Abstract
Background
Broad spectrum muscarinic receptor antagonists have represented the first available treatment for different movement disorders such as dystonia. However, the specificity of these drugs and their mechanism of action is not entirely clear.
Methods
We performed a systematic analysis of the effects of anticholinergic drugs on short- and long-term plasticity recorded from striatal medium spiny neurons from DYT1 dystonia knock-in (Tor1a+/Δgag) mice heterozygous for ΔE-torsinA and their controls (Tor1a+/+ mice).
Results
Antagonists were chosen that had previously been proposed to be selective for muscarinic receptor subtypes and included pirenzepine, trihexyphenydil, biperiden, orphenadrine, and a novel selective M1 antagonist, VU0255035. Tor1a+/Δgag mice exhibited a significant impairment of corticostriatal synaptic plasticity. Anticholinergics had no significant effects on intrinsic membrane properties and on short-term plasticity of striatal neurons. However, they exhibited a differential ability to restore the corticostriatal plasticity deficits. A complete rescue of both long-term depression (LTD) and synaptic depotentiation (SD) was obtained by applying the M1-preferring antagonists pirenzepine and trihexyphenidyl as well as VU0255035. Conversely, the non-selective antagonists orphenadrine produced only a partial rescue of synaptic plasticity, whereas biperiden and ethopropazine failed to restore plasticity. The selectivity for M1 receptors was further demonstrated by their ability to counteract the M1-dependent potentiation of NMDA current recorded from striatal neurons.
Conclusions
Our study demonstrate that selective M1 muscarinic receptor antagonism offsets synaptic plasticity deficits in the striatum of mice with the DYT1 dystonia mutation, providing a potential mechanistic rationale for the development of improved antimuscarinic therapies for this movement disorder.
Keywords: dystonia, striatum, muscarinic receptor antagonists, synaptic plasticity
Introduction
DYT1 dystonia is a severe form of inherited dystonia caused by a deletion in the gene encoding the protein torsinA. Currently the medical therapy for this disorder is still largely unsatisfactory. Synaptic plasticity abnormalities have been demonstrated both in patients1–3 as well as at corticostriatal synapses of multiple rodent models, either over-expressing the human mutant torsinA4,5 or in knock-in mice heterozygous for mutant torsinA6. These synaptic alterations were normalized by antimuscarinic agents, indicating a fundamental involvement of striatal acetylcholine in the pathogenesis of this disorder. Accordingly, broad spectrum muscarinic receptor (mAChR) antagonists have long been used to treat dystonia, but their side effects have significantly reduced their use7. The exact mechanism of action of anticholinergic drugs in the relief of dystonic symptoms remains undetermined, although it is believed that their effect is centrally mediated and that restores the imbalance between striatal dopamine and acetylcholine.
The mAChRs are G-protein-coupled receptors subdivided into two major classes according to their pharmacological and signalling properties8. The M1, M3, and M5 mAChRs couple to the Gq/G11-type G-proteins, leading to IP3 and DAG formation, whereas the M2 and M4 mAChRs activate Gi/Go-type G-proteins, inhibiting adenylyl cyclase. M1 mAChRs are expressed on dendrites and spines of medium spiny neurons (MSNs), and are therefore strategically positioned to influence motor control and synaptic plasticity8–11. The lack of subtype-selective ligands for the mAChRs has prevented a more comprehensive understanding of the role of mAChR subtypes in distinct brain regions.
More recently, novel compounds have been developed, displaying higher selectivity for single mAChRs15.
We performed a systematic characterization of the effects of antimuscarinic agents on short- and long-term synaptic plasticity of MSNs recorded from mice with the DYT1 dystonia mutation. We characterized the effects of antimuscarinic drugs such as trihexyphenydil, orphenadrine, biperiden and ethopropazine, and compared these results with those obtained with the novel, selective M1 mAChR antagonist N-[3-oxo-3-[4-(4-pyridinyl)-1-piperazinyl]propyl]-2,1,3-benzothiadiazole-4-sulfonamide (VU0255035)16. Clarifying the functions of mAChRs in striatum could indicate the direction for a modern and selective strategy for pharmacological intervention in dystonia.
Methods
Tissue preparation and physiology
The Animal Care and Use Committee of “Tor Vergata” University approved all experiments, in accord with EC, Internal Institutional Review Committee, EU directive and Italian rules (86/609/EEC; D.Lvo 116/1992, 63/2100 EU, 153/2001A–IHM and 5/2010UV). All efforts were made to reduce the number of animals used. Colonies of both knock-in Tor1a+/Δgag mice heterozygous for ΔE-torsinA and their controls (Tor1a+/+ mice)17 were bred at our animal house. Genotyping was performed as indicated17. Parahorizontal corticostriatal slices (180–200 μm) were cut in Krebs’ solution4 (in mM: 126 NaCl, 2.5 KCl, 1.3 MgCl2, 1.2 NaH2PO4, 2.4 CaCl2, 10 glucose, 18 NaHCO3, 95% O2, 5% CO2). Slices were transferred into a recording chamber, superfused with oxygenated Krebs’ medium (32–33°C). In these slices a knife-cut was made between the striatum and the thalamus, to prevent contamination from thalamostriatal inputs4. Sharp-microelectrode recordings were performed blindly with 2M KCl (40–60 MΩ). Signal acquisition and off-line analysis were performed with an Axoclamp 2B amplifier and pClamp9 software (Molecular Devices). MSNs were identified according to their electrophysiological characteristics4,6. Glutamatergic excitatory postsynaptic potentials (EPSPs) were evoked with a bipolar electrode in the cortex (V–VI layer), in picrotoxin (50 μM) to block GABAA receptors6. Synaptic stimuli were delivered at 0.1 Hz, and 6 events were averaged. Paired-pulse facilitation was assessed by presenting two stimuli (ISI 50 ms) and measuring the ratio (EPSP2/EPSP1). One neuron per slice was used for plasticity experiments. For high-frequency stimulation (HFS, 3 trains: 3 sec, 100 Hz, 20 s interval), stimulus intensity was raised to spike threshold. EPSP amplitude was averaged and plotted as % of control before HFS. Magnesium was removed to optimize LTP induction18. Synaptic depotentiation (SD) was induced by a low-frequency stimulation (LFS) protocol (2 Hz, 10 min), ~30 min after LTP stabilization. Whole-cell recordings were performed as described19, with a Multiclamp 700b amplifier, using borosilicate glass pipettes (resistance range: 2.5–5 MΩ). Membrane currents were continuously monitored and access resistance measured was between 5–30 MΩ prior to electronic compensation (60–80% routinely used). For NMDA-mediated currents, pipettes contained (mM): K+-gluconate (125), NaCl (10), CaCl2 (1.0), MgCl2 (2.0), 1,2-bis (2-aminophenoxy) ethane-N,N,N,N-tetra-acetic acid (BAPTA) (1), Hepes (10), GTP (0.3) Mg-ATP (2.0); pH adjusted to 7.3 with KOH. Cells were clamped at an holding potential (HP) of −80 mV. Data were analyzed offline (Clampfit 10.2; MiniAnalysis 6.0, Synaptosoft; Prism 3.02, GraphPad).
