Abstract
BACKGROUND
Previous studies have shown that exposure to inhaled anesthetics can cause cognitive dysfunction, suggesting that general anesthesia might be a risk factor for the development of Alzheimer disease. However, the underlying mechanisms remain to be elucidated. In the present study, we tested our hypothesis that enhanced tau protein phosphorylation in hippocampus contributes to isoflurane-induced cognitive dysfunction in a mouse model of Alzheimer disease.
METHODS
Fifty-four male wild-type (WT) mice (12 months old) and 54 male amyloid precursor protein 695 (APP695) mice (12 months old) were either anesthetized for 4 hours with 1.0 minimum alveolar concentration isoflurane or sham-anesthetized (control). Learning and memory behaviors were measured using the Morris Water Maze test for mice. Phosphorylation of hippocampal tau protein at Ser262 site was analyzed with quantitative Western blotting.
RESULTS
In the Morris Water Maze test, both WT and transgenic APP695 mice showed decreased latency times during a 4-day training period. Isoflurane exposure significantly increased the latency times on days 2 and 3 in WT mice as well as on days 3 and 4 in APP695 mice (WT: P = 0.005 for day 2 and P = 0.002 for day 3; APP695: P = 0.001 for day 3 and P < 0.0001 for day 4) and reduced platform quadrant times (WT: P < 0.0001; APP695: P < 0.0001) in both types of mice. Compared with WT mice, transgenic APP695 mice displayed worse learning and memory behaviors after isoflurane exposure (P = 0.0005 for escape latency testing on day 4 training; P = 0.009 for platform probe testing). Western blot analysis showed that the levels of phosphorylation of hippocampal tau protein at Ser262 site (tau[pS262]) in the transgenic APP695 mice were higher than those in WT mice (P < 0.0001) and that isoflurane exposure time dependently enhanced the hippocampal tau[pS262] levels in both types of mice, but this effect was much more significant in the transgenic APP695 mice (P < 0.0001). Our data also showed that isoflurane exposure had no effect on the expression of total tau protein in the hippocampi of all mice (P ≥ 0.54).
CONCLUSIONS
Isoflurane may induce cognitive dysfunction by enhancing phosphorylation of hippocampal tau protein at Ser262 site, and this effect is more significant in transgenic APP695 mice.
Inhaled anesthetics have been used in modern surgical procedures worldwide. However, there has been increasing concern about their involvement in postoperative cognitive dysfunction. Several lines of evidence have shown that inhaled anesthetics, such as isoflurane, cause learning and memory impairment.1–7 Meanwhile, it has been reported that isoflurane not only increases amyloid-β (Aβ) oligomerization and cytotoxicity,8 but also alters the processing of amyloid precursor protein (APP).9 Because altered APP processing that leads to the deposition of Aβ is a key event in the pathogenesis of Alzheimer disease (AD),10,11 these results suggest that isoflurane exposure might accelerate the process of AD and increase the risk of postoperative cognitive dysfunction in patients with AD. As one of the most serious neurodegenerative diseases, AD is characterized by progressive dementia and cognitive dysfunction. General anesthesia might be a risk factor for the development of AD. However, the underlying mechanisms remain to be elucidated.
Neurofibrillary tangles that are composed by insoluble deposits of hyperphosphorylated tau protein contribute to the neuropathogenesis of AD. Recent studies have shown that anesthetics including isoflurane induce tau protein phosphorylation.12–14 Tau protein is a microtubule-associated protein that is expressed abundantly in neuronal axons, and this protein is commonly phosphorylated at the Ser262 site (tau[pS262]), which is an important phosphorylation site connecting the tau protein and microtubule.15Hyperphosphorylated tau could dissociate from the microtubule and relocalize in the somatodendritic compartment, thereby destabilizing the microtubule and causing neuronal damage, neurodegeneration, and ultimately cognitive dysfunction.16 Therefore, it is possible that tau protein phosphorylation may be involved in the mechanism by which isoflurane exposure produces learning and memory impairment. In the central nervous system, the hippocampus is a critical region for learning and memory. Thus, assessing hippocampal tau protein phosphorylation will determine whether it correlates with isoflurane-induced cognitive dysfunction and will help us understand the importance of tau protein phosphorylation in the neuropathogenesis of AD. We hypothesized that enhanced tau protein phosphorylation in hippocampus contributes to isoflurane-induced cognitive dysfunction in a mouse model of AD.
In the present study, specific AD transgenic APP695 mice, in which the Aβ peptide is overexpressed and deposited,17,18 were used. We investigated the effects of isoflurane exposure on learning and memory behaviors as well as hippocampal tau protein phosphorylation in the mouse model of AD.
METHODS
Animals
All animal procedures performed in this study were approved by the Institutional Ethics Review Committee for Animal Care and Use at the Zhengzhou University in China. All experiments were performed in accordance with the National Institutes of Health guide for the Care and Use of Laboratory Animals (National Institutes of Health Publications No. 8023, revised 1978). Fifty-four 12-monthold male C57BL/6 wild-type (WT) mice and fifty-four 12-month-old male APP695 mice with C57BL/6 genetic background were used in this study. The mice were housed 4 per cage, using a 12/12-hour light/dark cycle, with free access to food and water. The mice were assigned as follows: 10 WT mice and 10 APP695 mice were used for minimum alveolar anesthetic concentration (MAC) testing; 20 WT mice and 20 APP695 mice were used for Morris Water Maze testing (10 for control group and 10 for isoflurane exposure group); 24 WT mice and 24 APP695 mice were used for Western blot analysis (6 for control group, 6 for day 1 after isoflurane, 6 for day 3 after isoflurane, and 6 for day 7 after isoflurane). All efforts were made to minimize animal suffering and to reduce the number of animals used. A timeline for animal treatment is listed in Figure S1 (Supplemental Digital Content 1, http://links.lww.com/AA/A924).
Anesthesia
Determination of the MAC value for isoflurane was performed as described previously with minor modification.19,20 Mice were placed into individual Plexiglas chambers (5 × 5 × 15 cm), and anesthesia was induced with 1.5 vol% isoflurane in 100% oxygen (1 L/min) through a regularly calibrated TEC3 isoflurane vaporizer (Datex-Ohmeda, GE Healthcare, Madison, WI). After an initial equilibration period of 30 minutes, MAC was determined by clamping the middle third of the tail to the first ratchet of a 15-cm hemostatic forceps for up to 1 minute. Motor activities (gross movements of the head, extremities, and/or body) were considered a positive response. Next, the anesthetic concentration was increased (or decreased) by 0.1 vol%. After 10 minutes of equilibration, the tail was stimulated again. Only the middle third of the tail was used for tail clamping, and the clamp was always placed proximal to the previous test site. The midpoint isoflurane concentration for negative and positive responses was considered the MAC. The Plexiglas chamber was cleaned with 5% ethanol.
