Abstract
Background
Adequate surgical field visualization is imperative for successful outcomes in endoscopic sinus surgery (ESS). The type of anesthetic administered can alter a patient’s hemodynamics and impact endoscopic visualization during surgery. We review the current evidence regarding the effect of total intravenous anesthesia (TIVA) compared to inhalational anesthesia (INA) on visualization of the surgical field during ESS.
Methods
A systematic review of the literature was performed. Ovid MEDLINE, Scopus, and Cochrane databases were searched from 1946 to January 2012. Citations from the primary search were reviewed and filtered to identify all relevant abstracts in English. Articles meriting full review included prospective controlled trials enrolling adult patients undergoing ESS that were randomized to a group receiving INA or TIVA with outcome measures focused on surgical field visualization.
‘Results
Seven eligible trials fulfilled inclusion criteria. Four of the seven articles demonstrated a statistically significant improvement in surgical field grade during ESS when receiving TIVA compared with INA. However, detailed INA concentrations were often not provided. High levels of INA may have been administered; therefore, side effects of INA rather than effects of an ideal INA administration were possibly represented. Analgesic administration also varied widely among the anesthetic groups further complicating interpretation of study results. The lack of power and the heterogeneity of the studies precluded a formal meta-analysis.
Conclusions
Although several studies reported that TIVA improve surgical conditions in ESS, however there are significant limitations. These findings prevent any definite recommendation at this point, emphasizing the need for further high quality studies.
Keywords: Endoscopic sinus surgery, total intravenous anesthesia, anesthetic, surgical field, endoscopy
Introduction
Endoscopic sinus surgery (ESS) is the currently accepted surgical intervention for treatment of refractory chronic rhinosinusitis and other conditions of the sinuses. Though relatively safe, there is potential for both minor and severe complications, including cerebral spinal fluid leak, orbital or intracranial injury, meningitis, synechiae, and bleeding. Minor complications have been noted to occur in less than 4% of cases, with major complications occurring in approximately 1%.1,2 Surgical field visualization is essential for successful outcomes from this procedure and to minimize development of these complications. Bleeding in the surgical field can be progressive and even detrimental, causing prolonged operative time, incomplete surgical interventions, and increased complications due to difficulty visualizing and identifying landmarks with subsequent injury of important anatomical structures.3–7 Many methods have been proposed to create a bloodless field, including patient positioning in reverse Trendelenburg, administration of topical vasoconstrictors, use of a laryngeal mask airway to decrease the hemodynamic response to endotracheal intubation, and manipulation of ventilator settings.8–12 Additionally, hypotensive medications have been utilized, such as beta blockers, magnesium sulfate, clonidine, and sodium nitroprusside.4,13–17 It is proposed that these medications reduce the mean arterial pressure (MAP) during the procedure and subsequently decrease blood flow to the nasal mucosal tissue undergoing manipulation. However, pure vasodilators, such as sodium nitroprusside, may lead to reflex tachycardia and increase cardiac output, worsening vasodilation and local bleeding.17 It has also been suggested that magnesium sulfate may negatively interact with the anesthetic and prolong recovery time.15 Beta-blockers, on the other hand, have been shown to be of advantage because they decrease blood pressure by lowering cardiac output rather than systemic vascular resistance.14
The use of different types of anesthetic during surgery was investigated as another method to reduce surgical site bleeding and to avoid some of the side effects of the prior medications.10,11 Total intravenous anesthesia (TIVA) has been proposed as a potential method to improve surgical conditions during ESS. It is thought that TIVA may be helpful in decreasing cardiac output without the significant decrease in systemic vascular resistance often seen with volatile agents.
However, there have been conflicting outcomes in the literature regarding the use of TIVA to achieve improved surgical field conditions compared with inhalational anesthesia (INA). Some studies have indicated improvement in surgical field visualization while other studies failed to demonstrate a difference. Because of its theoretical potential to improve surgical field visualization, there has been an increasing trend among surgeons to prefer TIVA over the past few years. Given the inconsistency among the currently available data in this regard, we conducted a systematic review of the pertinent literature on this topic evaluating the quality and results of current publications.
MATERIALS AND METHODS
Eligibility Criteria
We reviewed retrospective and prospective observational and controlled trials describing outcome data evaluating the influence of the anesthetic technique on blood loss and surgical field visualization during ESS. The studies were limited to human subjects and English language. Unpublished studies or protocols were excluded. Studies included were not limited by year. Studies that enrolled adult patients (≥ 16 years old) undergoing endoscopic sinus surgery receiving either TIVA or INA were included in our review. We excluded any study that involved patients with an underlying coagulopathy or on anticoagulation. Studies that discussed patients undergoing ESS under local anesthesia or that evaluated the outcomes of non-anesthetic medications on blood loss were excluded.
Outcomes included assessment of blood loss obtained from suction canister at the end of the procedure with or without hemoglobin calculations, and visual assessment of surgical field using either a 10-point visual analog scale (0=best possible operating conditions and dryness of the surgical field; 10= worst possible conditions) or a 6-point scale adapted from Fromme et al.18 and Boezaart et al.4 (Table 1).
Table 1.