Drug source and handling
Biperiden, ethopropazine, orphenadrine were from Sigma (Italy), pirenzepine from TOCRIS Bioscience (UK), VU0255035 was provided by Dr. Conn. Drugs were applied by bath perfusion.
Statistical analysis
Electrophysiological results are means ± SEM. Student’s t-test and non-parametric Mann-Whitney test were used to compare means pre- and post-HFS/drug. Analysis of variance (ANOVA) test with a post-hoc Tukey-test were performed among groups (p<0.05; α=0.01). P value <0.05 was considered statistically significant.
Results
Membrane and synaptic responses to antimuscarinic agents in striatal medium spiny neurons
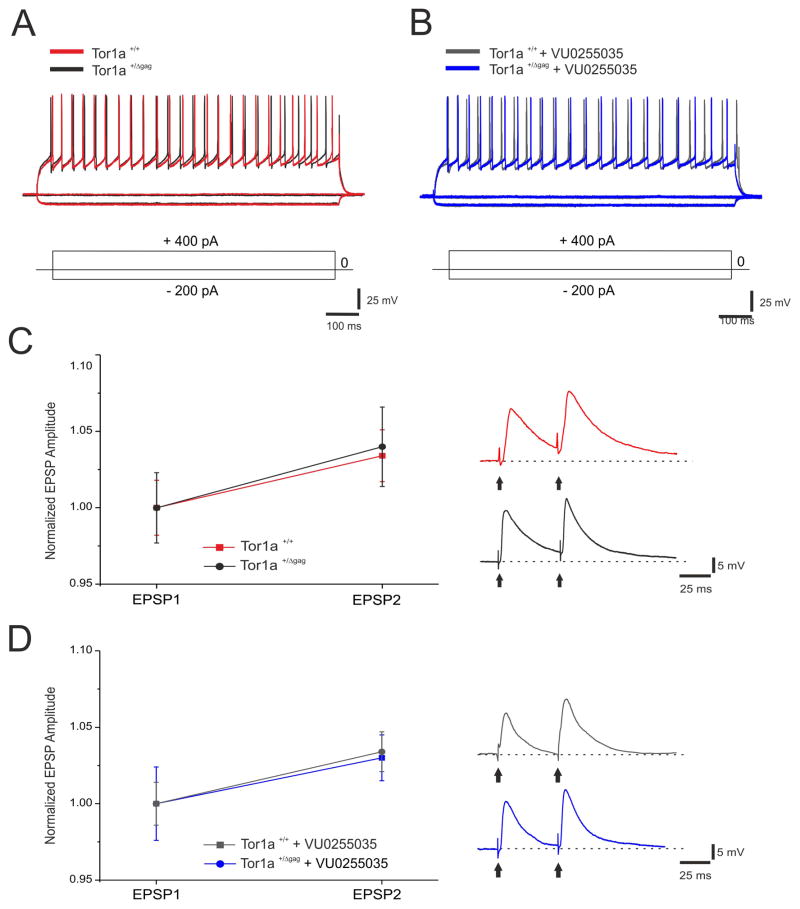
MSNs from both Tor1a+/+ and Tor1a+/Δgag mice had similar resting membrane potential, were silent at rest and, upon depolarizing current pulses showed membrane rectification and tonic action potential discharge6 (Fig. 1AC). Each of the tested drugs failed to modify intrinsic properties of MSNs (Suppl. Table 1). Then, we measured PPR as an indicator of presynaptic activity20. No significant differences in the PPR were found between Tor1a+/+ (Fig. 1C; n=8, 1.03±0.01%) and Tor1a+/Δgag neurons (Fig. 1C; n=9, 1.04±0.02%, p>0.05). The selective M1 mAChR antagonist, VU0255035 (0.05–1 μM), preserved the physiological I/V curve recorded in MSNs from both Tor1a+/+ (n=11) and Tor1a+/Δgag (n=15) mice (Fig 1B, p>0.05). Additionally, no difference in PPR was measured with VU0255035 (100–300 nM) in Tor1a+/+ (n=10, 1.02 ±0.01 %) and Tor1a+/Δgag (n=11, 1.03±0.02%) slices (Fig 1D, Suppl. Table 1; ANOVA p>0.05), indicating that M1 mAChR antagonism does not affect basal striatal glutamatergic transmission at the doses utilized.
Figure 1. Selective M1 mAChR antagonism does not modify intrinsic and synaptic properties in Tor1a+/+ and Tor1a+/Δgag. mice.
(A) Superimposed traces showing voltage responses to current steps in both depolarizing and hyperpolarizing direction from Tor1a+/+ (red, RMP=−89 mV) and Tor1a+/Δgag (black, RMP=−90 mV) MSNs. (B) Superimposed voltage responses to both depolarizing and hyperpolarizing current steps in MSN recorded from either Tor1a+/+ (grey, RMP=−89 mV) or Tor1a+/Δgag (blue, RMP =−89 mV) mice, in the presence of the selective M1 mAChR antagonist, VU0255035 (100 nM). (C) Paired-pulse facilitation (50 ms interstimulus interval) does not show significant differences between Tor1a+/+ and Tor1a+/Δgag mice. (Right) Representative paired recordings of EPSPs from both Tor1a+/+ and Tor1a+/Δgag MSNs (red and black, respectively). (D) Paired-pulse facilitation measured from both Tor1a+/+ and Tor1a+/Δgag MSNs is unaffected by VU0255035 (100 nM). (Right) Representative paired recordings of EPSPs from both genotypes MSNs (grey and blue, respectively). Each data point in the plot is the mean ± SEM of >8 independent recordings.
