Abstract
The dramatic cardiovascular mortality of chronic kidney disease patients is attributable in a significant proportion to endothelial dysfunction. Cyanate, a reactive species in equilibrium with urea, is formed in excess in chronic kidney disease. Cyanate is thought to have a causal role in promoting cardiovascular disease, but the underlying mechanisms remain unclear. Immunohistochemical analysis performed in the present study revealed that carbamylated epitopes associate mainly with endothelial cells in human atherosclerotic lesions. Cyanate treatment of human coronary artery endothelial cells reduced expression of endothelial nitric oxide synthase and increased tissue factor and plasminogen activator inhibitor-1 expression. In mice, administration of cyanate - promoting protein carbamylation at levels observed in uremic patients - attenuated arterial vasorelaxation of aortic rings in response to acetylcholine, without affecting sodium nitroprusside-induced relaxation. Total endothelial nitric oxide synthase and nitric oxide production were significantly reduced in aortic tissue of cyanate-treated mice. This coincided with a marked increase of tissue factor and plasminogen activator inhibitor-1 protein levels in aortas of cyanate-treated mice. These data provide evidence that cyanate compromises endothelial functionality in vitro and in vivo and may contribute to the dramatic cardiovascular risk of patients suffering from chronic kidney disease.
Keywords: renal disease, cyanate, endothelial nitric oxide synthase, tissue factor, plasminogen activator inhibitor-1
Introduction
Cardiac mortality of end-stage renal disease patients is several-fold increased in comparison to the general population (1, 2). However, the precise nature of the inflammatory and/or oxidative pathways involved remains unclear. As renal failure progresses, compounds accumulate in blood and tissues due to a decline in renal function. Several studies have postulated that chronic renal failure associated atherosclerosis and endothelial dysfunction result from accumulation of certain ‘uremic factors,’ the identities of which are still a matter of debate (3). A number of retention solutes may contribute to vascular damage in uremia, including urea, complement peptides, cytokines, phosphate, oxalate, para-cresol (4, 5) but may also originate from colonic microbial metabolism (6). Several lines of evidence suggest that break-down or oxidative modification of retained uremic solutes may potentiate their pathogenicity. While urea itself is innoxious, many molecules can be carbamylated through cyanate, a reactive decomposition product of urea. Due to the reactive nature of cyanate, its formation is best assessed by measuring serum levels of protein bound homocitrulline, a footprint of cyanate formation and protein carbamylation (7-10). Of particular interest, plasma protein bound homocitrulline levels were recently demonstrated to predict increased cardiovascular risk in patients with kidney failure (10, 11). Importantly, protein carbamylation also predicts cardiovascular risk in non-uremic subjects, given that cyanate is also generated via peroxidase-catalyzed oxidation of thiocyanate (7, 9). Urea levels may increase up to 100 mM in patients with renal failure and about 0.8 % of urea auto-decompose into cyanate (9, 12-15). In addition, myeloperoxidase-catalyzed oxidation of thiocyanate and myeloperoxidase-induced accelerated decomposition of urea may generate locally high cyanate levels (7, 9). Given that leukocyte-derived myeloperoxidase associates with endothelial cells, markedly increased formation of cyanate in close vicinity to endothelial cells is anticipated.
A key step and one of the earliest changes in the development of atherosclerosis is endothelial dysfunction, which is triggered – at least in part – by reactive metabolites leading to initiation and/or perpetuation of the inflammatory response. Here we assessed whether cyanate affects anti-atherogenic properties of endothelial cells and whether this mechanism is relevant under in vivo conditions.
Results
Carbamylated epitopes are mainly associated with endothelial cells in human atherosclerotic lesions
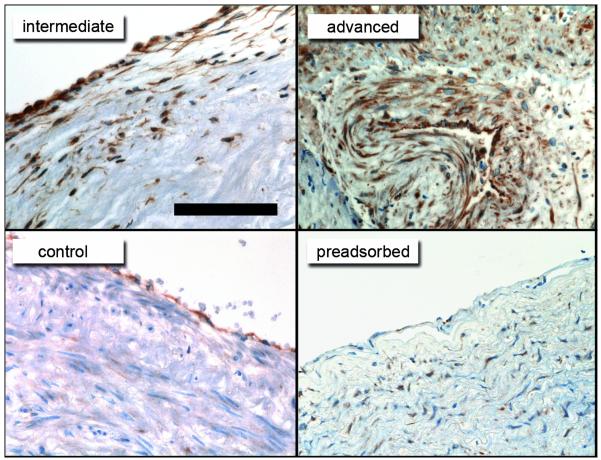
Augmented decomposition of urea/thiocyanate by myeloperoxidase is expected to generate high concentrations of cyanate in close vicinity to endothelial cells. In line with this assumption, we observed that homocitrulline-containing epitopes (carbamylated epitopes) are mainly associated with endothelial cells in intermediate human atherosclerotic lesions, whereas intense staining in several regions of atherosclerotic tissue was observed in advanced lesions (Figure 1). Control tissue showed weak staining mainly associated with endothelial cells. Interestingly, the immunohistochemical analysis was performed in atherosclerotic tissue of non-uremic subjects since tissue of uremic subjects was not available to us, suggesting that endothelial cells may be exposed to increased levels of cyanate also under conditions of normal urea levels. Pre-incubation of the anti-HCit antibody with carbamylated albumin almost completely abolished staining (Figure 1) and incubation with control non-immune IgG revealed no staining (Supplemental Figure 1)
Figure 1. Immunostaining for carbamylated proteins in human atherosclerotic tissue.
Reddish-brown immunostaining indicates accumulation of carbamylated epitopes detected with an anti-HCit antibody mainly within or around the endothelial lining of human control tissue and intermediate atherosclerotic lesions. Intense staining was observed in all regions of advanced atherosclerotic lesions. Pre-incubation of the anti-HCit antibody with carbamylated albumin (pre-adsorbed) almost completely abolished staining. Scale bar represents 100μm.