Endoscopic surgical field grading system
| Grade | Assessment |
|---|---|
| 0 | No bleeding (cadaveric conditions) |
| 1 | Slight bleeding, no suctioning required |
| 2 | Slight bleeding, occasional suctioning required |
| 3 | Slight bleeding, frequent suctioning required; bleeding threatens surgical field a few seconds after suction is removed |
| 4 | Moderate bleeding, frequent suctioning required, and bleeding threatens surgical field directly after suction is removed |
| 5 | Severe bleeding, constant suctioning required; bleeding appears faster than can be removed by suction; surgical field severely threatened and surgery usually not possible |
Study Identification and Selection Process
An electronic search was performed using the following databases: Ovid MEDLINE, Scopus, and Cochrane databases. The initial search of these three databases was performed by a single author in January 2012. Search terms included total intravenous anesthesia, endoscopic sinus surgery, or anesthesia. In Ovid MEDLINE, studies that contained search terms endoscopic sinus surgery or total intravenous anesthesia or propofol in the title or had search terms endoscopic sinus surgery and total intravenous anesthesia as keywords were included. The Cochrane database was searched using terms endoscopic sinus surgery or total intravenous anesthesia in the title, abstract, or as a keyword. Scopus database was also searched for citations including terms endoscopic sinus surgery and anesthesia in the title, abstract, or keyword.
The study selection algorithm adheres to that outlined in the PRISMA statement.19 Of the citations identified through this process, records identified for eligibility were chosen based on titles. Two authors (D.M.P. and E.A.K.) working independently reviewed the titles and the abstracts of each identified citation. Trials that did not meet the inclusion criteria of the review were excluded. If there were questions about a specific study, the full article was obtained for further review. The full-text manuscripts of all remaining studies were obtained and reviewed for eligibility. Additionally, reference lists of these identified studies were also reviewed to recognize any further potential studies. The quality of each study selected for the review was graded using the Jadad scale 20 (Table 2). The Jadad scale is an instrument using a 5-point scoring system determined by a study’s method of randomization, blinding, and reporting of subject withdrawals to measure likelihood of bias.
Table 2.
Assessment of quality of included studies
| Study Author | Jadad score*20 |
|---|---|
| Eberhart 2003 6 | 3 |
| Tirelli 2004 7 | 1 |
| Wormald 2005 24 | 2 |
| Beule 2007 3 | 2 |
| Ahn 2008 23 | 2 |
| Yoo 2010 22 | 2 |
| Ankichetty 2011 21 | 2 |
Jadad Score is an instrument utilized to measure reduction of bias in literature, scored 0–5 (0=poor, 5= excellent)
Data Collection and Extraction
The number of participants in each study, inclusion/exclusion criteria, mean age, and types of anesthetic were recorded from each study (Table 3). Intraoperative data obtained included range of anesthetic provided, dosages of hemodynamically active drugs, MAP, heart rate, assessment score of surgical field, and estimated blood loss during the procedure (Table 4). Due to the significant variability within and among studies, including anesthetic, narcotic, and hemodynamically active drugs used for the intervention and outcome measures, a meta-analysis was not performed.
Table 3.
Characteristics of Included Studies
| Study | n | Inclusion | Exclusion | Indication | Age (Mean in years) | Anesthetic and narcotic combination used |
|---|---|---|---|---|---|---|
| Eberhart 2003 18 | 88 | ASA grade 1 or 2, undergoing ESS | n/a | Diffuse polypoid pansinusitis, Chronic sinusitis | 33 | Propofol/remifentanil vs. isoflurane/alfentanil |
| Tirelli 2004 21 | 64 | ASA grade 1 or 2 undergoing ESS | n/a | Simple chronic sinusitis, chronic sinusitis with polyposis | TIVA- 48 INA- 45 |
Propofol/remifentanil vs. Isoflurane/fentanyl |
| Wormald 2005 23 | 56 | ASA grade 1 or 2, undergoing ESS | Receiving cardiovascular active medications and anticoagulation medications | Allergic fungal sinusitis, nasal polyposis, chronic rhinosinusitis | n/a | Propofol/remifentanil vs sevoflurane/fentanyl |
| Beule 2007 20 | 46 | ASA grade 1 or 2, 18–75 yo, Polypoid chronic pansinusitis without allergic mucin (LM ≥ 12), refractory to medical management | Noninflammatory disease of paranasal sinus, allergy to anesthetic, medication or condition associated with impaired hemostasis, altered coagulation factors, pre or intraoperative use of NSAIDs, sympathomimetics, colloids | Chronic rhinosinusitis with nasal polyposis | TIVA- 43 INA- 46 |
Propofol/fentanyl vs. sevoflurane/fentanyl |
| Ahn 2008 22 | 40 | ASA grade 1 or 2, undergoing ESS | Disease or medication related to coagulation or cardiovascular system | Chronic sinusitis with ≥ 2 sinuses involved | TIVA- 49 INA- 41 |
Propofol/remifentanil vs. sevoflurane/remifentanil |
| Yoo 2010 19 | 60 | ASA grade 1 or 2, undergoing ESS | BMI ≥30, alcohol/drug abuse, pregnancy, meds that impact MAC, communication problems, allergic fungal sinusitis, nasal polyposis | n/a | TIVA- 37 INA (sevo)- 43 INA (des)- 43 |
Propofol/remifentanil vs. sevoflurane/remifentanil vs. desflurane/remifentanil |
| Ankichetty 2011 17 | 40 | ASA grade 1 or 2, aged 16–60 years old, undergoing ESS | Bleeding disorder, receiving anticoagulation therapy, prior ESS, major hepatic, renal or CV disease | n/a | TIVA- 32 INA- 34 |
Propofol/fentanyl vs. isoflurane/fentanyl |
Abbreviations: ASA - American Society of Anesthesiologists, ESS - Endoscopic sinus surgery, n/a - not available, TIVA - total intravenous anesthesia, INA - inhaled anesthesia, CV -Cardiovascular, LM - Lund-Mackay score, BMI - body mass index, sevo- sevoflurane, des - desflurane.