For isoflurane exposure, mice assigned to the anesthesia groups were placed into the Plexiglas chambers and were anesthetized for 4 hours with 1.0 MAC isoflurane in 100% oxygen (1 L/min) through a regularly calibrated TEC3 isoflurane vaporizer. Mice in the control groups received 100% oxygen (1 L/min) without isoflurane for 4 hours. During MAC testing and isoflurane exposure, the isoflurane concentration at the Plexiglas chamber gas outlet was continuously monitored using an agent analyzer (Datex-Ohmeda, Helsinki, Finland). Rectal temperature of the mice was kept 36°C to 38°C throughout the experiment.
Learning and Memory Behavioral Testing
The Morris Water Maze test was performed to measure learning and memory behaviors as described previously21 with minor modification. The diameter and height of the pool were 1.2 and 0.6 m, respectively, and the depth of the water was 0.4 m. There was a Plexiglas escape platform (10 × 10 cm) in the pool, which was placed in the northwest quadrant of the pool and hidden 0.5 cm below the surface of the water. Water temperature was controlled at a steady level of 20°C to 22°C, and white titanium dioxide powder was added as a shield agent. The training session included environmental acclimation followed by an escape latency test for 4 days started from the next day after isoflurane exposure. In the escape latency test, each mouse underwent four 1-minute trials with a 5-minute break in between. Each trial began from a different point at the perimeter of the pool and ended when mice located the platform. If the beginning– ending duration time exceeded 60 seconds, mice were considered unsuccessful and would be led to the platform, remaining on the platform for 20 seconds.21 One day after the last training trial, each mouse was subjected to a spatial probe test, in which the mouse swam for 60 seconds without the platform in the pool. In the probe test, an experimenter removed the mouse and placed it in the water facing the maze wall. This test was recorded with a video camera. The data from the probe test were analyzed by calculating the total time spent in the platform quadrant of the pool.
Western Blot Analysis
Hippocampal tissues were harvested on days 1, 3, and 7 after isoflurane exposure, and total proteins were extracted. Tau protein phosphorylation and Aβ expression in the hippocampus were assessed using quantitative Western blotting as described previously.22 In brief, the proteins were separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane. After blocking with 5% skimmed milk in Tris-buffered saline containing 0.1% Tween (pH 7.6) for 2 hours, the membrane was incubated with the following primary antibodies overnight at 4°C: (1) polyclonal rabbit anti-tau antibody (1:200, Abcam, Cambridge, MA); (2) polyclonal rabbit anti–phospho-tau at Ser262 site (1:1000, Millipore, Billerica, MA); (3) polyclonal rabbit anti-Aβ(1–42) antibody (1:500, Abcam); (4) polyclonal rabbit anti–β-actin antibody (1:5000, Abcam). Next, the membranes were incubated with goat anti-rabbit horseradish peroxidase-conjugated secondary antibody (Amersham Pharmacia Biotech, Piscataway, NJ) for 2 hours at room temperature. Specific proteins were detected by enhanced chemiluminescence (Santa Cruz Biotechnology, Santa Cruz, CA). β-Actin served as a loading control.
Statistical Analysis
The data were expressed as mean ± SEM. The immunoblotting bands were quantified by densitometry using ImageJ software (National Institutes of Health, Bethesda, MD). For tau expression, the ratio of tau to β-actin was calculated for each group and then the difference of the ratio between control group and other groups was statistically analyzed. Comparisons among groups were performed by 1-way analysis of variance, 2-way, or 2-way repeated measures analysis of variance followed by post hoc comparisons with the Tukey method. All reported P values and confidence intervals are Tukey corrected. Student t test was used to compare the difference of the data from WT and APP695 mice. P < 0.01 was considered statistically significant. Ninety-nine percent confidence intervals for differences were calculated for all data in this study and shown in Table S1 (Supplemental Digital Content 2, http://links.lww.com/AA/A925). Statistical analysis was performed with SPSS version 11.0 software (SPSS Inc., Chicago, IL).
RESULTS
Expression of Aβ(1–42) in the Hippocampus of WT and Transgenic APP695 Mice
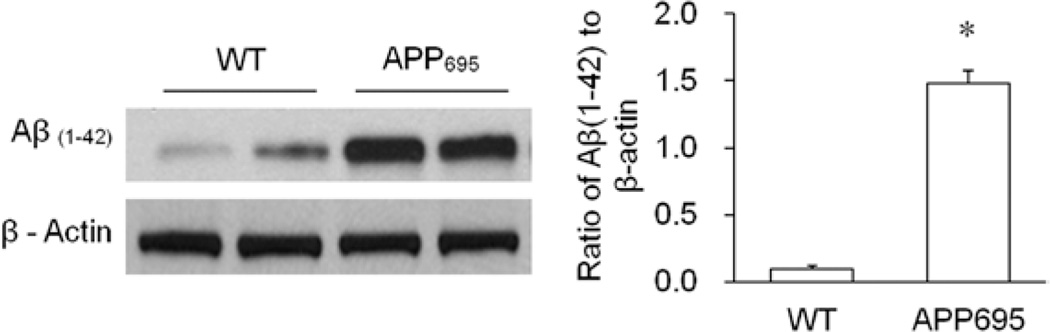
Western blotting showed that hippocampal Aβ(1–42) expression was markedly increased in APP695 mice compared with that in WT mice (Fig. 1, P < 0.0001). The transgenic APP695 mice were used as a mouse model of AD in this study.
Figure 1.
The expression of amyloid-β(1–42) (Aβ(1–42)) in the hippocampi of wild-type (WT) and transgenic amyloid precursor protein 695 (APP695) mice. Compared with WT mice, the APP695 mice showed markedly increased expression of Aβ(1–42) in the hippocampus. The bands shown were representative of 3 independent experiments. β-Actin served as a loading control. Statistical analysis indicated that the level of hippocampal Aβ(1–42) in the APP695 mice was significantly higher than that in WT mice (n = 6 per group, *P < 0.0001 versus WT mice).