Cyanate decreases endothelial nitric oxide synthase (eNOS) protein expression in human coronary artery endothelial cells (HCAECs)
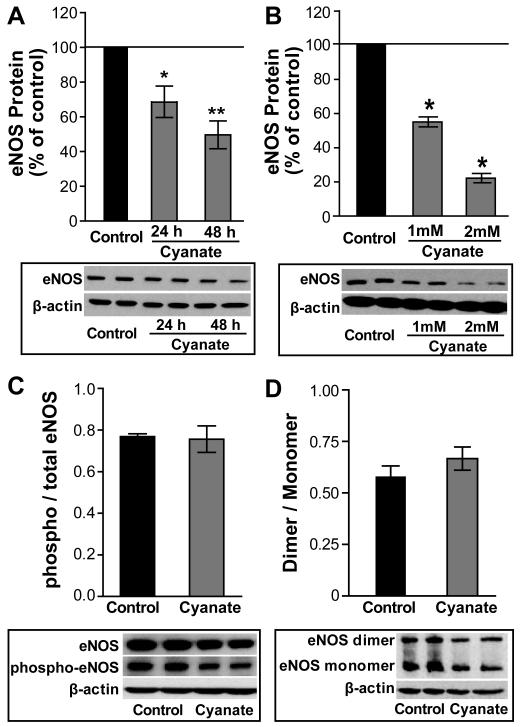
A key atheroprotective function of the vascular endothelium is the production of the vasorelaxing compound nitric oxide. Treatment of HCAECs with cyanate markedly decreased eNOS protein expression in a time-dependent and dose-dependent manner (Figure 2A, 2B) but did not affect cell viability (Supplemental Figure 2). Activity of eNOS is regulated by phosphorylation and dimerization. Exposure of HCAECs to cyanate significantly decreased total eNOS and phospho-eNOS in a similar fashion (Figure 2C). Dimer/monomer ratio of eNOS was not altered in endothelial cells upon cyanate exposure (Figure 2D).
Figure 2. Cyanate alters endothelial nitric oxide synthase (eNOS) protein in endothelial cells.
HCAECs were treated with sodium cyanate (1 or 2 mM) for 24h or 48 h. Cyanate elicits a (A) time-dependent and (B) dose-dependent decrease in eNOS protein expression. (C) Decreased phospho-eNOS in lysates from cyanate-treated cells (1 mM, 48 h) with quantification of phospho / total eNOS levels. (D) eNOS dimerization with quantification of eNOS dimer/monomer levels in lysates from cyanate-treated cells (1 mM, 48 h). Representative Western blots from HCAECs are shown. β-actin was used to normalize the data for equal protein loading and quantification of bands was done using ImageJ software. Data are expressed as mean ± SEM (n= 3-4). Statistical analysis was performed by One-Way ANOVA for more than two groups and with Student’s t-test for two groups. Significance was accepted at * p <0.05, ** p <0.01, versus control.
Cyanate decreases eNOS protein expression on the mRNA level
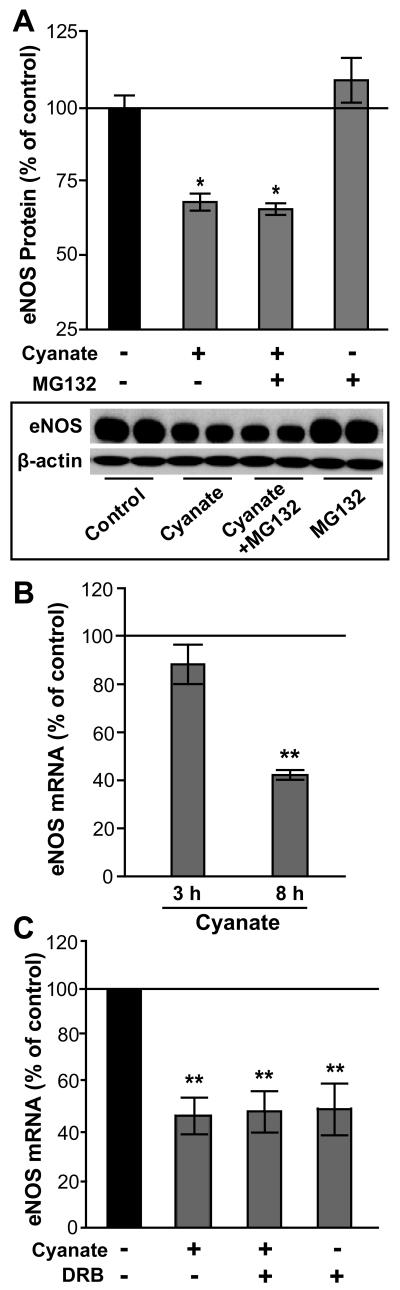
Cyanate might decrease eNOS protein levels via the ubiquitin-proteasome pathway. However, experiments performed in the presence of the proteasome inhibitor MG132 did not alter cyanate mediated effects (Figure 3A), suggesting that increased degradation via the ubiquitin-proteasome pathway was not involved. In addition, we observed a marked reduction in eNOS mRNA levels after 8 hours of cyanate exposure (Figure 3B), similar to that elicited by the transcriptional elongation inhibitor 5,6-dichloro-1-β-D-ribofuranosylbenzimidazole (Figure 3C), suggesting that cyanate inhibits eNOS expression on the transcriptional level and/or decreases the mRNA stability of eNOS.
Figure 3. Cyanate decreases eNOS at the transcriptional level in endothelial cells.
(A) HCAECs treated with cyanate (1 mM, 24 h) in the presence or absence of a proteasome inhibitor (MG132, 50 nM). A representative Western blot is shown. Blots were analyzed with ImageJ software and normalized to β-actin. (B) HCAECs were treated with 1 mM cyanate for 3 or 8 h and eNOS mRNA analyzed by qRT-PCR. (C) HCAECs were treated with cyanate (1 mM, 8 h) in the absence or presence of a transcriptional inhibitor; 5,6-dichloro-1-β-D-ribofuranosylbenzimidazole (DRB; 50 μM). Subsequently, eNOS mRNA expression was determined by qRT-PCR. Data are expressed as mean ± SEM (n = 3). Statistical analysis was performed by One-Way ANOVA. Significance was accepted at * p <0.05, versus control.