Table 4.
Surgical data outcomes of included studies
| Study | Type of anesthesia | Amount anesthetic used | MAP (mmHg) | Mean heart rate (beats/min) | Rating on VAS | Rating on 6-point scale | Estimated blood loss (mL) |
|---|---|---|---|---|---|---|---|
| Eberhart 2003 6 | Propofol/remifentanil | 5–8 mg/kg/hr | 65 | 55* | 2.8* | 1 | 100 |
| Isoflurane/alfentanil | 0.4–1.0 % | 67 | 72* | 4.9* | 2 | 170 | |
| Tirelli 2004 7 | Propofol/remifentanil | 32–45 ml/hr | 68 | 74 | n/a | 2.5* | n/a |
| Isoflurane/fentanyl | 1–2 % | 69 | 80 | n/a | 3* | n/a | |
| Wormald 2005 24 | Propofol/remifentanil | 2–4 ug/mL | 67** | 62 | n/a | 2.21* | n/a |
| Sevoflurane/fentanyl | 0.8–1.8 % end tidal | 71** | 57 | n/a | 2.8* | n/a | |
| Beule 2007 3 | Propofol/fentanyl | 6–10 mg/kg/hr | 71.5 | 64.6 | 4.9 | n/a | 276 ± 201 |
| Sevoflurane/fentanyl | 1–3% | 70 | 64.4 | 4.6 | n/a | 300 ±168 | |
| Ahn 2008 23 | Propofol/remifentanil | 1–3 ug/mL | 73 | 62** | 2.9** | n/a | 19** (mL/hr) |
| Sevoflurane/remifentanil | 1–3 % | 73 | 70** | 4.8** | n/a | 128** (mL/hr) | |
| Yoo 2010 22 | Propofol/remifentanil | 2–4 ug/mL | 69 | 77 | n/a | 2.05 | n/a |
| Sevoflurane/remifentanil | 0.8–2 % end tidal | 67 | 77 | n/a | 2.21 | n/a | |
| Desflurane/remifentanil | 3–5 % end tidal | 67 | 78 | n/a | 2.07 | n/a | |
| Ankichetty 2011 21 | Propofol/fentanyl | 8–12 mg/kg/hr | ~72*** | ~80*** | n/a | 0–1 (9pts) 2–3 (11pts) |
109 |
| Isoflurane/fentanyl | ≤2 % | ~70*** | ~77*** | n/a | 0–1 (12pts) 2–3 (8pts) |
132.5 |
P<0.001,
P< 0.05,
This value was obtained by estimating the mean from a graph displaying values from each patient in the study.
Abbreviations: VAS - visual analog scale, MAP - mean arterial pressure, pts - patients
RESULTS
Description of studies
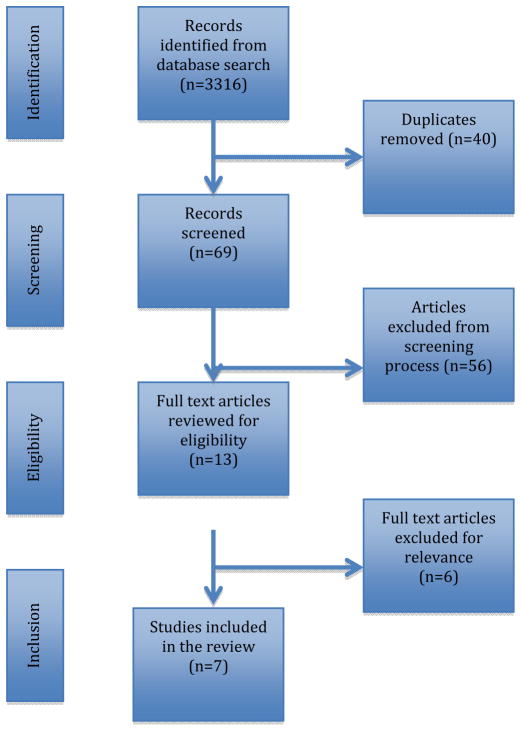
A total of 3316 articles were identified using the systematic search strategy, of which seven articles were included in the review. A breakdown of article selection is displayed in Figure 1. Of the 3316 citations identified through this process, records identified for eligibility were chosen based on titles that included types or methods of anesthesia utilized to control blood loss during sinus surgery. One hundred nine articles were identified for further review, including 40 duplicates. Two reviewers (D.M.P. and E.A.K.) independently screened the titles and abstracts of the retrieved 69 articles to identify eligible studies. The thirteen articles chosen for full text review focused on the intervention of different anesthetics to assess endoscopic visualization and blood loss. Conversely, articles that looked only at controlled hypotension, the use of other non-anesthetic agents, or review articles were excluded. An additional six articles were excluded: three retrospective studies, one review article, one study that switched anesthetic agents in several patients during the procedure yet included these patients in the data analysis, and one study that only used estimated blood loss as an outcome measure.
