Abstract
Opioids have an important place in pharmacology. While their clinical use as analgesics is fundamental in medicine, their use is constrained by their side-effects and abuse potential. Pharmacologists have sought analgesics lacking side-effects and the abuse liability of the current agents. The identification of the opioid receptors in 1973 marked the beginning of our understanding of the molecular mechanisms of these agents. The isolation of the opioid peptides quickly followed, along with the classification of three families of opioid receptors. Clinicians have long been aware of subtle differences among the mu opioids that were not easily reconciled with a single receptor and selective antagonists implied two subdivisions of mu receptors. However, the cloning of the mu opioid receptor MOR-1 has led to the realization of the extensive complexity of the mu opioid receptor gene and its vast array of splice variants. Many of these splice variants are truncated and do not conform to the structure of traditional G-protein coupled receptors. Yet, evidence now shows that they are quite important and may prove valuable targets in the development of potent analgesics lacking the undesirable properties of current opioids.
Keywords: opioid receptor, mu receptor, morphine, truncated, G-protein coupled receptor, splice variant, MOR-1
1.0 Opioid Receptors
Opiates have a special place in pharmacology due to their importance in the management of pain and the societal impact of their abuse. They have been at the leading edge of our understanding of the neuropeptides and their receptors for decades. Since the initial isolation of morphine in 1805, we have seen the generation of a wide range of analogs that have established strong structure-activity relationships, including some of the earliest synthetic pharmacological antagonists, starting with an N-allylnorcodeine in 1917 and evolving to the highly selective agents used today (Pasternak and Pan, 2013). Indeed, these antagonists have been crucial in defining the pharmacology of these drugs.
The synthesis of thousands of analogs and their strict structure-activity relationships led to the proposal of specific recognition sites, or receptors (Beckett and Casy, 1965; Portoghese, 1965; Portoghese, 1966) many years before their demonstration biochemically in 1973 (Pert et al., 1973; Simon et al., 1973; Terenius, 1973), based upon the concept of stereoselectivity described by Goldstein (Goldstein et al., 1971). Subsequent studies of opioid binding sites were the first to demonstrate the sodium effect and its ability to discriminate between the binding of agonists and antagonists, in addition to a variety of other treatments with similar effects (Pasternak et al., 1975b; Pasternak et al., 1975c; Pasternak and Snyder, 1975b; Wilson et al., 1975). These observations have since been extended to an array of other G-protein coupled receptors. Forty years later, crystal structures actually identified the sodium ion binding site within these receptors (Liu et al., 2012).
Since those initial descriptions, the field of opioid receptors has become increasingly complex. There is a long history of receptors in pharmacology, going back over a century, but the opiates were unique in that their receptor was identified without a known endogenous ligand. Looking back, it is remarkable how quickly the endogenous peptides were uncovered following those initial reports. Although several groups identified opioid-like materials in brain (Hughes, 1975; Pasternak et al., 1975a; Terenius and Wahlstrom, 1975), Kosterlitz was the first to identify the materials as pentapeptides (Hughes et al., 1975), followed soon afterwards by his identification of receptors selective for these enkephalins, which he termed delta (Lord et al., 1976). The enkephalins were soon followed by the isolation of dynorphin A (Goldstein et al., 1979) and β-endorphin (Li et al., 1976). Remarkably, these three classes of endogenous opioids share identical amino acid sequences at the first four positions, followed by either methionine ([Met5]enkephalin and β-endorphin) or leucine ([Leu5]enkephalin and the dynorphins). Dynorphin A has its own receptor (Chavkin and Goldstein, 1981), termed kappa1, which corresponds to the kappa receptor proposed by Martin (Martin et al., 1976), while β-endorphin has high affinity for both mu and delta receptors. Each of the three opioid peptides is generated by processing a different precursor confirming that they were separate families of peptides (Berezniuk and Fricker, 2011). Within the enkephalin and dynorphin precursors, there are a number of opioid-like peptides, raising the question as to whether or not these additional opioid-like peptides have their own distinct receptors. This would be quite intriguing since it might offer the opportunity of multiple new targets and drugs with unique pharmacologies.
2.0 Multiple Mu Receptors
There is a rich clinical history of opioids (Eddy, 1973). The opioid field is unique from most others in pharmacology due to the extensive clinical experience with a wide range of drugs, most of them mu agonists, prior to the identification of the receptors. Thus, with opioids, the clinical experience predated the studies of mechanism. This clinical experience provided a depth of understanding of the actions of these agents that was not possible with animal models due to the subtle differences among various drugs. For example, clinicians have long known that the opioids do not work equally well in every patient (Foley, 1985; Foley, 1996; Payne and Pasternak, 1992). Some patients respond better to one drug while another patient may be better managed with a different one. Side-effects seen with a specific drug also can vary from patient to patient independently of its analgesic activity. Finally, studies at the Addiction Research Center in Lexington Kentucky found that patients with a history of opioid abuse were able to distinguish one opioid from another (Eddy, 1973). Together, these observations raised the question of how to reconcile them with the existence of a single receptor, particularly since almost all the drugs were mu opioids.
The first experimental studies suggesting multiple mu opioid receptors came from detailed binding studies that revealed a second morphine binding site of even higher affinity with a distinctive selectivity profile (Lutz et al., 1985; Munson et al., 1984; Pasternak and Snyder, 1975a; Wolozin and Pasternak, 1981). The synthesis of antagonists capable of selectively blocking this second site provided pharmacological tools to distinguish the actions of the proposed subtypes of mu receptors. In brief, these antagonists, naloxonazine and naloxazone, dissociated the supraspinal and spinal analgesic actions of morphine (Ling et al., 1986; Ling and Pasternak, 1983; Paul et al., 1989), as well as separating analgesia from respiratory depression (Ling et al., 1983; Ling et al., 1985), most aspects of physical dependence (Ling et al., 1984), inhibition of gastrointestinal transit (Heyman et al., 1988; Paul and Pasternak, 1988) and even the release of prolactin and growth hormone (Spiegel et al., 1982). These were among the first examples that opioid analageisa could be dissociated from troublesome side-effects.
