Abstract
Osteoblasts, the chief bone-making cells in the body, are a focus of osteoporosis research. Although teriparatite, a synthetic fragment of the human parathyroid hormone (PTH), has been an effective bone anabolic drug, there remains a clinical need for additional therapeutics that safely stimulates osteoblast number and function. Work in the past several decades has provided unprecedented clarity about the roles of growth factors and transcription factors in regulating osteoblast differentiation and activity, but whether these factors may regulate cellular metabolism to influence cell fate and function has been largely unexplored. The past few years have witnessed a resurgence of interest in the cellular metabolism of osteoblasts, with the hope that elucidation of their metabolic profile may open new avenues for developing bone anabolic agents. Here we review the current understanding about glucose metabolism in osteoblasts.
Keywords: osteoblast, glucose metabolism, aerobic glycolysis, energy, bone formation
Introduction
Osteoblasts are the chief bone-making cells integral to skeletal health and disease in humans. They first appear in the embryo from mesodermal or neural crest cells, but are produced throughout life from mesenchymal progenitors during bone remodeling, remodeling and fracture healing 1. Osteoblasts engage in active bone formation for a limited time; subsequently the majority is believed to undergo apoptosis, whereas the remaining cells become either bone-lining cells on the bone surface, or osteocytes entombed in the bone matrix. In addition to making bone matrix, osteoblasts, together with osteocytes, also produce molecular signals to regulate osteoclastogenesis necessary for bone remodeling2,3. Osteocytes are also known to regulate phosphate homeostasis through the secretion of Fgf23 4. More recently, osteoblasts have been implicated in the regulation of systemic glucose metabolism in the mouse 5. Thus, elucidating osteoblast identity and regulation is important not only for advancing skeletal biology but also for a better understanding of whole-body physiology.
Osteoblasts synthesize a large amount of extracellular matrix proteins, and therefore have a high demand for both energy and building materials. However, the bioenergetics and carbon source for osteoblasts are poorly understood. A number of studies on glucose metabolism in bone slices and isolated osteoblasts were conducted between the 1950’s and 1980’s. These studies indicated that aerobic glycolysis, i.e., production of lactate from glucose despite the presence of oxygen, was the main mode of glucose metabolism in osteoblasts. Moreover, the calciotropic hormone parathyroid hormone (PTH) was shown to stimulate the production of lactic acid from glucose, prompting researchers to hypothesize that increased production of lactic acid is responsible for more active bone resorption. Subsequent rejection of this hypothesis may have contributed to the loss of interest in aerobic glycolysis in osteoblasts during the ensuing decades. A resurgent interest in cellular metabolism in recent years has led to the appreciation that lactate-producing glycolysis may play an important role in osteoblast differentiation and function.
Cellular glucose metabolism
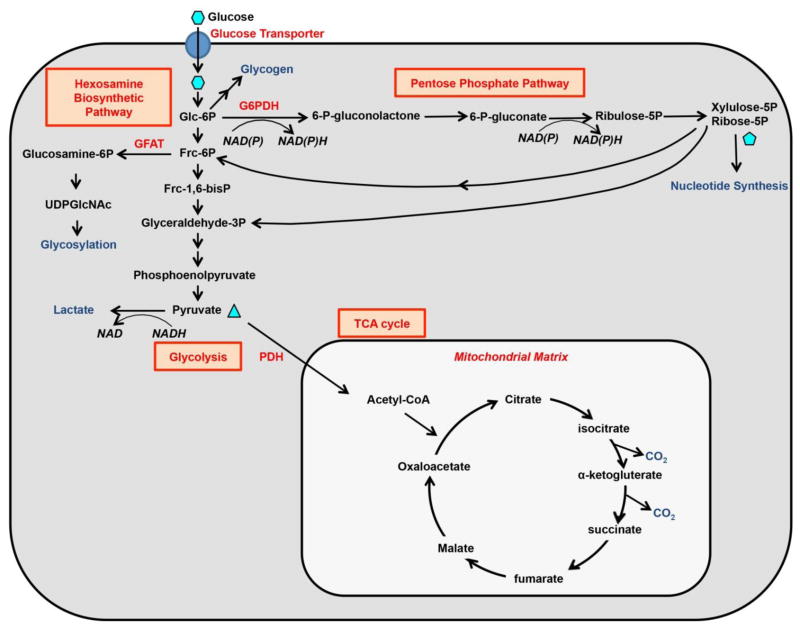
Glucose is a major energy and carbon source for mammalian cells. In most cells, glucose is transported via the GLUT family of facilitative transporters (GLUTs). This process does not require energy and can only transport glucose down a concentration gradient 6,7. Once inside the cell, glucose is phosphorylated to glucose-6-phosphate (G6P) by hexokinases, and is then either converted to glycogen for storage, or metabolized to produce ATP and intermediate metabolites for anabolic reactions. Glucose catabolism can follow multiple pathways, including glycolysis to produce pyruvate for further metabolism, entering pentose phosphate pathway (PPP), or fueling into hexosamine biosynthetic pathway (HBP) 8 (Figure 1). Overall, glucose is not only a unique fuel that can produce ATP with or without oxygen, but also a critical source for building blocks necessary for biosynthesis in the cell.