Effect of Isoflurane Exposure on Learning and Memory Behaviors
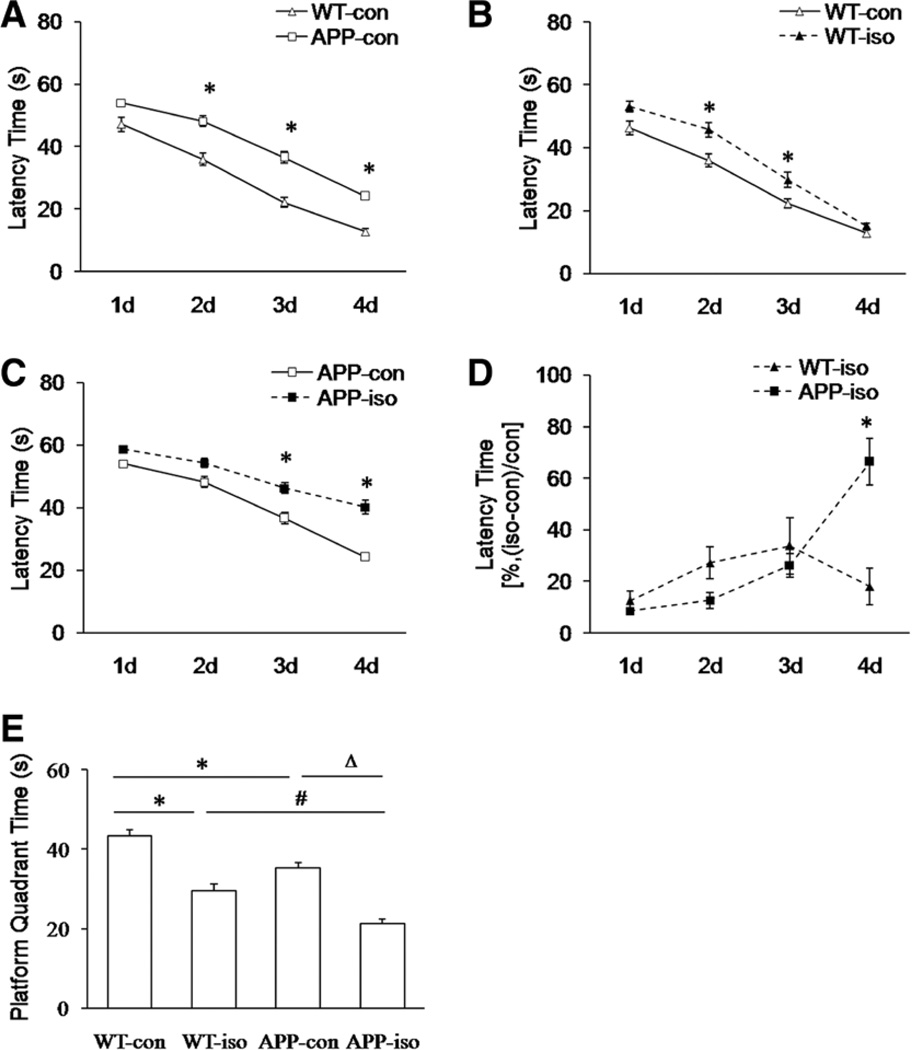
The MAC value for isoflurane in transgenic APP695 mice was significantly higher than that in WT mice (Table 1, P = 0.004). We treated both types of mice with 1 MAC of isoflurane (1.31 vol% isoflurane for WT mice and 1.48 vol% for APP695 mice) in this study. By conducting the Morris Water Maze test, we observed that exposure to 1 MAC of isoflurane for 4 hours impaired spatial learning and memory behaviors in both WT and APP695 mice (Fig. 2). Before isoflurane exposure, the latency times in APP695 mice were significantly longer than those in WT mice on days 2 to 4 (Fig. 2A, P = 0.0003 for day 2 and P < 0.0001 for days 3 and 4); after isoflurane exposure, the latency times were significantly increased on days 2 and 3 in WT mice as well as on days 3 and 4 in APP695 mice (Fig. 2, B and C, WT: P = 0.005 for day 2 and P = 0.002 for day 3; APP695: P = 0.001 for day 3 and P < 0.0001 for day 4). To statistically compare the decrement due to isoflurane between WT and APP695 mice with the decrement between the 2 control groups, we normalized the data from isoflurane exposure groups with their control groups and found that compared with WT mice, the transgenic APP695 mice showed markedly increased latency time on day 4 after isoflurane exposure (Fig. 2D, P = 0.0005). Moreover, isoflurane exposure significantly reduced platform quadrant times in both types of mice (Fig. 2E, P < 0.0001). Compared with WT mice, the transgenic APP695 mice displayed worse spatial memory before and after isoflurane exposure (Fig. 2E, P = 0.001 and P = 0.009 for before and after isoflurane exposure, respectively).
Table 1.
MAC Values of Isoflurane in WT and Transgenic APP695 Mice
| WT | APP695 | R2 | F | P value | |
|---|---|---|---|---|---|
| MAC values (vol%) | 1.31 ± 0.10 | 1.48 ± 0.13a | 0.144 | 10.616 | 0.004 |
Data expressed as mean and SEM. n = 10 per group.
APP695 = amyloid precursor protein 695; MAC = minimum alveolar anesthetic concentration; WT = wild type.
Significant difference between WT and APP695 mice (P = 0.004).
Figure 2.
The effect of isoflurane (iso) exposure on spatial learning and memory in both wild-type (WT) and transgenic amyloid precursor protein 695 (APP695) mice. All mice in iso-treated groups received 1 MAC of iso for 4 hours. A, During 4-day training period, the latency times in APP695 mice (APP-con) were significantly longer than those in WT mice (WT-con) before iso exposure (n = 10 per group, *P = 0.0003 for day 2, *P < 0.0001 for days 3 and 4 versus WT-con group). B, In isotreated WT mice (WT-iso), the latency times during 4-day training period were significantly increased compared with the control WT mice (n = 10 per group, *P = 0.005 for day 2 and *P = 0.002 for day 3 versus WT-con group). C, In iso-treated APP695 mice (APP-iso), the latency times during 4-day training period were significantly increased compared with the control APP695 mice (n = 10 per group, *P = 0.001 for day 3 and *P < 0.0001 for day 4 versus APP-con group). D, The data from iso exposure groups were normalized with their control (con) groups and expressed as percentage of latency time (%, [iso − con]/con). During 4-day training period, the latency times in APP695 mice were significantly longer than those in WT mice on day 4 after iso exposure (n = 10 per group *P = 0.0005 versus WT-iso group). E, Iso exposure significantly reduced platform quadrant times in both types of mice (n = 10 per group, *P < 0.0001 versus WT-con group and ΔP < 0.0001 versus APP-con group). Compared with WT mice, the transgenic APP695 mice displayed worse spatial memory before and after iso exposure (n = 10 per group, *P = 0.001 versus WT-con group and #P = 0.009 versus WT-iso group).