3.0 Molecular biology of mu opioid receptors
The concept of multiple opioid receptors was first proposed by Martin (Martin, 1967) based upon interactions between morphine and nalorphine (Houde and Wallenstein, 1956; Lasagna and Beecher, 1954). In this proposal, he suggested the existence of morphine, or “M”, receptors and nalorphine, or “N” receptors. He subsequently proposed that the “M” receptors be named mu and the “N” termed kappa, based upon the pharmacology of ketocyclazocine (Martin et al., 1976). The third member of the opioid receptor family was proposed by Kosterlitz based upon the enkephalins and termed delta (Lord et al., 1977), followed by the closely related peptide orphanin FQ/nociceptin (Meunier et al., 1995; Reinscheid et al., 1995) and its receptor, ORL1 (also known as KOR-3) (Bunzow et al., 1994; Chen et al., 1994; Fukuda et al., 1994; Keith, Jr. et al., 1994; Mollereau et al., 1994; Pan et al., 1994). Much effort has been focused upon targeting these additional receptor classes to develop analgesics lacking the problems of the traditional opiates used clinically – which are almost all mu. These efforts have not yet produced clinically useful agents. Kappa1 agents were found to have psychotomimetic actions while early delta compounds were complicated by seizure activity. However, recent results suggest that drugs selective for mu opioid receptor subtypes may prove valuable in the development of superior analgesics. Targeting the ORL1 receptor may yield interesting compounds, but they have not yet been examined clinically.
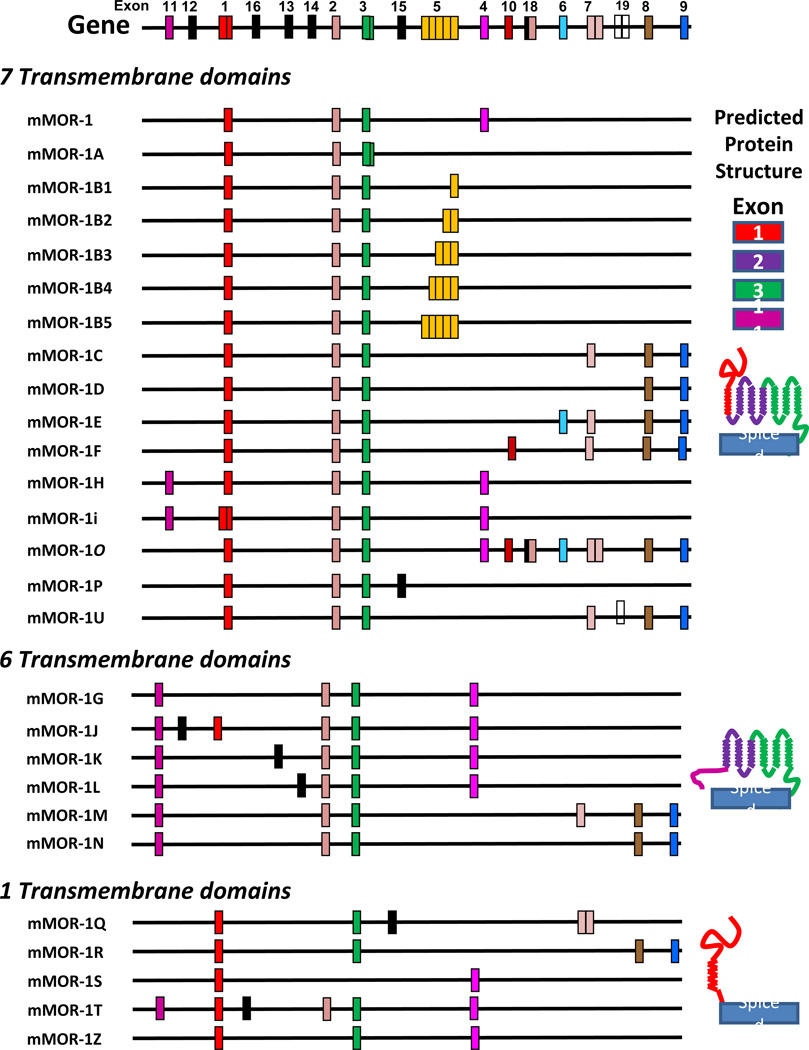
MOR-1 was first cloned in 1993 (Chen et al., 1993; Eppler et al., 1993; Thompson et al., 1993; Wang et al., 1993), soon after the delta receptor (Evans et al., 1992; Kieffer et al., 1992). The receptor was comprised of four exons (Fig. 1). The first exon encodes the N-terminus and the first transmembrane domain while the second and third exons each encoded an additional three transmembrane domains, yielding the seven transmembrane structure of traditional G-protein coupled receptors. The delta (DOR-1) and kappa1 (KOR-1) receptors have analogous structures with their three exons. However, MOR-1 differs from the others in that it also contains a fourth exon responsible for coding only 12 amino acids at the tip of the intracellular C-terminus. Over the years, a large array of 31 splice variants have been isolated from mice (Doyle et al., 2006; Doyle et al., 2007; Pan et al., 2009a; Pan et al., 1999; Pan et al., 2000; Pan et al., 2005b; Pan, 2005; Pan, 2000; Pan et al., 2001), sixteen from rats (Pasternak et al., 2004; Xu et al., 2011; Zimprich et al., 1994) and nineteen from humans (Bare et al., 1994; Cadet et al., 2003; Choi et al., 2006; Pan et al., 2003; Pan, 2005; Shabalina et al., 2009; Xu et al., 2009), with similar gene structures and splicing patterns in each species.
Figure 1. Schematic of the mouse Oprm1 gene and the MOR-1 splice variants.
There are three major classes of MOR-1 splice variants in mice, rats and humans. The first are the full length variants, in which 3’splicing leads to the replacement of the 12 amino acids encoded by exon 4 with alternative sets of exons which generate distinct amino acid sequences in the tip of the C-terminus (Fig. 1). The second set are associated with exon 11 and its promoter, located approximately 30 kbases upstream of exon 1. These variants, which lack exon 1 and thus the first transmembrane (TM) domain, are truncated and contain only the last 6 TM domains. The third set involve exon skipping with the loss of exon 2 or of exons 2 and 3 to generate a single TM protein encoded by exon 1. All three classes are functionally relevant.
4.0 Functional assessment of MOR-1 and its splice variants
There are many indications that these variants are functionally important. Their regional distributions at both the mRNA and protein levels are quite distinct from one another. Immunohistochemical studies using C-terminus epitopes clearly illustrated these differences (Abbadie et al., 2000a; Abbadie et al., 2000b; Abbadie et al., 2000c; Abbadie et al., 2004), as well as at the ultrastructural level (Abbadie et al., 2001). Whereas mMOR-1 was localized both presynaptically and postsynaptically, the splice variant mMOR-1C was almost exclusively presynaptic. Unlike many mu opioids, morphine does not internalize MOR-1 (Keith et al., 1996; Keith et al., 1998). However, morphine effectively internalizes MOR-1C in vivo, showing a clear difference in their trafficking (Abbadie and Pasternak, 2001). The full length variants also vary in their sensitivity towards activation by a series of opiates, with drugs showing varying efficacies and potencies among the variants as determined by stimulation of 35S-GTPγS binding (Bolan et al., 2004; Pan et al., 2005a; Pan et al., 2009a; Pan et al., 1999; Pan et al., 2000; Pan et al., 2003; Pan et al., 2001). However, a series of knockout models have provided the best insights into their actions.
