Abstract
We previously reported that ethanol (EtOH) stimulates endothelial angiogenic activity mediated via a Notch- and Angiopoientin-1 pathway. As crosstalk exists between Notch and vascular endothelial growth factor (VEGF) signaling, we examined whether the VEGF receptor Flk-1 mediates ethanol-stimulated Notch signaling and angiogenic activity.
Methods and Results
Treatment of human coronary artery endothelial cells (HCAEC) with EtOH (1–50 mM, 24 h) dose-dependently increased Flk-1 expression with a maximum increase observed at 25 mM EtOH. Ethanol treatment activated both Flk-1 and Flt-1 as indicated by their phosphorylation, and subsequent stimulation of Akt. EtOH activation of Flk-1 was inhibited by the VEGFR inhibitor SU5416. Gene silencing of Flk-1 using siRNA inhibited the EtOH-induced increase in Notch receptor 1 and 4 and Notch target gene (Hrt-1) mRNA. Knockdown of Flk-1 inhibited ethanol-induced Angiopoietin-1/Tie2 mRNA expression and blocked EtOH-induced HCAEC network formation on Matrigel, a response that was restored by Notch ligand, DII4, treatment. In vivo, moderate alcohol feeding increased vascular remodeling in mouse ischemic hindlimb.
Conclusions
These data demonstrate that ethanol activates Flk1 and Flt-1 receptors in HCAEC and promotes angiogenic activity via a Flk1/Notch pathway. These effects of ethanol may be relevant to the influence of moderate alcohol consumption on cardiovascular health.
Keywords: Alcohol, Angiogenesis, Vascular Endothelial, Notch, VEGF receptor, Vascular remodeling
Introduction
While the incidence of cardiovascular disease and morbidity increases with heavy alcohol (ethanol) consumption and/or binge drinking, studies indicate that moderate consumption is protective [1] [2] [3]. The precise cell targets and signaling mechanisms mediating these effects of ethanol are not fully elucidated. Angiogenesis, i.e., new blood vessel formation, is essential in both normal and pathological physiology and can be beneficial or deleterious depending on the circumstance [4]. With respect to cardiovascular disease, new vessel formation following heart attack or stroke is believed desirous as it can compensate for the resulting loss of perfusion and ischemia [4]. Stimulation of angiogenesis by ethanol in the setting of atherosclerotic-induced occlusion, therefore, represents a potential novel mechanism that may contribute, in part, to its cardiovascular protective effects.
Emerging evidence indicates that the Notch signaling pathway plays an important role in remodeling of the adult vasculature in general, and in angiogenesis in particular [5, 6] [7]. Indeed, adult vascular endothelial cells, whose migration, proliferation, differentiation and structural rearrangement are central to the angiogenic process, express Notch receptors (predominately Notch 1 and 4) though little is known to date of their regulation and function in this particular cell type. In general, upon ligand binding, the Notch receptor is cleaved allowing the intracellular portion (NIC) to translocate to the nucleus where it de-represses transcription and modulates the expression of Notch target genes that regulate cell fate decisions. These target genes include the `Hairy Enhancer of Split' (hes) gene and HES-related transcription factors (Hrts) [8, 9]. We have previously demonstrated that ethanol, at levels consistent with moderate consumption, stimulates endothelial angiogenic activity in vitro via a novel Notch/Angiopoietin-1/Tie-2-dependent pathway [5].
The vascular endothelial growth factor (VEGF) family of ligands and receptors are established regulators of angiogenesis [10, 11]. VEGF-A, B, C and D are ligands for receptor tyrosine kinases VEGFR1 (fms-like tryrosine kinase 1, Flt-1) and VEGFR2 (kinase domain-containing receptor, KDR, also called Flk-1). VEGF-A, the major ligand for angiogenesis, binds and activates both VEGFR1/Flt-1 and VEGFR2/Flk-1. On ligand binding, VEGF receptors initiate autophosphorylation and induce tyrosine kinase activity, stimulating cellular responses including the PLCγ-PKC-Raf kinase-MEK-MAPK pathway (stimulating cell growth) and the PI3K-Akt pathway (promoting cell survival) [12]. Both stimulatory and inhibitory effects of alcohol on the VEGF-VEGFR axis have been previously reported [13, 14]. Moreover, numerous studies in mammalian cell culture and in vivo support extensive crosstalk between Notch and VEGF [7, 15] [16]. Building on these reports in the literature and our previous studies, we investigated the involvement of the VEGF pathway in ethanol stimulated endothelial cell angiogenic activity. We report here that ethanol activates Flk-1 and Flt-1 receptors and promotes angiogenic activity via a Flk1/Notch pathway. These data add new mechanistic information as to how ethanol affects vascular endothelial cells and may be relevant to the cardiovascular protective effect of moderate alcoholic beverage consumption.
Materials and Methods
Cell Culture
Human Coronary Artery Endothelial Cells (HCAEC) were obtained from Lonza (Walkersville, MD) and cultured in optimized endothelial cell medium (CloneticsR, Lonza). Cells were assessed for endothelial cell phenotype by morphology and for the expression of von Willebrand Factor antigen and platelet-endothelial cell adhesion molecule (PECAM). HCAEC between passages 3 and 12 were used in all experiments. EtOH, (200 proof, ACS/USP Grade, Pharmco Products, Brookfield, CT) was diluted in media to achieve the desired concentrations (1 – 50 mM) before being added to HCAEC in culture for either 24h (protein and mRNA expression analysis) or 5 – 30 min (receptor activation experiments) as indicated. Alternatively, HCAEC were treated with hrVEGF165 (30 ng/ml, R&D Systems, Minneapolis, MN) for 5 – 30 mins.