Effect of Isoflurane Exposure on Hippocampal Tau Protein Phosphorylation
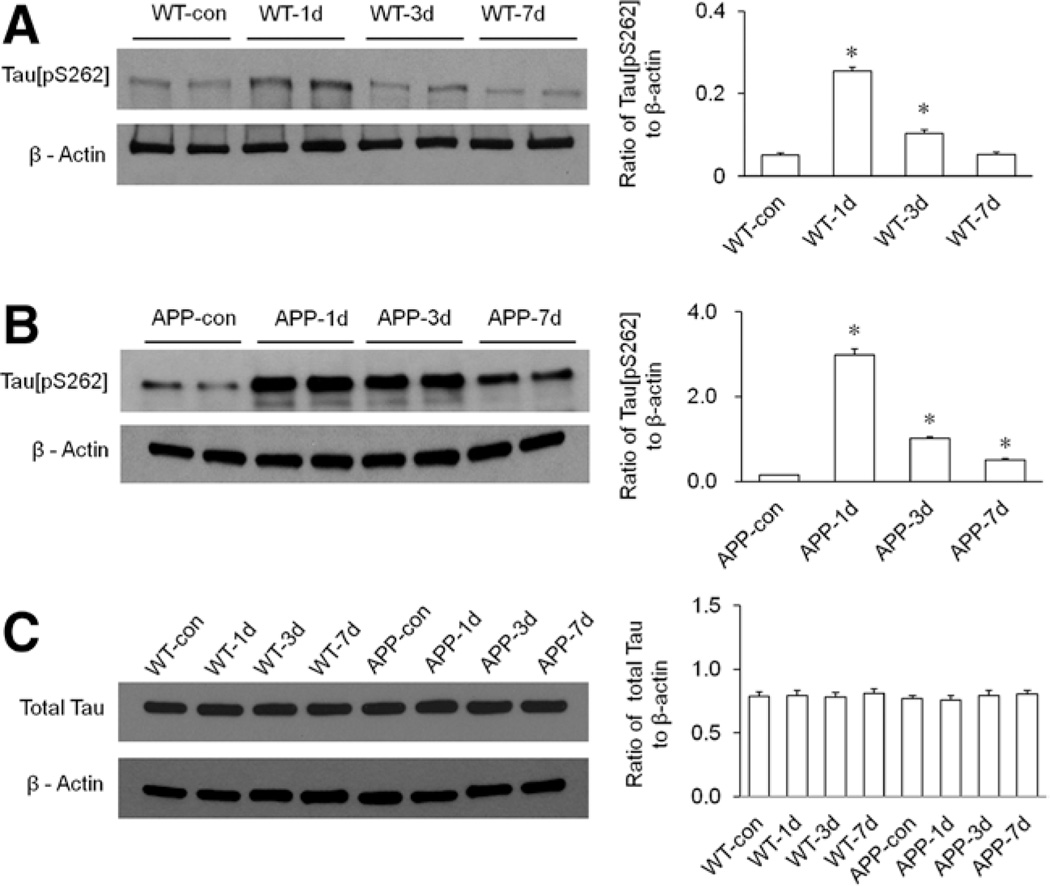
Western blot analysis showed that isoflurane exposure time dependently altered phosphorylation of hippocampal tau protein at the Ser262 site (tau[pS262]) in both WT and transgenic APP695 mice (Fig. 3, A and B). However, isoflurane exposure had no significant effect on the expression of total tau protein in the hippocampi of all mice (Fig. 3C, P ≥ 0.54). The widths of the 99% confidence intervals are all <46% of the corresponding means and an associated SE is 2% (Table S2, Supplemental Digital Content 3, http://links.lww.com/AA/A926). In WT mice, the levels of hippocampal tau[pS262] were significantly increased on days 1 and 3 after isoflurane exposure (Fig. 3A, P < 0.0001 for day 1 and P = 0.0008 for day 3), and the tau phosphorylation reached peak level on day 1 and returned to baseline (levels in control group) on day 7 after isoflurane (Fig. 3A, P = 0.8 for day 7). In the transgenic APP695 mice, the levels of hippocampal tau[pS262] were markedly increased on days 1, 3, and 7 after isoflurane exposure (Fig. 3B, P < 0.0001 for all 3 time points), and the tau phosphorylation also reached peak level on day 1 but did not return to baseline (levels in control group) on day 7 after isoflurane (Fig. 3B).
Figure 3.
The effect of isoflurane exposure on hippocampal tau protein phosphorylation in both wild-type (WT) and transgenic amyloid precursor protein 695 (APP695) mice. All mice in isoflurane-treated groups received 1 minimum alveolar anesthetic concentration (MAC) of isoflurane for 4 hours. A, Isoflurane exposure time dependently altered the expression of tau[pS262] in the hippocampus of WT mice. Statistical analysis indicated that the levels of hippocampal tau[pS262] in WT mice were significantly increased on days 1 (WT-1d) and 3 (WT-3d) after isoflurane exposure (n = 6 per group, *P < 0.0001 for day 1 and *P = 0.0008 for day 3 versus WT-con group), and the tau phosphorylation returned to control level on day 7 (WT-7d) post-isoflurane (n = 6 per group, P = 0.8 versus WT-con group). B, Isoflurane exposure time dependently altered the expression of tau[pS262] in the hippocampus of the APP695 mice. Statistical analysis indicated that the levels of hippocampal tau[pS262] in the APP695 mice were significantly increased on days 1 (APP-1d), 3 (APP-3d), and 7 (APP-7d) after isoflurane exposure (n = 6 per group, *P < 0.0001 versus APP-con group). C, Isoflurane exposure had no effect on the expression of total tau protein in the hippocampi of both types of mice. Statistical analysis indicated that the levels of total tau protein in the hippocampi of all mice were not significantly different (n = 6 per group, WT: P = 0.95 for day 1, P = 0.87 for day 3, and P = 0.65 for day 7 versus WT-con group; APP695: P = 0.54 for day 1, P = 0.90 for day 3, and P = 0.75 for day 7 versus APP-con group). β-Actin served as a loading control for all Western blotting experiments.