Figure 1.
PRISMA flow diagram for systematic review manuscript selection.
Bias of Included Studies
The design and methodology of the included studies varied. All studies reported a randomized treatment allocation. The blinding of each study varied. In one study only the anesthesia personnel assessing intraoperative blood loss was blinded to the type of anesthetic used, not the surgeon.21 In four studies the surgeon was blinded to the type of anesthetic. Blinding was often performed by having a covered infusion pump for propofol set up in every room and a covered volatile anesthetic vaporizer.3,6,7,22,23 However, this was not described in detail in every study. In one study there were attempts to blind the surgeon, by indicating that he attempted not to notice the type of anesthetic used and infusion pump was not consistently set up.24
Individual Study Review
Eberhart et al.6 designed a prospective randomized, controlled trial with a total of 88 patients. They included patients with chronic rhinosinusitis, and American Society of Anesthesiologists (ASA) grade 1 or 2 undergoing ESS. Degree of sinusitis was reported to be equivalent among all the patients in the study. All patients received a 20 mg dose of clonidine the night before and morning of surgery. Forty-five patients were randomly allocated into a group receiving TIVA including propofol with a continuous infusion of remifentanil. Forty-three patients were allocated to the balanced anesthesia group, receiving INA with isoflurane with repetitive doses of alfentanil. Both groups were topically decongested with naphozoline nitrate (1:1000) soaked neurosurgical gauze in the nasal cavity. Additionally they were provided 100 mg diclofenac suppository and 1 g metamizole IV after induction of anesthesia. MAPs were maintained between 60 and 70 mmHg by adjusting anesthetic concentration and administering small doses (10 mg) of urapidil, an α1-adrenoceptor antagonist, as needed. MAP was similar in the two groups, and the TIVA group had an overall lower heart rate than the INA group (P ≤ 0.001). Outcomes measured included blood loss and impact of visualization of surgical field due to blood loss. Blood loss was estimated from the amount obtained from suction canister during the case. Surgical field was assessed by a 10-cm visual analog scale and the 6-point scale described by Fromme et al.18 and Boezaart et al.4 and completed by the surgeon at the end of the case. The patient group that received TIVA did have a drier surgical field as rated on the 10-cm visual analog scale (P = 0.0001). The propofol/remifentanil group had less recorded total blood loss, however this was not statistically significant. There were no perioperative complications in either group.
Tirelli et al.7 designed a prospective randomized control trial with a total of 64 patients. The patients had a diagnosis of simple chronic sinusitis or chronic sinusitis with polyposis and underwent ESS. The patients were divided into three groups depending on the extent of surgery performed. The patients included in the study were in ASA class 1 or 2. There were 27 patients in the TIVA group that received propofol and remifentanil. The other 37 patients were randomized in the INA group and received isoflurane and fentanyl. Both groups were decongested with placement of adrenaline soaked pledgets (1:1000) in the nasal cavity. Nasal mucosa was then locally anesthetized with carbocaine with adrenaline (1:1000). All patients were pre-medicated with midazolam and atropine. Outcomes measured include assessment of quality of surgical field using a 6-point scale adapted from Fromme et al.18 and Boezaart et al.4 There were no significant differences between the two groups with regards to age, ASA class, baseline MAP and heart rate. The levels of bleeding and disruption in the surgical field were statistically significant, with worsening visualization in the INA group (P = 0.001). There were no differences noted in outcomes between patients with more extensive surgery compared to those with less extensive surgery. There were no severe complications noted during the study.
Wormald et al.24 performed a prospective randomized controlled trial including a total of 56 patients. Patients had a diagnosis of allergic fungal disease, nasal polyposis, or chronic rhinosinusitis. All patients undergoing ESS (primary or revision surgery) were eligible for the study. Patients that were >18 years old and with ASA physical status 1 or 2 were included in the study. Those patients receiving medications affecting cardiovascular activity or blood coagulation were excluded. The two groups overall had similar Lund-Mackay (LM)25 scores determined by computed tomography (CT) prior to surgery. Twenty-eight patients were randomized into an INA group that received sevoflurane with bolus doses of fentanyl. The other 28 patients were randomized to the TIVA group and received propofol with a continuous infusion of remifentanil. Metoprolol and clonidine were also provided if patients needed additional control to maintain MAPs between 55–70 mmHg and a heart rate < 60 beats per minute. The surgeon was not blinded to the type of anesthesia provided. Surgical field assessment was made every 15 minutes using the scale introduced by Fromme et al.18 and adapted by Boezaart et al.4 Patients in the propofol/remifentanil group had an overall lower mean value for MAP compared to the sevoflurane/fentanyl group (P = 0.042). The two groups had comparable heart rates during the procedure, however a lower heart rate was associated with reduced Boezaart score. In both groups, a higher LM score was associated with a higher Boezaart score. When comparing Boezaart scores at equivalent MAPs, the propofol/remifentanil group had a lower score than the sevoflurane/fentanyl group (P < 0.001). However, the analysis performed to determine a significant difference in Boezaart score between these two groups may not have been ideal. The Boezaart scale is an ordinal scale and therefore not typically transformed to fit into the mixed linear model that was used in the analysis.