Quantitative real-time RT-PCR
Total RNA (0.5–1 μg), isolated from cells using Qiagen RNeasy kit (Valencia, CA) was reverse-transcribed using iscript™ cDNA Synthesis kit from BIO-RAD (Carlsbad, CA). The gene-specific oligonucleotide sequences were designed using NCBI/Primer blast and primers that specifically spanned exon-exon junctions, utilized to eliminate genomic DNA contamination. Comparative Ct analysis using Syber Green-based Real-Time RT-PCR was performed where calibrator controls were selected using the Stratagene MX3005 machine and the SYBER green jumpstart PCR kit (Sigma, St. Louis, MO) as described by the manufacturer.
Western Blot Analysis
Proteins from cell lysates (12–15 μg) were resolved on SDS-PAGE (12% resolving, 5% stacking) prior to transfer onto nitrocellulose membrane (Amersham Biosciences, Piscataway, NJ). Membranes were stained with Ponceau S and probed for Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) to ensure equal protein loading and transfer and rinsed in wash buffer (PBS containing 0.05% Tween-20) before being probed using commercially available antibodies from Abcam (Cambridge, MA). All primary antibody dilutions were 1:500 (overnight) with corresponding 1:10,000 secondary dilutions (2 h). Following secondary antibody incubation, membranes were rinsed in wash buffer (PBS containing 0.05% Tween-20) before being stained with Clarity™ ECL Western Substrate Solution (Bio-Rad, Hercules, CA, USA). Membranes were then analyzed for protein expression using the Chemidoc™ XRS+ System (Bio-Rad, Hercules, CA, USA). Band Intensity was quantified and normalized for protein concentration (GAPDH) using the Image Lab™ software (Bio-Rad, Hercules, CA, USA).
Angiogenic Activity (Network Formation on Matrigel)
96-well tissue culture plates were coated with BD Matrigel™ (100μL per well, Becton–Dickinson, Franklin lakes, NJ, USA), which was allowed to solidify at 37°C for 30min before plating the cells. HCAEC (3 × 103 cells), which had been treated with or without ethanol for 24 h, were then plated at 125 μL per well onto the surface of the Matrigel and incubated at 37° C. After 16 h, the cells were photographed with the use of a CCD digital camera (Spot RT, Diagnostics Instruments, Inc., Sterling Heights, MI, USA) at 4X magnification. Network formation was quantified by measuring the length of the network of connected cells in each well by drawing a line over them and measuring the length of the line in pixels with the use of SpotSoftware Version 4.6 (Diagnostic Instruments, Inc.) essentially as described by us previously [5, 17]. In parallel experiments Angiotensin-1 and Tie-2 mRNA and protein expression was also determined as an additional measurement of angiogenic activity.
siRNA Transfection
For gene silencing studies, the Gene Pulser XcellTM system (Bio-Rad, Hercules, CA, USA) was used for transient transfection of HCAEC with gene-specific siRNA (60–70% transfection efficiency achieved). Briefly, 2 × 105 cells were transfected with 2 μg of siRNA targeting Flk-1 (Ambion, Austin, TX, USA) or a scrambled negative control siRNA (Ambion, cat #4611) in 75μL of siRNA electroporation buffer. Following transfection, cells were treated with or without ethanol for 24 h and/or Notch ligand Delta like ligand 4 (DII4, 2.0 μg).
Alcohol gavage
8–10 wk old male C57BL6 mice were divided into 2 groups; `control' and `moderate ethanol.' For 1 week the `moderate ethanol' group received by oral gavage 0.8 g/kg of 200 proof ethanol in a total volume of 200 μl water, giving a peak BAC of ~15 mM [18], whereas the `control' group were gavaged daily with an isocaloric water/cornstarch mixture. After the 1 week equilibration period, hindlimb ischemia was achieved in all mice (as described below), and the control or alcohol feeding regimens continued up to 7 days post surgery.
Hindlimb ischemia was achieved in mice by unilateral ligation of the femoral artery [19]. The common femoral artery was ligated twice proximal to the popliteal and the caudal femoral artery. The epigastric artery was also ligated. After confirming successful limb ischemia by checking the severe paleness of the foot, the overlying skin was closed with sutures. Monitoring of hindlimb blood flow. Before, and at day 3 and 7 after femoral ligation, ischemic (right)/normal (left) limb blood flow ratio was measured by use of a Moor Infrared Laser Doppler Imager (MOORLDI12-IR, Moor Instruments Inc, Wilmington, DE). Before scanning was initiated, mice were placed on a heating plate at 37°C to minimize temperature variation. After Laser Doppler color images had been recorded twice, the average perfusion of the ischemic and nonischemic limbs was calculated on the basis of colored histogram pixels. The data was analyzed with Moor LDI image processing software V3.09. Perfusion was expressed as the ratio of flow in the ischemic/nonischemic hindlimb after background subtraction.
Data Analysis
Results are expressed as mean ± SEM. Experimental points were performed in triplicate, with a minimum of 3 independent experiments. For hind limb ischemia study, 4–6 mice were used per experimental point. An ANOVA test was performed on network formation data and a Wilcoxon Signed rank test was used for comparison of two groups when compared to normalized control. A value of p≤0.05 was considered significant.