Several groups have generated knockouts of the OPRM1 gene, targeting exon 1(Schuller et al., 1999; Sora et al., 1997), exon 2 or exons 2/3 (Loh et al., 1998; Matthes et al., 1996), or exon 11 (Pan et al., 2009b). All the knockout models targeting exons 1, 2 or 3 eliminated morphine actions. However, one knockout animal targeting exon 1 retained heroin and morphine-6β-glucuronide (M6G) analgesia, implying that their analgesic mechanisms were distinct from those of morphine (Schuller et al., 1999). This knockout model still expressed a second set of MOR-1 variants associated with exon 11 that did not contain exon 1, suggesting that these variants may play a role in heroin and M6G analgesia. If so, disruption of exon 11 and its associated variants should selectively diminish heroin and M6G analgesia. When this hypothesis was tested in an exon 11 knockout mouse (Pan et al., 2009b), morphine analgesia was fully retained despite the loss of the exon 11-associated splice variants. Yet, the analgesic activity of heroin and M6G were significantly reduced, as predicted.
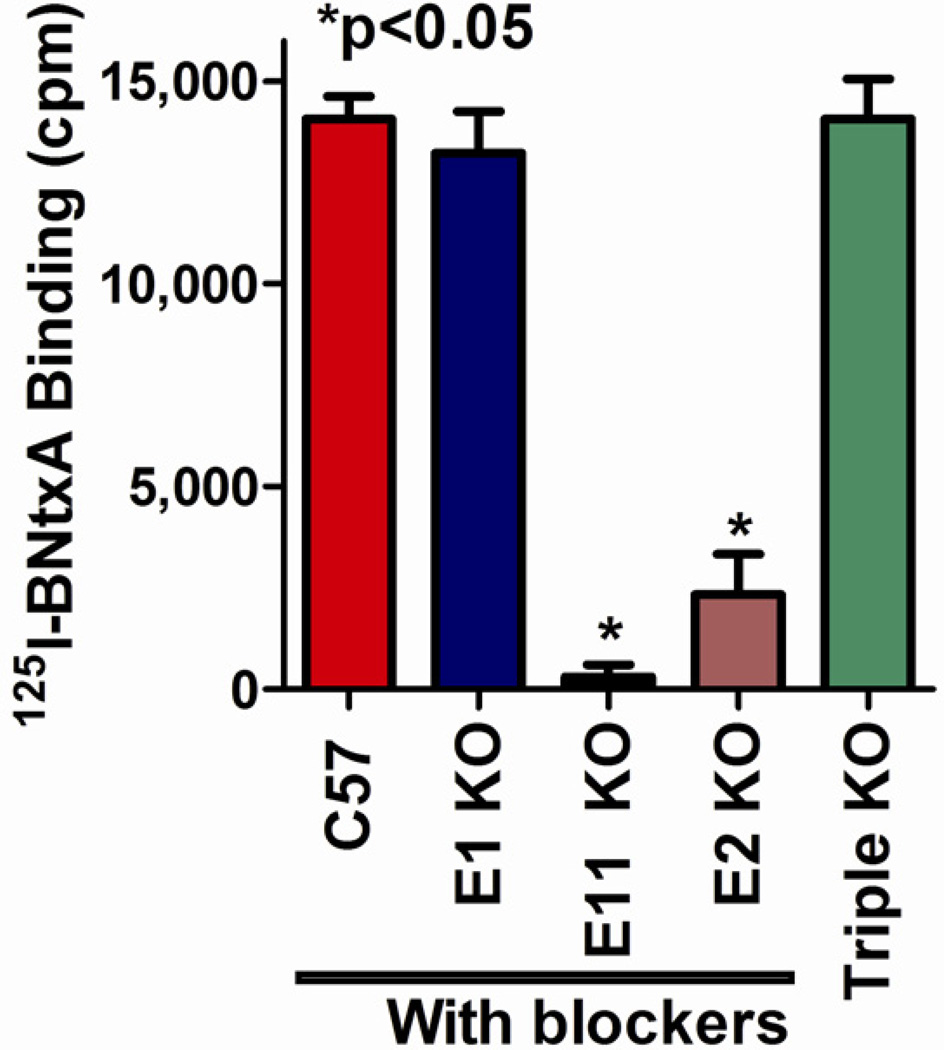
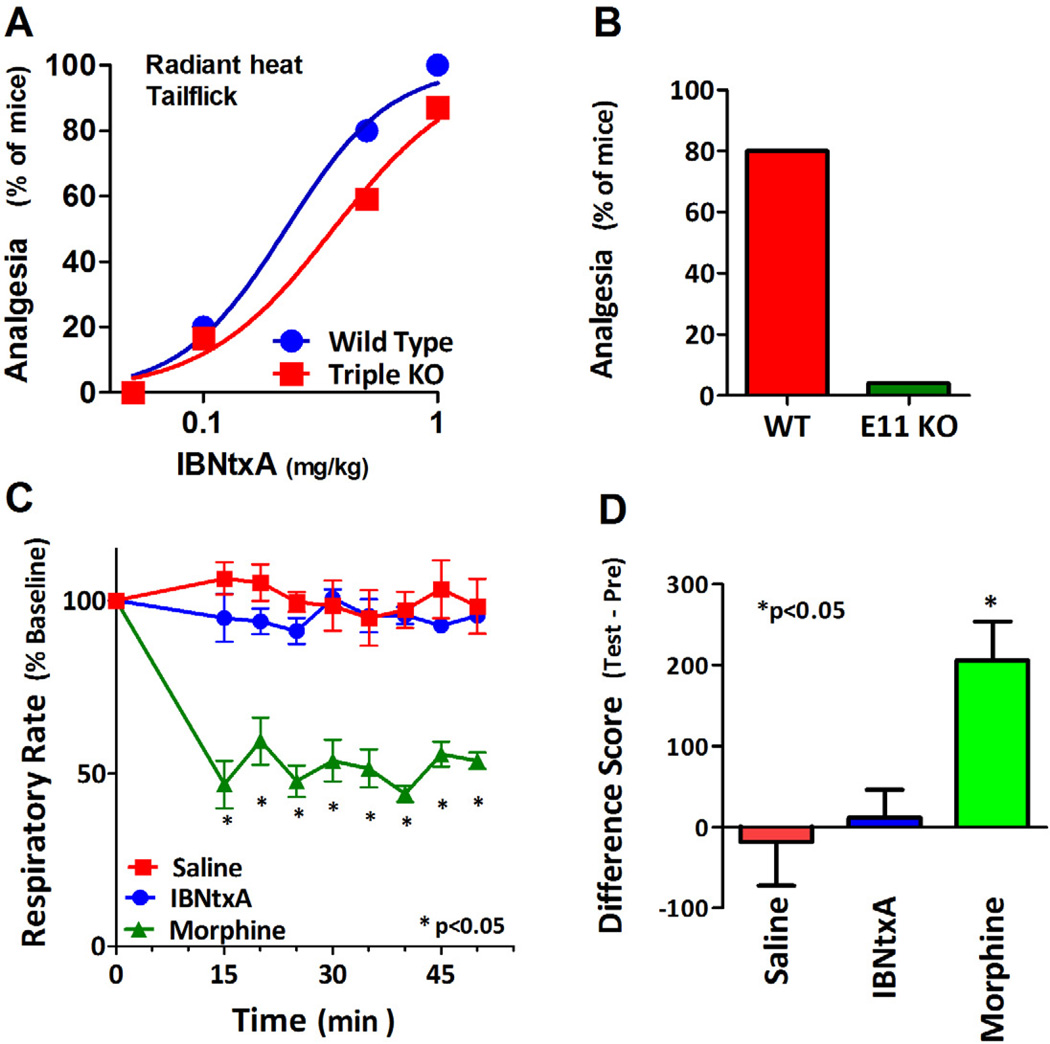
The importance of the truncated 6TM exon 11-associated variants became clearer with the development of a novel series of ligands (Majumdar et al., 2011; Majumdar et al., 2012). The significance of the truncated 6TM variants initially was uncertain. Lacking the full 7 transmembrane domains associated with traditional G-protein coupled receptors, initial studies examining the variants expressed in cell lines failed to demonstrate binding. However, the pharmacology of these variants was clarified by a recently developed compound, 3-iodobenzoyl-6β-naltrexamide (IBNtxA). Using an 125I-radiolabeled version of the compound, we demonstrated a very high affinity binding site in brain with a pharmacological profile unlike any of the traditional opioid receptors. Furthermore, the binding was still present in an exon 1 knockout mouse lacking all the full length MOR-1 splice variants as well as in a “triple knockout mouse” in which the delta and kappa1 receptors were also eliminated (Majumdar et al., 2011; Majumdar et al., 2012). However, disruption of exon 11 with the loss of the exon 11-associated variants eliminated the binding (Fig. 2). Although it was structurally related to the antagonist naltrexone, IBNtxA was a potent analgesic and it retained full analgesic activity in the triple knockout mice (Fig. 3a,b). Like the binding site, IBNtxA analgesia was completely lost in the exon 11 knockout mouse, clearly showing that the 6TM exon 11-associated variants played a critical role in both the binding site and its functions. Pharmacologically, IBNtxA had other distinctions. While IBNtxA and a number of related analogs were potent analgesics, they lacked respiratory depressant activity (Fig. 3c), had minimal effect on gastrointestinal transit and showed no evidence of physical dependence with chronic dosing. They showed no cross tolerance to morphine analgesia and, perhaps most intriguing, showed no reinforcing or aversive activity in a conditioned place preference paradigm (Fig. 3d) (Majumdar et al., 2011; Majumdar et al., 2012). These studies clearly established the relevance of these truncated receptors, but many questions remain. While the composition of the target of IBNtxA clearly contains exon 11-associated variants, it appears that in brain it may be a heterodimer between the 6TM variants and other G-protein coupled receptors. Since the binding site and the analgesic actions of IBNtxA remained in the triple knockout mice remained, it seems unlikely that these partners involve any of the traditional opioid receptors, raising interesting questions on the potential partner(s). ORL1 is among the possibilities. While IBNtxA does not label ORL1 receptors with high affinity, we already know that a heterodimer between ORL1 and MOR-1 yield displays differing binding profiles than either receptor alone. Finally, it is important to consider that there may be more than one partner for the exon 11 variants and to remember that there are multiple 6TM exon 11 variants in all the species.