Beule et al.3 designed a prospective randomized, controlled trial with 46 patients. The patients had a diagnosis of chronic rhinosinusitis with nasal polyps involving all paranasal sinuses and a LM CT score ≥ 12. Patients with abnormal coagulation, perioperative blood pressure outside the range of 70–140 mmHg systolic and 50–90 mmHg diastolic, and perioperative use of non-steroidal anti-inflammatory drugs, sympathomimetics, and colloids were excluded. Twenty-two patients received sevoflurane/fentanyl in one arm and 24 patients received propofol/fentanyl in the other arm. The main outcome measures were total blood loss, blood loss per minute, endoscopic vision using visual analog scale at fifteen minute intervals during the procedure, and platelet function. Blood loss was calculated using hemoglobin levels and volume in the suction canister at the end of the case. All patients were positioned with head of bed at 30 degrees and bilateral nasal packs soaked with 3 mL adrenaline 1:10,000 were placed for ten minutes prior to procedure. At the completion of the procedure, each ethmoid cavity was packed. Mean arterial pressure and heart rate were similar in both groups. There was no statistically significant difference between the two groups in total calculated blood loss, blood loss per minute, or endoscopic visual field. Impaired platelet function was seen in both groups. There was a greater impairment of platelet function in the propofol group compared to the sevoflurane group (P = 0.0006).
Ahn et al.23 designed a prospective randomized study involving 40 patients. The patients had a diagnosis of chronic sinusitis involving a minimum of two paranasal sinuses. Those patients with ASA class 1 or 2 undergoing ESS were included in the study. Each patient was assigned a LM score. Patients with a disease process or prescribed medication related to coagulation or cardiovascular disease were excluded. No power analysis was performed in the study. Twenty patients were randomly assigned to the anesthetic group receiving sevoflurane/remifentanil and the other 20 patients were assigned to the group receiving propofol/remifentanil. Patients were not pre-medicated. Each patient was positioned in 20 degrees reverse Trendelenburg and the nasal mucosa was decongested with 1:1 solution of epinephrine (1:1000) and lidocaine (2 %). The main outcome measures included calculated total blood loss (determined from amount of volume in suction canister from procedure, hemoglobin level in suction canister, and patient’s hemoglobin levels), blood loss per minute, and rating of surgical field conditions using a 10-point visual analog scale. Patient demographics and LM scores were similar amongst the two groups. Similar amounts of local epinephrine (1:100,000) were injected into the surgical site in both groups. Two patients were excluded from the sevoflurane group as the anesthetic was switched during the procedure from sevoflurane to propofol. An improvement in visual analog score was noted in both patients after switching anesthetics. These patients were not included in further statistical analysis in the study. Although not statistically significant (P = 0.33), the propofol group appeared to have a 40% higher rate of remifentanil infusion (0.150 mcg/kg/min) compared to the sevoflurane group (0.107 mcg/kg/min). Intraoperative MAP was similar. Patients in the propofol group had a lower mean intraoperative heart rate compared with the sevoflurane group (P = 0.008). The median amount of blood loss per hour was also significantly lower in the propofol group (P = 0.004). The surgical field conditions in the propofol group had a better visual analog score (P = 0.021). After further analysis, decreased blood loss and improved visual analog score were specifically demonstrated in the patients in the propofol group with a high LM score (>12). Patients with a low LM score had similar blood loss and surgical conditions between the two groups.
Yoo et al.22 designed a prospective randomized, controlled trial involving 60 patients. Patients with an ASA class of 1 or 2 undergoing ESS were included in the study. Those patients with a body mass index (BMI) ≥ 30, history of alcohol or drug abuse, pregnancy, taking medications that impact minimum alveolar anesthetic concentration, allergic fungal sinusitis, or nasal polyposis were excluded from the study. The patients were randomly assigned to one of three of the following anesthetic groups: propofol/remifentanil group, sevoflurane/remifentanil group, desflurane/remifentanil group. There were 20 patients in each group. Ephedrine was given as needed for hypotensive events. Outcome measures included a surgical grading scale initially described by Fromme et al.18 and adapted by Boezaart et al.4 Surgeons began scoring the operative field at intermittent time points starting within 60 minutes of the procedure. The three groups had similar LM scores (all scores were ≤ 12) and operative times. There were no significant differences amongst MAP or heart rate during the procedure between the groups. No significant differences in Boezaart score noted among the three groups.