Results
Ethanol increases expression and activates Flk-1 in HCAEC
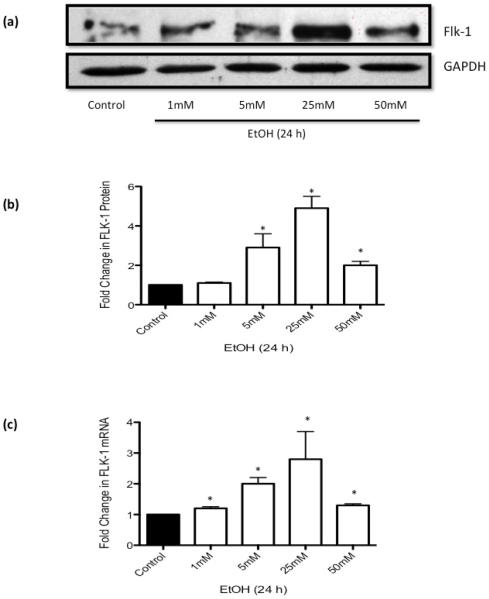
Exposure of HCAEC to ethanol (EtOH 1-50 mM, 24 h) did not affect VEGF-A levels (data not shown), but dose-dependently increased Flk-1 (i.e., VEGFR2) expression in the moderate range up to 25 mM while showing only a subtle increase at the higher dose of 50 mM (Figure 1). Thus, a maximum response was observed at 25 mM EtOH with a 4.9±0.7 and 2.8±0.9 fold increase in Flk-1 protein and mRNA expression, respectively. Flt1 (VEGFR1) mRNA expression was also increased by EtOH, but to a lesser extent (data not shown). EtOH (1-50 mM, 24 h) had no effect on HCAEC viability as determined by trypan blue exclusion (data not shown).
Figure 1. Ethanol Stimulates Flk-1 Expression in HCAEC.
HCAEC were treated with or without ethanol (EtOH, 1–50mM) for 24 h. (a) Representative western blots and (b) cumulative data, showing changes in Flk-1 protein expression following ethanol treatment. (c) QRTPCR analysis of Flk-1 mRNA expression following ethanol treatment. Data were normalized to GAPDH and represent the mean ± SEM values from four independent experiments. * P <0.05 vs. control.
Exposure of HCAEC to VEGF (hrVEGF165, 30 ng/ml, 5 and 10 min) caused the expected activation of Flk-1 as evidence by increased Flk-1 Tyr1054 and Tyr1059 phosphorylation on Western Blot (Figure 2). Acute EtOH treatment (25 mM, 5 and 10 min) activated both Flk-1 and Flt-1 receptors in these cells (Figure 2). Interestingly, combined EtOH/VEGF treatment at the same concentrations and time showed no effect on Flk1 activation (Figure 2). The VEGF receptor inhibitor SU5416 significantly attenuated the ethanol-induced Flk-1 receptor activation (Figure 2 c). In parallel cultures, EtOH treatment stimulated AKT, as indicated by increased AKTSer473 phosphorylation, to an extent greater than the stimulation by VEGF treatment (Figure 2b). In addition, there was no increase in p-AKT, compared to control, with combined EtOH/VEGF treatment (Figure 2d).
Figure 2. Ethanol Activates Flt-1 and Flk-1 receptors in HCAEC.
(a) Representative western blot data showing phospho-Flk-1 following Ethanol (25mM), VEGF (30ng) or combined Ethanol/VEGF treatment for 0 (control), 5, 10 and 30 mins. Representative western blot of (b) phospho-Flt-1 +/− EtOH, and (c) EtOH-induced p-Flk1 expression, in the absence or presence of the VEGF receptor inhibitor SU5416 (10 μM, 30 min pretreatment). (d) Phospho-AKT following Ethanol (25mM), VEGF (30ng) or combined Ethanol/VEGF treatment for 0 (control), 5, 10 and 30 mins.
Flk-1 knockdown inhibits ethanol-stimulated Notch signaling in HCAEC
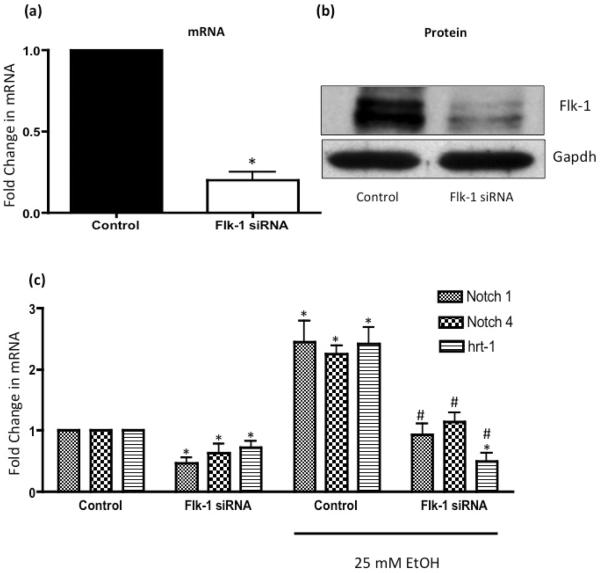
We previously reported that EtOH stimulates Notch expression and signaling in human umbilical vein endothelial cells [5]. Gene silencing of Flk-1 in HCAEC with specific targeted siRNA duplexes was confirmed at the mRNA and protein level; > 80% decrease in Flk-1 mRNA and protein expression when compared to scrambled controls (Figure 3a, b). Flk1 siRNA significantly decreased Notch receptors 1 and 4, and Notch target gene Hrt1 mRNA in control, untreated HCAEC (Figure 3c). Moreover, Flk-1 knockdown strongly attenuated ethanol-induced Notch 1 and 4 and Hrt-1 mRNA expression in these cells (Figure 3c).