Cyanate promotes a prothrombogenic endothelial phenotype
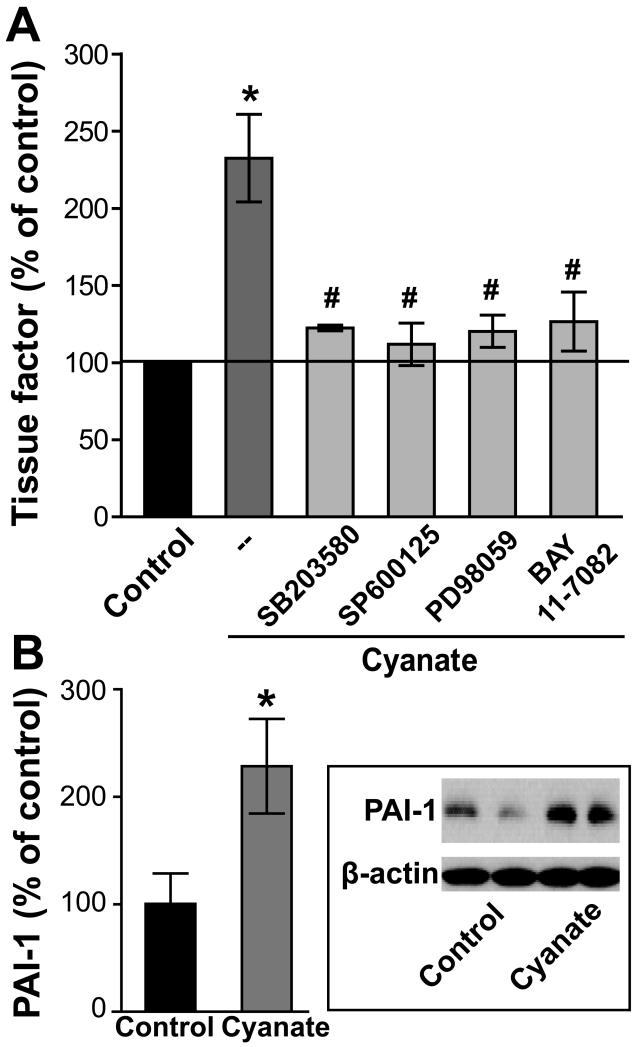
Tissue factor is critical to a range of pathological processes including atherosclerosis, coagulation and neointimal hyperplasia (16, 17). Tissue factor is the key initiator of the coagulation cascade; it binds factor VIIa resulting in activation of factor IX and factor X, ultimately leading to fibrin formation. Despite their diversity, most of these mediators share similar signal transduction pathways regulating tissue factor induction via the MAP kinases p38 MAPK, JNK, ERK and NF-κB (16, 17). Of particular interest, treatment of HCAECs with cyanate markedly promoted tissue factor expression as assessed by flow cytometric analyses (Figure 4A). The up-regulation of tissue factor elicited by cyanate was markedly attenuated by specific inhibitors of p38 MAPK, JNK, ERK and NF-κB (SB203580, SP600125, PD98059, and BAY 11-7082, respectively) (Figure 4A).
Figure 4. Increased expression of tissue factor and plasminogen activator inhibitor-1 (PAI-1) in cyanate-stimulated endothelial cells.
(A) Cyanate-treated HCAECs (1 mM, 4 h) show an increased expression of tissue factor, which is recovered in the presence of specific MAPK family member inhibitors (p38 MAPK inhibitor [SB203580; 5 μM] - JNK-2 inhibitor [SP600125, 5 μM] - ERK1/2 inhibitor [PD98059; 10 μM]) or the NF-kB inhibitor (BAY 11-7082; 5 μM). Tissue factor expression was determined by flow cytometry as described in Methods. Control was set at 100% and values are expressed as % of control. Results are shown as mean ± SEM (n= 4-5). Statistical analysis was performed by One-way ANOVA. Significance was accepted at * p <0.05 versus control, # p <0.05 versus cyanate-treated cells. (B) HCAECs showing enhanced PAI-1 expression after cyanate treatment (1 mM, 24 h). A representative Western blot is shown. Blots were analyzed with ImageJ software and normalized to β-actin. Data expressed as mean ± SEM (n=3). Student’s t-test was used for comparison. Significance was accepted at * p <0.05 versus control.
Plasminogen activator inhibitor-1 (PAI-1) is a multifunctional protein expressed mainly by endothelial cells with the ability to regulate fibrinolysis through inhibition of plasminogen activation. Elevated plasma levels of PAI-1 have been shown to have profound effects on the development and progression of cardiovascular disease (18). Interestingly, cyanate effectively induced endothelial PAI-1 expression (Figure 4B) in HCAECs, suggesting that cyanate promotes a prothrombogenic endothelial phenotype.
Cyanate, rather than carbamylated (lipo)proteins, modulates eNOS and tissue factor expression
Given that our experiments were performed in the presence of serum, cyanate-driven carbamylation of (lipo)proteins may have contributed to the effects observed. To test whether carbamylated (lipo)proteins alter eNOS, tissue factor and PAI-1 expression in our experiments, serum-containing cell culture medium was incubated with cyanate (1mM) for 48 hours in the absence of cells to induce protein carbamylation. After 48 hours homocitrulline formation was saturated (Supplemental Figure 3), indicating that a large fraction of cyanate was consumed by serum proteins. To remove residual cyanate, low molecular-weight substances (i.e., cyanate) were removed by dialysis (molecular-weight cut off of 3000 Da). Subsequently, HCAECs were treated with dialysed preconditioned medium. Of note, preconditioned medium failed to alter eNOS and tissue factor expression, whereas addition of cyanate to preconditioned medium was effective (Supplemental Figure 4). Only PAI-1 expression was induced (albeit to a lower extent) by preconditioned medium. These data suggest that cyanate, rather than carbamylated proteins affects eNOS and tissue factor expression, whereas carbamylated proteins may also induce PAI-1 expression.
Cyanate inhalation of mice promotes protein carbamylation
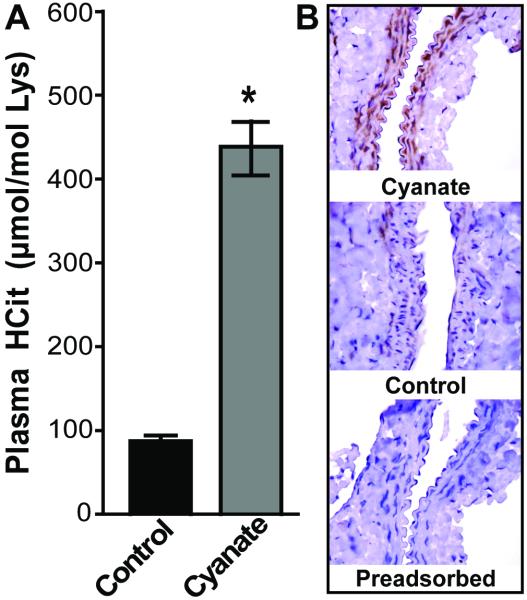
Prompted by the marked effects of cyanate on endothelial functionality, we next assessed whether cyanate impacts endothelial functionality in vivo. For that purpose, five week old male wild-type C57BL/6 mice were exposed to cyanate or vehicle inhalation for a period of 3 weeks. As a marker of cyanate formation and increased cyanate exposure, protein-associated HCit levels were assessed by mass spectrometric analysis of hydrolyzed plasma proteins. Cyanate treatment resulted in an approximately four-fold increase in plasma HCit levels (Figure 5A), reaching levels previously observed in patients with renal (8, 10, 11) and cardiovascular disease (7, 9). Aortas of cyanate-treated mice showed marked immunostaining for carbamylated epitopes (Figure 5B).
Figure 5. Cyanate administration induces protein carbamylation in mice.