Figure 1. Metabolic fates of glucose in mammalian cells.
G6PDH: Glucose-6-phosphate dehydrogenase. GFAT: Glutamine fructose-6-phosphate amidotransferase. PDH: Pyruvate dehydrogenase complex.
Glycolysis is the predominant route for cellular glucose utilization. The process occurs in the cytosol and breaks down one molecule of glucose into two molecules of pyruvate. Pyruvate can be converted to lactate by the enzyme lactate dehydrogenase (LDH) in the cytosol. This reaction regenerates NAD+ necessary for further glycolysis, and can occur with or without oxygen. LDH is a tetrameric enzyme consisting of subunits A, B or C expressed from different genes. The A and B subunits are expressed ubiquitously and can form five different tetrameric enzyme forms, namely LDH1-5, through different combinations. On the other hand, the C subunit is specific to the testis and sperms 9. An alternative fate for pyruvate is to participate in the tricarboxylic acid (TCA) cycle (also known as Krebs cycle) within the matrix of mitochondria. This process produces the most ATP per glucose molecule but requires molecular oxygen. The predominant route for mitochondrial pyruvate to enter the TCA cycle is through decarboxylation by pyruvate dehydrogenase complex (PDH) to form acetyl-coA. This entry point is tightly regulated as elevated levels of ATP and pyruvate dehydrogenase kinase (PDK) can inactivate PDH. Acetyl-coA enters the TCA cycle by condensing with oxaloacetate to form citrate, which then sequentially releases two CO2 molecules to regenerate oxaloacetate. The TCA cycle not only produces ATP through oxidative phosphorylation, but also provides intermediate molecules (e.g., citrate) that exit mitochondria to participate in biosynthesis (e.g., fatty acid synthesis) elsewhere in the cell.
During glycolysis, a small portion of the intermediates is diverted into HBP and PPP. 5–30% of glucose is estimated to enter PPP that is the source of NAPDH and ribose-5-phosphate (R5P). Whereas NADPH provides the reducing equivalents for fatty acid and cholesterol synthesis, R5P is the precursor for nucleotide synthesis. PPP starts with an oxidative phase including the rate-limiting, irreversible dehydrogenation of G6P by glucose 6-phosphate dehydrogenase (G6PD). This is followed by further oxidation and decarboxylation into ribose-5 phosphate (R5P). In the non-oxidative phase, PPP yields fructose 6-phosphate and glyceraldehyde 3-phosphate, both of which can enter the glycolytic pathway 10,11. Approximately 2–5% of cellular glucose is metabolized through HBP. Here, fructose-6-phosphate together with glutamine is catalyzed by GFPT1 (also known as GFAT) to form glucosamine-6-phosphate in the very first and rate-limiting step. Glucosamine-6-phosphate is further used to generate uridine diphospho-N-acetylglucosamine (UDP-GlcNAc), which is a precursor necessary for protein glycosylation 12. Overall, PPP and HBP consume a relatively small percentage of cellular glucose but provide critical intermediates for various anabolic reactions. The extent to which glycolytic intermediates are diverted to PPP or HBP is determined by the biosynthetic needs of the cell.
Aerobic glycolysis in osteoblasts
Members of the GLUT glucose transporter family are expressed in osteoblast-lineage cells. GLUT1 and GLUT3 were detected in an osteosarcoma cell line, UMR 106-01 13,14. There is also evidence for the expression of GLUT1 in rodent osteoblastic cells (PyMS) 15. Further investigation is necessary to characterize the specific expression pattern and function of each GLUT protein in osteoblasts in vivo.