M1 mAChR antagonism rescues striatal synaptic plasticity in knock-in mice
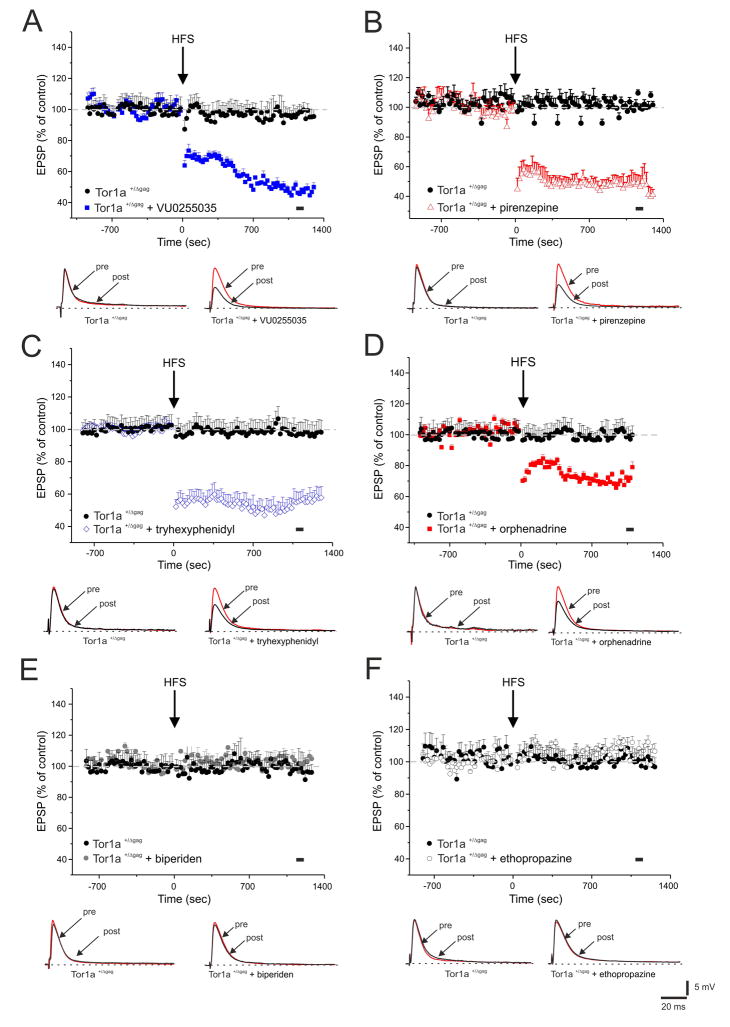
A bidirectional impairment of synaptic plasticity has been described in Tor1a+/Δgag mice6. As expected, HFS of corticostriatal afferents led invariably to the induction of long-term depression (LTD) in MSNs recorded from Tor1a+/+ mice (data not shown; n=12, 52.8 ±6.4% of control, measured 20 min post-HFS; t-test p<0.05), whereas HFS failed to elicit LTD in Tor1a+/Δgag MSNs (Fig. 2; n=10, 98.04 ±5.8% of control, 20 min post-HFS; t-test p>0.05). Endogenous acetylcholine modulates corticostriatal synaptic plasticity through M1 mAChRs11,21. Each of the mAChR antagonists tested did not affect physiological LTD in Tor1a+/+ mice (data not shown; VU0255035: n=6, 50.6 ±4.3%; t-test p<0.05; pirenzepine: n=6, 49.1 ±5.9%; t-test p<0.05; tryhexyphenidyl: n=5, 54.7 ±7.7%; Mann-Whitney: p<0.05; orphenadrine: n=6, 51.9 ±6.5%; t-test p<0.05; biperiden: n=5, 50.6 ±4.3%; Mann-Whitney: p<0.05; ethopropazine: n=6, 51.1 ±6.2%; t-test p<0.05).
Figure 2. Corticostriatal LTD is restored by M1-preferring mAChRs antagonists in Tor1a+/Δgag MSNs.
(A) HFS (arrow) fails to induce LTD in Tor1a+/Δgag mice (black circles), whereas preincubation with the selective M1 mAChR antagonist, VU0255035 (100 nM, 20 min, blue squares), fully restores LTD in Tor1a+/Δgag MSNs. (B, C) Similarly, M1-preferring antagonists, pirenzepine (100 nm, 20 min, red triangles) and tryhexyphenidyl (3 μM, 15 min, blue diamonds) rescue LTD in Tor1a+/Δgag mice. (D) Conversely, slice preincubation with orphenadrine (100 nM, 15 min, red squares), only partially offsets LTD deficits in Tor1a+/Δgag mice. (E, F) In slices treated with biperiden (20 μM, 20 min, grey filled circles) and ethopropazine (100 μM, 20 min, grey open circles), HFS fails to induce LTD. Below each plot, superimposed traces of EPSPs recorded before (pre) and 20 min after HFS (post). The black spot indicates at which time point samples were measured. Each data point represents the mean ± SEM of ≥10 independent observations.
Conversely, in Tor1a+/Δgag mice, anticholinergic agents displayed a distinct profile, according to their ability to antagonize M1 mAChRs, without affecting basal EPSP amplitude. The novel selective M1 mAChRs antagonist, VU0255035 (100 nM, 20 min) fully restored LTD (Fig. 2A; n=16, 47.9 ±6.2%; t-test p<0.05). Similarly, both M1-preferring antagonists pirenzepine (100 nM, 20 min) and trihexyphenidyl (3 μM, 20 min) were able to rescue a physiological LTD in Tor1a+/Δgag MSNs (Fig. 2B,C; pirenzepine: n=12, 48.2 ±6.6%; t-test p<0.05; tryhexyphenidyl: n=13, 53.87 ±5.4%; Mann-Whitney: p<0.05). Conversely, the non-selective mAChR antagonist orphenadrine (100 nM, 20 min) produced only a partial rescue of LTD in MSNs from Tor1a+/Δgag mice (Fig. 2D; n=12, 69.57 ±2.5%; t-test p<0.05). Additionally, both biperiden (10–50 μM, 20 min) and ethopropazine (100 μM, 20 min) failed to restore LTD in Tor1a+/Δgag mice (Fig 2E,F; biperiden: n=10, 105.8 ±4.7%; ethopropazine: n=10, 102.2 ±6.9%; Mann-Whitney: p>0.05 for both).
