Abstract
Methylphenidate (MPD) is clinically effective in treating symptoms of attention-deficit/hyperactivity disorder; however, its relatively wide availability has raised public health concerns for non-medical use of MPD among certain adult populations. Most preclinical studies investigate whether presumed therapeutically relevant doses of MPD alter sensitivity to the reinforcing effects of other drugs, but it remains unclear whether doses of MPD likely exceeding therapeutic relevance impact the subsequent reinforcing effects of drugs. To begin to address this question, the effect of prior MPD self-administration (0.56 mg/kg/infusion) on the subsequent reinforcing effects of methamphetamine (METH, 0.032 or 0.1 mg/kg/infusion) was investigated in male, Sprague-Dawley rats. For comparison, it was also determined whether prior experimenter-administered MPD, injected daily at a presumed therapeutically-relevant dose (2 mg/kg), altered the subsequent reinforcing effects of METH. Results indicate that under the current conditions, only a history of MPD self-administration increased sensitivity to the subsequent reinforcing effects of METH. Furthermore, MPD did not impact food-maintained responding, suggesting that the effect of MPD might be specific to drug reinforcers. These data suggest that short-term, non-medical use of MPD might alter the positive reinforcing effects of METH in a manner relevant to vulnerability to drug use in humans.
Keywords: Methylphenidate, self-administration, methamphetamine, rat
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a common neuropsychiatric disorder that affects an estimated 9.0% of American children age 13 to 18 years (Merikangas et al., 2010) and 4.1% of American adults age 18 years and older (Kessler et al., 2005). Although the etiology of the disorder is not well understood, treatment of ADHD generally involves pharmacotherapy with psychostimulants, such as methylphenidate (MPD). MPD, like cocaine, inhibits the dopamine (DA) transporter (DAT; Ritz et al., 1987) and thereby increases extracellular DA (Hurd and Ungerstedt, 1989; Butcher et al., 1991) that, in turn, binds to various dopaminergic receptor subtypes. Indeed, MPD is one of the most commonly prescribed psychostimulants in the United States (Zito et al., 2000; Olfson et al., 2002; Kaye and Darke, 2012) and because ADHD often persists into adulthood (Wilens et al., 1995; Wilens and Dodson, 2004; Spencer et al., 2007), prolonged treatment with medications like MPD is common (Kolar et al., 2008).
Although medications, such as MPD, are clinically effective in treating symptoms of ADHD, its relatively wide availability has raised public health concerns (Wilens et al., 2008; for review, see Kaye and Darke, 2012) for its abuse potential and its non-medical use among adults in general, and in college students in particular (Teter et al., 2003; DeSantis et al., 2008; Johnston et al., 2012). For instance, one study reported that 7.5% of college students reported pharmaceutical stimulant misuse (including MPD) within the past 30 days (Weyandt et al., 2009; also see Dupont et al., 2008 and for review Kaye and Darke, 2012). A wealth of both animal and human literature have demonstrated that MPD produces subjective and reinforcing effects similar to other drugs of abuse like cocaine and amphetamine (for review see Kollins et al., 2001), providing evidence for the potential abuse liability of MPD. Furthermore, a number of studies exist in the literature describing intranasal or intravenous misuse of MPD (Levine et al., 1986; Jaffe, 1991; Parran and Jasinski, 1991; Garland 1998; Massello and Carpenter, 1999; Morton et al., 2000; Gautschi and Zellweger, 2006).
Despite the misuse of prescription MPD among certain adult populations (Teter et al., 2006; Kaye and Darke, 2012), the majority of clinical studies have focused on whether long-term use of clinically relevant doses of MPD leads to an increased risk of a subsequent substance abuse disorder. For instance, in clinical studies involving ADHD subjects, results suggest that treatment does not increase (Biederman et al., 2008; Mannuzza et al., 2008), and may even decrease (Biederman et al., 1999; Barkley et al., 2003), the likelihood of developing a substance abuse disorder. However, one study showed an increased risk of tobacco and cocaine use in adults with ADHD who were treated with stimulants as children (Lambert and Hartsough, 1998). These mixed results may be due to numerous factors including differences in treatment (i.e. duration, dosing), diagnoses, and comorbidities with other disorders in human subjects with ADHD.