Despite the incomplete understanding of glucose transporters, numerous studies have demonstrated active glycolysis in osteoblasts. The initial studies with bone slices in the early 1960’s showed that bone not only consumed a large amount of glucose, but also produced lactate as the major end product while the TCA cycle played a minor role even in aerobic conditions 16,17. It was estimated that >80% of glucose consumed by bone was converted to lactate 16 Providing excess amino acid had no effect on the amount of cellular CO2 or lactate production from glucose 18. The metabolic features of bone slices were later confirmed in osteoblasts enzymatically digested from calvaria 19. More recently, calvarial osteoblastic cells were shown to utilize both aerobic glycolysis and oxidative phosphorylation during differentiation in vitro, but relied more on the former than the latter when they became mature osteoblasts 20. Moreover, forced activation of glycolysis by overexpressing a stabilized form of HIF1α led to more osteoblasts and a higher bone mass in the mouse 21. Overall, osteoblasts use glucose as a major nutrient and metabolize it mostly to lactate even in the presence of oxygen. This process is known as aerobic glycolysis.
The aerobic glycolysis in osteoblasts is akin to the Warburg effect observed in cancer cells 22. In cancer cells, aerobic glycolysis has been postulated to provide the necessary glycolytic intermediates to support uncontrolled proliferation, but this is unlikely to be the case for normal somatic cells such as osteoblasts 23,24. It is possible that aerobic glycolysis is necessary to fulfill the specific metabolic needs of osteoblasts. For instance, osteoblasts have been long known to produce and secrete a large amount of citrate 25,26. Citrate in bone was recently shown to be critical for the structure of the apatite nanocrystal, providing stability, strength, and resistance to fracture 27–29. The mechanism for the high citrate output by osteoblasts is not fully understood, but may be partly due to a relatively low activity of isocitrate dehydrogenase (IDH) which converts isocitrate to α-ketoglutarate for the continuation of the TCA cycle 25. In addition, the essential trace element zinc is known to attenuate the activity of aconidase, the enzyme responsible for isomerizing citrate to isocitrate, in prostate epithelial cells 30. Although a similar role has not been demonstrated in osteoblasts, zinc is abundant in bone and mice lacking a zinc transporter, ZNT5, manifest poor growth and decreased bone density due to impairment of osteoblast maturation 31,32. Moreover, zinc uptake and the level of ZIP1 zinc transporter were shown to increase during osteoblast differentiation, and overexpression ZIP1 enhanced osteoblastogenesis in vitro 33. If indeed a high level of citrate accumulates in the mitochondria of osteoblasts, it may suppress pyruvate entering the TCA cycle and promote the conversion to lactate.
Regulation of osteoblast glycolysis by PTH
Intermittent use of parathyroid hormone (PTH) or its N-terminal fragment known as teriparatite effectively promotes bone formation, but the mechanism underlying such anabolic function is incompletely understood. Interestingly, long before PTH was used as a bone anabolic agent, it was shown to stimulate aerobic glycolysis in osteoblasts. Experiments with metaphyseal bone explants of the long bones showed that injections of parathyroid extracts prior to sacrifice of the mice increased lactate production by the explants in culture under aerobic conditions 34. This effect was only observed in the presence but not absence of glucose, and estradiol did not have the same effect. Later, PTH was shown to increase lactate production by calvarium explants 35,36. More recently, PTH was reported to induce glucose uptake in rat osteoblastic cells (PyMS) 37. Overall, PTH promotes lactate-producing glycolysis in osteoblasts, but the mechanism for such regulation is unclear. Moreover, whether increased aerobic glycolysis contributes to the bone anabolic effect of PTH has not been explored.
Wnt stimulates aerobic glycolysis during osteoblast differentiation
Although all major developmental signals have been shown to regulate osteoblast differentiation, their potential effects on cellular metabolism have been largely unexplored 1. Wnt signaling has emerged as a major promoter of bone accretion 38. Recently, Wnt proteins were shown to reprogram cellular glucose metabolism during osteoblast differentiation 39. In particular, Wnt3a, 7b and 10b, each known to induce osteoblast differentiation, stimulated aerobic glycolysis in both cell lines and primary cultures of osteogenic cells. Mechanistically, Wnt3a rapidly increased the protein levels of several glycolytic enzymes independent of GSK3-β and β-catenin but downstream of mTORC2 and Akt. Suppressing the Wnt3a-induced glycolytic enzymes impaired osteoblast differentiation in vitro. Moreover, deletion of Lrp5 in mice reduced mTORC2 activity and the glycolytic enzymes in bone, concurrent with a decrease in postnatal bone mass. Conversely, mice harboring a high-bone-mass allele of Lrp5 expressed higher levels of glycolytic enzymes in bone. Thus, direct stimulation of aerobic glycolysis may contribute to bone anabolism in response to Wnt-Lrp5 signaling.