(A) Increased plasma levels of homocitrulline (HCit) in cyanate-treated mice. Plasma levels of HCit in control and cyanate-treated mice were quantified by LC-MS/MS and expressed as μmol HCit per mol lysine (Lys). Results are shown as mean ± SEM (n = 8). Student’s t-test was used for comparison. Significance was accepted at * p <0.05 versus control. (B) Aortas of cyanate-treated mice show increased cyanate-induced protein carbamylation. Sections of paraffin-embedded aortas were stained with an anti-HCit antibody. In contrast to control mice, mice receiving cyanate showed marked staining of carbamylated epitopes in the descending aorta, whereas preadsorption of the anti HCit antibody with carbamylated albumin abolished staining. Positive immunohistochemical staining is indicated by a reddish-brown immunoreaction product. Images are representatives of the treatment groups (n=5). (Magnification 40x)
Cyanate impacts vascular reactivity in vivo
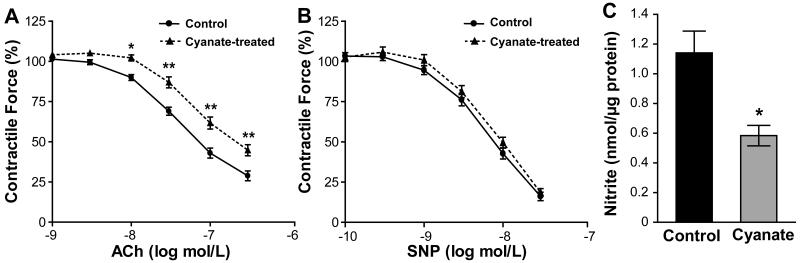
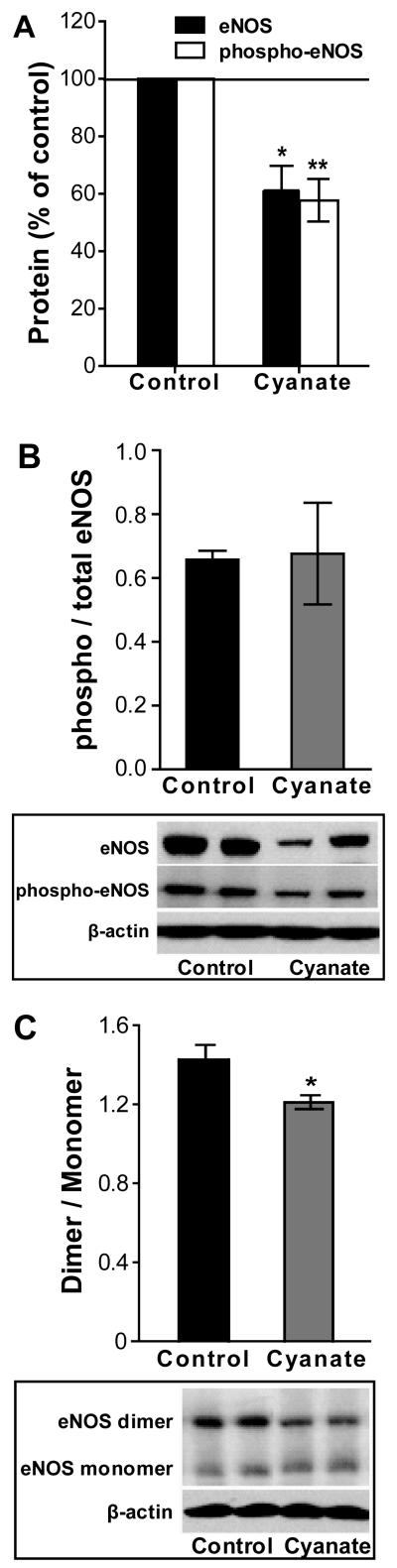
To assess whether cyanate impacts vascular reactivity, aortic rings from cyanate- and vehicle-treated control mice were exposed to increasing concentrations of acetylcholine (ACh). Compared with control mice, ACh-induced vasorelaxation was attenuated in aortic rings from cyanate-treated mice at all applied ACh concentrations (Figure 6A). EC50 values were found to be 84 nM (74 – 96, 95% confidence intervals) for control and 192 nM (157 – 234, 95% confidence intervals) for cyanate-treated mouse aortic tissues, respectively. In contrast, no significant difference in relaxation was observed in response to the nitric oxide (NO)-donor sodium nitroprusside (SNP) (Figure 6B). Of note, the contractile response of aortic smooth muscles to 60 mM KCl and norepinephrine was unaltered in cyanate-treated mice when compared to the control group (Supplemental Figure 5A). These findings suggest that a decreased production of endothelium-derived vasodilator substance(s) is responsible for the impaired vasorelaxation observed in cyanate-treated mice. In line with this result, we observed that the impaired vasorelaxation of aortic rings of cyanate-treated animals was accompanied by markedly lower nitrite levels (indicative of NO production) released from aortic rings, when compared with control mice (Figure 6C). Pre-incubation of aortic rings of control mice with an eNOS inhibitor (L-NNA; 200 μM) almost completely abolished the vasorelaxation to ACh (Supplemental Figure 5B), suggesting that NO is the predominant mediator of ACh-induced relaxation in mouse aortic rings. Based on the decreased nitrite release from cyanate-treated rings (Figure 6C) and the principal role of NO in aortic vasorelaxation (Supplemental Figure 5B), we assume that the inhibitory effect(s) of cyanate on eNOS protein accounts for the impaired vasorelaxation. In support with this assumption, immunoblot analysis revealed decreased total- and phospho-eNOS (S1177) protein levels in aortas of cyanate-treated mice when compared to control mice (Figure 7A). However, the ratio of phospho-eNOS/eNOS remained unaltered (Figure 7B) suggesting that the decreased protein expression of eNOS and the modest decrease in dimer/monomer ratio (Figure 7C) account for the decreased NO production in aortas of cyanate-treated animals.
Figure 6. Cyanate-induced impairment of vascular function is accompanied by decreased nitric oxide production.
To test vascular function in cyanate-treated mice, aortic rings were pre-constricted with norepinephrine, and relaxant responses to “endothelium-dependent” acetylcholine (ACh) and “endothelium-independent” sodium nitroprusside (SNP) vasodilating agents were measured. (A) Vasorelaxation in response to ACh was markedly attenuated in cyanate-treated mice. EC50 values were found to be 84 nM for control and 192 nM for cyanate-treated mouse aortic tissues. (B) Vasorelaxation in response to SNP (nitric oxide-donor) indicated no “endothelium-independent” difference between treatment groups. Data are expressed as mean ± SEM (n=7-9). Two-Way ANOVA for repeated measurements was used for comparison. Significance was accepted at * p < 0.05, ** p <0.01 versus control. (C) Total nitrite (indicative of nitric oxide production) was quantified via HPLC in aliquots of the bath solution taken after ACh-induced aortic ring relaxation of mouse aortic rings. Data are expressed as mean ± SEM (n=7-9). Student’s t-test was used for comparison between treatment groups. Significance was accepted at * p < 0.05, ** p <0.01 versus control.
Figure 7. Cyanate exposure decreases eNOS in mouse aorta.