In the majority of preclinical studies, adolescent animals are treated with doses thought to mimic clinically relevant doses of MPD for periods ranging from a few weeks to several months and are subsequently tested in adulthood for altered sensitivity to the effects of pharmacologically similar drugs such as cocaine. Of these studies, in adolescent rats treated with MPD, some have shown increased sensitivity to the reinforcing (i.e., self-administration) effects of cocaine (Brandon et al., 2001; Schenk et al., 2002; Crawford et al., 2011), while at least one study has shown decreased sensitivity to the reinforcing effects of cocaine (Thanos et al., 2007). In non-human primates treated with a dose of MPD to mimic therapeutic levels, there was no difference in cocaine self-administration, compared to those treated with placebo (Gill et al., 2012).
MPD diversion and misuse appears to be most prevalent and problematic among adult populations (Teter et al., 2006; Kaye and Darke, 2012); however, there is a paucity of data on the relationship between short-term, large doses of MPD and subsequent sensitivity to the abuse-related effects of other drugs. Of the available literature, some clinical data suggests that people who misuse stimulant medication such as MPD are more likely to misuse/abuse a variety of other drugs (e.g. marijuana, hallucinogens, cocaine; SAMSHA, 2009; Sweeney et al., 2013). Furthermore, a recent study demonstrated that short-term history of a large dose of MPD (i.e. 5 days of MPD self-administration) enhances sensitivity to the reinforcing effects of amphetamine (Calipari et al., 2013). The current study extends these studies to examine the effects of short-term MPD self-administration on the subsequent reinforcing effects of two doses of methamphetamine (METH). Thus, the goals of the current study were to examine: 1) whether a large dose of MPD (as observed in intravenous use; Levine et al., 1986; Parran and Jasinski, 1991; Morton and Stockton, 2000; Gautschi and Zellweger, 2006) impacts the subsequent reinforcing effects of METH; and 2) whether MPD alters the subsequent reinforcing effects of a non-drug reinforcer (i.e., food). Finally, and for comparison, experimenter-administered MPD, given at a relatively small dose thought to reflect therapeutic levels (Gerasimov et al., 2000; Brandon et al., 2001) was also examined for its subsequent impact on the effects of METH.
Methods
Subjects
A total of sixty Male Sprague-Dawley rats (275-300 g; Charles River Breeding Laboratories) were housed individually and maintained in a temperature and humidity controlled environment on a 14:10 h light/dark cycle with free access to water (see Table 1 for experimental groups). Twenty-four hours prior to the initiation of operant training, all rats were food-restricted to 90% of their free-feeding body weight for the duration of the experiments (with the exception of recovery time after surgery). All experiments were approved by the University of Utah Institutional Animal Care and Use Committee, in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Table 1.
Experimental design
| First condition | Second condition | |
|---|---|---|
| Experiment 1 | Saline (i.v.) | Saline (i.v.) |
| Saline (i.v.) | METH (0.032 mg/kg/inf; i.v.) | |
| MPD (0.56 mg/kg/inf; i.v.) | METH (0.032 mg/kg/inf; i.v.) | |
| Saline (i.v.) | Saline (i.v.) | |
| Saline (i.v.) | METH (0.1 mg/kg/inf; i.v.) | |
| MPD (0.56 mg/kg/inf; i.v.) | METH (0.1 mg/kg/inf; i.v.) | |
| Experiment 2 | Saline (i.p.) | Saline (i.v.) |
| Saline (i.p.) | METH (0.1 mg/kg/inf; i.v.) | |
| MPD (2 mg/kg; i.p.) | METH (0.1 mg/kg/inf; i.v.) |
Apparatus
All experimental sessions were conducted in an operant chamber (30.5 × 25.5 × 30.5 cm; Coulbourn Instruments, Allentown, PA) located within a sound-attenuating cubicle (79 × 53 × 53 cm; Coulbourn Instruments). Each chamber was equipped with a food pellet hopper, two retractable levers, and a house light. An infusion pump connected to a liquid swivel suspended outside of the operant chamber delivered drug or saline through a polyethylene tube located within a spring leash tethered to a rat.