Regulation of osteoblasts by insulin and IGFs
Insulin is a critical hormone controlling whole-body glucose homeostasis. Type I diabetes mellitus, caused by insulin deficiency, has been linked with decreased bone density, increased risk as well as early onset for osteoporosis, increased fracture risk and poor fracture healing in human patients 40–43. Consistent with the clinical observations, studies in diabetic animal models revealed defects in osteoblast function associated with insulin deficiency 44,45. Delayed fracture healing in diabetic rats could be overcome by insulin delivery at the fracture site without affecting the systemic blood glucose level 46. Moreover, genetic deletion of insulin receptor (IR) in either mature osteoblasts or osteoprogenitors impaired bone formation in postnatal mice 47–49. These findings indicate that insulin likely functions as an anabolic signal in the osteoblast lineage. However, it is not known at present whether insulin may promote bone formation through reprogramming of cellular metabolism.
Insulin-like growth factors (IGF1 and 2) have also been implicated in bone formation 50. Whereas IGF2 mainly regulates fetal growth via both IGF1R and IR, IGF1 controls postnatal growth and homeostasis through IGF1 receptor (IGF1R) 51. IGF1 is abundantly associated with the postnatal bone matrix and has been extensively studied as a bone anabolic signal. IGF1 knockout mice exhibit defects in bone formation, whereas overexpression of IGF1 in osteoblasts enhanced their activity 52,53. Removal of IGF1 at early stages of osteoblast differentiation produced a more severe defect in bone accretion 54. Similarly, deletion of IGF1R in either mature osteoblasts or osteoprogenitors impaired bone formation 55,56. There is evidence that reduced IGF signaling may relate to age-dependent bone loss. Both serum and bone-matrix levels of IGF1 appeared to decrease with aging in humans 57,58. Aging was also shown to impair IGF1R responsiveness to IGF1 in bone marrow stromal cells of mice 59. Moreover, injection of IGF1 together with IGF binding protein 3 (IGFBP3) ameliorated aging-related bone loss in rats 60. Thus, IGF signaling stimulates bone formation and plays important roles in bone homeostasis.
Apart from its importance in basal bone physiology, IGF1 signaling appears to mediate the bone anabolic effect of intermittent PTH. Removal of either IGF1 or IGF1R in osteoblasts essentially abolished the PTH response 61–63. The mechanism underlying the bone anabolic effect of IGF signaling is not fully understood. It is worth noting that like PTH, IGF1 also induces glucose uptake in osteoblastic cells 37. Future studies are necessary to determine the role of glycolysis in bone anabolism induced by IGF signaling.
Conclusions
Understanding the mechanisms governing osteoblast differentiation and function is key to developing novel bone anabolic therapeutics. Although transcriptional factors and developmental signals have been extensively studied over the past several decades, relatively little is known about the status of cellular metabolism during osteoblast differentiation. Future studies are necessary to define the metabolic phenotype of osteoblasts, including the energy and carbon sources as well as their metabolic fates. Work to date has mostly focused on glucose metabolism, but studies of fatty acids and amino acids are clearly necessary. In addition, to understand how osteoblasts achieve their specific metabolic profile, it is important to explore whether other developmental signals besides Wnt, or the known transcription factors reprogram cellular metabolism. The molecular basis for the effect of metabolic reprogramming on osteoblast differentiation needs to be elucidated. Finally, technical advances are necessary to monitor cellular metabolic changes in bone in vivo. Overall, a clear understanding of cellular metabolism in osteoblasts will not only advance basic biology, but may also provide mechanistic insights about bone frailty associated with metabolic diseases and aging.
Acknowledgments
Work in the Long lab was supported by NIH grants AR060456, AR055923 and DK065789.
Footnotes
Conflict of Interest
E Esen and F Long both declare no conflicts of interest.
Human and Animal Rights and Informed Consent
All studies by the authors involving animal and/or human subjects were performed after approval by the appropriate institutional review boards. When required, written informed consent was obtained from all participants.