Figure 3. Flk-1 Mediates Ethanol-stimulated Notch Signaling in HCAEC.
HCAEC transfected with scrambled RNA (Control), or with an siRNA targeted to Flk-1 were treated with or without EtOH (25mM, 24 h) before mRNA expression for Notch 1, 4 and Hrt-1 was determined. (a) Cumulative data for Flk-1 mRNA expression and (b) Representative western blot data in HCAEC transfected with scrambled RNA (Control), or with an siRNA targeted to Flk-1. (c) QRTPCR analysis of Notch 1, 4 and Hrt-1 mRNA expression following ethanol treatment in HCAEC transfected with scrambled RNA (Control), or with an siRNA targeted to Flk-1. Data were normalized to GAPDH and represent the mean + SEM values from four independent experiments. *P< 0.05 vs. scrambled control, #P<0.05 vs EtOH Control.
Ethanol-induced angiogenic response is Flk-1-dependent
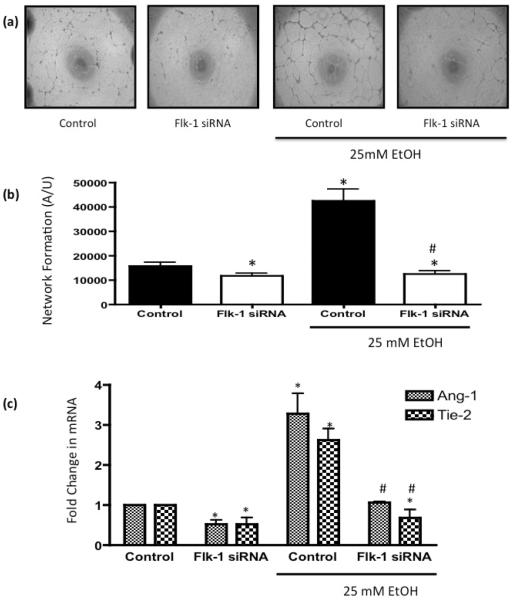
EtOH treatment (25 mM, 24h) significantly stimulated HCAEC angiogenic activity as indicated by increased network formation on Matrigel (Figure 4). As an in vivo correlate, we used the mouse hindlimb ischemia model. There was significantly greater perfusion in the ischemic hindlimbs of moderate ethanol gavaged mice compared to controls, at 3 days and 7 days post femoral artery ligation, as determined by Laser Doppler imaging (Figure 5), indicative of increased vascular remodeling which may include angiogenesis. Knockdown of Flk-1 with siRNA attenuated network formation compared to cells treated with scrambled RNA control, and completely inhibited ethanol-stimulated HCAEC network formation (Figure 4a, b). Moreover, Flk-1 siRNA reduced Angiopoetin-1 (Ang-1) and Tie-2 mRNA expression in control HCAEC by 51.0±5 % and 50.0±7% respectively and completely inhibited ethanol-induced Ang-1/Tie-2 mRNA expression (Figure 4c).
Figure 4. Flk-1 Gene Knockdown inhibits Ethanol-induced Angiogenic Activity.
HCAEC transfected with scrambled RNA (Control), or with an siRNA targeted to Flk-1 were treated with or without EtOH (25mM, 24 h) before (a, b) network formation on Matrigel was assessed (representative images and cumulative data from 3 separate experiments conducted in triplicate was shown. (c) Ang-1 and Tie-2 mRNA levels were analyzed by QRTPCR in HCAEC transfected with scrambled RNA (Control), or with an siRNA targeted to Flk-1 treated with or without EtOH (25mM, 24 h). Data were normalized to GAPDH and represent the mean + SEM values from three independent experiments. *P< 0.05 vs. scrambled control, #P>0.05 vs EtOH control.
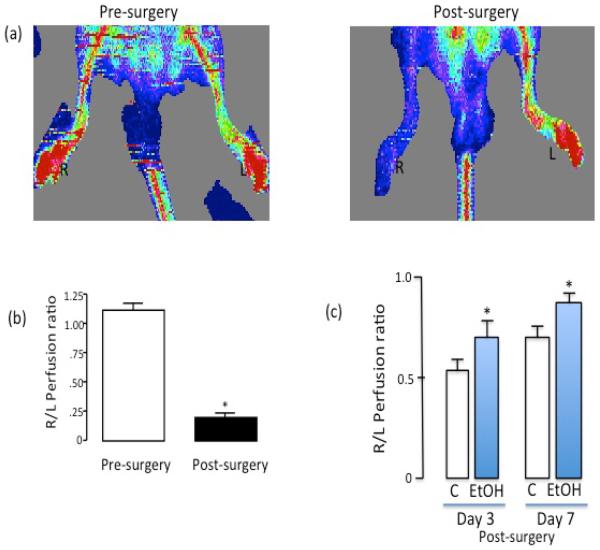
Figure 5.
(a) Representative laser doppler flow images of a C57Bl/6 mouse pre- and post-surgery (i.e., femoral artery ligation). R (right) and L (left) foot. Low perfusion is depicted as blue color, whereas red color represents high perfusion. (b) Changes in perfusion after ligation shown as a ratio of right to left hindlimb (R/L ratio). Cumulative data, n=3. (c) Greater perfusion 3 days and 7 days post femoral artery ligation in moderate ethanol fed animals, compared to controls. n=4–6. *p<0.05 vs control.