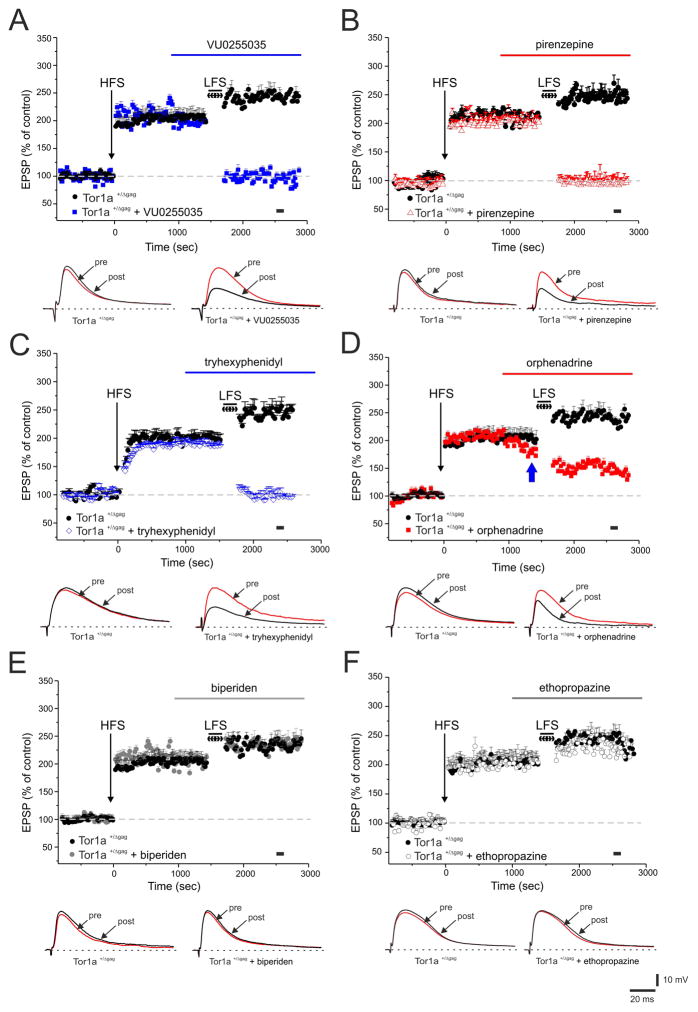
A physiological LTP was measured in Tor1a+/+ mice (data not shown, n=6, 170.9 ±8.3% of control, measured 20 min post-HFS; t-test p<0.05), whereas an LTP of significantly increased amplitude was recorded in MSNs from Tor1a+/Δgag mice (Fig. 3; n=10, 202.6 ±6.2% of control, 20 min post-HFS; Mann-Whitney: p<0.05). Once LTP is stabilized, a LFS protocol reverts synaptic activity to resting levels, a phenomenon termed synaptic depotentiation (SD)22. LFS caused a normal SD in Tor1a+/+ mice (data not shown, n=6, 105.5 ±7.9% of control, 10 min post-LFS; t-test p<0.05) but was unable to depotentiate corticostriatal synapses in Tor1a+/Δgag MSNs (Fig. 3; n=10, 242.4 ±7.8% of control, 10 min post-LFS; Mann-Whitney: p<0.05).
Figure 3. Antagonists of mAChRs restore synaptic depotentiation (SD) in Tor1a+/Δgag MSNs.
(A) After LTP induction, a low-frequency stimulation, LFS (2 Hz, 10 min) protocol fails to induce SD in Tor1a+/Δgag MSNs (black circles). Before LFS, bath-application of VU0255035 (100 nM, blue bar) restores SD in Tor1a+/Δgag mice. (B, C) Similarly, both pirenzepine (100 nM, red triangles) and tryhexyphenidyl (3 μM, blue diamonds), restore SD. Conversely, bath-applied orphenadrine (100 nM, red bar) causes only a partial rescue of SD in Tor1a+/Δgag MSNs (D) (blue arrow indicates the decrease in EPSP amplitude by orphenadrine). Biperiden (20 μM, grey filled circles) and ethopropazine (100 μM, grey open circles), fail to restore the LFS-induced SD (E, F). Below each plot representative EPSPs recorded before (pre) and 15 min after (post) LFS protocol. The black spot indicates at which time point samples were measured. Each data point represents the mean ± SEM of ≥8 independent observations.
In Tor1a+/+ mice, a physiological SD was measured, without significant effects by the tested drugs (data not shown; VU0255035: n=5, 98.8 ±6.2%; Mann-Whitney: p<0.05; pirenzepine: n=6, 99.8 ±9.1%; t-test p<0.05. tryhexyphenidyl: n=5, 100.3 ±4.9%; Mann-Whitney: p<0.05; biperiden: n=5, 101.1 ±5.5%; Mann-Whitney: p<0.05; ethopropazine: n=6, 102.9 ±8.2%; t-test p<0.05). However, in knock-in mice, VU0255035 (100 nM, 20 min) was able to completely rescue SD in Tor1a+/Δgag mice (Fig. 3A; n=10, 95.1 ±6.8%; Mann-Whitney: p<0.05) as well as both pirenzepine (100 nM, 20 min) and trihexyphenidyl (3 μM, 20 min) (Fig 3B,C; pirenzepine: n=10, 96.4 ±8.9%; t-test p<0.05; tryhexyphenidyl: n=8, 102.87 ±9.36%; Mann-Whitney: p<0.05).
Orphenadrine has been shown to inhibit NMDA responses23. Indeed, when bath-applied in low-magnesium solution, which relieves the Mg2+-dependent NMDA receptor blockade18, orphenadrine (n=6), prior to LFS protocol, reduced the amplitude of the recorded EPSP (Fig. 3D, blue arrow; 30% of control). Under these conditions, LFS caused a partial SD, although this could well be related to the NMDA antagonism; therefore, although a significant difference emerges compared to the pre-LFS values (Fig 3D; n=8, 153.19 ±5.9%; t-test p<0.05), the efficacy of orphenadrine in rescuing SD cannot be ascribed solely to muscarinic antagonism.
SD deficit was not normalized by treatment with both biperiden (20 μM, 20 min) and ethopropazine (100 μM, 20 min) in Tor1a+/Δgag mice (Fig 3E,F; biperiden: n=6, 231.5 ±5.02%; t-test p>0.05; ethopropazine: n=6, 228.1 ±9.3%; t-test p>0.05).
Muscarinic antagonists preferentially target M1 mAChR-dependent responses in striatal MSNs
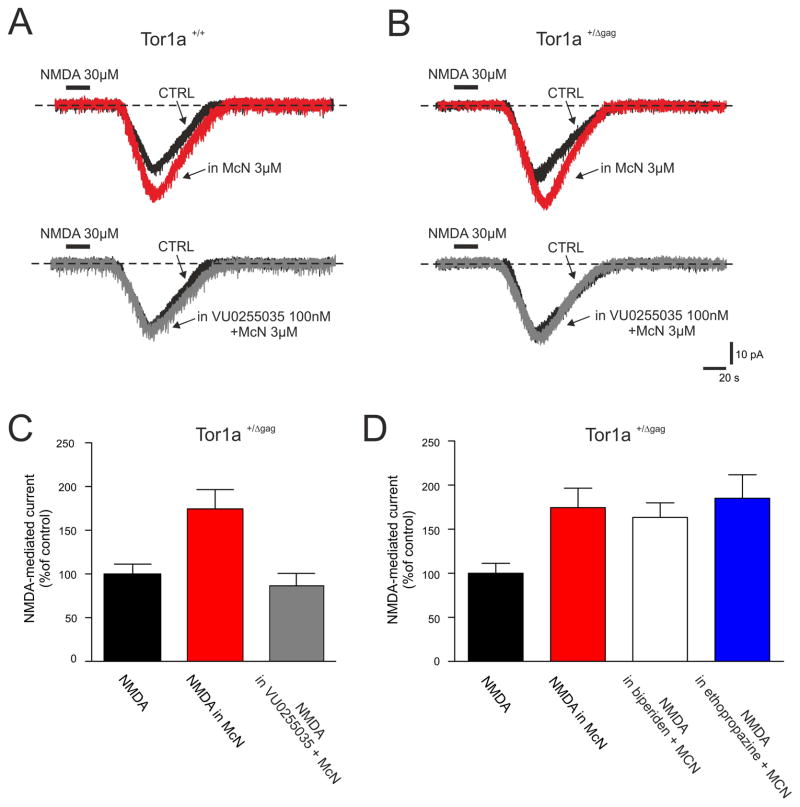
Selective activation of M1 mAChRs results in the potentiation of NMDA receptor-mediated currents in MSNs24. To demonstrate the selectivity of VU0255035, we recorded NMDAR-mediated currents in MSNs from both Tor1a+/+ and Tor1a+/Δgag mice (Fig. 4). Whole-cell recordings were performed in picrotoxin (50 μM) plus TTX (1 μM). Bath-application of NMDA (20 s, 30 μM) produced a transient and reversible inward current in MSNs recorded from both Tor1a+/+ (n=7) and Tor1a+/Δgag (n=10) mice (Fig. 4A,B). Preincubation with the selective M1 mAChR agonist McN-A-343 (3 μM), potentiated the NMDA-induced current in all the recorded MSNs from both Tor1a+/+ (n=7) and Tor1a+/Δgag (n=10) mice (Fig. 4A–C; 175.2 ±6.8%; 174.5 ±6.3%, respectively; p<0.05). In the presence of 100 nM VU0255035 (15 min), the McN-A-343-induced potentiation of NMDA currents was fully prevented in MSNs from both Tor1a+/+ and Tor1a+/Δgag mice (Fig. 4A–C; 83.7 ±3.9% and 86.6 ±3.9%, respectively; p>0.05). Similarly, in both genotypes, pirenzepine completely prevented the potentiation of NMDA-mediated currents caused by McN-A-343 (3 μM; 86 ±3.4%, n=5; p>0.05; not shown).