Figure 2. 125I-BNtxA binding in knockout mice.
Mice with the indicated exon disruption were tested for 125I-BNtxA binding. Wildtype and mice with disruptions of exons containing within MOR-1 were assayed in the presence of blockers to eliminate binding to the traditional opioid receptors (mu: CTAP 1 µM; delta: DPDPE 1 µM; kappa1: U50,488H 1 µM). Blockers were not used in the triple knockout mice since they had no traditional opioid binding due to the knockouts. From the literature (Majumdar et al., 2011).
Figure 3. Pharmacological characterization of IBNtxA actions in vivo.
a) IBNtxA analgesia was assessed in wildtype mice and triple knockout mice at the indicated dose. The ED50 values for the two groups were not significantly different. b) IBNtxA (0.5 mg/kg, s.c.) was given and analgesia assessed using the radiant heat tailflick assay in wildtype and exon 11 knockout mice. No observable analgesia could be detected in the knockout animals. c) Respiratory rate was determined in groups of mice given saline or high equianalgesic doses of morphine (20 mg/kg, s.c.)or IBNtxA (2.5 mg/kg, s.c.). d) Conditioned place preference was carried out and the activity of morphine (10 mg/kg, s.c.) and IBNtxA (1 mg/kg, s.c.) compared to saline. Results are from the literature (Majumdar et al., 2011).
5.0 Conclusions
Much has happened since the initial biochemical description of opioid receptors forty years ago. Research has uncovered a complexity far exceeding early estimates. Pharmacological approaches using receptor binding and selective antagonists initially suggested mu1 and mu2 receptors, but molecular biological approaches have now identified over 30 splice variants of the mu receptor in mice, 16 in rats and 19 in humans. Much work will be needed to understand the full significance of all these variants.
The most intriguing aspect of the field, however, remains our continued progress towards the “Holy Grail” – opioid analgesics with potent analgesic activity that lack side-effects and abuse potential. With the identification of delta and kappa1 receptors, efforts focused upon agents targeting these sites. Although these led to many candidates, their clinical potential has been impaired by psychotomimetic or epileptogenic activity. Ironically, it appears that the most promising agents target mu opioid receptor splice variants, but not necessarily the traditional ones initially observed in 1973. Biased agonism might prove useful in drug development (Raehal et al., 2011), but the truncated variants may prove better targets to yield highly potent analgesics lacking most of the typical side-effects of opioids as well as physical dependence and reward and aversive behavior, at least in a conditioned place preference assay. This is a major step forward, both in drug development and in our understanding of G-protein coupled receptors in general where a vast array of truncated forms of these receptors have been uncovered over the years.
Are we there yet? Maybe we are close….
Highlights.
Morphine and most clinical opiates act through mu opioid receptors
The mu opioid receptor MOR-1 was cloned twenty years ago
The mu opioid receptor undergoes extensive splicing to generate three major classes of variants
MOR-1 splice variants may prove useful in the development of analgesics lacking the undesirable actions of current opioid drugs.