Food Training
Before surgery, rats were trained to lever press for food under a fixed ratio (FR) 1 schedule of reinforcement whereby pressing on either lever resulted in the delivery of a food pellet. In daily 1-h sessions, rats could receive a maximum of 100 pellets. When at least 50 pellets were received in a session, only responding on the active lever (for some rats the active lever was the left lever and for others, the right lever) was reinforced. Responses on the inactive lever were counted but had no programmed consequence. Food training was completed after rats received at least 50 pellets in each of 3 consecutive sessions, while responding under the FR 1 schedule (this training required ~5-8 sessions).
Surgery
Rats were surgically implanted with a chronic indwelling jugular catheter (constructed in the laboratory as described previously, Frankel et al., 2011) in the right jugular vein, under ketamine:xyalzine (90: 7 mg/kg; i.p.) anesthesia. The outlet of the catheter was implanted subcutaneously in the back, and the free end of the Silastic tubing was inserted ~25 mm into the right jugular vein and secured to the surrounding tissue with sutures. Each rat received flunixin meglumine (s.c.) on the day of the surgery. Immediately after surgery and daily thereafter, catheters were infused with 0.05 ml of heparinized saline prior to the start of each session, and with 0.1 ml of the antibiotic cefazaolin followed by 0.05 ml of heparinized saline and heparinized glycerol after the completion of each session. If at any point during the experiment catheter leaks or abnormal shifts in self-administration behavior were observed, rats received xylazine through the catheter. Rats with patent catheters exhibited clear loss of muscle tone within a few seconds of the i.v. injection. Data collected from animals with nonpatent catheters were excluded from the data analyses (a total of 3 rats were excluded). All rats were allowed to recover for a minimum of 3 days after surgery before the start of self-administration sessions.
Drug Self-Administration (Experiment 1)
First, all rats were tested in daily 1-h sessions, during which illumination of the house light signaled availability of intravenous MPD (0.56 mg/kg/infusion, Marusich et al., 2010) or saline. A single response on the active lever resulted in the delivery of drug or saline (infusion duration 5-6 s, corresponding to 0.01-0.02 ml). Each infusion was followed by a 20-s timeout during which the chamber was dark and lever presses had no programmed consequence. Rats self-administered either MPD or saline for 7 consecutive sessions, a sufficient period to allow for stabilization of responding, defined as three consecutive sessions in which the mean number of infusions for an individual rat did not change by more than ±20% and there was no increasing or decreasing trend in overall group mean responding. After the 7-day period and to examine whether MPD history altered food-maintained responding, rats were placed in the operant chamber for daily 1-h sessions for 14 days and allowed to respond for food under the same schedule of reinforcement as described under food training (i.e. FR1 schedule of reinforcement, response on active lever delivered a food pellet, maximum of 100 pellets). Although all rats earned 100 food pellets after the first session, they were given 14 days of food-maintained responding to control for the number of days between MPD and METH across experiments. Subsequently, and to assess the influence of drug history on the reinforcing effects of METH, rats were allowed to self-administer either METH (0.032 or 0.1 mg/kg/infusion) or saline, depending on the group. The conditions for METH self-administration were identical to MPD self-administration with the exception that the duration of METH self-administration sessions was 8 h (i.e. a duration thought to better model METH-taking behaviors in humans; Kitamura et al., 2006; Krasnova et al., 2010).
Pretreatment Studies (Experiment 2)
A separate study investigated the influence of an experimenter-administered dose of MPD, thought to reflect therapeutic levels (Gerasimov et al., 2000; Brandon et al., 2001), or saline on the subsequent reinforcing effects of METH. Rats were administered either MPD (2 mg/kg/day; i.p.) or saline for 7 consecutive days. Subsequently, rats were food-trained and surgically implanted with catheters (as described above). Then, 14 days after the last MPD administration (i.e. the same number of days between the last MPD exposure and the first METH self-administration day in the above studies), rats were allowed to self-administer either METH (0.1 mg/kg/infusion) or saline for 7 consecutive days under the same conditions as described above.