Figure 4. VU0255035 prevents the M1 mAChR-mediated potentiation of NMDA-responses in MSNs.
(A, B) Whole-cell recordings in the voltage-clamp mode, showing that bath-applied NMDA (30 μM, 20 s) induces an inward current in the recorded MSN from both Tor1a+/+ and Tor1a+/Δgag mice. In both genotypes, pretreatment with the selective M1 mAChR agonist McN-A-343 (3 μM, red traces) significantly potentiates the NMDA-induced current, which returns to control levels in the presence of VU0255035 (100 nM, grey traces). H.P.: −80 mV. (C, D). Summary plots of the effects (expressed as % of the NMDA-induced control current) of anticholinergic agents on the McN-A-343-induced potentiation of NMDA currents in Tor1a+/Δgag mice.
In keeping with the hypothesis that selective M1 antagonism normalizes synaptic plasticity deficits, we performed similar whole-cell recordings with biperiden, ethopropazine and orphenadrine. In Tor1a+/Δgag MSNs, both biperiden and ethopropazine failed to prevent the enhancement of NMDA-mediated currents caused by McN-A-343 (Fig. 4D; biperiden: 163.3 ±4.6%; n=6; p<0.05; ethopropazine: 185.1 ±7.5%; n=6; p<0.05). A final set of recordings was performed with orphenadrine, which acts as non-competitive NMDA antagonist in cultured neurons23. Orphenadrine (100 nM, 10–15 min) per sè reduced significantly NMDA currents in MSNs (not shown, 44.7 ±3.1% inhibition of NMDA-induced current; n=7; p<0.05). Such inhibitory effect did not allow to test further its potential efficacy on M1-induced potentiation.
Anticholinergics do not affect the M2/M4-mediated responses in striatal neurons
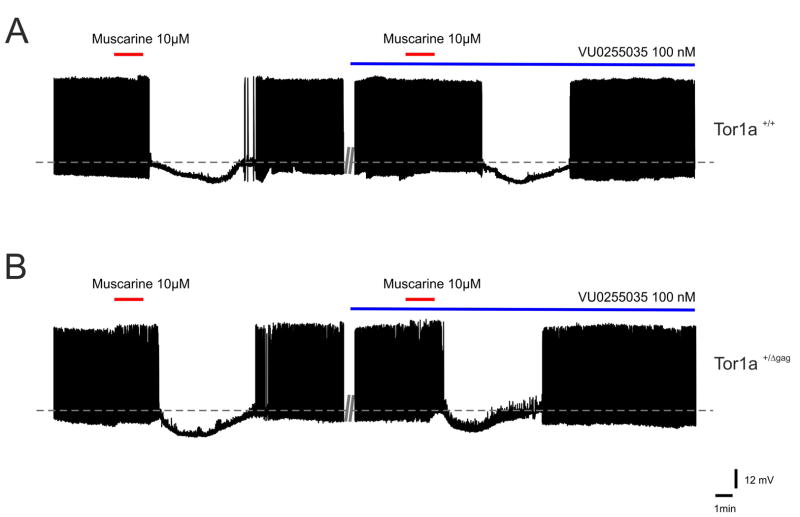
M2/M4 mAChRs mediate two responses in distinct striatal neuron subtypes. In cholinergic interneurons, M2/M4 autoreceptor activation generates a membrane hyperpolarization/outward current, by closing CaV2 Ca2+ channels, and by increasing opening of Kir3 potassium channels, which hyperpolarize terminals and further reduce Ca2+ channel opening25. In addition, the cortical glutamatergic drive to MSNs is reduced by presynaptic M2/M4 mAChRs located at corticostriatal terminals14,26. This latter effect was excluded since no change in EPSP amplitude was measured, as described above for plasticity experiments. Then, we tested the effects of antimuscarinic agents on cholinergic interneurons, identified by means of their electrophysiological properties27,28. Intrinsic membrane properties of these interneurons were not significantly different between genotypes, as reported6. In cells from both strains, muscarine (10 μM, 90s) induced a membrane hyperpolarization and interrupted their firing activity, an effect that was blocked by the M2/M4 receptor-preferring antagonist methoctramine (300 nM, 10 min; not shown). No significant difference was measured between genotypes, showing that the muscarinic autoreceptor function is preserved in Tor1a+/Δgag mice. To exclude a non-specific involvement of M2/M4 mAChRs, we tested pirenzepine, trihexyphenydil and VU0255035 on the response to muscarine. VU0255035 affected neither the intrinsic properties nor the inhibitory response to muscarine, ruling out a recruitment of M2/M4 mAChRs (Fig. 5; n=6, p>0.05). Similar results were obtained with pirenzepine and tryhexyphenydil (n=4 for each drug, p>0.05; not shown).
Figure 5. VU0255035 does not affect M2/M4 mAChR-mediated responses.