Acknowledgements
This work was supported by grants from the National Institute on Drug Abuse of the National Institutes of Health to GWP (DA02615, DA07242, DA06241) and a core grant to MSKCC from the National Cancer Institute (CA08748).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbadie C, Gultekin SH, Pasternak GW. Immunohistochemical localization of the carboxy terminus of the novel mu opioid receptor splice variant MOR-1C within the human spinal cord. Neuroreport. 2000a;11:1953–1957. doi: 10.1097/00001756-200006260-00029. [DOI] [PubMed] [Google Scholar]
- Abbadie C, Pan Y-X, Drake CT, Pasternak GW. Comparative immunhistochemical distributions of carboxy terminus epitopes from the mu opioid receptor splice variants MOR-1D, MOR-1 and MOR-1C in the mouse and rat central nervous systems. Neuroscience. 2000b;100:141–153. doi: 10.1016/s0306-4522(00)00248-7. [DOI] [PubMed] [Google Scholar]
- Abbadie C, Pan Y-X, Pasternak GW. Differential distribution in rat brain of mu opioid receptor carboxy terminal splice variants MOR-1C and MOR-1-like immunoreactivity: Evidence for region-specific processing. J.Comp.Neurol. 2000c;419:244–256. doi: 10.1002/(sici)1096-9861(20000403)419:2<244::aid-cne8>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Abbadie C, Pan Y-X, Pasternak GW. Immunohistochemical study of the expression of exon11-containing mu opioid receptor variants in the mouse brain. Neuroscience. 2004:419–430. doi: 10.1016/j.neuroscience.2004.03.033. i. [DOI] [PubMed] [Google Scholar]
- Abbadie C, Pasternak GW. Differential in vivo internalization of MOR-1 and MOR-1C by morphine. Neuroreport. 2001;12:3069–3072. doi: 10.1097/00001756-200110080-00017. [DOI] [PubMed] [Google Scholar]
- Abbadie C, Pasternak GW, Aicher SA. Presynaptic localization of the carboxy-terminus epitopes of the mu opioid receptor splice variants MOR-1C and MOR-1D in the superficial laminae of the rat spinal cord. Neuroscience. 2001;106:833–842. doi: 10.1016/s0306-4522(01)00317-7. [DOI] [PubMed] [Google Scholar]
- Bare LA, Mansson E, Yang D. Expression of two variants of the human m opioid receptor mRNA in SK-N-SH cells and human brain. FEBS Lett. 1994;354:213–216. doi: 10.1016/0014-5793(94)01129-x. [DOI] [PubMed] [Google Scholar]
- Beckett AH, Casy AF. Analgesics and their antagonists: biochemical aspects and structure-activity relationships. Prog.Med.Chem. 1965;4:171–218. doi: 10.1016/s0079-6468(08)70169-3. [DOI] [PubMed] [Google Scholar]
- Berezniuk I, Fricker LD. Endogenous opioids. (2nd) 2011:93–120. [Google Scholar]
- Bolan EA, Pasternak GW, Pan Y-X. Functional analysis of MOR-1 splice variants of the mu opioid receptor gene, Oprm . Synapse. 2004;51:11–18. doi: 10.1002/syn.10277. [DOI] [PubMed] [Google Scholar]
- Bunzow JR, Saez C, Mortrud M, Bouvier C, Williams JT, Low M, Grandy DK. Molecular cloning and tissue distribution of a putative member of the rat opioid receptor gene family that is not a µ, d or kappa opioid receptor type. FEBS Lett. 1994;347:284–288. doi: 10.1016/0014-5793(94)00561-3. [DOI] [PubMed] [Google Scholar]
- Cadet P, Mantione KJ, Stefano GB. Molecular identification and functional expression of mu 3, a novel alternatively spliced variant of the human mu opiate receptor gene. J.Immunol. 2003;170:5118–5123. doi: 10.4049/jimmunol.170.10.5118. [DOI] [PubMed] [Google Scholar]
- Chavkin C, Goldstein A. Specific receptor for the opioid peptide dynorphin: Structure-activity relationships. Proc.Natl.Acad.Sci.USA. 1981;78:6543–6547. doi: 10.1073/pnas.78.10.6543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Fan Y, Liu J, Mestek A, Tian M, Kozak CA, Yu L. Molecular cloning, tissue distribution and chromosomal localization of a novel member of the opioid receptor gene family. FEBS Lett. 1994;347:279–283. doi: 10.1016/0014-5793(94)00560-5. [DOI] [PubMed] [Google Scholar]
- Chen Y, Mestek A, Liu J, Hurley JA, Yu L. Molecular cloning and functional expression of a µ-opioid receptor from rat brain. Mol.Pharmacol. 1993;44:8–12. [PubMed] [Google Scholar]
- Choi HS, Kim CS, Hwang CK, Song KY, Wang W, Qiu Y, Law PY, Wei LN, Loh HH. The opioid ligand binding of human mu-opioid receptor is modulated by novel splice variants of the receptor. Biochem.Biophys.Res Commun. 2006;343:1132–1140. doi: 10.1016/j.bbrc.2006.03.084. [DOI] [PubMed] [Google Scholar]
- Doyle GA, Rebecca, Sheng X, Lin SS, Press DM, Grice DE, Buono RJ, Ferraro TN, Berrettini WH. Identification of three mouse mu-opioid receptor (MOR) gene (Oprm1) splice variants containing a newly identified alternatively spliced exon. Gene. 2007;388:135–147. doi: 10.1016/j.gene.2006.10.017. [DOI] [PubMed] [Google Scholar]
- Doyle GA, Sheng XR, Schwebel CL, Ferraro TN, Berrettini WH, Buono RJ. Identification and functional significance of polymorphisms in the mu-opioid receptor gene (Oprm) promoter of C57BL/6 and DBA/2 mice. Neurosci.Res. 2006;55:244–254. doi: 10.1016/j.neures.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Eddy NB. The National Research Council Involvement in the Opiate Problem. 1973:1928–1971. [Google Scholar]
- Eppler CM, Hulmes JD, Wang J-B, Johnson B, Corbett M, Luthin DR, Uhl GR, Linden J. Purification and partial amino acid sequence of a m opioid receptor from rat brain. J.Biol.Chem. 1993;268:26447–26451. [PubMed] [Google Scholar]
- Evans CJ, Keith DE, Jr, Morrison H, Magendzo K, Edwards RH. Cloning of a delta opioid receptor by functional expression. Science. 1992;258:1952–1955. doi: 10.1126/science.1335167. [DOI] [PubMed] [Google Scholar]
- Foley KM. The treatment of cancer pain. N.Engl.J.Med. 1985;313:84–95. doi: 10.1056/NEJM198507113130205. [DOI] [PubMed] [Google Scholar]