Drugs
(±)-Methamphetamine hydrochloride and methylphenidate hydrochloride (Research Triangle Institute, Research Triangle Park, NC) were dissolved in 0.9% sterile saline, with the dose described as the free-base form. Ketamine (Hospira Inc., Lake Forest, IL) and xylazine (Sigma-Aldrich, St. Louis, MO) were used to anesthetize animals. The antibiotic cefazolin (10 mg/ml; Schein Pharmaceutical, Florham Park, NJ) was dissolved in heparinized saline (63.33 U/ml; Sigma-Aldrich). Flunixin meglumine (1.1 mg/kg; MWI Veterinary Supply, Meridian, ID) was used for postsurgical analgesia.
Data Analyses
Self-administration data are expressed as the mean (± S.E.M.) reinforcers earned and plotted as a function of session. Dose-response curves represent the mean (± S.E.M.) number of infusions or intake during the last three sessions for each dose of drug. For the saline data point, the mean (± S.E.M.) number of infusions during the last three sessions was averaged across the two experiments wherein rats had prior MPD or saline reinforcement. Statistical analyses were conducted with an ANOVA with post hoc Bonferroni's tests for multiple comparisons. For all tests, significance was set at P≤0.05.
Results
Experiment 1
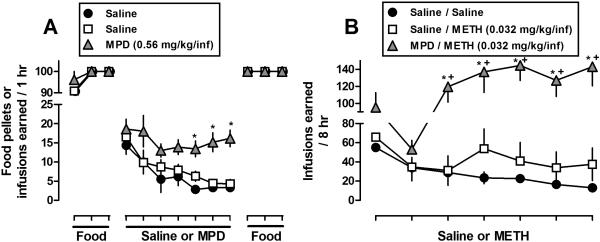
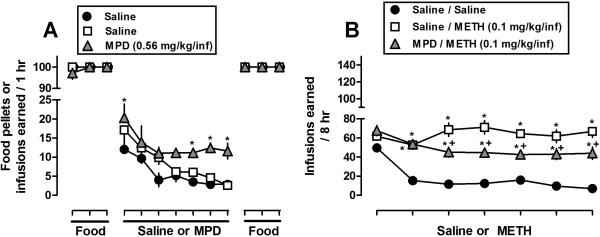
Prior MPD self-administration increased and decreased subsequent responding for METH when administered at doses of 0.032 (Fig. 1), and 0.1 (Fig. 2) mg/kg/infusion, respectively, compared to METH self-administration in rats with no drug history. Furthermore, prior MPD self-administration did not alter subsequent food-maintained responding. Specifically, the results presented in Figs. 1A and 2A illustrate the food and saline/MPD components of the experimental paradigm. For clarity, only the last three sessions of each food component are shown. In the first food component, the rate of acquisition for food-maintained responding did not differ between groups or across experiments. In the subsequent self-administration component, responding maintained by MPD (0.56 mg/kg/infusion) was greater than for saline during sessions 5-7. In the second food component, prior MPD did not alter subsequent food-maintained responding (i.e., both in terms of the number of food pellets earned and the time required to earn) between groups. For instance, the mean amount of time in minutes (± SEM) required to earn at or near 100 food pellets in the first session did not vary over 14 daily sessions [Fig. 1A, Session 1: Saline/Saline 6.8 min (± 0.4), Saline/METH 6.6 (± 0.5), MPD/METH 6.9 (± 0.3); Session 14: Saline/Saline 7.0 (± 0.3), Saline/METH 6.4 (± 0.5), MPD/METH 7.1 (± 0.3)]. Finally, the results in Fig. 1B demonstrate that prior MPD exposure increased subsequent levels of METH self-administration when METH was available at a dose of 0.032 mg/kg/infusion, a dose that was not readily self-administered by rats previously exposed to saline. However, in contrast to Fig. 1B, the results presented in Figure 2B indicate that prior MPD self-administration decreased subsequent levels of METH self-administration when METH was available at a dose of 0.1 mg/kg/infusion.
Fig. 1.