Flk-1 mediates ethanol-stimulated HCAEC angiogenic activity via a Notch-dependent pathway
Treatment with Notch ligand Delta like ligand 4 (DII4, 2.0 μg, 24 h) partly recovered the Flk-1 siRNA inhibition of ethanol-induced network formation (Figure 6). In parallel cultures, treatment with DII4 recovered the Flk-1 siRNA inhibition of ethanol-induced expression of Notch 1 and 4 and Ang-1/Tie-2 mRNA. These data indicate that Flk-1 mediates ethanol-stimulated HCAEC Notch signaling and angiogenic activity, and that Notch is downstream of VEGF.
Figure 6. Flk-1 Mediates Ethanol–stimulated HCAEC Angiogenic Activity Via a Notch-dependent Pathway.
HCAEC transfected with scrambled RNA (Control), or with an siRNA targeted to Flk-1 were treated with or without EtOH (25mM, 24 h) and/or DLL4 (2.0μg, 24 h) before (A and B) network formation on Matrigel was assessed (representative images and cumulative data from 3 separate experiments conducted in triplicate was shown). (C) Notch 1 and 4, Hrt-1, Ang-1 and Tie-2 mRNA levels were analyzed by QRTPCR in HCAEC transfected with scrambled RNA (Control), or with an siRNA targeted to Flk-1 treated with or without EtOH (25mM, 24 h) and/or DLL4 (2.0μg, 24 h). Data were normalized to GAPDH and represent the mean + SEM values from three independent experiments. *P< 0.05 vs. scrambled control, #P>0.05 vs Flk-1 siRNA/EtOH.
Discussion
While heavy and/or binge drinking is deleterious to health, epidemiological studies describe moderate or `low-risk' drinking (defined for women as no more than 3 drinks on any single day and no more than 7 drinks per week; NIAAA) as a negative risk factor for atherosclerosis and its clinical consequences, heart attack and stroke [3]. In the USA, 1 unit of alcohol (i.e., 12 oz beer, 5 oz wine or 1.5 oz of liquor) contains 14g of pure alcohol. Moderate alcohol consumption in the range 1–3 drinks per day gives rise to blood alcohol levels of 5–25 mM [1] [20]. The main type of alcohol found in alcoholic beverages is ethanol (EtOH) and a better understanding of the cellular and molecular mechanisms mediating its effects is beginning to emerge. Here we report that EtOH, at concentrations found in moderate drinkers, increases angiogenesis in vivo and stimulates the expression and activation of the VEGF receptor Flk-1/KDR in cultured human coronary artery endothelial cells in vitro. Moreover, Flk-1 is critical in mediating EtOH-stimulated Notch signaling and subsequent angiogenic activity in these cells. These effects of ethanol may be relevant to the influence of moderate alcohol consumption on cardiovascular health.
Migration and proliferation of endothelial cells is critical to angiogenesis which is associated with a variety of pathologies and can be beneficial or harmful, depending on the clinical situation [4]. Thus, control of angiogenesis represents an area of considerable therapeutic potential. With regards to cardiovascular disease, the role of angiogenesis is complex and context-dependent. For example, atherosclerotic plaque angiogenesis seems to characterize the inflammatory, more vulnerable plaque and a positive association between neovessel density and plaque rupture has been reported [21]. In contrast, coronary collateral angiogenesis in response to occlusion ischemia can compensate for loss of perfusion following myocardial infarct or stroke and in this way can protect tissues from ischemic damage [22] [23]. Clinical observations indicate that the extent of collateralization among patients with cardiovascular disease varies considerably [23–25], with the factors responsible for this variation unclear. An understanding of the contributing causes, which may include genetic elements or lifestyle habits such as drinking, is therefore desirable. Indeed, recent research studies support a modulatory effect of alcohol consumption on neovessel formation in the heart. Lassaletta et al., reported that diet supplementation with moderate doses of EtOH increased arteriolar density and improved myocardial perfusion in chronically ischemic swine myocardium [26]. Louboutin et al., reported in a rat model that alcohol feeding stimulated VEGF production, increased the number of capillaries in the heart, and protected the heart from myocardial ischemia/reperfusion injury [27]. The effects of ethanol in that study were inhibited by VEGF neutralizing antibody [27]. Similarly, our data in the hindlimb ischemia mouse model suggests that ethanol feeding increases vascular remodeling in response to ischemia, which is usually a combination of arteriogenesis and angiogenesis, as indicated by significantly greater perfusion post ligation in the moderate ethanol fed group compared to controls (no alcohol). In apparent contrast, ethanol consumption reportedly diminished the protective effect of estrogen on infarct size and myocardial capillary density in ovariectomized mice [28]. Despite these contradictory reports, available evidence supports a modulatory effect of alcohol on angiogenesis in the heart.