References
Papers of particular interest, published recently, have been highlighted as:
•• Of major importance
• Of importance
- 1.Long F. Building strong bones: molecular regulation of the osteoblast lineage. Nature reviews. Molecular cell biology. 2012 Jan;13(1):27–38. doi: 10.1038/nrm3254. [DOI] [PubMed] [Google Scholar]
- 2.O’Brien CA, Nakashima T, Takayanagi H. Osteocyte control of osteoclastogenesis. Bone. 2013 Jun;54(2):258–263. doi: 10.1016/j.bone.2012.08.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teitelbaum SL, Ross FP. Genetic regulation of osteoclast development and function. Nat Rev Genet. 2003 Aug;4(8):638–649. doi: 10.1038/nrg1122. [DOI] [PubMed] [Google Scholar]
- 4.Feng JQ, Clinkenbeard EL, Yuan B, White KE, Drezner MK. Osteocyte regulation of phosphate homeostasis and bone mineralization underlies the pathophysiology of the heritable disorders of rickets and osteomalacia. Bone. 2013 Jun;54(2):213–221. doi: 10.1016/j.bone.2013.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clemens TL, Karsenty G. The osteoblast: an insulin target cell controlling glucose homeostasis. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2011 Apr;26(4):677–680. doi: 10.1002/jbmr.321. [DOI] [PubMed] [Google Scholar]
- 6.Bell GI, Burant CF, Takeda J, Gould GW. Structure and function of mammalian facilitative sugar transporters. J Biol Chem. 1993 Sep 15;268(26):19161–19164. [PubMed] [Google Scholar]
- 7.Mueckler M. Facilitative glucose transporters. Eur J Biochem. 1994 Feb 1;219(3):713–725. doi: 10.1111/j.1432-1033.1994.tb18550.x. [DOI] [PubMed] [Google Scholar]
- 8.Bouche C, Serdy S, Kahn CR, Goldfine AB. The cellular fate of glucose and its relevance in type 2 diabetes. Endocr Rev. 2004 Oct;25(5):807–830. doi: 10.1210/er.2003-0026. [DOI] [PubMed] [Google Scholar]
- 9.Kopperschlager G, Kirchberger J. Methods for the separation of lactate dehydrogenases and clinical significance of the enzyme. J Chromatogr B Biomed Appl. 1996 Sep 20;684(1–2):25–49. doi: 10.1016/0378-4347(96)00133-8. [DOI] [PubMed] [Google Scholar]
- 10.Perl A, Hanczko R, Telarico T, Oaks Z, Landas S. Oxidative stress, inflammation and carcinogenesis are controlled through the pentose phosphate pathway by transaldolase. Trends Mol Med. 2011 Jul;17(7):395–403. doi: 10.1016/j.molmed.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wamelink MM, Struys EA, Jakobs C. The biochemistry, metabolism and inherited defects of the pentose phosphate pathway: a review. J Inherit Metab Dis. 2008 Dec;31(6):703–717. doi: 10.1007/s10545-008-1015-6. [DOI] [PubMed] [Google Scholar]
- 12.Teo CF, Wollaston-Hayden EE, Wells L. Hexosamine flux, the O-GlcNAc modification, and the development of insulin resistance in adipocytes. Mol Cell Endocrinol. 2010 Apr 29;318(1–2):44–53. doi: 10.1016/j.mce.2009.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas DM, Rogers SD, Ng KW, Best JD. Dexamethasone modulates insulin receptor expression and subcellular distribution of the glucose transporter GLUT 1 in UMR 106–01, a clonal osteogenic sarcoma cell line. J Mol Endocrinol. 1996 Aug;17(1):7–17. doi: 10.1677/jme.0.0170007. [DOI] [PubMed] [Google Scholar]
- 14.Thomas DM, Maher F, Rogers SD, Best JD. Expression and regulation by insulin of GLUT 3 in UMR 106–01, a clonal rat osteosarcoma cell line. Biochem Biophys Res Commun. 1996 Jan 26;218(3):789–793. doi: 10.1006/bbrc.1996.0140. [DOI] [PubMed] [Google Scholar]
- 15.Rolland F, Winderickx J, Thevelein JM. Glucose-sensing mechanisms in eukaryotic cells. Trends Biochem Sci. 2001 May;26(5):310–317. doi: 10.1016/s0968-0004(01)01805-9. [DOI] [PubMed] [Google Scholar]
- 16.Borle AB, Nichols N, Nichols G., Jr Metabolic studies of bone in vitro. I. Normal bone. The Journal of biological chemistry. 1960 Apr;235:1206–1210. [PubMed] [Google Scholar]
- 17.Cohn DV, Forscher BK. Aerobic metabolism of glucose by bone. The Journal of biological chemistry. 1962 Mar;237:615–618. [PubMed] [Google Scholar]