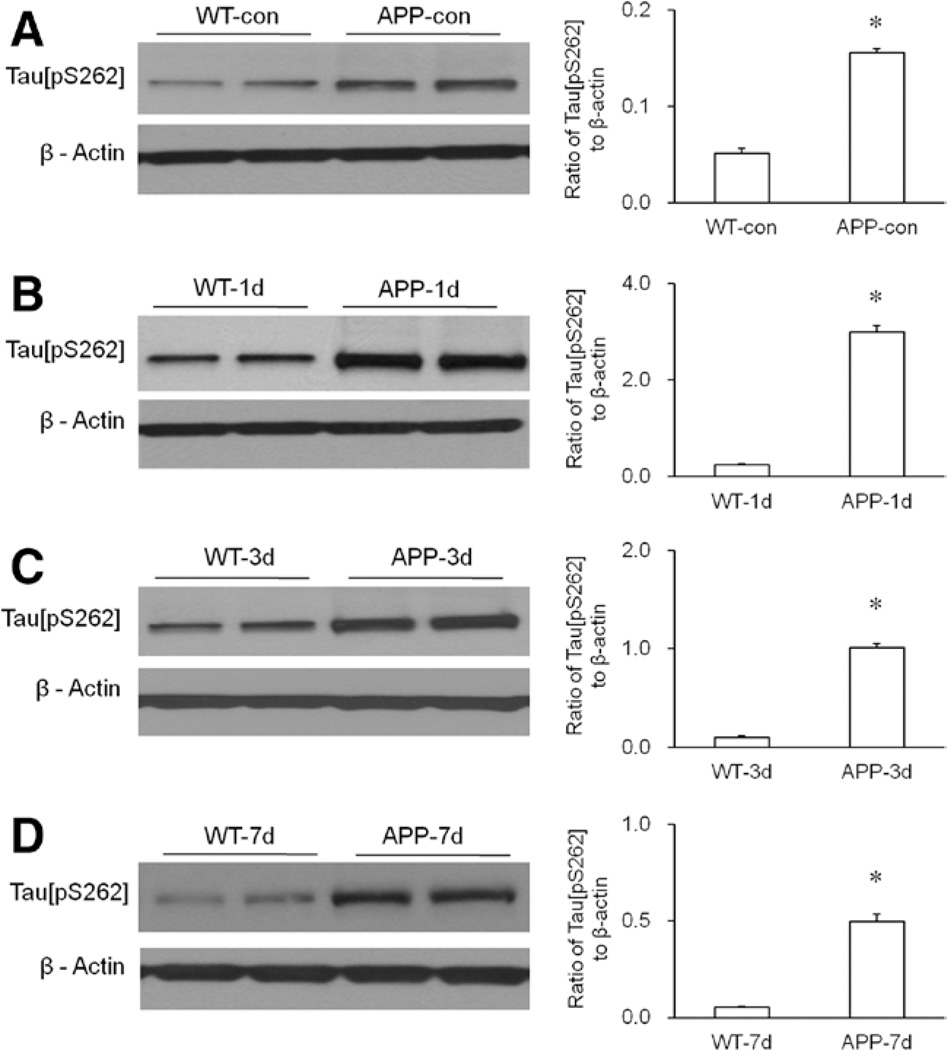
Compared with WT mice, transgenic APP695 mice displayed increased levels of hippocampal tau[pS262] before and after isoflurane exposure (Fig. 4). In normal conditions, the level of hippocampal tau[pS262] in the APP695 mice was significantly higher than in WT mice (Fig. 4A, P < 0.0001). On days 1, 3, and 7 after isoflurane exposure, the levels of hippocampal tau[pS262] in the APP695 mice were also significantly higher than those in WT mice (Fig. 4, B−D, P < 0.0001).
Figure 4.
Enhanced phosphorylation of hippocampal tau protein in the transgenic APP695 mice. A, Compared with wild-type (WT) mice, the amyloid precursor protein 695 (APP695) mice showed increased hippocampal tau[pS262] in normal condition. Statistical analysis indicated that the level of hippocampal tau[pS262] in the APP695 mice was significantly higher than that in WT mice (n = 6 per group, *P < 0.0001 versus WT-con group). B, Compared with WT mice, the APP695 mice showed increased hippocampal tau[pS262] on day 1 (APP-1d) after isoflurane exposure. Statistical analysis indicated that the level of hippocampal tau[pS262] in the APP695 mice was significantly higher than that in WT mice (n = 6 per group, *P < 0.0001 versus WT mice on day 1 [WT-1d] group). C, Compared with WT mice, the APP695 mice showed increased hippocampal tau[pS262] on day 3 (APP-3d) after isoflurane exposure. Statistical analysis indicated that the level of hippocampal tau[pS262] in the APP695 mice was significantly higher than that in WT mice (n = 6 per group, *P < 0.0001 versus WT-3d group). D, Compared with WT mice, the APP695 mice showed increased hippocampal tau[pS262] on day 7 (APP-7d) after isoflurane exposure. Statistical analysis indicated that the level of hippocampal tau[pS262] in the APP695 mice was significantly higher than that in WT mice (n = 6 per group, *P < 0.0001 versus WT-7d group). β-Actin served as a loading control for all Western blotting experiments.
DISCUSSION
In the present study, we investigated the role of hippocampal tau protein phosphorylation in isoflurane-induced cognitive dysfunction in a transgenic mouse model of AD. We found that isoflurane exposure markedly impairs spatial learning and memory in transgenic APP695 mice. Isoflurane-induced cognitive dysfunction may be correlated with phosphorylation of hippocampal tau protein at the Ser262 site. Our results demonstrate that isoflurane may induce cognitive impairment by enhancing phosphorylation of hippocampal tau protein at the Ser262 site, and this effect is more significant in APP695 mice, suggesting isoflurane might have a detrimental effect on the development of AD by enhancing hippocampal tau protein phosphorylation and cognitive dysfunction.
Our data showed that isoflurane MAC was higher in transgenic APP695 mice than in WT mice (Table 1), which is consistent with 2 studies from other laboratories that used other AD mouse models.23,24 Previous studies have shown that Aβ oligomers directly activate N-methyl-d-aspartate (NMDA) receptors25 and that Aβ also induces astrocytic glutamate release, which in turn activates extrasynaptic NMDA receptors on neurons.26 An increase in the cytosolic concentration of Ca2+ induced by Aβ oligomers in cortical neurons is prevented by AP5, a broad spectrum NMDA receptor antagonist.25 In our study, we observed that Aβ(1–42) expression was markedly increased in APP695 mice (Fig. 1). Therefore, the excitatory effect of Aβ may contribute to increased isoflurane MAC in APP695 mice. To investigate the effect of isoflurane exposure on cognitive function in our AD mouse model, we treated both APP695 and WT mice with 1 MAC isoflurane to reach the same anesthesia depth. MAC is defined as the concentration of inhaled anesthetic that is needed to prevent movement in 50% of subjects in response to surgical (pain) stimulus. MAC value is accepted as a valid measure of potency for inhaled anesthetics, which remains fairly constant for a given species. Thus, by treating animals with 1 MAC isoflurane, we can avoid overdose of isoflurane-produced off-target effects in the 2 types of mice. However, APP695 mice may have been overdosed with isoflurane relative to WT mice for tau phosphorylation, because it is well known that not all anesthetic end points have the same sensitivity.