Ankichettey et al.21 designed a prospective randomized controlled trial with 40 patients during a one year time period. Patients included in the study had an ASA class 1 or 2, aged 16–60 years old, and undergoing ESS. Patients with any bleeding disorder, receiving anticoagulation therapy, history of prior ESS, or any major hepatic, renal or cardiovascular disease were excluded from the study. Twenty patients were randomly allocated to the inhaled anesthetic group receiving isoflurane during the procedure and 20 patients to the group receiving propofol. Each patient was pre-medicated with oral diazepam prior to induction of anesthesia and also received an infusion of fentanyl following intubation. Outcome measures include a 6-point grading scale of surgical field visualization proposed by Fromme et al.18 and Boezaart et al.4 (Table 1). In general, the two groups were comparable in demographic data with a higher mean weight in patients in the isoflurane group. The authors found no statistically significant difference between the two groups in regards to heart rate or time to reach target mean arterial pressure (60–70 mmHg). There was no difference in amount of intraoperative blood loss between the two groups. The operative field conditions in both groups achieved a score of three or less on the 6-point surgical field grading system (P = 0.34). There was an increased mean fentanyl requirement in the isoflurane group (P = 0.026). Additionally, the isoflurane group also had an overall longer procedure time. There were no perioperative complications.
DISCUSSION
There have been a variety of studies investigating different interventions that influence the visualization of the surgical field during ESS. In this review, we have assessed the literature that discusses inhalational versus intravenous anesthetics to optimize surgical conditions during ESS. Numerous studies have attempted to elucidate the best anesthetic option for surgical field visualization; however, the results remain inconclusive.
When reviewing the results of the seven studies, four studies indicated improved visualization of surgical field in the group receiving TIVA compared to INA.6,7,23,24 In two of these four studies, the improved surgical field was only noted in patients with more severe sinus disease and a higher LM scores (>12).23,24 In one of those two studies the TIVA group also had an overall lower heart rate,23 while the second study had an overall lower MAP in the TIVA group.24 Both of these factors can result in decreased blood loss suggesting that propofol and/or remifentanil decrease blood loss indirectly rather than intrinsically. The remaining three studies did not show a significant difference between the two types of anesthesia. Unfortunately, a possible type II error due to lack of power cannot be excluded given the small sample sizes in each of these studies.
Reducing MAP has been the focus of some interventions to reduce surgical field impairment. Wormald et al.24 did demonstrate that both the TIVA group and the INA group had a positive correlation between surgical field grade and MAP. However, the INA group had to achieve significantly lower MAPs to obtain a similar Boezaart score as the TIVA group. The TIVA group overall had a significantly better Boezaart score.24 One of the limitations of the studies included in this review involves the administration of the inhaled anesthetic. Most studies adjusted the anesthetic to achieve a target MAP prior to administering any additional medications to control MAP. When using inhalational anesthetics, the volatile agent is often increased to reduce the MAP.6,23,24 There is likely a dose dependent vasodilation associated with the inhaled anesthetics.26,27 Higher concentrations of inhaled anesthetic cause peripheral vasodilation, possibly resulting in tachycardia, and increase capillary bleeding. The studies in our review did not adequately compare the two anesthetic techniques. Most of the studies only provided a range of inhaled anesthetic administered, with the quoted upper limit far exceeding one minimal alveolar concentration. In fact, high dose inhaled anesthetics are not needed to achieve adequate amnesia and may inaccurately suggest that all patients receiving INA would have increased bleeding. Therefore, one cannot adequately conclude that an ideal inhaled anesthetic was provided to all patients in these studies. However, excluding patients that did receive higher concentrations of inhaled anesthetic would likely contribute to an even higher type II error.
Additionally, the use of narcotic analgesic may also contribute significantly to surgical field visualization. Fentanyl is often given in bolus doses, whereas remifentanil is a short acting fentanyl derivative administered as a continuous infusion that often has a synergistic effect with propofol in TIVA.28 Remifentanil can cause hypotension by decreasing cardiac output without the accompanied peripheral vasodilation.29 It has been associated with some improvement in surgical field conditions compared with fentanyl administration, even when used as an adjunct with an inhaled anesthetic. This finding is thought to be related to its’ hypotensive and bradycardic effects.30 In Ahn et al. 23, there was a higher rate of remifentanil infusion in the TIVA group compared to the INA group, although it was not statistically significant likely due to small sample size and lack of power. The TIVA group also had a lower mean heart rate and likely decreased cardiac output, which may have contributed to the improved visual analog score in the TIVA group. Furthermore, the reviewed studies were heterogenous in how “TIVA” was defined: some studies contrasted propofol vs a volatile anesthetic using the same opioid 3,21–23, while others additionally used a different opioid regimen between the study groups 6,7,24 making it difficult to correlate outcome to intervention and to differentiate the effects of the anesthetic from the effects of remifentanil.
Another limitation in the studies was the lack of a consistent measuring tool to assess surgical field visualization. Five of the seven studies used a similar scale, however there were two studies that utilized a different visual analog scale to assess surgical field conditions. The 6-point scale used in the majority of the studies has been validated with relatively high inter-rater and intra-rater reliability.31 However, there has been a more sensitive 11-point scale, the Wormald scale, recently introduced that has also been show to have better inter-rater and intra-rater reliability than the 6-point scale.31 This scale may be better at identifying changes in the surgical field that would be clinically relevant.