- 18.Flanagan B, Nichols G., Jr Metabolic Studies of Bone in Vitro. V. Glucose Metabolism and Collagen Biosynthesis. J Biol Chem. 1964 Apr;239:1261–1265. [PubMed] [Google Scholar]
- 19.Peck WA, Birge SJ, Jr, Fedak SA. Bone Cells: Biochemical and Biological Studies after Enzymatic Isolation. Science. 1964 Dec 11;146(3650):1476–1477. doi: 10.1126/science.146.3650.1476. [DOI] [PubMed] [Google Scholar]
- •20.Guntur AR, Le PT, Farber CR, Rosen CJ. Bioenergetics during calvarial osteoblast differentiation reflect strain differences in bone mass. Endocrinology. 2014 May;155(5):1589–1595. doi: 10.1210/en.2013-1974. This work provides evidence that oxygen consumption rates in calvarial osteoblast cultures positively correlate with bone formation rate in vivo across different inbred mouse strains. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •21.Regan JN, Lim J, Shi Y, et al. Up-regulation of glycolytic metabolism is required for HIF1alpha-driven bone formation. Proceedings of the National Academy of Sciences of the United States of America. 2014 Jun 10;111(23):8673–8678. doi: 10.1073/pnas.1324290111. This work provides evidence that increased glycolysis contributes to more robust cancellous bone formation in the mouse. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warburg O. On the origin of cancer cells. Science. 1956 Feb 24;123(3191):309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 23.Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004 Nov;4(11):891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 24.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009 May 22;324(5930):1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dixon TF, Perkins HR. Citric acid and bone metabolism. Biochem J. 1952 Oct;52(2):260–265. doi: 10.1042/bj0520260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Costello LC, Franklin RB, Reynolds MA, Chellaiah M. The Important Role of Osteoblasts and Citrate Production in Bone Formation: “Osteoblast Citration” as a New Concept for an Old Relationship. Open Bone J. 2012:4. doi: 10.2174/1876525401204010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu YY, Rawal A, Schmidt-Rohr K. Strongly bound citrate stabilizes the apatite nanocrystals in bone. Proc Natl Acad Sci U S A. 2010 Dec 28;107(52):22425–22429. doi: 10.1073/pnas.1009219107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kenny AD, Draskoczy PR, Goldhaber P. Citric acid production by resorbing bone in tissue culture. Am J Physiol. 1959 Aug;197:502–504. doi: 10.1152/ajplegacy.1959.197.2.502. [DOI] [PubMed] [Google Scholar]
- 29.Taylor TG. The nature of bone citrate. Biochim Biophys Acta. 1960 Mar 25;39:148–149. doi: 10.1016/0006-3002(60)90131-1. [DOI] [PubMed] [Google Scholar]
- 30.Costello LC, Liu Y, Franklin RB, Kennedy MC. Zinc inhibition of mitochondrial aconitase and its importance in citrate metabolism of prostate epithelial cells. J Biol Chem. 1997 Nov 14;272(46):28875–28881. doi: 10.1074/jbc.272.46.28875. [DOI] [PubMed] [Google Scholar]
- 31.Alhava EM, Olkkonen H, Puittinen J, Nokso-Koivisto VM. Zinc content of human cancellous bone. Acta Orthop Scand. 1977 May;48(1):1–4. doi: 10.3109/17453677708985102. [DOI] [PubMed] [Google Scholar]
- 32.Inoue K, Matsuda K, Itoh M, et al. Osteopenia and male-specific sudden cardiac death in mice lacking a zinc transporter gene, Znt5. Human molecular genetics. 2002 Jul 15;11(15):1775–1784. doi: 10.1093/hmg/11.15.1775. [DOI] [PubMed] [Google Scholar]
- 33.Tang Z, Sahu SN, Khadeer MA, Bai G, Franklin RB, Gupta A. Overexpression of the ZIP1 zinc transporter induces an osteogenic phenotype in mesenchymal stem cells. Bone. 2006 Feb;38(2):181–198. doi: 10.1016/j.bone.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 34.Borle AB, Nichols N, Nichols G., Jr Metabolic studies of bone in vitro. II. The metabolic patterns of accretion and resorption. The Journal of biological chemistry. 1960 Apr;235:1211–1214. [PubMed] [Google Scholar]