- Foley KM. Controlling the pain of cancer. Sci.Am. 1996;275:164–165. doi: 10.1038/scientificamerican0996-164. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Kato S, Mori K, Nishi M, Takeshima H, Iwabe N, Miyata T, Houtani T, Siguimoti T. cDNA cloning and regional distribution of a novel member of the opioid receptor family. FEBS Lett. 1994;343:42–46. doi: 10.1016/0014-5793(94)80603-9. [DOI] [PubMed] [Google Scholar]
- Goldstein A, Lowney LI, Pal BK. Stereospecific and nonspecific interactions of the morphine congener levorphanol in subcellular fractions of mouse brain. Proc.Natl.Acad.Sci.USA. 1971;68:1742–1747. doi: 10.1073/pnas.68.8.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein A, Tachibana S, Lowney LI, Hunkapiller M, Hood L. Dynorphin-(1–13), an extraordinarily potent opioid peptide. Proc.Natl.Acad.Sci.USA. 1979;76:6666–6670. doi: 10.1073/pnas.76.12.6666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyman JS, Williams CL, Burks TF, Mosberg HI, Porreca F. Dissociation of opioid antinociception and central gastrointestinal propulsion in the mouse: Studies with naloxonazine. J.Pharmacol.Exp.Ther. 1988;245:238–243. [PubMed] [Google Scholar]
- Houde RW, Wallenstein SL. Clinical studies of morphine-nalorphine combinations. Fed.Proc. 1956;15:440–441. [Google Scholar]
- Hughes J. Isolation of an endogenous compound from the brain with pharmacological properties similar to morphine. Brain Res. 1975;88:295–308. doi: 10.1016/0006-8993(75)90391-1. [DOI] [PubMed] [Google Scholar]
- Hughes J, Smith TW, Kosterlitz HW, Fothergill LA, Morgan BA, Morris HR. Identification of two related pentapeptides from the brain with potent opiate agonist activity. Nature. 1975;258:577–579. doi: 10.1038/258577a0. [DOI] [PubMed] [Google Scholar]
- Keith D, Jr, Maung T, Anton B, Evans C. Isolation of cDNA clones homologous to opioid receptors. Regul.Pept. 1994;54:143–144. [Google Scholar]
- Keith DE, Anton B, Murray SR, Zaki PA, Chu PC, Lissin DV, Monteillet-Agius G, Stewart PL, Evans CJ, Von Zastrow M. µ-opioid receptor internalization: Opiate drugs have differential effects on a conserved endocytic mechanism in vitro and in the mammalian brain. Mol.Pharmacol. 1998;53:377–384. [PubMed] [Google Scholar]
- Keith DE, Murray SR, Zaki PA, Chu PC, Lissin DV, Kang L, Evans CJ, Von Zastrow M. Morphine activates opioid receptors without causing their rapid internalization. J.Biol.Chem. 1996;271:19021–19024. doi: 10.1074/jbc.271.32.19021. [DOI] [PubMed] [Google Scholar]
- Kieffer BL, Befort K, Gaveriaux-Ruff C, Hirth CG. The δ-opioid receptor: Isolation of a cDNA by expression cloning and pharmacological characterization. Proc.Natl.Acad.Sci.USA. 1992;89:12048–12052. doi: 10.1073/pnas.89.24.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasagna L, Beecher HK. The analgesic effectiveness of nalorphine and nalorphine-morphine combinations in man. J.Pharmacol.Exp.Ther. 1954;112:356–363. [PubMed] [Google Scholar]
- Li CH, Chung D, Doneen BA. Isolation, characterization and opiate activity of beta-endorphin from human pituitary glands. Biochem.Biophys.Res.Commun. 1976;72:1542–1547. doi: 10.1016/s0006-291x(76)80189-1. [DOI] [PubMed] [Google Scholar]
- Ling GSF, MacLeod JM, Lee S, Lockhart SH, Pasternak GW. Separation of morphine analgesia from physical dependence. Science. 1984;226:462–464. doi: 10.1126/science.6541807. [DOI] [PubMed] [Google Scholar]
- Ling GSF, Pasternak GW. Spinal and supraspinal opioid analgesia in the mouse: the role of subpopulations of opioid binding sites. Brain Res. 1983;271:152–156. doi: 10.1016/0006-8993(83)91376-8. [DOI] [PubMed] [Google Scholar]
- Ling GSF, Simantov R, Clark JA, Pasternak GW. Naloxonazine actions in vivo. Eur.J.Pharmacol. 1986;129:33–38. doi: 10.1016/0014-2999(86)90333-x. [DOI] [PubMed] [Google Scholar]
- Ling GSF, Spiegel K, Lockhart SH, Pasternak GW. Separation of opioid analgesia from respiratory depression: evidence for different receptor mechanisms. J.Pharmacol.Exp.Ther. 1985;232:149–155. [PubMed] [Google Scholar]
- Ling GSF, Spiegel K, Nishimura S, Pasternak GW. Dissociation of morphine's analgesic and respiratory depressant actions. Eur.J.Pharmacol. 1983;86:487–488. doi: 10.1016/0014-2999(83)90203-0. [DOI] [PubMed] [Google Scholar]
- Liu W, Chun E, Thompson A, Chubukov P. Structural Basis for Allosteric Regulation of GPCRs by Sodium Ions. Science. 2012;337:232–236. doi: 10.1126/science.1219218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh HH, Liu HC, Cavalli A, Yang WL, Chen YF, Wei LN. m opioid receptor knockout in mice: effects on ligand-induced analgesia and morphine lethality. Mol.Brain Res. 1998;54:321–326. doi: 10.1016/s0169-328x(97)00353-7. [DOI] [PubMed] [Google Scholar]
- Lord JAH, Waterfield AA, Hughes J, Kosterlitz HW. Multiple opiate receptors. Opiates and Endogenous Opioid Peptides. 1976:275–280. [Google Scholar]
- Lord JAH, Waterfield AA, Hughes J, Kosterlitz HW. Endogenous opioid peptides: multiple agonists and receptors. Nature. 1977;267:495–499. doi: 10.1038/267495a0. [DOI] [PubMed] [Google Scholar]
- Lutz RA, Cruciani RA, Munson PJ, Rodbard D. Mu1: A very high affinity subtype of enkephalin binding sites in rat brain. Life Sci. 1985;36:2233–2338. doi: 10.1016/0024-3205(85)90334-0. [DOI] [PubMed] [Google Scholar]