Finally, there is a great heterogeneity among the studies with regards to those parameters that were fixed and those parameters that were used to measure the outcome. Interventions that focus on maintaining a constant MAP by using antihypertensive drugs or varying anesthetic/opioid concentrations6,7,21,23,24 introduces an unnecessary confounding factor whose role is neither dependent nor independent and makes correlating the type of anesthetic with bleeding impossible. Further investigation is indicated to eliminate those confounding factors. To test the hypothesis that TIVA causes less bleeding and creates improved surgical field visualization, a fixed concentration of a volatile anesthetic should be compared to a fixed propofol infusion rate. Additionally outcomes should be consistently measured by a validated subjective assessment and objective measurements.
The strength of this systematic review includes a comprehensive literature search utilizing multiple databases. Our study does provide a thorough review of the current published literature evaluating the impact of different anesthetics on surgical field outcomes. However it also has some limitations, including the exclusion of non-English publications. Most importantly, the majority of papers included in the study have a small sample size and lower quality of evidence on Jadad scoring system.
CONCLUSION
Although several studies suggest that TIVA may improve surgical conditions during ESS, others did not show a difference, and more so, those that did, often had severe limitations in design and/or power that preclude an overall recommendation to use TIVA over INA in order to decrease bleeding during ESS. A series of well defined interventions including a combination of analgesic and a limited amount of volatile anesthetic and/or propofol may together help create optimal surgical field visualization, likely through limiting systemic blood pressure by decreasing cardiac output. Nevertheless, there remains a definite need for sufficiently powered, randomized control studies to delineate the role of different anesthetic techniques and interventions in aiding to improved outcome in ESS.
References
- 1.Ramakrishnan VR, Kingdom TT, Nayak JV, Hwang PH, Orlandi RR. Nationwide incidence of major complications in endoscopic sinus surgery. International Forum of Allergy & Rhinology. 2011;2(1):34–39. doi: 10.1002/alr.20101. [DOI] [PubMed] [Google Scholar]
- 2.Siedek V, Pilzweger E, Betz C, Berghaus A, Leunig A. Complications in endonasal sinus surgery: a 5-year retrospective study of 2,596 patients. Eur Arch Otorhinolaryngol. 2012 doi: 10.1007/s00405-012-1973-z. [DOI] [PubMed] [Google Scholar]
- 3.Beule AG, Wilhelmi F, Kühnel TS, et al. Propofol versus sevoflurane: bleeding in endoscopic sinus surgery. Otolaryngol Head Neck Surg. 2007;136(1):45–50. doi: 10.1016/j.otohns.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Boezaart AP, van der Merwe J, Coetzee A. Comparison of sodium nitroprusside- and esmolol-induced controlled hypotension for functional endoscopic sinus surgery. Can J Anaesth. 1995;42(5 Pt 1):373–376. doi: 10.1007/BF03015479. [DOI] [PubMed] [Google Scholar]
- 5.Danielsen A, Gravningsbråten R, Olofsson J. Anaesthesia in endoscopic sinus surgery. European Archives of Oto-Rhino-Laryngology. 2003;260(9):481–486. doi: 10.1007/s00405-003-0613-z. [DOI] [PubMed] [Google Scholar]
- 6.Eberhart LHJ, Folz BJ, Wulf H, Geldner G. Intravenous anesthesia provides optimal surgical conditions during microscopic and endoscopic sinus surgery. Laryngoscope. 2003;113(8):1369–1373. doi: 10.1097/00005537-200308000-00019. [DOI] [PubMed] [Google Scholar]
- 7.Tirelli G, Bigarini S, Russolo M, Lucangelo U, Gullo A. Total intravenous anaesthesia in endoscopic sinus-nasal surgery. Acta Otorhinolaryngol Ital. 2004;24(3):137–144. [PubMed] [Google Scholar]
- 8.Ko M-T, Chuang K-C, Su C-Y. Multiple analyses of factors related to intraoperative blood loss and the role of reverse Trendelenburg position in endoscopic sinus surgery. Laryngoscope. 2008;118(9):1687–1691. doi: 10.1097/MLG.0b013e31817c6b7c. [DOI] [PubMed] [Google Scholar]
- 9.Atef A, Fawaz A. Comparison of laryngeal mask with endotracheal tube for anesthesia in endoscopic sinus surgery. Am J Rhinol. 2008;22(6):653–657. doi: 10.2500/ajr.2008.22.3247. [DOI] [PubMed] [Google Scholar]
- 10.Baker AR, Baker AB. Anaesthesia for endoscopic sinus surgery. Acta Anaesthesiol Scand. 2010;54(7):795–803. doi: 10.1111/j.1399-6576.2010.02259.x. [DOI] [PubMed] [Google Scholar]
- 11.Ramachandran R, Singh PM, Batra M, Pahwa D. Anaesthesia for endoscopic endonasal surgery. Trends in Anaesthesia and Critical Care. 2011;1(2):79–83. [Google Scholar]
