496–1501. doi: 10.1126/science.1157535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Christie GR, Hajduch E, Hundal HS, et al. Intracellular sensing of amino acids in Xenopus laevis oocytes stimulates p70 S6 kinase in a target of rapamycin-dependent manner. J Biol Chem. 2002;277:9952–9957. doi: 10.1074/jbc.M107694200. [DOI] [PubMed] [Google Scholar]
- [92].Sinclair LV, Rolf J, Emslie E, et al. Control of amino-acid transport by antigen receptors coordinates the metabolic reprogramming essential for T cell differentiation. Nat Immunol. 2013;14:500–508. doi: 10.1038/ni.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *This report integrates TCR stimulation with the upregulation and function of the Large Neutral Amino Acid transporter Slc7a5.
- [93].Nicklin P, Bergman P, Zhang B, et al. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell. 2009;136:521–534. doi: 10.1016/j.cell.2008.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Hidayat S, Yoshino K, Tokunaga C, et al. Inhibition of amino acid-mTOR signaling by a leucine derivative induces G1 arrest in Jurkat cells. Biochem Biophys Res Commun. 2003;301:417–423. doi: 10.1016/s0006-291x(02)03052-8. [DOI] [PubMed] [Google Scholar]
- [95].Nath N, Giri S, Prasad R, et al. 5-aminoimidazole-4-carboxamide ribonucleoside: a novel immunomodulator with therapeutic efficacy in experimental autoimmune encephalomyelitis. J Immunol. 2005;175:566–574. doi: 10.4049/jimmunol.175.1.566. [DOI] [PubMed] [Google Scholar]
- [96].Tamas P, Hawley SA, Clarke RG, et al. Regulation of the energy sensor AMP-activated protein kinase by antigen receptor and Ca2+ in T lymphocytes. J Exp Med. 2006;203:1665–1670. doi: 10.1084/jem.20052469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Mayer A, Denanglaire S, Viollet B, et al. AMP-activated protein kinase regulates lymphocyte responses to metabolic stress but is largely dispensable for immune cell development and function. Eur J Immunol. 2008;38:948–956. doi: 10.1002/eji.200738045. [DOI] [PubMed] [Google Scholar]
- [98].Rolf J, Zarrouk M, Finlay DK, et al. AMPKalpha1: A glucose sensor that controls CD8 T-cell memory. Eur J Immunol. 2013 doi: 10.1002/eji.201243008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Brugarolas J, Lei K, Hurley RL, et al. Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex. Genes Dev. 2004;18:2893–2904. doi: 10.1101/gad.1256804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Shoshani T, Faerman A, Mett I, et al. Identification of a novel hypoxia-inducible factor 1-responsive gene, RTP801, involved in apoptosis. Mol Cell Biol. 2002;22:2283–2293. doi: 10.1128/MCB.22.7.2283-2293.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Sitkovsky M, Lukashev D. Regulation of immune cells by local-tissue oxygen tension: HIF1 alpha and adenosine receptors. Nat Rev Immunol. 2005;5:712–721. doi: 10.1038/nri1685. [DOI] [PubMed] [Google Scholar]
- [102].Sayegh MH, Carpenter CB. Transplantation 50 years later--progress, challenges, and promises. N Engl J Med. 2004;351:2761–2766. doi: 10.1056/NEJMon043418. [DOI] [PubMed] [Google Scholar]
- [103].Wissing KM, Pipeleers L. Obesity, metabolic syndrome and diabetes mellitus after renal transplantation: Prevention and treatment. Transplant Rev (Orlando) 2014;28:37–46. doi: 10.1016/j.trre.2013.12.004. [DOI] [PubMed] [Google Scholar]
- [104].Hernandez-Diaz S, Rodriguez LA. Steroids and risk of upper gastrointestinal complications. American journal of epidemiology. 2001;153:1089–1093. doi: 10.1093/aje/153.11.1089. [DOI] [PubMed] [Google Scholar]
- [105].Arnold R, Pussell BA, Pianta TJ, et al. Association between calcineurin inhibitor treatment and peripheral nerve dysfunction in renal transplant recipients. Am J Transplant. 2013;13:2426–2432. doi: 10.1111/ajt.12324. [DOI] [PubMed] [Google Scholar]
- [106].Chapman JR. Chronic calcineurin inhibitor nephrotoxicity-lest we forget. Am J Transplant. 2011;11:693–697. doi: 10.1111/j.1600-6143.2011.03504.x. [DOI] [PubMed] [Google Scholar]
- [107].Gao W, Lu Y, El Essawy B, et al. Contrasting effects of cyclosporine and rapamycin in de novo generation of alloantigen-specific regulatory T cells. Am J Transplant. 2007;7:1722–1732. doi: 10.1111/j.1600-6143.2007.01842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Hsieh MM, Kang EM, Fitzhugh CD, et al. Allogeneic hematopoietic stem-cell transplantation for sickle cell disease. N Engl J Med. 2009;361:2309–2317. doi: 10.1056/NEJMoa0904971. [DOI] [PMC free article] [PubMed] [Google Scholar]