(A) Western blot analysis of protein from mouse aorta probed with antibodies recognizing phospho-eNOS (S1177) and total eNOS protein with (B) quantification of phospho/total eNOS levels. (C) Western blot analysis showing eNOS dimers and monomers in mouse aorta. To detect eNOS dimers and monomers, low-temperature SDS-PAGE (4°C) was performed. Shown are representative Western blots, in which bands were normalized to β-actin for equal protein loading and quantified using ImageJ software. Data expressed as mean ± SEM (n=5-7). Student’s t-test was used for comparison between groups. Significance was accepted at * p <0.05 versus control.
Enhanced tissue factor and PAI-1 protein expression in aortas of cyanate-treated mice
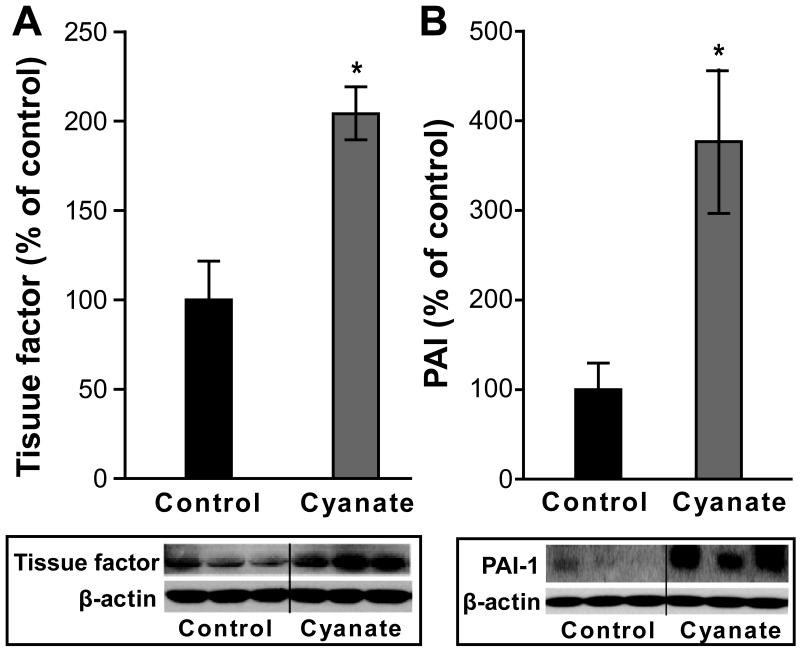
Prompted by our in vitro observation that cyanate induced tissue factor and PAI-1 expression in HCAECs, we were interested whether cyanate shows similar activities in vivo. Of particular interest, we observed markedly increased protein levels of tissue factor (Figure 8A) as well as PAI-1 (Figure 8B) in aortas of cyanate-treated mice by immunoblot analysis.
Figure 8. Cyanate exposure increases tissue factor and plasminogen activator inhibitor-1 (PAI-1) in mice.
Western blot analysis indicating enhanced expression of tissue factor (A) and PAI-1 (B) in aortas from cyanate-treated mice. Shown are representative Western blots, in which bands were normalized to β-actin for equal protein loading and quantified using ImageJ software. Data expressed as mean ± SEM (n=5). Student’s t-test was used for comparison between groups. Significance was accepted at * p < 0.05 versus control.
Discussion
Much of the pathophysiology seen in patients with chronic kidney disease is believed to be the result of increased inflammation and accumulation of uremic toxins (19, 20). It is of particular interest that the biochemical process of protein carbamylation resides at the nexus of both of these pathways, considering that leukocyte-derived myeloperoxidase and other peroxidases promotes cyanate formation through decomposition of thiocyanate and urea that accumulate in uremic patients (7, 9, 21). Consistently, carbamylated proteins are highly enriched in human atherosclerotic plaques, even in non-uremic subjects, as observed in the present and previous studies (7, 9, 15, 21). Recently, we could show that oral administration of cyanate up-regulates vascular ICAM-1 expression and that soluble ICAM-1 plasma levels in end-stage renal disease patients significantly correlated with plasma concentrations of plasma protein-bound HCit, a foot-print of cyanate formation and increased cyanate exposure (8). Most importantly, subsequent studies have clearly demonstrated that serum levels of carbamylated proteins predict mortality risk in chronic kidney disease patients (10, 11). Therefore, the localization of phagocytes in the immediate vicinity of endothelial cells at sites of inflammation may contribute to cyanate-induced endothelial activation.
The impressive cardiovascular mortality of chronic kidney disease patients is attributable in a significant proportion to endothelial dysfunction, arterial stiffness, and vascular calcifications (22). Reduced endothelial nitric oxide release and abnormal vascular reactivity occurs early on in the course of chronic kidney disease, but remains undiagnosed in the usual clinical setting (23-25). Nitric oxide is one of the most important vasodilating substances released by the endothelium that also inhibits inflammation, and shows anti-aggregatory effects on platelets. A reduction in nitric oxide bioavaibility has been reported to be a hallmark of impaired endothelial function (26-28). Since the vascular endothelium represents the largest organ in the body, decreased total nitric oxide production in renal disease likely reflects endothelial dysfunction. In the present study, we made the remarkable observation that cyanate suppressed endothelial nitric oxide synthase expression in a time- and concentration-dependent manner. Our results suggest that cyanate-induced inhibition of eNOS expression in HCAECs is mediated on the transcriptional level and/or by a decrease in mRNA stability, but not by increased degradation via the ubiquitin-proteasome pathway. In line with our in vitro results, administration of cyanate in mice decreased endothelial nitric oxide synthase expression in aortic vessels, accompanied by a significant reduction of vascular reactivity. Importantly, plasma levels of protein-bound HCit, a marker of cyanate exposure, reached levels in mice that we and others have observed in patients undergoing hemodialysis (8, 10), demonstrating that the plasma cyanate concentrations reached in our in vivo experiments are biologically relevant. Interestingly, our data suggest that cyanate, rather than carbamylated proteins, affects expression of endothelial nitric oxide synthase.
Increasing attention has been paid to the role of haemostatic factors in the pathogenesis of cardiovascular events in uremia (29). Although uremia has long been recognized as thrombogenic, the mechanisms remain poorly elucidated, thus limiting the ability to risk stratify and treat these patients effectively. Interestingly, renal insufficiency is independently associated with elevations in inflammatory and procoagulant biomarkers (30). These pathways may be important mediators leading to the increased cardiovascular risk of patients with kidney disease. Increased levels of tissue factor, the primary initiator of coagulation, and the serine protease inhibitor PAI-1 are considered key factors in haemostatic abnormalities. Of particular interest, impaired endogenous thrombolysis was recently identified as a novel risk factor in end-stage renal disease patients which was strongly associated with cardiovascular events (31). A main finding of our present study is that cyanate markedly up-regulates endothelial tissue factor and PAI-1 expression in vitro and in vivo, which could potentially contribute to the high incidence of thrombotic complications and atherosclerosis in chronic kidney disease (31).