- 12.Gilbey P, Kukuev Y, Samet A, Talmon Y, Ivry S. The quality of the surgical field during functional endoscopic sinus surgery--the effect of the mode of ventilation--a randomized, prospective, double-blind study. Laryngoscope. 2009;119(12):2449–2453. doi: 10.1002/lary.20614. [DOI] [PubMed] [Google Scholar]
- 13.Nair S, Collins M, Hung P, et al. The effect of beta-blocker premedication on the surgical field during endoscopic sinus surgery. Laryngoscope. 2004;114(6):1042–1046. doi: 10.1097/00005537-200406000-00016. [DOI] [PubMed] [Google Scholar]
- 14.Shen P-H, Weitzel EK, Lai J-T, Wormald P-J, Ho C-S. Intravenous esmolol infusion improves surgical fields during sevoflurane-anesthetized endoscopic sinus surgery: a double-blind, randomized, placebo-controlled trial. Am J Rhinol Allergy. 2011;25(6):e208–11. doi: 10.2500/ajra.2011.25.3701. [DOI] [PubMed] [Google Scholar]
- 15.Elsharnouby NM, Elsharnouby MM. Magnesium sulphate as a technique of hypotensive anaesthesia. Br J Anaesth. 2006;96(6):727–731. doi: 10.1093/bja/ael085. [DOI] [PubMed] [Google Scholar]
- 16.Mohseni M, Ebneshahidi A. The effect of oral clonidine premedication on blood loss and the quality of the surgical field during endoscopic sinus surgery: a placebo-controlled clinical trial. J Anesth. 2011;25(4):614–617. doi: 10.1007/s00540-011-1157-9. [DOI] [PubMed] [Google Scholar]
- 17.Jacobi KE, Böhm BE, Rickauer AJ, Jacobi C, Hemmerling TM. Moderate controlled hypotension with sodium nitroprusside does not improve surgical conditions or decrease blood loss in endoscopic sinus surgery. Journal of Clinical Anesthesia. 2000;12(3):202–207. doi: 10.1016/s0952-8180(00)00145-8. [DOI] [PubMed] [Google Scholar]
- 18.Fromme GA, MacKenzie RA, Gould AB, Lund BA, Offord KP. Controlled hypotension for orthognathic surgery. Anesth Analg. 1986;65(6):683–686. [PubMed] [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 20.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 21.Ankichetty SP, Ponniah M, Cherian V, et al. Comparison of total intravenous anesthesia using propofol and inhalational anesthesia using isoflurane for controlled hypotension in functional endoscopic sinus surgery. J Anaesthesiol Clin Pharmacol. 2011;27(3):328–332. doi: 10.4103/0970-9185.83675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoo H-S, Han JH, Park SW, Kim KS. Comparison of surgical condition in endoscopic sinus surgery using remifentanil combined with propofol, sevoflurane, or desflurane. Korean J Anesthesiol. 2010;59(6):377–382. doi: 10.4097/kjae.2010.59.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahn HJ, Chung S-K, Dhong H-J, et al. Comparison of surgical conditions during propofol or sevoflurane anaesthesia for endoscopic sinus surgery. Br J Anaesth. 2008;100(1):50–54. doi: 10.1093/bja/aem304. [DOI] [PubMed] [Google Scholar]
- 24.Wormald PJ, van Renen G, Perks J, Jones JA, Langton-Hewer CD. The effect of the total intravenous anesthesia compared with inhalational anesthesia on the surgical field during endoscopic sinus surgery. Am J Rhinol. 2005;19(5):514–520. [PubMed] [Google Scholar]
- 25.Hopkins C, Browne JP, Slack R, Lund V, Brown P. The Lund-Mackay staging system for chronic rhinosinusitis: how is it used and what does it predict? Otolaryngol Head Neck Surg. 2007;137(4):555–561. doi: 10.1016/j.otohns.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 26.Ebert TJ. Cardiovascular and autonomic effects of sevoflurane. Acta Anaesthesiol Belg. 1996;47(1):15–21. [PubMed] [Google Scholar]
- 27.Patel SS, Goa KL. Sevoflurane. A review of its pharmacodynamic and pharmacokinetic properties and its clinical use in general anaesthesia. Drugs. 1996;51(4):658–700. doi: 10.2165/00003495-199651040-00009. [DOI] [PubMed] [Google Scholar]
- 28.Scott LJ, Perry CM. Remifentanil: a review of its use during the induction and maintenance of general anaesthesia. Drugs. 2005;65(13):1793–1823. doi: 10.2165/00003495-200565130-00007. [DOI] [PubMed] [Google Scholar]
- 29.Manola M, De Luca E, Moscillo L, Mastella A. Using remifentanil and sufentanil in functional endoscopic sinus surgery to improve surgical conditions. ORL J Otorhinolaryngol Relat Spec. 2005;67(2):83–86. doi: 10.1159/000084576. [DOI] [PubMed] [Google Scholar]
- 30.Laguna D, Lopez-Cortijo C, Millan I, Gonzalez FM, Garcia-Berrocal JR. Blood loss in endoscopic sinus surgery: assessment of variables. J Otolaryngol Head Neck Surg. 2008;37(3):324–330. [PubMed] [Google Scholar]
- 31.Athanasiadis T, Beule A, Embate J, et al. Standardized video-endoscopy and surgical field grading scale for endoscopic sinus surgery: a multi-centre study. Laryngoscope. 2008;118(2):314–319. doi: 10.1097/MLG.0b013e318157f764. [DOI] [PubMed] [Google Scholar]