Effects of prior MPD (0.56 mg/kg/inf) or saline self-administration on (A) food-maintained responding and (B) subsequent METH self-administration (0.032 mg/kg/inf). Each condition represents the mean ± SEM of 6-7 rats. Horizontal axes: Ticks indicate daily consecutive sessions. For clarity, only the last 3 sessions of the food components and all sessions for drug components are shown. Vertical axes: mean (± SEM) food pellets or infusions earned. * p<0.05, values significantly different compared with saline or saline/saline. ± p<0.05, values significantly different compared with saline/METH.
Fig. 2.
Effects of prior MPD (0.56 mg/kg/inf) or saline self-administration on (A) food-maintained responding and (B) subsequent METH self-administration (0.1 mg/kg/inf). Each condition represents the mean ± SEM of 6-7 rats. Horizontal axes: Ticks indicate daily consecutive sessions. For clarity, only the last 3 sessions of the food components and all sessions for drug components are shown. Vertical axes: mean (± SEM) food pellets or infusions earned. * p<0.05, values significantly different compared with saline or saline/saline. ± p<0.05, values significantly different compared with saline/METH.
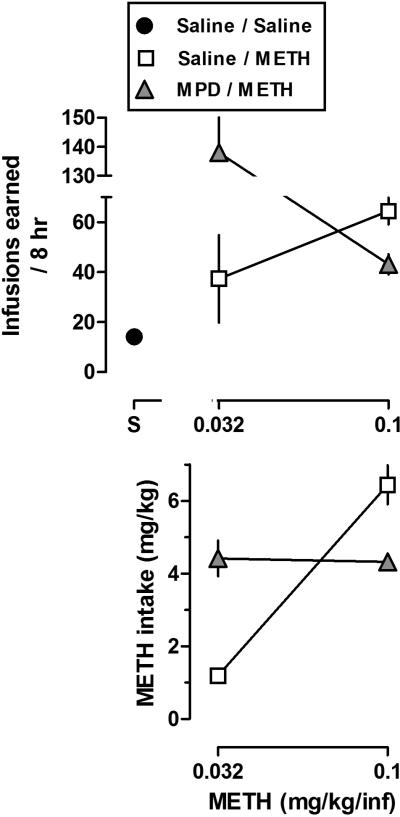
Dose-response curves for infusions and METH intake were generated from data presented in Figs. 1B and 2B by averaging the last three sessions of METH self-administration (Fig. 3). In rats with no drug history, there was a dose-dependent increase in the mean number of infusions (Fig. 3, open squares, upper panel). However, in rats with prior MPD self-administration, there was a dose-dependent decrease in the mean number of infusions (Fig. 3, gray triangles, upper panel). These data suggest that in rats with a history of prior MPD self-administration, the dose-response curve for METH infusions shifted leftward and upward, compared to rats with no MPD history (Fig. 3, upper panel; see below for additional discussion). The corresponding dose-response curves for METH intake indicate that in rats with no drug history, there was a dose-dependent increase in average METH intake (Fig. 3, open squares, lower panel). However, in rats with prior MPD self-administration, the mean METH intake was similar between the two doses (Fig. 3, gray triangles, lower panels).
Fig. 3.
Dose-response curves for METH infusions (upper panel) or intake (lower panel) generated from data presented in Figs. 1B and 2B. Data represent the mean ± SEM of the last three sessions for each dose of drug. For the saline data point, the mean ± SEM number of infusions during the last three sessions was averaged across the two experiments. Horizontal axes: dose in milligrams per kilogram of body weight. Vertical axes: mean (± SEM) infusions or METH intake (mg/kg).