(A, B) Sample traces of striatal cholinergic interneurons recorded from both genotypes, showing a typical spontaneous, pacemaking firing activity. Bath-application of muscarine (10 μM, 90 s) causes a transient membrane hyperpolarization and cessation of firing activity in the recorded interneurons, recorded from both Tor1a+/+ and Tor1a+/Δgag mice. This inhibitory response, mediated by M2/M4 mAChRs, is not prevented by pretreatment with VU0255035 (100 nM) in both strains of mice.
Discussion
Anticholinergic treatments are used in different forms of dystonia, although the existing drugs are non-selective muscarinic antagonists, associated with a broad array of undesirable central and peripheral side effects7,29. Therefore, it is important to develop a detailed understanding of the roles of individual mAChR subtypes in basal ganglia function. Surprisingly, few studies analyzed the mechanism of action of this class of drugs, despite the evidence that it still represents one of the few medical options for the treatment of dystonia7,29. Our study demonstrates that M1 mAChR antagonism offsets striatal synaptic plasticity deficits in mice with the DYT1 dystonia mutation, providing a potential mechanistic rationale for the development of improved antimuscarinic therapies for this movement disorder.
Striatal acetylcholine and dystonia
Acetylcholine plays key roles in the striatal regulation of normal voluntary movement, as well as the motor dysfunction that occurs in different movement disorders, such as Parkinson’s disease and dystonia30. The striatum contains a small percentage of interneurons which provide this area with one of the highest levels of acetylcholine in the brain31. Besides the local striatal innervation, a number of cholinergic projections arise from nuclei of the basal forebrain, as well as from the pedunculopontine-lateral dorsal tegmental nuclei32, therefore caution is required in assuming that striatal mAChR antagonism might fully explain the therapeutic efficacy of antimuscarinic agents. However, emerging common themes in DYT1 dystonia indicate that disruption of synaptic plasticity processes represents a reproducible feature in multiple models, and might underlie the motor learning abnormalities observed in patients1–3. In different rodent models of DYT1 dystonia, striatal MSNs exhibit a loss of LTD, whereas LTP is enhanced in magnitude. Low-frequency stimulation (LFS) can normally revert potentiated synapses to resting levels, a phenomenon termed synaptic depotentiation (SD)22. However, LFS fails to induce SD both in mice and rats overexpressing mutant torsinA, as well as in DYT knock-in mice4–6. Collectively, a loss of inhibitory plasticity phenomena (LTD and SD) and concomitant increase in LTP amplitude emerges from these models. Consistently, the current pathophysiological hypothesis for dystonia suggests that dystonia may result from a deficient “surround inhibition” of competing motor patterns, coupled to an enhanced plasticity in motor areas33,34. In such context, cholinergic transmission is profoundly involved, as it plays a key role in the impairment of corticostriatal synaptic plasticity4–6,35.
Muscarinic receptor antagonism in dystonia
MAChR subtypes M1, M2 and M4, appear to be dominant in the striatum12,13. MSNs express primarily M1, variable levels of M4, but very low levels of M2, M3 and M5 mAChRs10. Cholinergic interneurons by contrast have dominant expression and function of M2 and M4 mAChRs11,36. Some of the adverse effects of mAChR inhibitors are mediated by peripheral M2 and M3 mAChRs, whereas M1 is responsible for the effects on cognition and motor function8,9,11,37. M1 receptor activation increases MSN excitability by reducing KCNQ and Kir2 currents38–40, and exert a central role in striatal long-term plasticity11,21.
Our results indicate that M1 mAChR antagonism is specifically required to offset plasticity deficits in mice with mutant torsinA. Although a morphological characterization was not performed between MSNs of the direct and indirect pathways, MSNs express homogeneously M1 mAChRs and no specific difference was measured in their responses to muscarinic agents. Moreover, we had previously shown that the vast majority of morphologically-identified MSNs had a similar response to muscarinic modulation4. At the doses utilized, trihexyphenydil, pirenzepine, VU0255035 restored a normal LTD, as well as SD. Conversely, biperiden and ethopropazine failed to rescue LTD and SD. Orphenadrine should not be considered in this experimental setting, since its antagonistic action on NMDA receptor function prevents a reliable evaluation23. We further demonstrated the specificity of effect on M1 mAChR by testing their ability to prevent the M1–dependent potentiation of NMDA currents. Only VU0255035, pirenzepine and tryhexyphenydil blocked the enhancement induced by M1 agonists, whereas biperiden, ethopropazine were ineffective. Accordingly, VU0255035 antagonizes potentiating NMDA receptor currents induced by muscarinic agonists in hippocampal CA1 cells16.
Additionally, we also ruled out a potential involvement of M2/M4 mAChRs, by testing antimuscarinic agents on the autoreceptor response that was unaffected by all the drugs tested. Although firing activity of other striatal interneurons41,42 may partially resemble that of cholinergic interneurons, we are confident that the electrophysiological and pharmacological profile allows to unequivocally identify cholinergic interneurons. Moreover, these drugs failed to affect the presynaptic inhibition by M2/M4 mAChRs14,26,43, indicating the specificity for M1 mAChRs. Dystonic reactions can also appear as a consequence of treatment with neuroleptic drugs44. Recently, in a model of haloperidol-induced catalepsy, anticholinergic drugs displaying a higher affinity for M1 mAChRs were more potent in counteracting catalepsy45. Collectively, our data suggest that irrespective of the specific form of dystonia, M1 mAChRs may represent a preferred target for relieving dystonic symptoms.
A note of caution is required in translating these effects into their therapeutic efficacy. Indeed, the observation that biperiden, orphenadrine and ethopropazine were unable to rescue synaptic plasticity is in disagreement with their clinical efficacy. Our work does not address this issue, but demonstrates that their mechanism of action cannot be attributed to a M1 mAChR antagonism, rather it may rely on their ability to act at different transmitter receptors simultaneously. This is consistent with previous observations reporting their anti- NMDA, anti-histamine and anti-nicotinic receptor properties7,46,47. Further work is required to address these issues.
In conclusion, our results indicate that subtype selectivity will be crucial to achieving clinical efficacy without adverse effects.
Supplementary Material
Intrinsic membrane properties of medium spiny neurons recorded from both genotypes in the presence of muscarinic receptor (mAChR) antagonists.
Acknowledgments
Support grants: Ministero Salute (Progetto Finalizzato RF-2010-2311657 to AP, G.R. to GS), Dystonia Medical Research Foundation (DMRF) and Federation for Dystonia Research (FDR) to AP, Italian Ministry of Instruction, University and Research (PRIN 2011 to AP; FIRB to GS); COST (European Cooperation in Science and Technology) Action BM1101 (AP) for support in promoting networking; grants from NIMH and the NIH Common fund to P.J.C.
We wish to thank Mr. Massimo Tolu and Vladimiro Batocchi for their excellent technical support.
Footnotes
Conflict of interest: nothing to report.
Author roles: AP, PB (P. Bonsi): designed and wrote the paper. MM, GM (G. Madeo), GM (G. Martella), IF, AT, GP, GS performed the experiments and statistical analysis. MM, GP prepared illustrations. PJC, PB (P. Burbaud): reviewed critically the manuscript providing comments and revisions.