Several other groups have also investigated the effect of ethanol on angiogenesis, not only in the context of cardiovascular disease but also in tumorogenesis and wound healing, in a variety of in vitro and in vivo models. We previously reported that EtOH, at levels consistent with moderate consumption, stimulated endothelial cell growth and migration, key processes in angiogenesis [5]. In that study, ethanol promoted angiogenic activity in human umbilical vein endothelial cells by stimulating a novel Notch/Angiopoietin-1 pathway [5]. These in vitro data, and the new data in human coronary artery endothelial cells presented here, are in general agreement with the in vivo studies mentioned above demonstrating a stimulatory effect of alcohol consumption on myocardial neovessel development [26, 27] and with several other studies reporting a stimulatory effect of EtOH on angiogenesis, some looking at tumor angiogenesis [29] [30] [31] [13]. At least one group describes an inhibitory effect of EtOH on angiogenesis in the context of wound healing [14] [32]. These apparently contradictory findings are likely due to differing experimental doses, exposure times and/or models used. Nevertheless, our data support a stimulatory effect of ethanol on endothelial cell (both HUVEC and HCAEC) angiogenic activity as determined by Matrigel in vitro angiogenesis assay which models the tube formation `morphogenesis' stage of angiogenesis [33].
The VEGF family of ligands and receptors are established regulators of angiogenesis [10, 11] and both stimulatory and inhibitory effects of EtOH on VEGF have been reported (e.g., [13, 27, 32]). Given that there is evidence of extensive crosstalk between Notch and VEGF signaling pathways [15] [7] [34] [35] [16] [36], we investigated the possibility that stimulation of Notch and subsequent angiogenesis in endothelial cells by EtOH is mediated via its effect on VEGF. Ethanol dose-dependently increased Flk-1 expression, in the absence of any effect on VEGF-A levels, and activated Flk-1 and downstream Akt. Gene knockdown experiments illustrated the critical requirement for Flk-1 in mediating the EtOH-induced Notch signaling and endothelial angiogenic response. Moreover, treatment with a Notch ligand partly recovered the EtOH Notch and angiogenic responses in cells in which Flk-1 was knocked down illustrating the directional hierarchy of the pathway; i.e., VEGF upstream of Notch.
While others have also demonstrated a stimulatory effect of EtOH on VEGF and on VEGF receptor expression [37] [38] [13], few have reported whether EtOH acts to stimulate Flk-1 receptor activation like our data indicate. Precisely how EtOH activates Flk-1, an effect blocked by the VEGFR inhibitor SU5416, is not clear at present. Of note EtOH stimulates endothelial nitric oxide synthase activity resulting in increased NO [39]. Nitric oxide has previously been reported to cause Flk-1 receptor phosphorylation in cardiomyocytes [40]. Thus, EtOH activation of the Flk-1 receptor in HCAEC could be an indirect effect via NO. Moreover, we found it interesting that despite their individual stimulatory effects, a combination of both ethanol and VEGF had essentially no effect on Flk-1 receptor activation/auto-phosphorylation. The reasons for this unexpected result are not obvious at present. It is possible that some direct chemical interaction occurs between the two agonists that negates an agonist effect of either at the Flk-1 receptor. Alternatively, because EtOH promotes the proangiogenic action of Angiopoietin-1 and Angiopoietins have been shown to inhibit VEGF-A actions in endothelial cells [41], it is also possible that Ang-1 inhibits VEGF activation of Flk-1 when EtOH is present. Further investigation is warranted to fully understand this effect.
In conclusion, our findings illuminate a critical role for the VEGF receptor 2 (Flk-1/KDR) in mediating the stimulatory effect of ethanol on Notch signaling and subsequent angiogenic activity in HCAEC. This mechanistic information adds to our understanding of how alcohol affects vascular endothelial cells whose function is critical not only in angiogenesis but also in vascular homeostasis and vascular pathologies such as atherosclerosis.
Acknowledgements
This study was supported in part by funds from the National Institutes of Health (RO1AA12610 and R21AA020365 to E.M.R. and R00HL095650 to D.M.) and the Science Foundation Ireland (SFI-11PI/1128 to P.A.C).
References
- 1.Thun MJ, Peto R, Lopez AD, Monaco JH, Henley SJ, Heath CW, Jr., Doll R. Alcohol consumption and mortality among middle-aged and elderly u.S. Adults. N Engl J Med. 1997;337:1705–1714. doi: 10.1056/NEJM199712113372401. [DOI] [PubMed] [Google Scholar]
- 2.Pearson TA. Alcohol and heart disease. Circulation. 1996;94:3023–3025. doi: 10.1161/01.cir.94.11.3023. [DOI] [PubMed] [Google Scholar]
- 3.Di Castelnuovo A, Costanzo S, Bagnardi V, Donati MB, Iacoviello L, de Gaetano G. Alcohol dosing and total mortality in men and women: An updated meta-analysis of 34 prospective studies. Arch Intern Med. 2006;166:2437–2445. doi: 10.1001/archinte.166.22.2437. [DOI] [PubMed] [Google Scholar]