Experiment 2
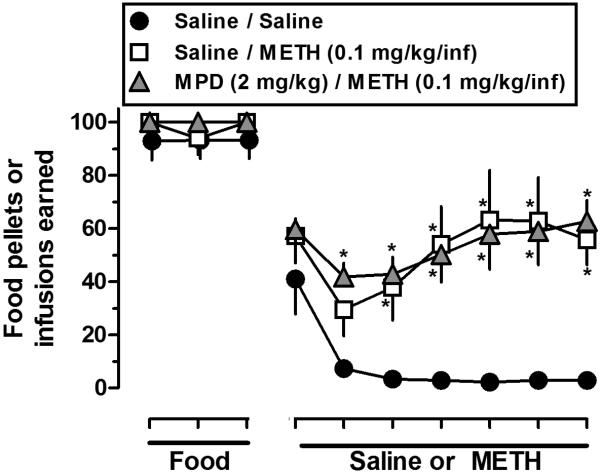
Fig. 4 shows that prior experimenter-administered MPD (2 mg/kg/day for 7 days; i.p.) did not impact the rate of acquisition for food-maintained responding (only the last three sessions of 14-day food component are shown) or subsequent levels of METH self-administration. For example, responding maintained by METH (0.1 mg/kg/infusion) was greater than for saline and there were no differences in the number of infusions received between rats treated previously with either saline or MPD (Fig. 4, compare squares and triangles). The mean (± SEM) number of infusions during the last three sessions for METH or saline was: saline/saline 3 (± 0.5), saline/METH 61 (± 14.4), and MPD/METH 60 (± 7.0); and the mean (± SEM) drug intakes (mg/kg) during the last three sessions for METH were: saline/METH 6.0 (± 1.4) and MPD/METH 6.0 (± 0.7).
Fig. 4.
Effects of prior experimenter-administered MPD (2 mg/kg/day for 7 days) or saline on food- and METH-maintained (0.1 mg/kg/inf) responding. Each condition represents the mean ± SEM of 6-7 rats. Horizontal axis: Ticks indicate consecutive sessions. For clarity, only the last 3 sessions of the food and all sessions for drug components are shown. Rats were first pretreated with MPD, then underwent food training and surgery, and then were allowed to self-administer METH such that the same number of days occurred between MPD and METH (i.e. 14 days) as in Figs. 1 and 2. Vertical axis: mean (± SEM) food pellets or infusions earned. * p<0.05, values significantly different compared with saline/saline.
Discussion
The major finding of the current study is that, under the current conditions, a history of MPD self-administration appears to increase the subsequent reinforcing effects of METH. Of significance is that the duration of MPD self-administration was relatively short (i.e. 7 days) and yet this still enhanced the reinforcing effects of METH long-term (i.e., 14 days) after the cessation of MPD. These findings in rats are similar to the effects reported by Calipari et al. (2013), where prior MPD reinforcement increased responding for amphetamine. Furthermore, in the current study, prior experimenter-administered MPD did not alter subsequent METH self-administration, compared to rats with no drug history. Taken together, these data indicate that short-term, non-medical use (i.e. large doses) of MPD might increase sensitivity of individuals to the abuse-related effects of METH.
Drug self-administration data are typically plotted as reinforcers earned as a function of drug dose and the curve is best described as an inverse u-shaped (although the shape can depend on the schedule of reinforcement). In the self-administration curve, increased or decreased sensitivity to the reinforcing effects of drugs is evident by a leftward/upward or a rightward/downward shift, respectively. In the current study and in rats with no drug history, there was a dose-dependent increase in mean number of infusions (Fig. 3, open squares, upper panel), consistent with findings that this represents the ascending limb of the inverted U-shaped dose-response curve associated with METH self-administration, with its peak at 0.1 mg/kg/infusion (see Stefanski et al., 1999). However, in rats with prior MPD self-administration, there was a dose-dependent decrease (corresponding to the descending limb of the dose-response curve with the peak at 0.032 mg/kg/infusion) in mean number of infusions (Fig. 3, gray triangles, upper panel). These data are consistent with the possibility that in rats with prior MPD self-administration history, the U-shaped dose-response curve for METH infusions shifted leftward and upward, compared to rats with no drug history. In contrast, the dose-effect curve for drug intake in mg/kg as a function of the unit dose of drug is typically a monotonic increasing function. In rats with no drug history, there was a dose-dependent increase in average METH intake (Fig. 3, open squares, lower panel). However, in rats with prior MPD self-administration, the average METH intake was similar between the two doses, reflecting the top of the monotonic function and a shift leftwards compared to rats with no drug history (Fig. 3, gray triangles, lower panels).