Financial disclosures: AP is employee at the University of Rome “Tor Vergata”, Italy; PB (P. Bonsi) is employee at Fondazione Santa Lucia; PB (P. Burbaud) is employee at Université Victor Segalen, Bordeaux, France; PJC is employee at Vanderbilt University, Nashville, USA; other authors: none. PJC is an inventor on multiple patents protecting allosteric modulators of muscarinic acetylcholine receptors, including patents that are licensed to AstraZeneca. PJC receives research support from AstraZeneca and Bristol Myers Squibb; AP, PB hold grants that are not related to the subject of the present study.
References
- 1.Ghilardi MF, Carbon M, Silvestri G, et al. Impaired sequence learning in carriers of the DYT1 dystonia mutation. Ann Neurol. 2003;54:102–9. doi: 10.1002/ana.10610. [DOI] [PubMed] [Google Scholar]
- 2.Edwards MJ, Huang YZ, Mir P, et al. Abnormalities in motor cortical plasticity differentiate manifesting and nonmanifesting DYT1 carriers. Mov Disord. 2006;21:2181–6. doi: 10.1002/mds.21160. [DOI] [PubMed] [Google Scholar]
- 3.Quartarone A, Pisani A. Abnormal plasticity in dystonia: Disruption of synaptic homeostasis. Neurobiol Dis. 2011;42(2):162–70. doi: 10.1016/j.nbd.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 4.Martella G, Tassone A, Sciamanna G, et al. Impairment of bidirectional synaptic plasticity in the striatum of a mouse model of DYT1 dystonia: role of endogenous acetylcholine. Brain. 2009;132:2336–2349. doi: 10.1093/brain/awp194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grundmann K, Glöckle N, Martella G, et al. Generation of a novel rodent model for DYT1 dystonia. Neurobiol Dis. 2012;47:61–74. doi: 10.1016/j.nbd.2012.03.024. [DOI] [PubMed] [Google Scholar]
- 6.Martella G, Maltese M, Nisticò R, et al. Regional specificity of synaptic plasticity deficits in a knock-in mouse model of DYT1 dystonia. Neurobiol Dis. 2014;65:124–32. doi: 10.1016/j.nbd.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 7.Jankovic J. Treatment of dystonia. Lancet Neurol. 2006;5:864–72. doi: 10.1016/S1474-4422(06)70574-9. [DOI] [PubMed] [Google Scholar]
- 8.Wess J, Eglen RM, Gautam D. Muscarinic acetylcholine receptors: mutant mice provide new insights for drug development. Nat Rev Drug Discov. 2007;6:721–733. doi: 10.1038/nrd2379. [DOI] [PubMed] [Google Scholar]
- 9.Conn PJ, Jones CK, Lindsley CW. Subtype-selective allosteric modulators of muscarinic receptors for the treatment of CNS disorders. Trends Pharmacol Sci. 2009;30(3):148–55. doi: 10.1016/j.tips.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hersch SM, Gutekunst CA, Rees HD, et al. Distribution of m1-m4 muscarinic receptor proteins in the rat striatum: light and electron microscopic immunocytochemistry using subtype-specific antibodies. J Neurosci. 1994;14:3351–63. doi: 10.1523/JNEUROSCI.14-05-03351.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonsi P, Martella G, Cuomo D, et al. Loss of muscarinic autoreceptor function impairs long-term depression, but not long-term potentiation in the striatum. J Neurosci. 2008;28:6258–63. doi: 10.1523/JNEUROSCI.1678-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang W, Basile AS, Gomeza J, et al. Characterization of central inhibitory muscarinic autoreceptors by the use of muscarinic acetylcholine receptor knock-out mice. J Neurosci. 2002;22:1709–1717. doi: 10.1523/JNEUROSCI.22-05-01709.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou FM, Wilson CJ, Dani JA. Cholinergic interneuron characteristics and nicotinic properties in the striatum. J Neurobiol. 2002;53:590–605. doi: 10.1002/neu.10150. [DOI] [PubMed] [Google Scholar]
- 14.Pancani T, Bolarinwa C, Smith Y, et al. M4 mAChR-Mediated Modulation of Glutamatergic Transmission at Corticostriatal Synapses. ACS Chem Neurosci. 2014;5(4):318–24. doi: 10.1021/cn500003z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiang Z, Thompson AD, Jones CK, et al. Roles of the M1 muscarinic acetylcholine receptor subtype in the regulation of basal ganglia function and implications for the treatment of Parkinson’s disease. J Pharmacol Exp Ther. 2012;340(3):595–603. doi: 10.1124/jpet.111.187856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sheffler DJ, Williams R, Bridges TM, et al. A novel selective muscarinic acetylcholine receptor subtype 1 antagonist reduces seizures without impairing hippocampus-dependent learning. Mol Pharmacol. 2009;76(2):356–68. doi: 10.1124/mol.109.056531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodchild RE, Kim CE, Dauer WT. Loss of the dystonia-associated protein torsinA selectively disrupts the neuronal nuclear envelope. Neuron. 2005;48(6):923–32. doi: 10.1016/j.neuron.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 18.Calabresi P, Pisani A, Mercuri NB, et al. Long-term potentiation in the striatum is unmasked by removing the voltage-dependent magnesium block of NMDA receptor channels. Eur J Neurosci. 1992;4:929–35. doi: 10.1111/j.1460-9568.1992.tb00119.x. [DOI] [PubMed] [Google Scholar]
- 19.Sciamanna G, Bonsi P, Tassone A, et al. Impaired striatal D2 receptor function leads to enhanced GABA transmission in a mouse model of DYT1 dystonia. Neurobiol Dis. 2009;34(1):133–45. doi: 10.1016/j.nbd.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schulz PE, Cook EP, Johnston D. Changes in paired-pulse facilitation suggest presynaptic involvement in long-term potentiation. J Neurosci. 1994;14(9):5325–5337. doi: 10.1523/JNEUROSCI.14-09-05325.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Z, Kai L, Day M, et al. Dopaminergic control of corticostriatal long-term synaptic depression in medium spiny neurons is mediated by cholinergic interneurons. Neuron. 2006;50(3):443–52. doi: 10.1016/j.neuron.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 22.Rioult-Pedotti MS, Donoghue JP, Dunaevsky A. Plasticity of the synaptic modification range. J Neurophysiol. 2007;98(6):3688–95. doi: 10.1152/jn.00164.2007. [DOI] [PubMed] [Google Scholar]
- 23.Kornhuber J, Parsons CG, Hartmann S, et al. Orphenadrine is an uncompetitive N-methyl-D-aspartate (NMDA) receptor antagonist: binding and patch clamp studies. J Neural Transm Gen Sect. 1995;102(3):237–46. doi: 10.1007/BF01281158. [DOI] [PubMed] [Google Scholar]