- 35.Neuman WF, Neuman MW, Brommage R. Aerobic glycolysis in bone: lactate production and gradients in calvaria. The American journal of physiology. 1978 Jan;234(1):C41–50. doi: 10.1152/ajpcell.1978.234.1.C41. [DOI] [PubMed] [Google Scholar]
- 36.Rodan GA, Rodan SB, Marks SC., Jr Parathyroid hormone stimulation of adenylate cyclase activity and lactic acid accumulation in calvaria of osteopetrotic (ia) rats. Endocrinology. 1978 May;102(5):1501–1505. doi: 10.1210/endo-102-5-1501. [DOI] [PubMed] [Google Scholar]
- 37.Zoidis E, Ghirlanda-Keller C, Schmid C. Stimulation of glucose transport in osteoblastic cells by parathyroid hormone and insulin-like growth factor I. Mol Cell Biochem. 2011 Feb;348(1–2):33–42. doi: 10.1007/s11010-010-0634-z. [DOI] [PubMed] [Google Scholar]
- 38.Maupin KA, Droscha CJ, Williams BO. A comprehensive overview of skeletal phenotypes associatred with alterations in Wnt/b-catenin signaling in humans and mice. Bone Research. 2013;1(1):27–71. doi: 10.4248/BR201301004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •39.Esen E, Chen J, Karner CM, Okunade AL, Patterson BW, Long F. WNT-LRP5 signaling induces Warburg effect through mTORC2 activation during osteoblast differentiation. Cell metabolism. 2013 May 7;17(5):745–755. doi: 10.1016/j.cmet.2013.03.017. This work provides evidence that WNT signaling directly stimulates aerobic glycolysis during osteoblast differentiation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kemink SA, Hermus AR, Swinkels LM, Lutterman JA, Smals AG. Osteopenia in insulin-dependent diabetes mellitus; prevalence and aspects of pathophysiology. J Endocrinol Invest. 2000 May;23(5):295–303. doi: 10.1007/BF03343726. [DOI] [PubMed] [Google Scholar]
- 41.Nicodemus KK, Folsom AR. Type 1 and type 2 diabetes and incident hip fractures in postmenopausal women. Diabetes Care. 2001 Jul;24(7):1192–1197. doi: 10.2337/diacare.24.7.1192. [DOI] [PubMed] [Google Scholar]
- 42.Loder RT. The influence of diabetes mellitus on the healing of closed fractures. Clinical orthopaedics and related research. 1988 Jul;(232):210–216. [PubMed] [Google Scholar]
- 43.Levin ME, Boisseau VC, Avioli LV. Effects of diabetes mellitus on bone mass in juvenile and adult-onset diabetes. The New England journal of medicine. 1976 Jan 29;294(5):241–245. doi: 10.1056/NEJM197601292940502. [DOI] [PubMed] [Google Scholar]
- 44.Verhaeghe J, van Herck E, Visser WJ, et al. Bone and mineral metabolism in BB rats with long-term diabetes. Decreased bone turnover and osteoporosis. Diabetes. 1990 Apr;39(4):477–482. doi: 10.2337/diab.39.4.477. [DOI] [PubMed] [Google Scholar]
- 45.Lu H, Kraut D, Gerstenfeld LC, Graves DT. Diabetes interferes with the bone formation by affecting the expression of transcription factors that regulate osteoblast differentiation. Endocrinology. 2003 Jan;144(1):346–352. doi: 10.1210/en.2002-220072. [DOI] [PubMed] [Google Scholar]
- 46.Gandhi A, Beam HA, O’Connor JP, Parsons JR, Lin SS. The effects of local insulin delivery on diabetic fracture healing. Bone. 2005 Oct;37(4):482–490. doi: 10.1016/j.bone.2005.04.039. [DOI] [PubMed] [Google Scholar]
- 47.Ferron M, Wei J, Yoshizawa T, et al. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell. 2010 Jul 23;142(2):296–308. doi: 10.1016/j.cell.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fulzele K, Riddle RC, DiGirolamo DJ, et al. Insulin receptor signaling in osteoblasts regulates postnatal bone acquisition and body composition. Cell. 2010 Jul 23;142(2):309–319. doi: 10.1016/j.cell.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thrailkill K, Bunn RC, Lumpkin C, Jr, et al. Loss of insulin receptor in osteoprogenitor cells impairs structural strength of bone. J Diabetes Res. 2014;2014:703589. doi: 10.1155/2014/703589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kawai M, Rosen CJ. The insulin-like growth factor system in bone: basic and clinical implications. Endocrinol Metab Clin North Am. 2012 Jun;41(2):323–333. vi. doi: 10.1016/j.ecl.2012.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakae J, Kido Y, Accili D. Distinct and overlapping functions of insulin and IGF-I receptors. Endocr Rev. 2001 Dec;22(6):818–835. doi: 10.1210/edrv.22.6.0452. [DOI] [PubMed] [Google Scholar]