- 4.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 5.Morrow D, Cullen JP, Cahill PA, Redmond EM. Ethanol stimulates endothelial cell angiogenic activity via a notch- and angiopoietin-1-dependent pathway. Cardiovasc Res. 2008;79:313–321. doi: 10.1093/cvr/cvn108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ridgway J, Zhang G, Wu Y, Stawicki S, Liang WC, Chanthery Y, Kowalski J, Watts RJ, Callahan C, Kasman I, Singh M, Chien M, Tan C, Hongo JA, de Sauvage F, Plowman G, Yan M. Inhibition of dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature. 2006;444:1083–1087. doi: 10.1038/nature05313. [DOI] [PubMed] [Google Scholar]
- 7.Takeshita K, Satoh M, Ii M, Silver M, Limbourg FP, Mukai Y, Rikitake Y, Radtke F, Gridley T, Losordo DW, Liao JK. Critical role of endothelial notch1 signaling in postnatal angiogenesis. Circ Res. 2007;100:70–78. doi: 10.1161/01.RES.0000254788.47304.6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lai EC. Keeping a good pathway down: Transcriptional repression of notch pathway target genes by csl proteins. EMBO Rep. 2002;3:840–845. doi: 10.1093/embo-reports/kvf170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iso T, Kedes L, Hamamori Y. Hes and herp families: Multiple effectors of the notch signaling pathway. J Cell Physiol. 2003;194:237–255. doi: 10.1002/jcp.10208. [DOI] [PubMed] [Google Scholar]
- 10.Breen EC. Vegf in biological control. J Cell Biochem. 2007;102:1358–1367. doi: 10.1002/jcb.21579. [DOI] [PubMed] [Google Scholar]
- 11.Yla-Herttuala S, Rissanen TT, Vajanto I, Hartikainen J. Vascular endothelial growth factors: Biology and current status of clinical applications in cardiovascular medicine. J Am Coll Cardiol. 2007;49:1015–1026. doi: 10.1016/j.jacc.2006.09.053. [DOI] [PubMed] [Google Scholar]
- 12.Ellis LM. The role of neuropilins in cancer. Mol Cancer Ther. 2006;5:1099–1107. doi: 10.1158/1535-7163.MCT-05-0538. [DOI] [PubMed] [Google Scholar]
- 13.Tan W, Bailey AP, Shparago M, Busby B, Covington J, Johnson JW, Young E, Gu JW. Chronic alcohol consumption stimulates vegf expression, tumor angiogenesis and progression of melanoma in mice. Cancer Biol Ther. 2007;6:1211–1217. doi: 10.4161/cbt.6.8.4406. [DOI] [PubMed] [Google Scholar]
- 14.Radek KA, Matthies AM, Burns AL, Heinrich SA, Kovacs EJ, Dipietro LA. Acute ethanol exposure impairs angiogenesis and the proliferative phase of wound healing. Am J Physiol Heart Circ Physiol. 2005;289:H1084–1090. doi: 10.1152/ajpheart.00080.2005. [DOI] [PubMed] [Google Scholar]
- 15.Liu ZJ, Shirakawa T, Li Y, Soma A, Oka M, Dotto GP, Fairman RM, Velazquez OC, Herlyn M. Regulation of notch1 and dll4 by vascular endothelial growth factor in arterial endothelial cells: Implications for modulating arteriogenesis and angiogenesis. Mol Cell Biol. 2003;23:14–25. doi: 10.1128/MCB.23.1.14-25.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morrow D, Cullen JP, Liu W, Guha S, Sweeney C, Birney YA, Collins N, Walls D, Redmond EM, Cahill PA. Sonic hedgehog induces notch target gene expression in vascular smooth muscle cells via vegf-a. Arterioscler Thromb Vasc Biol. 2009;29:1112–1118. doi: 10.1161/ATVBAHA.109.186890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morrow D, Cullen JP, Cahill PA, Redmond EM. Cyclic strain regulates the notch/cbf-1 signaling pathway in endothelial cells: Role in angiogenic activity. Arterioscler Thromb Vasc Biol. 2007;27:1289–1296. doi: 10.1161/ATVBAHA.107.142778. [DOI] [PubMed] [Google Scholar]
- 18.Morrow D, Cullen JP, Liu W, Cahill PA, Redmond EM. Alcohol inhibits smooth muscle cell proliferation via regulation of the notch signaling pathway. Arterioscler Thromb Vasc Biol. 2010;30:2597–2603. doi: 10.1161/ATVBAHA.110.215681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tirziu D, Moodie KL, Zhuang ZW, Singer K, Helisch A, Dunn JF, Li W, Singh J, Simons M. Delayed arteriogenesis in hypercholesterolemic mice. Circulation. 2005;112:2501–2509. doi: 10.1161/CIRCULATIONAHA.105.542829. [DOI] [PubMed] [Google Scholar]
- 20.Mukamal KJ, Conigrave KM, Mittleman MA, Camargo CA, Jr., Stampfer MJ, Willett WC, Rimm EB. Roles of drinking pattern and type of alcohol consumed in coronary heart disease in men. N Engl J Med. 2003;348:109–118. doi: 10.1056/NEJMoa022095. [DOI] [PubMed] [Google Scholar]
- 21.Moreno PR, Purushothaman KR, Fuster V, Echeverri D, Truszczynska H, Sharma SK, Badimon JJ, O'Connor WN. Plaque neovascularization is increased in ruptured atherosclerotic lesions of human aorta: Implications for plaque vulnerability. Circulation. 2004;110:2032–2038. doi: 10.1161/01.CIR.0000143233.87854.23. [DOI] [PubMed] [Google Scholar]
- 22.Koerselman J, van der Graaf Y, de Jaegere PP, Grobbee DE. Coronary collaterals: An important and underexposed aspect of coronary artery disease. Circulation. 2003;107:2507–2511. doi: 10.1161/01.CIR.0000065118.99409.5F. [DOI] [PubMed] [Google Scholar]