The underlying mechanism(s) whereby administration of MPD might alter sensitivity to METH remains unclear. MPD binds to DAT and increases extracellular concentrations of DA (Schweri et al., 1985, Kuczenski and Segal 1997) that, in turn, binds to a number of DA receptor subtypes (i.e., D1, D2 and D3) important in mediating the behavioral effects of MPD (Botly et al., 2008). Moreover, ex vivo voltammetry studies have demonstrated that MPD self-administration increased DAT activity in the nucleus accumbens, compared to control rats (Calipari et al., 2013, 2014). Thus, it might be reasonable to speculate that MPD-induced increases in DAT activity (i.e., DA clearance) leads to a reduction in extracellular DA, compensatory upregulation of post-synaptic DA receptors, and increased sensitivity to drugs acting indirectly at those receptors (i.e., METH). In support of the possibility that increased sensitivity of MPD-treated rats to the reinforcing effects of METH is related to increased expression/activity of DA receptors, rats treated with drugs like MPD are more sensitive to the effects of direct-acting D2/D3 agonists (Collins et al., 2011) and expression of DA receptor subtypes important in mediating the effects of MPD are greater, under some conditions (Thanos et al., 2007; Collins et al., 2011).
Although this study is not the first to describe differences in the reinforcing properties of drugs following substitution from different maintenance drugs, including MPD (e.g. Brandon et al., 2001; Thanos et al., 2007; Calipari et al., 2013), it is the first to address systematically the impact of varying drug and reinforcement histories on the capacity of METH to function as a reinforcer. That is, two conditions of MPD were evaluated, a small dose purportedly reflecting a therapeutic dose and a larger dose that might exceed therapeutic relevance, and only the larger dose of MPD increased the subsequent reinforcing effects of METH. In addition, a history of MPD self-administration did not impact responding maintained by a nondrug reinforcer (i.e. food; Figs. 1A and 2A), highlighting that MPD selectively alters the reinforcing properties of at least some drug reinforcers such as METH. Other studies have demonstrated that a history of drug reinforcement impacts subsequent reinforcing effects of drugs and not food (Collins and Woods, 2007). Although not tested in the current study, future studies might address whether a history of MPD reinforcement selectively alters the subsequent reinforcing effects of amphetamines. Previous studies, for example, have demonstrated that a prior history of MPD self-administration selectively enhanced the reinforcing effects of amphetamine, but not cocaine (Calipari et al., 2013; Calipari and Jones, 2014), suggesting that prior MPD does not similarly impact responding maintained by all drugs acting at DAT. In this regard, MPD self-administration might change DA or even non-DA neurotransmitter systems (e.g. norepinephrine) in a manner that selectively alters the reinforcing effects of DA releasers, such as METH, but not DA blockers.
Finally, the current findings might suggest that prior exposure to non-medicinal MPD sensitizes animals to the reinforcing effects of drug reinforcers. However, it is reasonable to speculate that sensitization alone does not account for the differences in METH self-administration because experimenter-administered MPD failed to alter subsequent METH self-administration (Fig. 4), as would have been expected if MPD exposure had been sufficient to sensitize the rats to the reinforcing effects of METH. In addition, even when a larger, experimenter-administered dose of MPD was used in another study (i.e. 2 injections of 5 mg/kg/day for 14 days, Calipari et al., 2013), the subsequent reinforcing effects of amphetamine were not altered. Thus, although it seems unlikely that increasing the dose of experimenter-administered MPD would impact sensitivity to METH self-administration, future studies might vary the dose and route of administration.
In summary, misuse and abuse of prescription drugs, including medications such as MPD, have increased in recent years among certain adult populations (for review see Wilens et al., 2008; Bogle and Smith, 2009). There is still much to be learned regarding interactions among drug history, sensitivity to drugs, and DA systems. The current study provides evidence that suggests short-term use of large doses of MPD might confer long-lasting changes to DA systems that impact subsequent sensitivity to the abuse-related effects of METH.
Acknowledgements
This work was supported by grants from the National Institute of Health DA031883, DA11389, DA019447, DA00869, DA00378, and DA013367.