- 52.Bikle D, Majumdar S, Laib A, et al. The skeletal structure of insulin-like growth factor I-deficient mice. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2001 Dec;16(12):2320–2329. doi: 10.1359/jbmr.2001.16.12.2320. [DOI] [PubMed] [Google Scholar]
- 53.Zhao G, Monier-Faugere MC, Langub MC, et al. Targeted overexpression of insulin-like growth factor I to osteoblasts of transgenic mice: increased trabecular bone volume without increased osteoblast proliferation. Endocrinology. 2000 Jul;141(7):2674–2682. doi: 10.1210/endo.141.7.7585. [DOI] [PubMed] [Google Scholar]
- 54.Govoni KE, Wergedal JE, Florin L, Angel P, Baylink DJ, Mohan S. Conditional deletion of insulin-like growth factor-I in collagen type 1alpha2-expressing cells results in postnatal lethality and a dramatic reduction in bone accretion. Endocrinology. 2007 Dec;148(12):5706–5715. doi: 10.1210/en.2007-0608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xian L, Wu X, Pang L, et al. Matrix IGF-1 maintains bone mass by activation of mTOR in mesenchymal stem cells. Nature medicine. 2012 Jun 24; doi: 10.1038/nm.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang M, Xuan S, Bouxsein ML, et al. Osteoblast-specific knockout of the insulin-like growth factor (IGF) receptor gene reveals an essential role of IGF signaling in bone matrix mineralization. J Biol Chem. 2002 Nov 15;277(46):44005–44012. doi: 10.1074/jbc.M208265200. [DOI] [PubMed] [Google Scholar]
- 57.Brabant G, von zur Muhlen A, Wuster C, et al. Serum insulin-like growth factor I reference values for an automated chemiluminescence immunoassay system: results from a multicenter study. Horm Res. 2003;60(2):53–60. doi: 10.1159/000071871. [DOI] [PubMed] [Google Scholar]
- 58.Seck T, Scheppach B, Scharla S, et al. Concentration of insulin-like growth factor (IGF)-I and -II in iliac crest bone matrix from pre- and postmenopausal women: relationship to age, menopause, bone turnover, bone volume, and circulating IGFs. J Clin Endocrinol Metab. 1998 Jul;83(7):2331–2337. doi: 10.1210/jcem.83.7.4967. [DOI] [PubMed] [Google Scholar]
- 59.Cao JJ, Kurimoto P, Boudignon B, Rosen C, Lima F, Halloran BP. Aging impairs IGF-I receptor activation and induces skeletal resistance to IGF-I. J Bone Miner Res. 2007 Aug;22(8):1271–1279. doi: 10.1359/jbmr.070506. [DOI] [PubMed] [Google Scholar]
- 60.Xian L, Wu X, Pang L, et al. Matrix IGF-1 maintains bone mass by activation of mTOR in mesenchymal stem cells. Nature medicine. 2012 Jul;18(7):1095–1101. doi: 10.1038/nm.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bikle DD, Sakata T, Leary C, et al. Insulin-like growth factor I is required for the anabolic actions of parathyroid hormone on mouse bone. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2002 Sep;17(9):1570–1578. doi: 10.1359/jbmr.2002.17.9.1570. [DOI] [PubMed] [Google Scholar]
- 62.Wang Y, Nishida S, Boudignon BM, et al. IGF-I receptor is required for the anabolic actions of parathyroid hormone on bone. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2007 Sep;22(9):1329–1337. doi: 10.1359/jbmr.070517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miyakoshi N, Kasukawa Y, Linkhart TA, Baylink DJ, Mohan S. Evidence that anabolic effects of PTH on bone require IGF-I in growing mice. Endocrinology. 2001 Oct;142(10):4349–4356. doi: 10.1210/endo.142.10.8436. [DOI] [PubMed] [Google Scholar]