Endothelial tissue factor is induced by cytokines such as tumor necrosis factor-α, interleukin-1β and by mediators such as thrombin, oxidized low-density lipoprotein, or vascular endothelial growth factor (16, 17). Despite their diversity, most of these mediators share similar signal transduction pathways regulating tissue factor induction via the mitogen activated protein kinases p38 JNK, ERK and nuclear factor-κB (16, 17). In line with those reports, up-regulation of tissue factor expression elicited by cyanate in our experiments was markedly attenuated by specific inhibitors of mitogen activated protein kinases and nuclear factor-κB.
We have assessed protein carbamylation in the media during the treatment conditions and observed that 1 mM cyanate induced carbamylation of about 0.4 % lysine residues, similar levels of carbamyllysine were recently observed in lipoproteins extracted from atherosclerotic tissue (9, 21). Interestingly, our in vitro data suggest that cyanate, rather than carbamylated proteins affects eNOS and tissue factor expression, whereas carbamylated proteins may also induce PAI-1 expression.
Summing up, the present findings provide previously unreported insights into the underlying mechanisms that contribute to the enhanced cardiovascular risk associated with chronic renal failure. Given that plasma levels of protein-bound HCit, which is a marker of the time-average concentration of cyanate in plasma, are independently associated with an increased mortality, interventions aimed at reducing levels of cyanate are potential promising approaches in reducing cardiovascular disease.
Material and Methods
An extended Materials and Methods Section can be found in the Supplementary Material
Western blot analysis
Denatured proteins were resolved on 4% – 20% SDS-PAGE reducing gels (Invitrogen, CA, USA), transferred to PVDF membranes (Bio-Rad Lab., CA, USA) and later probed with the corresponding primary antibodies: anti-eNOS (1:1000; BD Bioscience), anti-PAI-1 (1:1000; American Diagnostica Inc., CT, USA), and anti-β-actin (Abcam Cambridge, UK) in 5% fat-free milk or mouse anti-eNOS (pS1177) (1:1000; BD Bioscience) in 5% bovine serum albumin overnight at 4°C. Membranes were further incubated with HRP-conjugated anti-rabbit (1:10,000; Sigma) or anti-mouse IgG (1:1000; Thermo Fisher Scientific, IL, USA) for 2 hours at room temperature and protein bands were visualized with Immobilon™ Western chemiluminescence HRP substrate (Millipore Corp., MA, USA) according to the manufacturer’s instructions. When used for re-blotting, immunoblots were stripped with stripping buffer (Tris-HCl 62.5 mM, pH 6.9, 2% SDS, 2-mercapatoethanol 100 mM) at 50°C for 30 min. To determine eNOS dimer protein expression, samples were not denatured by heat, and low-temperature SDS-PAGE at 4°C (cold electrophoresis) under reducing conditions was performed as described previously (32, 33). Immunoblotting for eNOS dimmers with detection and visualization was continued as mentioned above. Quantification of bands was performed using ImageJ software (NIH) for all blots. Values were calculated as ratio of the protein of interest to β-actin.
Cell Culture
HCAEC were purchased from Lonza (Verviers, Belgium) and cultured in EGM-2 MV Bullet medium (Lonza) containing 5% FBS at 37°C in humidified 5% CO2. All experiments were performed without serum starvation. Endothelial cells from five different HCAEC donors were passaged at 80 – 90% confluence and were used within 4 passages for experiments. 200000 cells were plated on 6-well plates (Greiner, Germany) reaching 60 – 70 % confluency within 2 days. At the end of experiments (eg 48hr culture treatment) the cells reached 90% confluency (≈500,000 cells per well). Cyanate treatment did not alter cell growth.
Nitrite determination
As an indicator for NO production, nitrite was determined according to a previously described fluorometric HPLC method (34) utilizing the reaction of nitrite with 2,3-diaminonaphthalene. In brief, after the ACh-induced aortic ring relaxation, 100 μL of the bath solution was incubated at 24°C with 10 μL of 316 μM 2,3-diaminonaphthalene (in 0.62 M HCl) for 10 min, followed by addition of 10 μL NaOH (2.8 M). This reaction mixture was directly used for chromatographic separation (injection volume: 20 μL) of the formed 2, 3-naphthotriazole (NAT). Nitrite standards (range: 0 – 2 μM) were derivatized accordingly. NAT was isocratically separated on a 5-μm ODS hypersil column (150 × 4.6 mm) guarded by a 5-μm ODS hypersil column (10 × 4.6 mm; Uniguard holder) with a 30 mM sodium phosphate buffer (pH 7.5) containing 50% methanol (flow rate: 0.8 mL/min). Fluorescence was monitored at an excitation wavelength of 375 nm and an emission wavelength of 415 nm. The HPLC apparatus consisted of an L-2200 autosampler, L-2130 HTA pump and L-2480 fluorescence detector (VWR Hitachi, Tokyo, Japan). Detector signals were recorded with a personal computer. The program EZchrom Elite (Scientific Software Inc., San Ramon, CA USA) was used for data acquisition and analysis. The detection limit for nitrite was 10 pM. After collecting buffer for nitrite measurement, aortic rings were homogenized for protein quantification and nitrite levels were normalised to protein content of respective tissues.
Determination of tissue factor protein expression
Quiescent human coronary artery endothelial cells (HCAEC) were stimulated with cyanate (1mM) for 3 hours after which cells were harvested using an EDTA buffer (10 mM) and subsequently incubated with PE-conjugated antibody against tissue factor (anti-CD142, 1:30) or isotype control antibody at 4°C for 30 min. Cells were then rinsed, fixed and analyzed using a FACSCalibur flow cytometer (Becton-Dickinson, Mountain View, CA, USA).
In vivo studies
C57BL/6 mice (males, 20 – 22 g, 5 weeks old) were purchased from Charles River (Sulzfeld, Germany). Mice were housed in plastic sawdust floor cages under controlled temperature (22°C), humidity (40%) and a 12:12-hour light-dark cycle with access ad libitum to both food and water. Experimental protocols were approved by the Animal Care Committee of the Austrian State Department of Science and Research. To expose mice to cyanate, an inhalation model was selected based on a small pilot trial which revealed low mouse to mouse variability in plasma HCit levels and resulted in protein carbamylation levels similar to those previously observed in patients with cardiovascular (7) or renal (8) disease.