Inhaled anesthesia has a variety of effects, both short term and long term, on central nervous system function. Isoflurane exposure has been shown to influence cognitive function in rodent animals, but previous studies have reported controversial results regarding the effect of isoflurane anesthesia on cognitive function.4,22,27–30 For instance, Rammes et al.28 showed that isoflurane reversibly improves cognitive function and long-term enhancement, and Su et al.29 also observed that repeated isoflurane exposure improves spatial memory. However, several other studies have illustrated that isoflurane exposure leads to cognitive dysfunction.4,22,27,30 These discrepancies may have been due to different ages of animals used in different studies. It has been reported that isoflurane anesthesia impairs acquisition of hippocampus-dependent spatial memory 2 weeks after cessation of anesthesia in aged rats,31 but in adult rats, previous isoflurane exposure produces improved spatial memory performance 2 weeks later.32 Isoflurane treatment with different concentrations and exposure times might also produce differential effects. In our study, 12-month-old male mice received 1.0 MAC of isoflurane treatment for 4 hours, and our data in the Morris Water Maze test showed that isoflurane exposure significantly impaired spatial learning and memory. Compared with WT mice, transgenic AD mice (APP695 mice) displayed enhanced impairment of spatial learning and memory after isoflurane exposure, suggesting that isoflurane may promote AD development by aggravating cognitive dysfunction.
Tau protein can functionally bind microtubules, which is crucial for the processes of neuronal outgrowth and axonal integrity.33 Tau protein plays an important role in synaptic plasticity and memory formation, and tau hyperphosphorylation may contribute to cognitive dysfunction in tauopathies.34 The hyperphosphorylated form of tau protein is present in neurofibrillary tangles.35 The formation of neurofibrillary tangles is considered to be a common mechanism underlying neurodegeneration in the pathogenesis of AD.36,37 In addition, the progression of tau phosphorylation has been shown to correlate with the evolution of tau pathology.38 The development of intraneuronal lesions as a result of the progressive deposition of hyperphosphorylated tau at specific brain regions (such as hippocampus and cortex) plays a key role in the pathological process of AD. It has been reported that tau phosphorylation at Ser262 is relevant to the pathogenesis of AD.39,40 It is noteworthy that phosphorylation at this residue is modified in the brain of patients with AD.39 In double transgenic mice (APPSwe/tau-VLW) that reproduce Aβ and tau pathologies, tau phosphorylation at Ser262 correlates with an increase in the formation of filamentous tau aggregates showing a diameter similar to that of tau filaments observed in AD.40Moreover, tau hyperphosphorylation has been associated with memory impairment after isoflurane exposure.22 Tan et al.22 observed that isoflurane anesthesia combined with hypothermia, but not isoflurane anesthesia alone, increases tau phosphorylation at the Thr205 and Ser396 sites in the hippocampus.22 They also found that approximately 45% of protein phosphatase 2A are inhibited in isoflurane-anesthetized rats without temperature control.22 Their results indicate that tau hyperphosphorylation is not induced by isoflurane anesthesia directly, but may be a consequence of isoflurane anesthesia-produced hypothermia, because the inhibition of phosphatase activity and subsequent tau hyperphosphorylation could occur in hypothermic rats. Thus, tau hyperphosphorylation may be associated with isoflurane-induced spatial learning and memory impairment through isoflurane anesthesiaproduced hypothermia. Recently, Dong et al.12 observed that isoflurane exposure increases tau phosphorylation at the Ser262 site both in vitro and in vivo. In their in vitro study, primary neurons were exposed to 2% isoflurane for 6 hours and were harvested at the end of isoflurane exposure12; in their in vivo study, 5- to 8-month-old mice were treated with 1.4% isoflurane for 2 hours, and whole brain tissues were harvested at 6, 12, and 24 hours after isoflurane anesthesia.12 By conducting Western blot analysis, they showed that isoflurane exposure increases the level of phosphotau at the Ser262 site in both primary neurons and whole brain tissues.12 They also found that isoflurane-induced tau phosphorylation is mediated by caspase activation and Aβ generation.12 To further investigate the relationship between hippocampus-dependent cognitive deficits and hippocampal tau phosphorylation after isoflurane anesthesia in aged animals, we determined whether isoflurane exposure alters tau phosphorylation in the hippocampus of 12-month-old mice. We found that isoflurane exposure time dependently increases hippocampal tau phosphorylation at the Ser262 site. Compared with WT mice, transgenic AD mice (APP695 mice) displayed greater enhancement of hippocampal tau phosphorylation after isoflurane exposure. Our results suggest that isoflurane-induced cognitive dysfunction may be correlated with phosphorylation of hippocampal tau protein at the Ser262 site. Because of its role in tau phosphorylation at the Ser262 site, the effect of isoflurane exposure on the pathology of AD should be studied in the future.
In conclusion, our study demonstrates that isoflurane may induce cognitive dysfunction by enhancing phosphorylation of hippocampal tau protein at the Ser262 site and this effect is more significant in transgenic APP695 mice. Tau phosphorylation at the Ser262 site has been illustrated to prevent tau from binding and stabilizing microtubules, which is associated with increasing severity of neuronal cytopathology in AD.41–43 Therefore, hippocampal tau phosphorylation might be involved in the molecular mechanisms by which isoflurane anesthesia impairs cognitive function and promotes AD development, and isoflurane might be a deleterious factor contributing to the neuropathogenesis of AD.
Supplementary Material
ACKNOWLEDGMENTS
The authors are grateful to the invaluable assistance and insight from Professor Haiyan Yang (Department of Epidemiology and Biostatistics, Zhengzhou University College of Public Health, Zhengzhou, Henan, China) with regard to statistical analysis.
Funding: This work is made possible by the grant from National Natural Science Fund of China 30940025 to Dr. Xing and National Institutes of Health grant R01DE022880 to Dr. Tao.
Footnotes
The authors declare no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (http://www.anesthesia-analgesia.org).
DISCLOSURES
Name: Changsheng Li, MD.
Contribution: This author helped design and conduct the study, analyze the data, and write the manuscript.
Attestation: Changsheng Li has seen the original study data, reviewed the analysis of the data, and approved the final manuscript.
Name: Sufang Liu, MD.
Contribution: This author helped design and conduct the study, analyze the data, and write the manuscript.
Attestation: Sufang Liu has seen the original study data, reviewed the analysis of the data, and approved the final manuscript.
Name: Ying Xing, MD, PhD.
Contribution: This author helped design the study, analyze the data, and write the manuscript.
Attestation: Ying Xing has seen the original study data, reviewed the analysis of the data, and approved the final manuscript.
Name: Feng Tao, MD, PhD.
Contribution: This author helped design the study, analyze the data, and write the manuscript.
Attestation: Feng Tao has seen the original study data, reviewed the analysis of the data, approved the final manuscript, and is the author responsible for archiving the study files.
This manuscript was handled by: Gregory J. Crosby, MD.