- 23.Rubanyi GM. Mechanistic, technical, and clinical perspectives in therapeutic stimulation of coronary collateral development by angiogenic growth factors. Mol Ther. 2013;21:725–738. doi: 10.1038/mt.2013.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koerselman J, de Jaegere PP, Verhaar MC, Grobbee DE, van der Graaf Y. Coronary collateral circulation: The effects of smoking and alcohol. Atherosclerosis. 2007;191:191–198. doi: 10.1016/j.atherosclerosis.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 25.Hansen JF. Coronary collateral circulation: Clinical significance and influence on survival in patients with coronary artery occlusion. Am Heart J. 1989;117:290–295. doi: 10.1016/0002-8703(89)90771-0. [DOI] [PubMed] [Google Scholar]
- 26.Lassaletta AD, Elmadhun NY, Liu Y, Feng J, Burgess TA, Karlson NW, Laham RJ, Sellke FW. Ethanol promotes arteriogenesis and restores perfusion to chronically ischemic myocardium. Circulation. 2013;128:S136–143. doi: 10.1161/CIRCULATIONAHA.112.000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Louboutin JP, Marusich E, Gao E, Agrawal L, Koch WJ, Strayer DS. Ethanol protects from injury due to ischemia and reperfusion by increasing vascularity via vascular endothelial growth factor. Alcohol. 2012;46:441–454. doi: 10.1016/j.alcohol.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 28.Mackie AR, Krishnamurthy P, Verma SK, Thorne T, Ramirez V, Qin G, Abramova T, Hamada H, Losordo DW, Kishore R. Alcohol consumption negates estrogen-mediated myocardial repair in ovariectomized mice by inhibiting endothelial progenitor cell mobilization and function. J Biol Chem. 2013;288:18022–18034. doi: 10.1074/jbc.M113.468009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones MK, Sarfeh IJ, Tarnawski AS. Induction of in vitro angiogenesis in the endothelial-derived cell line, ea hy926, by ethanol is mediated through pkc and mapk. Biochem Biophys Res Commun. 1998;249:118–123. doi: 10.1006/bbrc.1998.9095. [DOI] [PubMed] [Google Scholar]
- 30.Gu JW, Elam J, Sartin A, Li W, Roach R, Adair TH. Moderate levels of ethanol induce expression of vascular endothelial growth factor and stimulate angiogenesis. Am J Physiol Regul Integr Comp Physiol. 2001;281:R365–372. doi: 10.1152/ajpregu.2001.281.1.R365. [DOI] [PubMed] [Google Scholar]
- 31.Wang S, Xu M, Li F, Wang X, Bower KA, Frank JA, Lu Y, Chen G, Zhang Z, Ke Z, Shi X, Luo J. Ethanol promotes mammary tumor growth and angiogenesis: The involvement of chemoattractant factor mcp-1. Breast Cancer Res Treat. 2012;133:1037–1048. doi: 10.1007/s10549-011-1902-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Radek KA, Kovacs EJ, Gallo RL, DiPietro LA. Acute ethanol exposure disrupts vegf receptor cell signaling in endothelial cells. Am J Physiol Heart Circ Physiol. 2008;295:H174–184. doi: 10.1152/ajpheart.00699.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goodwin AM. In vitro assays of angiogenesis for assessment of angiogenic and anti-angiogenic agents. Microvasc Res. 2007;74:172–183. doi: 10.1016/j.mvr.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lobov IB, Renard RA, Papadopoulos N, Gale NW, Thurston G, Yancopoulos GD, Wiegand SJ. Delta-like ligand 4 (dll4) is induced by vegf as a negative regulator of angiogenic sprouting. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:3219–3224. doi: 10.1073/pnas.0611206104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siekmann AF, Covassin L, Lawson ND. Modulation of vegf signalling output by the notch pathway. Bioessays. 2008;30:303–313. doi: 10.1002/bies.20736. [DOI] [PubMed] [Google Scholar]
- 36.Li JL, Harris AL. Crosstalk of vegf and notch pathways in tumour angiogenesis: Therapeutic implications. Front Biosci (Landmark Ed) 2009;14:3094–3110. doi: 10.2741/3438. [DOI] [PubMed] [Google Scholar]
- 37.Das SK, Mukherjee S, Vasudevan DM. Effects of long term ethanol consumption mediated oxidative stress on neovessel generation in liver. Toxicol Mech Methods. 2012;22:375–382. doi: 10.3109/15376516.2012.666651. [DOI] [PubMed] [Google Scholar]
- 38.Neves DR, Tomada IM, Assuncao MM, Marques FA, Almeida HM, Andrade JP. Effects of chronic red wine consumption on the expression of vascular endothelial growth factor, angiopoietin 1, angiopoietin 2, and its receptors in rat erectile tissue. J Food Sci. 2010;75:H79–86. doi: 10.1111/j.1750-3841.2010.01527.x. [DOI] [PubMed] [Google Scholar]
- 39.Hendrickson RJ, Cahill PA, Sitzmann JV, Redmond EM. Ethanol enhances basal and flow-stimulated nitric oxide synthase activity in vitro by activating an inhibitory guanine nucleotide binding protein. J Pharmacol Exp Ther. 1999;289:1293–1300. [PubMed] [Google Scholar]
- 40.Kuwabara M, Kakinuma Y, Ando M, Katare RG, Yamasaki F, Doi Y, Sato T. Nitric oxide stimulates vascular endothelial growth factor production in cardiomyocytes involved in angiogenesis. The journal of physiological sciences : JPS. 2006;56:95–101. doi: 10.2170/physiolsci.rp002305. [DOI] [PubMed] [Google Scholar]
- 41.Gavard J, Patel V, Gutkind JS. Angiopoietin-1 prevents vegf-induced endothelial permeability by sequestering src through mdia. Dev Cell. 2008;14:25–36. doi: 10.1016/j.devcel.2007.10.019. [DOI] [PubMed] [Google Scholar]