Mice were assigned equally to two groups and were exposed to either water (bi-distilled sterile water pH 7.4; control group) or cyanate aqueous solution (5 mg/mL sodium cyanate with pH adjusted to 7.4; cyanate-treated group). Treatment was delivered using a compressor-assisted nebulizer (PARI JuniorBOY® S, PARI GmbH, Starnberg, Germany) to generate fine aerosol particles with a mass median diameter of 2.9 μm at the air flow rate of 12 L/min according to the manufacturer. Nebulizations were delivered daily early in the morning over a duration of 2.5 hours as five consecutive 30-min aerosols (with short breaks in between). During exposure mice were kept in sealed chambers under laminar flow conditions and treatment continued for a period of 3 weeks after which the mice were deeply anesthetized with an intraperitoneal injection of ketamine (80 mg/kg BW) and xylazine (12 mg/kg BW) and the chest and peritoneal cavity were opened. Blood was collected by cardiac puncture and plasma was isolated and stored at −70°C for further analysis (HCit quantification).
Aortas were removed, cleaned of surrounding tissue under a dissection microscope and processed for all assays. For immunohistochemistry, the ascending aorta was isolated and fixed immediately in 4% paraformaldehyde, whereas descending thoracic aorta was either immediately used for vascular function studies or snap-frozen in liquid nitrogen and stored at −70°C for Western blot analysis in which thoracic aortas were homogenized in ice-cold RIPA buffer (Sigma) supplemented with a mixture of protease and phosphatase inhibitors (Roche Diagnostics). Tissue protein extraction was performed as described previously (35).
Plasma homocitrulline quantification
Free amino acids of plasma were removed by gel filtration and plasma proteins were hydrolyzed as described previously (36). As a measure for plasma protein carbamylation, homocitrulline was quantified by mass spectrometry as described previously (21).
Assessment of endothelium-dependent relaxation in aortic rings
Aortic rings approximately 2 mm in length were cut from murine descending thoracic aorta and the aortic rings were positioned in small wire myograph chambers (Danish MyoTechnology, Aarhus, Denmark), which contained a physiological salt solution (114 mM NaCl, 4.7 mM KCl, 0.8 mM KH2PO4, 1.2 mM MgCl2, 2.5 mM CaCl2, 25 mM NaHCO3 and 11 mM D-glucose pH 7.4) aerated with 5% CO2/95% O2 at 37°C. The myograph chambers were connected to force transducers for isometric tension recording (PowerLab, ADInstruments). The rings were heated in physiological salt solution to 37°C. Physiological salt solution containing 60 mM KCl was used to determine maximum contractility of the tissue. When the tension attained its peak value, the rings were relaxed by rinsing with the buffer. Next, the rings were pre-contracted with increasing concentrations of norepinephrine (1 nM – 0.3 μM) to produce 80% of the maximum contraction achieved by 60 mM KCl (EC80), followed by endothelium-dependent relaxation to cumulatively increasing concentrations of acetylcholine chloride (1 nM – 0.3 μM) till maximum relaxation was reached. Relaxation values were expressed as a percentage of the norepinephrine -induced contraction. Endothelium-independent relaxation was examined by exposure of rings to increasing concentrations of sodium nitroprusside (SNP; 0.1 nM – 30 nM), a nitric oxide donor. In a control experiment, aortic rings were incubated with 200 μM L-NNA (a NOS inhibitor) for 30 min followed by norepinephrine pre-contraction and cumulative addition of acetylcholine; wherein endothelium-dependent relaxation was monitored for control and L-NNA treated tissues.
Statistical analyses
Data are shown as mean ± SEM for n observations with the exception of EC50 values (ACh concentrations required to achieve 50% of maximal relaxation), which were expressed as mean with 95% confidence intervals. Comparison of multiple groups was performed using One-Way ANOVA with Tukey’s Multiple Comparison post-hoc test (HCAEC experiments). Student’s t-test was used to test for differences between control and cyanate-treated mice while for vascular function studies Two-Way ANOVA for repeated measurements followed by a Bonferroni’s post-hoc test was used. Significance was accepted at p<0.05. Statistical analyses were performed with Prism Version 4.03 (GraphPad Software, USA).
Supplementary Material
Sources of Funding
This work was supported by the Austrian Science Fund FWF (Grants W1241, P 27069-B19, P-22521-B18, P22771-B18 and P22976-B18) and by the Österreichische Nationalbank, Jubiläumsfond (Grants No. 14853, 13533).
Footnotes
Disclosures The authors declare that they have nothing to disclose.
Supplementary information is available at Kidney International’s website
References
- 1.Lindner A, Charra B, Sherrard DJ, Scribner BH. Accelerated atherosclerosis in prolonged maintenance hemodialysis. N Engl J Med. 1974;290:697–701. doi: 10.1056/NEJM197403282901301. [DOI] [PubMed] [Google Scholar]
- 2.Stenvinkel P. Interactions between inflammation, oxidative stress, and endothelial dysfunction in end-stage renal disease. J Ren Nutr. 2003;13:144–148. doi: 10.1053/jren.2003.50018. [DOI] [PubMed] [Google Scholar]
- 3.Dobre M, Meyer TW, Hostetter TH. Searching for uremic toxins. Clin J Am Soc Nephrol. 2013;8:322–327. doi: 10.2215/CJN.04260412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kraus LM, Kraus AP., Jr. The search for the uremic toxin: the case for carbamoylation of amino acids and proteins. Wien Klin Wochenschr. 1998;110:521–530. [PubMed] [Google Scholar]
- 5.Lameire N, Vanholder R, De Smet R. Uremic toxins and peritoneal dialysis. Kidney Int Suppl. 2001;78:S292–7. doi: 10.1046/j.1523-1755.2001.59780292.x. [DOI] [PubMed] [Google Scholar]
- 6.Evenepoel P, Meijers BK, Bammens BR, Verbeke K. Uremic toxins originating from colonic microbial metabolism. Kidney Int Suppl. 2009;(114):S12–9. doi: 10.1038/ki.2009.402. doi: S12-9. [DOI] [PubMed] [Google Scholar]
- 7.Wang Z, Nicholls SJ, Rodriguez ER, et al. Protein carbamylation links inflammation, smoking, uremia and atherogenesis. Nat Med. 2007;13:1176–1184. doi: 10.1038/nm1637. [DOI] [PubMed] [Google Scholar]