References
- 1.Jildenstål PK, Hallén JL, Rawal N, Gupta A, Berggren L. Effect of auditory evoked potential-guided anaesthesia on consumption of anaesthetics and early postoperative cognitive dysfunction: a randomised controlled trial. Eur J Anaesthesiol. 2011;28:213–219. doi: 10.1097/EJA.0b013e328340dbb9. [DOI] [PubMed] [Google Scholar]
- 2.Iselin-Chaves IA, Willems SJ, Jermann FC, Forster A, Adam SR, Van der Linden M. Investigation of implicit memory during isoflurane anesthesia for elective surgery using the process dissociation procedure. Anesthesiology. 2005;103:925–933. doi: 10.1097/00000542-200511000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Carr ZJ, Torjman MC, Manu K, Dy G, Goldberg ME. Spatial memory using active allothetic place avoidance in adult rats after isoflurane anesthesia: a potential model for postoperative cognitive dysfunction. J Neurosurg Anesthesiol. 2011;23:138–145. doi: 10.1097/ANA.0b013e3182049f19. [DOI] [PubMed] [Google Scholar]
- 4.Su D, Zhao Y, Wang B, Xu H, Li W, Chen J, Wang X. Isoflurane-induced spatial memory impairment in mice is prevented by the acetylcholinesterase inhibitor donepezil. PLoS One. 2011;6:e27632. doi: 10.1371/journal.pone.0027632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang H, Xu Z, Feng C, Wang Y, Jia X, Wu A, Yue Y. Changes of learning and memory in aged rats after isoflurane inhalational anaesthesia correlated with hippocampal acetylcholine level. Ann Fr Anesth Reanim. 2012;31:e61–e66. doi: 10.1016/j.annfar.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Callaway JK, Jones NC, Royse CF. Isoflurane induces cognitive deficits in the Morris water maze task in rats. Eur J Anaesthesiol. 2012;29:239–245. doi: 10.1097/EJA.0b013e32835103c1. [DOI] [PubMed] [Google Scholar]
- 7.Lin D, Zuo Z. Isoflurane induces hippocampal cell injury and cognitive impairments in adult rats. Neuropharmacology. 2011;61:1354–1359. doi: 10.1016/j.neuropharm.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eckenhoff RG, Johansson JS, Wei H, Carnini A, Kang B, Wei W, Pidikiti R, Keller JM, Eckenhoff MF. Inhaled anesthetic enhancement of amyloid-beta oligomerization and cytotoxicity. Anesthesiology. 2004;101:703–709. doi: 10.1097/00000542-200409000-00019. [DOI] [PubMed] [Google Scholar]
- 9.Xie Z, Dong Y, Maeda U, Alfille P, Culley DJ, Crosby G, Tanzi RE. The common inhalation anesthetic isoflurane induces apoptosis and increases amyloid beta protein levels. Anesthesiology. 2006;104:988–994. doi: 10.1097/00000542-200605000-00015. [DOI] [PubMed] [Google Scholar]
- 10.Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology. 1992;42:631–639. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- 11.Mattson MP. Pathways towards and away from Alzheimer’s disease. Nature. 2004;430:631–639. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong Y, Wu X, Xu Z, Zhang Y, Xie Z. Anesthetic isoflurane increases phosphorylated tau levels mediated by caspase activation and Aβ generation. PLoS One. 2012;7:e39386. doi: 10.1371/journal.pone.0039386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Le Freche H, Brouillette J, Fernandez-Gomez FJ, Patin P, Caillierez R, Zommer N, Sergeant N, Buée-Scherrer V, Lebuffe G, Blum D, Buée L. Tau phosphorylation and sevoflurane anesthesia: an association to postoperative cognitive impairment. Anesthesiology. 2012;116:779–787. doi: 10.1097/ALN.0b013e31824be8c7. [DOI] [PubMed] [Google Scholar]
- 14.Whittington RA, Virág L, Marcouiller F, Papon MA, El Khoury NB, Julien C, Morin F, Emala CW, Planel E. Propofol directly increases tau phosphorylation. PLoS One. 2011;6:e16648. doi: 10.1371/journal.pone.0016648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buée L, Bussière T, Buée-Scherrer V, Delacourte A, Hof PR. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res Brain Res Rev. 2000;33:95–130. doi: 10.1016/s0165-0173(00)00019-9. [DOI] [PubMed] [Google Scholar]
- 16.Trojanowski JQ, Lee VM. Paired helical filament tau in Alzheimer’s disease. The kinase connection. Am J Pathol. 1994;144:449–453. [PMC free article] [PubMed] [Google Scholar]
- 17.Games D, Adams D, Alessandrini R, Barbour R, Berthelette P, Blackwell C, Carr T, Clemens J, Donaldson T, Gillespie F. Alzheimer-type neuropathology in transgenic mice overexpressing V717F beta-amyloid precursor protein. Nature. 1995;373:523–527. doi: 10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- 18.Czech C, Masters C, Beyreuther K. Alzheimer’s disease and transgenic mice. J Neural Transm Suppl. 1994;44:219–230. doi: 10.1007/978-3-7091-9350-1_17. [DOI] [PubMed] [Google Scholar]
- 19.Engelhardt T, Lowe PR, Galley HF, Webster NR. Inhibition of neuronal nitric oxide synthase reduces isoflurane MAC and motor activity even in nNOS knockout mice. Br J Anaesth. 2006;96:361–366. doi: 10.1093/bja/ael010. [DOI] [PubMed] [Google Scholar]
- 20.Tao F, Skinner J, Yang Y, Johns RA. Effect of PSD-95/SAP90 and/ or PSD-93/chapsyn-110 deficiency on the minimum alveolar anesthetic concentration of halothane in mice. Anesthesiology. 2010;112:1444–1451. doi: 10.1097/ALN.0b013e3181dcd3dc. [DOI] [PubMed] [Google Scholar]
- 21.Gallagher JJ, Minogue AM, Lynch MA. Impaired performance of female APP/PS1 mice in the Morris water maze is coupled with increased Aβ accumulation and microglial activation. Neurodegener Dis. 2013;11:33–41. doi: 10.1159/000337458. [DOI] [PubMed] [Google Scholar]
- 22.Tan W, Cao X, Wang J, Lv H, Wu B, Ma H. Tau hyperphosphorylation is associated with memory impairment after exposure to 1.5% isoflurane without temperature maintenance in rats. Eur J Anaesthesiol. 2010;27:835–841. doi: 10.1097/EJA.0b013e32833a6561. [DOI] [PubMed] [Google Scholar]