- Majumdar S, Grinnell S, Le Rouzic V, Burgman M, Polikar L, Ansonoff M, Pintar J, Pan YX, Pasternak GW. Truncated G protein-coupled mu opioid receptor MOR-1 splice variants are targets for highly potent opioid analgesics lacking side effects. Proc.Natl.Acad.Sci.U.S.A. 2011;108:19776–19783. doi: 10.1073/pnas.1115231108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar S, Subrath J, Le Rouzic V, Polikar L, Burgman M, Nagakura K, Ocampo J, Haselton N, Pasternak AR, Grinnell S, Pan Y-X, Pasternak GW. Synthesis and evaluation of aryl-naloxamide opiate analgesics targeting truncated exon 11-associated mu opioid receptor (MOR-1) splice variants. J.Med.Chem. 2012;55:6352–6362. doi: 10.1021/jm300305c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin WR. Opioid antagonists. Pharmacol.Rev. 1967;19:463–521. [PubMed] [Google Scholar]
- Martin WR, Eades CG, Thompson JA, Huppler RE, Gilbert PE. The effects of morphine and nalorphine-like drugs in the nondependent and morphine-dependent chronic spinal dog. J.Pharmacol.Exp.Ther. 1976;197:517–532. [PubMed] [Google Scholar]
- Matthes HWD, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, Befort K, Dierich A, Le Meur M, Dollé P, Tzavara E, Hanoune J, Roques BP, Kieffer BL. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the µ-opioid-receptor gene. Nature. 1996;383:819–823. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, Butour JL, Guillemot JC, Ferrara P, Monsarrat B, Mazargull H, Vassart G, Parmentier M, Costentin J. Isolation and structure of the endogenous agonist of the opioid receptor like ORL1 receptor. Nature. 1995;377:532–535. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- Mollereau C, Parmentier M, Mailleux P, Butour JL, Moisand C, Chalon P, Caput D, Vassart G, Meunier JC. ORL-1, a novel member of the opioid family: cloning, functional expression and localization. FEBS Lett. 1994;341:33–38. doi: 10.1016/0014-5793(94)80235-1. [DOI] [PubMed] [Google Scholar]
- Munson PJ, Cruciani RA, Lutz RA, Rodbard D. New methods for characterization of complex receptor systems involving 3 or more biniding sites: Application to brain opiate receptors. J.Recept.Res. 1984;4:339–355. doi: 10.3109/10799898409042560. [DOI] [PubMed] [Google Scholar]
- Pan L, Xu J, Yu R, Xu MM, Pan YX, Pasternak GW. Identification and characterization of six new alternatively spliced variants of the human mu opioid receptor gene, Oprm. Neuroscience. 2005a;133:209–220. doi: 10.1016/j.neuroscience.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Pan Y-X, Xu JY, Xu MM, Yu R, Bolan E, Gilbert AK, Pasternak GW. Isolation and expression of three new splice variants, mMOR-1A, mMOR-1O and mMOR-1P, from mouse mu opioid receptor gene Oprm . Submitted. 2009a [Google Scholar]
- Pan YX. Diversity and complexity of the mu opioid receptor gene: alternative pre-mRNA splicing and promoters. DNA Cell Biol. 2005;24:736–750. doi: 10.1089/dna.2005.24.736. [DOI] [PubMed] [Google Scholar]
- Pan YX, Xu J, Bolan E, Chang A, Mahurter L, Rossi G, Pasternak GW. Isolation and expression of a novel alternatively spliced mu opioid receptor isoform, MOR-1F. FEBS Lett. 2000;466:337–340. doi: 10.1016/s0014-5793(00)01095-4. [DOI] [PubMed] [Google Scholar]
- Pan YX, Xu J, Bolan E, Moskowitz HS, Xu M, Pasternak GW. Identification of four novel exon 5 splice variants of the mouse mu-opioid receptor gene: functional consequences of C-terminal splicing. Mol.Pharmacol. 2005b;68:866–875. doi: 10.1124/mol.105.011858. [DOI] [PubMed] [Google Scholar]
- Pan YX, Xu J, Bolan EA, Abbadie C, Chang A, Zuckerman A, Rossi GC, Pasternak GW. Identification and characterization of three new alternatively spliced mu opioid receptor isoforms. Mol.Pharmacol. 1999;56:396–403. doi: 10.1124/mol.56.2.396. [DOI] [PubMed] [Google Scholar]
- Pan YX, Xu J, Mahurter L, Xu M, Gilbert AK, Pasternak GW. Identification and characterization of two new human mu opioid receptor splice variants, hMOR-1O and hMOR-1X. Biochem.Biophys.Res.Commun. 2003;301:1057–1061. doi: 10.1016/s0006-291x(03)00089-5. [DOI] [PubMed] [Google Scholar]
- Pan YX, Xu J, Xu M, Rossi GC, Matulonis JE, Pasternak GW. Involvement of exon 11-associated variants of the mu opioid receptor MOR-1 in heroin, but not morphine, actions. Proc.Natl.Acad.Sci.U.S.A. 2009b;106:4917–4922. doi: 10.1073/pnas.0811586106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y-X. Identification and characterization of a novel promoter of a mouse mu opioid receptor gene (Oprm) that generates eight splice variants. Gene. 2000;295:97–108. doi: 10.1016/s0378-1119(02)00825-9. [DOI] [PubMed] [Google Scholar]
- Pan Y-X, Cheng J, Xu J, Pasternak GW. Cloning, expression and classification of a kappa3-related opioid receptor using antisense oligodeoxynucleotides. Regul.Pept. 1994;54:217–218. [Google Scholar]
- Pan Y-X, Xu J, Mahurter L, Bolan EA, Xu MM, Pasternak GW. Generation of the mu opioid receptor (MOR-1) protein by three new splice variants of the Oprm gene. Proc.Natl.Acad.Sci.U.S.A. 2001;98:14084–14089. doi: 10.1073/pnas.241296098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak DA, Pan L, Xu J, Yu R, Xu MM, Pasternak GW, Pan YX. Identification of three new alternatively spliced variants of the rat mu opioid receptor gene: dissociation of affinity and efficacy. J.Neurochem. 2004;91:881–890. doi: 10.1111/j.1471-4159.2004.02767.x. [DOI] [PubMed] [Google Scholar]
- Pasternak GW, Goodman R, Snyder SH. An endogenous morphine like factor in mammalian brain. Life Sci. 1975a;16:1765–1769. doi: 10.1016/0024-3205(75)90270-2. [DOI] [PubMed] [Google Scholar]
- Pasternak GW, Pan Y-X. Mu opioids and their receptors: Evolution of a concept. Pharmacol.Rev. 2013 doi: 10.1124/pr.112.007138. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak GW, Snowman AS, Snyder SH. Selective enhancement of [3H]opiate agonist binding by divalent cations. Mol.Pharmacol. 1975b;11:478–484. [PubMed] [Google Scholar]