- 24.Calabresi P, Centonze D, Gubellini P, et al. Endogenous ACh enhances striatal NMDA-responses via M1-like muscarinic receptors and PKC activation. Eur J Neurosci. 1998;10(9):2887–95. doi: 10.1111/j.1460-9568.1998.00294.x. [DOI] [PubMed] [Google Scholar]
- 25.Yan Z, Surmeier DJ. Muscarinic (m2/m4) receptors reduce N- and P-type Ca2+ currents in rat neostriatal cholinergic interneurons through a fast membrane-delimited, G-protein pathway. J Neurosci. 1996:2592–2604. doi: 10.1523/JNEUROSCI.16-08-02592.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pakhotin P, Bracci E. Cholinergic interneurons control the excitatory input to the striatum. J Neurosci. 2007;27:391–400. doi: 10.1523/JNEUROSCI.3709-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawaguchi Y. Physiological, morphological, and histochemical characterization of three classes of interneurons in rat neostriatum. J Neurosci. 1993;13:4908–4923. doi: 10.1523/JNEUROSCI.13-11-04908.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sciamanna G, Tassone A, Mandolesi G, et al. Cholinergic dysfunction alters synaptic integration between thalamostriatal and corticostriatal inputs in DYT1 dystonia. J Neurosci. 2012;32(35):11991–2004. doi: 10.1523/JNEUROSCI.0041-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katzenschlager R, Sampaio C, Costa J, et al. Anticholinergics for symptomatic management of Parkinson’s disease. Cochrane Database Syst Rev. 2003;(2):CD003735. doi: 10.1002/14651858.CD003735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pisani A, Bernardi G, Ding J, et al. Re-emergence of striatal cholinergic interneurons in movement disorders. Trends Neurosci. 2007;30:545–53. doi: 10.1016/j.tins.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 31.Bolam JP, Wainer BH, Smith AD. Characterization of cholinergic interneurons in the rat neostriatum. A combination of choline acetyltransferase immunocytochemistry, Golgi-impregnation and electron microscopy. Neuroscience. 1984;12:711–718. doi: 10.1016/0306-4522(84)90165-9. [DOI] [PubMed] [Google Scholar]
- 32.Everit BJ, Robbins TW. Central cholinergic systems and cognition. Ann Rev Psychol. 1997;48:649–684. doi: 10.1146/annurev.psych.48.1.649. [DOI] [PubMed] [Google Scholar]
- 33.Mink JW. The Basal Ganglia and involuntary movements: impaired inhibition of competing motor patterns. Arch Neurol. 2003;60(10):1365–8. doi: 10.1001/archneur.60.10.1365. [DOI] [PubMed] [Google Scholar]
- 34.Sohn YH, Hallett M. Disturbed surround inhibition in focal hand dystonia. Ann Neurol. 2004;56(4):595–9. doi: 10.1002/ana.20270. [DOI] [PubMed] [Google Scholar]
- 35.Dang MT, Yokoi F, Cheetham CC, et al. An anticholinergic reverses motor control and corticostriatal LTD deficits in Dyt1 ΔGAG knock-in mice. Behav Brain Res. 2012;226:465–72. doi: 10.1016/j.bbr.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ding J, Guzman JN, Tkatch T, et al. RGS4-dependent attenuation of M4 autoreceptor function in striatal cholinergic interneurons following dopamine depletion. Nat Neurosci. 2006;9(6):832–42. doi: 10.1038/nn1700. [DOI] [PubMed] [Google Scholar]
- 37.Bymaster FP, McKinzie DL, Felder CC, et al. Use of M1-M5 muscarinic receptor knockout mice as novel tools to delineate the physiological roles of the muscarinic cholinergic system. Neurochem Res. 2003;28(3–4):437–42. doi: 10.1023/a:1022844517200. [DOI] [PubMed] [Google Scholar]
- 38.Galarraga E, Hernandez-Lopez S, Reyes A, et al. Cholinergic modulation of neostriatal output: a functional antagonism between different types of muscarinic receptors. J Neurosci. 1999;19:3629–3638. doi: 10.1523/JNEUROSCI.19-09-03629.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shen W, Hamilton SE, Nathanson NM, et al. Cholinergic suppression of KCNQ channel currents enhances excitability of striatal medium spiny neurons. J Neurosci. 2005;25:7449–58. doi: 10.1523/JNEUROSCI.1381-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shen W, Tian X, Day M, et al. Cholinergic modulation of Kir2 channels selectively elevates dendritic excitability in striatopallidal neurons. Nat Neurosci. 2007;10(11):1458–66. doi: 10.1038/nn1972. [DOI] [PubMed] [Google Scholar]
- 41.Beatty JA, Sullivan MA, Morikawa H, Wilson CJ. Complex autonomous firing patterns of striatal low-threshold spike interneurons. J Neurophysiol. 2012;108(3):7. doi: 10.1152/jn.00283.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ibáñez-Sandoval O, Tecuapetla F, Unal B, Shah F, Koós T, Tepper JM. Electrophysiological and morphological characteristics and synaptic connectivity of tyrosine hydroxylase-expressing neurons in adult mouse striatum. J Neurosci. 2010;30(20):6999–7016. doi: 10.1523/JNEUROSCI.5996-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Calabresi P, Centonze D, Gubellini P, et al. Activation of M1-like muscarinic receptors is required for the induction of corticostriatal LTP. Neuropharmacology. 1999;38:323–6. doi: 10.1016/s0028-3908(98)00199-3. [DOI] [PubMed] [Google Scholar]
- 44.Teo JT, Edwards MJ, Bhatia K. Tardive dyskinesia is caused by maladaptive synaptic plasticity: a hypothesis. Mov Disord. 2012;27(10):1205–15. doi: 10.1002/mds.25107. [DOI] [PubMed] [Google Scholar]
- 45.Erosa-Rivero HB, Bata-García JL, Alvarez-Cervera FJ, et al. The potency and efficacy of anticholinergics to inhibit haloperidol-induced catalepsy in rats correlates with their rank order of affinities for the muscarinic receptor subtypes. Neuropharmacology. 2014;81:176–87. doi: 10.1016/j.neuropharm.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 46.Horn S, Comella CL. Treatment of dystonia. In: Jankovic J, Tolosa E, editors. Parkinson’s disease and movement disorders. 5. 2007. pp. 348–355. [Google Scholar]
- 47.Lang AE, Lees A. Anticholinergic therapies in the treatment of Parkinson’s disease. Management of Parkinson’s disease: an evidence-based review. Mov Disord. 2002;17 (Suppl 4):S7–S12. doi: 10.1002/mds.5556. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Intrinsic membrane properties of medium spiny neurons recorded from both genotypes in the presence of muscarinic receptor (mAChR) antagonists.