- 8.El-Gamal D, Holzer M, Gauster M, et al. Cyanate is a novel inducer of endothelial icam-1 expression. Antioxid Redox Signal. 2012;16:129–137. doi: 10.1089/ars.2011.4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holzer M, Zangger K, El-Gamal D, et al. Myeloperoxidase-Derived Chlorinating Species Induce Protein Carbamylation Through Decomposition of Thiocyanate and Urea: Novel Pathways Generating Dysfunctional High-Density Lipoprotein. Antioxid Redox Signal. 2012;17:1043–1052. doi: 10.1089/ars.2011.4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koeth RA, Kalantar-Zadeh K, Wang Z, et al. Protein Carbamylation Predicts Mortality in ESRD. J Am Soc Nephrol. 2013 doi: 10.1681/ASN.2012030254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berg AH, Drechsler C, Wenger J, et al. Carbamylation of serum albumin as a risk factor for mortality in patients with kidney failure. Sci Transl Med. 2013;5 doi: 10.1126/scitranslmed.3005218. 175ra29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bell JD, Lee JA, Lee HA, et al. Nuclear-Magnetic-Resonance Studies of Blood-Plasma and Urine from Subjects with Chronic-Renal-Failure - Identification of Trimethylamine-N-Oxide. Biochim Biophys Acta. 1991;1096:101–107. doi: 10.1016/0925-4439(91)90046-c. [DOI] [PubMed] [Google Scholar]
- 13.Blackmore DJ, Elder WJ, Bowden CH. Urea distribution in renal failure. J Clin Pathol. 1963;16:235–243. doi: 10.1136/jcp.16.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dirnhuber P, Schutz F. The isomeric transformation of urea into ammonium cyanate in aqueous solutions. Biochem J. 1948;42:628–632. [PubMed] [Google Scholar]
- 15.Marsche G, Saemann MD, Heinemann A, Holzer M. Inflammation alters HDL composition and function: Implications for HDL-raising therapies. Pharmacol Ther. 2013;137:341–351. doi: 10.1016/j.pharmthera.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Steffel J, Luscher TF, Tanner FC. Tissue factor in cardiovascular diseases: molecular mechanisms and clinical implications. Circulation. 2006;113:722–731. doi: 10.1161/CIRCULATIONAHA.105.567297. [DOI] [PubMed] [Google Scholar]
- 17.Westrick RJ, Bodary PF, Xu Z, et al. Deficiency of tissue factor pathway inhibitor promotes atherosclerosis and thrombosis in mice. Circulation. 2001;103:3044–3046. doi: 10.1161/hc2501.092492. [DOI] [PubMed] [Google Scholar]
- 18.Ploplis VA. Effects of altered plasminogen activator inhibitor-1 expression on cardiovascular disease. Curr Drug Targets. 2011;12:1782–1789. doi: 10.2174/138945011797635803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vanholder R, De Smet R, Glorieux G, et al. Review on uremic toxins: classification, concentration, and interindividual variability. Kidney Int. 2003;63:1934–1943. doi: 10.1046/j.1523-1755.2003.00924.x. [DOI] [PubMed] [Google Scholar]
- 20.Vanholder RC, Glorieux GL. An overview of uremic toxicity. Hemodial Int. 2003;7:156–161. doi: 10.1046/j.1492-7535.2003.00034.x. [DOI] [PubMed] [Google Scholar]
- 21.Holzer M, Gauster M, Pfeifer T, et al. Protein Carbamylation Renders High-Density Lipoprotein Dysfunctional. Antioxid Redox Signal. 2011;14:2337–2346. doi: 10.1089/ars.2010.3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hogas SM, Voroneanu L, Serban DN, et al. Methods and potential biomarkers for the evaluation of endothelial dysfunction in chronic kidney disease: a critical approach. J Am Soc Hypertens. 2010;4:116–127. doi: 10.1016/j.jash.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 23.Baigent C, Burbury K, Wheeler D. Premature cardiovascular disease in chronic renal failure. Lancet. 2000;356:147–152. doi: 10.1016/S0140-6736(00)02456-9. [DOI] [PubMed] [Google Scholar]
- 24.Landray MJ, Wheeler DC, Lip GY, et al. Inflammation, endothelial dysfunction, and platelet activation in patients with chronic kidney disease: the chronic renal impairment in Birmingham (CRIB) study. Am J Kidney Dis. 2004;43:244–253. doi: 10.1053/j.ajkd.2003.10.037. [DOI] [PubMed] [Google Scholar]
- 25.Thambyrajah J, Landray MJ, McGlynn FJ, et al. Abnormalities of endothelial function in patients with predialysis renal failure. Heart. 2000;83:205–209. doi: 10.1136/heart.83.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shu L, Park JL, Byun J, et al. Decreased nitric oxide bioavailability in a mouse model of Fabry disease. J Am Soc Nephrol. 2009;20:1975–1985. doi: 10.1681/ASN.2008111190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feletou M, Kohler R, Vanhoutte PM. Nitric oxide: Orchestrator of endothelium-dependent responses. Ann Med. 2011 doi: 10.3109/07853890.2011.585658. [DOI] [PubMed] [Google Scholar]
- 28.Yetik-Anacak G, Catravas JD. Nitric oxide and the endothelium: history and impact on cardiovascular disease. Vascul Pharmacol. 2006;45:268–276. doi: 10.1016/j.vph.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 29.Sagripanti A, Barsotti G. Bleeding and thrombosis in chronic uremia. Nephron. 1997;75:125–139. doi: 10.1159/000189522. [DOI] [PubMed] [Google Scholar]
- 30.Shlipak MG, Fried LF, Crump C, et al. Elevations of inflammatory and procoagulant biomarkers in elderly persons with renal insufficiency. Circulation. 2003;107:87–92. doi: 10.1161/01.cir.0000042700.48769.59. [DOI] [PubMed] [Google Scholar]
- 31.Sharma S, Farrington K, Kozarski R, et al. Impaired thrombolysis: a novel cardiovascular risk factor in end-stage renal disease. Eur Heart J. 2013;34:354–363. doi: 10.1093/eurheartj/ehs300. [DOI] [PubMed] [Google Scholar]
- 32.Andelid K, Bake B, Rak S, et al. Myeloperoxidase as a marker of increasing systemic inflammation in smokers without severe airway symptoms. Respir Med. 2007;101:888–895. doi: 10.1016/j.rmed.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 33.Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation. 2007;115:1285–1295. doi: 10.1161/CIRCULATIONAHA.106.652859. [DOI] [PubMed] [Google Scholar]
- 34.de Sousa MG, Yugar-Toledo JC, Rubira M, et al. Ascorbic acid improves impaired venous and arterial endothelium-dependent dilation in smokers. Acta Pharmacol Sin. 2005;26:447–452. doi: 10.1111/j.1745-7254.2005.00069.x. [DOI] [PubMed] [Google Scholar]
- 35.Cacicedo JM, Gauthier MS, Lebrasseur NK, et al. Acute exercise activates AMPK and eNOS in the mouse aorta. Am J Physiol Heart Circ Physiol. 2011;301:H1255–65. doi: 10.1152/ajpheart.01279.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Damm M, Holzer M, Radspieler G, et al. Microwave-assisted high-throughput acid hydrolysis in silicon carbide microtiter platforms-A rapid and low volume sample preparation technique for total amino acid analysis in proteins and peptides. J Chromatogr A. 2010;1217:7826–7832. doi: 10.1016/j.chroma.2010.10.062. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.