- 23.Bianchi SL, Caltagarone BM, Laferla FM, Eckenhoff RG, Kelz MB. Inhaled anesthetic potency in aged Alzheimer mice. Anesth Analg. 2010;110:427–430. doi: 10.1213/ANE.0b013e3181b5a292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eckel B, Richtsfeld M, Starker L, Blobner M. Transgenic Alzheimer mice have a larger minimum alveolar anesthetic concentration of isoflurane than their nontransgenic littermates. Anesth Analg. 2010;110:438–441. doi: 10.1213/ANE.0b013e3181b76383. [DOI] [PubMed] [Google Scholar]
- 25.Texidó L, Martín-Satué M, Alberdi E, Solsona C, Matute C. Amyloid β peptide oligomers directly activate NMDA receptors. Cell Calcium. 2011;49:184–190. doi: 10.1016/j.ceca.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 26.Talantova M, Sanz-Blasco S, Zhang X, Xia P, Akhtar MW, Okamoto S, Dziewczapolski G, Nakamura T, Cao G, Pratt AE, Kang YJ, Tu S, Molokanova E, McKercher SR, Hires SA, Sason H, Stouffer DG, Buczynski MW, Solomon JP, Michael S, Powers ET, Kelly JW, Roberts A, Tong G, Fang-Newmeyer T, Parker J, Holland EA, Zhang D, Nakanishi N, Chen HS, Wolosker H, Wang Y, Parsons LH, Ambasudhan R, Masliah E, Heinemann SF, Piña-Crespo JC, Lipton SA. Aβ induces astrocytic glutamate release, extrasynaptic NMDA receptor activation, and synaptic loss. Proc Natl Acad Sci U S A. 2013;110:E2518–E2527. doi: 10.1073/pnas.1306832110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bianchi SL, Tran T, Liu C, Lin S, Li Y, Keller JM, Eckenhoff RG, Eckenhoff MF. Brain and behavior changes in 12-monthold Tg2576 and nontransgenic mice exposed to anesthetics. Neurobiol Aging. 2008;29:1002–1010. doi: 10.1016/j.neurobiolaging.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rammes G, Starker LK, Haseneder R, Berkmann J, Plack A, Zieglgänsberger W, Ohl F, Kochs EF, Blobner M. Isoflurane anaesthesia reversibly improves cognitive function and longterm potentiation (LTP) via an up-regulation in NMDA receptor 2B subunit expression. Neuropharmacology. 2009;56:626–636. doi: 10.1016/j.neuropharm.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 29.Su D, Zhao Y, Wang B, Li W, Xiao J, Chen J, Wang X. Repeated but not single isoflurane exposure improved the spatial memory of young adult mice. Acta Anaesthesiol Scand. 2011;55:468–473. doi: 10.1111/j.1399-6576.2010.02385.x. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Xu Z, Wang H, Dong Y, Shi HN, Culley DJ, Crosby G, Marcantonio ER, Tanzi RE, Xie Z. Anesthetics isoflurane and desflurane differently affect mitochondrial function, learning, and memory. Ann Neurol. 2012;71:687–698. doi: 10.1002/ana.23536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Culley DJ, Baxter MG, Crosby CA, Yukhananov R, Crosby G. Impaired acquisition of spatial memory 2 weeks after isoflurane and isoflurane-nitrous oxide anesthesia in aged rats. Anesth Analg. 2004;99:1393–1397. doi: 10.1213/01.ANE.0000135408.14319.CC. [DOI] [PubMed] [Google Scholar]
- 32.Crosby C, Culley DJ, Baxter MG, Yukhananov R, Crosby G. Spatial memory performance 2 weeks after general anesthesia in adult rats. Anesth Analg. 2005;101:1389–1392. doi: 10.1213/01.ANE.0000180835.72669.AD. [DOI] [PubMed] [Google Scholar]
- 33.Weingarten MD, Lockwood AH, Hwo SY, Kirschner MW. A protein factor essential for microtubule assembly. Proc Natl Acad Sci U S A. 1975;72:1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boekhoorn K, Terwel D, Biemans B, Borghgraef P, Wiegert O, Ramakers GJ, de Vos K, Krugers H, Tomiyama T, Mori H, Joels M, van Leuven F, Lucassen PJ. Improved long-term potentiation and memory in young tau-P301L transgenic mice before onset of hyperphosphorylation and tauopathy. J Neurosci. 2006;26:3514–3523. doi: 10.1523/JNEUROSCI.5425-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI. Abnormal phosphorylation of the microtubuleassociated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci U S A. 1986;83:4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Binder LI, Guillozet-Bongaarts AL, Garcia-Sierra F, Berry RW. Tau, tangles, and Alzheimer’s disease. Biochim Biophys Acta. 2005;1739:216–223. doi: 10.1016/j.bbadis.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 37.Lee VM, Goedert M, Trojanowski JQ. Neurodegenerative tauopathies. Annu Rev Neurosci. 2001;24:1121–1159. doi: 10.1146/annurev.neuro.24.1.1121. [DOI] [PubMed] [Google Scholar]
- 38.Delacourte A, David JP, Sergeant N, Buée L, Wattez A, Vermersch P, Ghozali F, Fallet-Bianco C, Pasquier F, Lebert F, Petit H, Di Menza C. The biochemical pathway of neurofibrillary degeneration in aging and Alzheimer’s disease. Neurology. 1999;52:1158–1165. doi: 10.1212/wnl.52.6.1158. [DOI] [PubMed] [Google Scholar]
- 39.Morishima-Kawashima M, Hasegawa M, Takio K, Suzuki M, Yoshida H, Titani K, Ihara Y. Proline-directed and nonproline- directed phosphorylation of PHF-tau. J Biol Chem. 1995;270:823–829. doi: 10.1074/jbc.270.2.823. [DOI] [PubMed] [Google Scholar]
- 40.Pérez M, Ribe E, Rubio A, Lim F, Morán MA, Ramos PG, Ferrer I, Isla MT, Avila J. Characterization of a double (amyloid precursor protein-tau) transgenic: tau phosphorylation and aggregation. Neuroscience. 2005;130:339–347. doi: 10.1016/j.neuroscience.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 41.Fischer D, Mukrasch MD, Biernat J, Bibow S, Blackledge M, Griesinger C, Mandelkow E, Zweckstetter M. Conformational changes specific for pseudophosphorylation at serine 262 selectively impair binding of tau to microtubules. Biochemistry. 2009;48:10047–10055. doi: 10.1021/bi901090m. [DOI] [PubMed] [Google Scholar]
- 42.Lauckner J, Frey P, Geula C. Comparative distribution of tau phosphorylated at Ser262 in pre-tangles and tangles. Neurobiol Aging. 2003;24:767–776. doi: 10.1016/s0197-4580(02)00228-2. [DOI] [PubMed] [Google Scholar]
- 43.Alonso AD, Di Clerico J, Li B, Corbo CP, Alaniz ME, Grundke-Iqbal I, Iqbal K. Phosphorylation of tau at Thr212, Thr231, and Ser262 combined causes neurodegeneration. J Biol Chem. 2010;285:30851–30860. doi: 10.1074/jbc.M110.110957. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.