- Pasternak GW, Snyder SH. Identification of a novel high affinity opiate receptor binding in rat brain. Nature. 1975a;253:563–565. doi: 10.1038/253563a0. [DOI] [PubMed] [Google Scholar]
- Pasternak GW, Snyder SH. Opiate receptor binding: Enzymatic treatments and discrimination between agonists and antagonists. Mol.Pharmacol. 1975b;11:735–744. [Google Scholar]
- Pasternak GW, Wilson HA, Snyder SH. Differential effects of protein-modifying reagants on receptor binding of opiate agonists and antagonists. Mol.Pharmacol. 1975c;11:340–351. [PubMed] [Google Scholar]
- Paul D, Bodnar RJ, Gistrak MA, Pasternak GW. Different m receptor subtypes mediate spinal and supraspinal analgesia in mice. Eur.J.Pharmacol. 1989;168:307–314. doi: 10.1016/0014-2999(89)90792-9. [DOI] [PubMed] [Google Scholar]
- Paul D, Pasternak GW. Differential blockade by naloxonazine of two m opiate actions: analgesia and inhibition of gastrointestinal transit. Eur.J.Pharmacol. 1988;149:403–404. doi: 10.1016/0014-2999(88)90680-2. [DOI] [PubMed] [Google Scholar]
- Payne R, Pasternak GW. Pain. 1992:268–301. [Google Scholar]
- Pert CB, Pasternak GW, Snyder SH. Opiate agonists and antagonists discriminated by receptor binding in brain. Science. 1973;182:1359–1361. doi: 10.1126/science.182.4119.1359. [DOI] [PubMed] [Google Scholar]
- Portoghese PS. A new concept on the mode of interaction of narcotic analgesics with receptors. Unknown. 1965;8:609–616. doi: 10.1021/jm00329a013. [DOI] [PubMed] [Google Scholar]
- Portoghese PS. Stereochemical factors and receptor interactions associated with narcotic analgesics. J.Pharmac.Sciences. 1966;55:865–887. doi: 10.1002/jps.2600550902. [DOI] [PubMed] [Google Scholar]
- Raehal KM, Schmid CL, Groer CE, Bohn LM. Functional selectivity at the mu-opioid receptor: implications for understanding opioid analgesia and tolerance. Pharmacol.Rev. 2011;63:1001–1019. doi: 10.1124/pr.111.004598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsen RA, Bunzow JR, Grandy DK, Langen H, Monsma FJ, Civelli O. Orphanin FQ: a neuropeptide that activates an opioidlike G protein-coupled receptor. Science. 1995;270:792–794. doi: 10.1126/science.270.5237.792. [DOI] [PubMed] [Google Scholar]
- Schuller AG, King MA, Zhang J, Bolan E, Pan YX, Morgan DJ, Chang A, Czick ME, Unterwald EM, Pasternak GW, Pintar JE. Retention of heroin and morphine-6 beta-glucuronide analgesia in a new line of mice lacking exon 1 of MOR-1. Nat.Neurosci. 1999;2:151–156. doi: 10.1038/5706. [DOI] [PubMed] [Google Scholar]
- Shabalina SA, Zaykin DV, Gris P, Ogurtsov AY, Gauthier J, Shibata K, Tchivileva IE, Belfer I, Mishra B, Kiselycznyk C, Wallace MR, Staud R, Spiridonov NA, Max MB, Goldman D, Fillingim RB, Maixner W, Diatchenko L. Expansion of the human mu-opioid receptor gene architecture: novel functional variants. Hum.Mol.Genet. 2009;18:1037–1051. doi: 10.1093/hmg/ddn439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon EJ, Hiller JM, Edelman I. Stereospecific binding of the potent narcotic analgesic [3H]etorphine to rat-brain homogenate. Proc.Natl.Acad.Sci.USA. 1973;70:1947–1949. doi: 10.1073/pnas.70.7.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sora I, Takahashi N, Funada M, Ujike H, Revay RS, Donovan DM, Miner LL, Uhl GR. Opiate receptor knockout mice define m receptor roles in endogenous nociceptive responses and morphine-induced analgesia. Proc.Natl.Acad.Sci.USA. 1997;94:1544–1549. doi: 10.1073/pnas.94.4.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel K, Kourides I, Pasternak GW. Prolactin and growth hormone release by morphine in the rat: different receptor mechanisms. Science. 1982;217:745–747. doi: 10.1126/science.6285470. [DOI] [PubMed] [Google Scholar]
- Terenius L. Stereospecific interaction between narcotic analgesics and a synaptic plasma membrane fraction of rat cerebral cortex. Acta Pharmacol.et toxicol. 1973;32:317–320. doi: 10.1111/j.1600-0773.1973.tb01477.x. [DOI] [PubMed] [Google Scholar]
- Terenius L, Wahlstrom A. Search for an endogenous ligand for the opiate receptor. Acta Physiol Scand. 1975;94:74–81. doi: 10.1111/j.1748-1716.1975.tb05863.x. [DOI] [PubMed] [Google Scholar]
- Thompson RC, Mansour A, Akil H, Watson SJ. Cloning and pharmacological characterization of a rat m opioid receptor. Neuron. 1993;11:903–913. doi: 10.1016/0896-6273(93)90120-g. [DOI] [PubMed] [Google Scholar]
- Wang JB, Imai Y, Eppler CM, Gregor P, Spivak CE, Uhl GR. m opiate receptor: cDNA cloning and expression. Proc.Natl.Acad.Sci.USA. 1993;90:10230–10234. doi: 10.1073/pnas.90.21.10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson HA, Pasternak GW, Snyder SH. Differentiation of opiate agonist and antagonist receptor binding by protein-modifying reagants. Nature. 1975;256:448–450. doi: 10.1038/253448a0. [DOI] [PubMed] [Google Scholar]
- Wolozin BL, Pasternak GW. Classification of multiple morphine and enkephalin binding sites in the central nervous system. Proc.Natl.Acad.Sci.USA. 1981;78:6181–6185. doi: 10.1073/pnas.78.10.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Xu M, Hurd YL, Pasternak GW, Pan YX. Isolation and characterization of new exon 11-associated N-terminal splice variants of the human mu opioid receptor gene. J.Neurochem. 2009;108:962–972. doi: 10.1111/j.1471-4159.2008.05833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Xu M, Rossi GC, Pasternak GW, Pan YX. Identification, characterization of seven new exon 11-associated splice variants of the rat mu opioid receptor gene, OPRM1. Mol.Pain. 2011;7:9. doi: 10.1186/1744-8069-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimprich A, Bacher B, Höllt V. Cloning and expression of an isoform of the rmu-opioid receptor (rmuOR1B) Regul.Pept. 1994;54:347–348. [Google Scholar]