References
- Barkley RA, Fischer M, Smallish L, Fletcher K. Does the treatment of attention-deficit/hyperactivity disorder with stimulants contribute to drug use/abuse? A 13-year prospective study. Pediatrics. 2003;111:97–109. doi: 10.1542/peds.111.1.97. [DOI] [PubMed] [Google Scholar]
- Biederman J, Monuteaux MC, Spencer T, Wilens TE, Macpherson HA, Faraone SV. Stimulant therapy and risk for subsequent substance use disorders in male adults with ADHD: a naturalistic controlled 10-year follow-up study. The American journal of psychiatry. 2008;165:597–603. doi: 10.1176/appi.ajp.2007.07091486. [DOI] [PubMed] [Google Scholar]
- Biederman J, Wilens T, Mick E, Spencer T, Faraone SV. Pharmacotherapy of attention-deficit/hyperactivity disorder reduces risk for substance use disorder. Pediatrics. 1999;104:e20. doi: 10.1542/peds.104.2.e20. [DOI] [PubMed] [Google Scholar]
- Bogle KE, Smith BH. Illicit methylphenidate use: a review of prevalence, availability, pharmacology, and consequences. Current drug abuse reviews. 2009;2:157–176. doi: 10.2174/1874473710902020157. [DOI] [PubMed] [Google Scholar]
- Botly LC, Burton CL, Rizos Z, Fletcher PJ. Characterization of methylphenidate self-administration and reinstatement in the rat. Psychopharmacology. 2008;199:55–66. doi: 10.1007/s00213-008-1093-z. [DOI] [PubMed] [Google Scholar]
- Brandon CL, Marinelli M, Baker LK, White FJ. Enhanced reactivity and vulnerability to cocaine following methylphenidate treatment in adolescent rats. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2001;25:651–661. doi: 10.1016/S0893-133X(01)00281-0. [DOI] [PubMed] [Google Scholar]
- Butcher SP, Liptrot J, Aburthnott GW. Characterisation of methylphenidate and nomifensine induced dopamine release in rat striatum using in vivo brain microdialysis. Neuroscience letters. 1991;122:245–248. doi: 10.1016/0304-3940(91)90869-u. [DOI] [PubMed] [Google Scholar]
- Calipari ES, Ferris MJ, Salahpour A, Caron MG, Jones SR. Methylphenidate amplifies the potency and reinforcing effects of amphetamines by increasing dopamine transporter expression. Nature communications. 2013;4:2720. doi: 10.1038/ncomms3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Ferris MJ, Melchior JR, Bermejo K, Salahpour A, Roberts DC, Jones SR. Methylphenidate and cocaine self-administration produce distinct dopamine terminal alterations. Addiction biology. 2014;19:145–155. doi: 10.1111/j.1369-1600.2012.00456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Woods JH. Drug and reinforcement history as determinants of the response-maintaining effects of quinpirole in the rat. The Journal of pharmacology and experimental therapeutics. 2007;323:599–605. doi: 10.1124/jpet.107.123042. [DOI] [PubMed] [Google Scholar]
- Collins GT, Truong YN, Levant B, Chen J, Wang S, Woods JH. Behavioral sensitization to cocaine in rats: evidence for temporal differences in dopamine D3 and D2 receptor sensitivity. Psychopharmacology. 2011;215:609–620. doi: 10.1007/s00213-010-2154-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford CA, Baella SA, Farley CM, Herbert MS, Horn LR, Campbell RH, Zavala AR. Early methylphenidate exposure enhances cocaine self-administration but not cocaine-induced conditioned place preference in young adult rats. Psychopharmacology. 2011;213:43–52. doi: 10.1007/s00213-010-2011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSantis AD, Webb EM, Noar SM. Illicit use of prescription ADHD medications on a college campus: a multimethodological approach. J Am Coll Health. 2008;57:315–324. doi: 10.3200/JACH.57.3.315-324. [DOI] [PubMed] [Google Scholar]
- Dupont RL, Coleman JJ, Bucher RH, Wilford BB. Characteristics and motives of college students who engage in nonmedical use of methylphenidate. The American journal on addictions / American Academy of Psychiatrists in Alcoholism and Addictions. 2008;17:167–171. doi: 10.1080/10550490802019642. [DOI] [PubMed] [Google Scholar]
- Frankel PS, Hoonakker AJ, Alburges ME, McDougall JW, McFadden LM, Fleckenstein AE, Hanson GR. Effect of methamphetamine self-administration on neurotensin systems of the basal ganglia. The Journal of pharmacology and experimental therapeutics. 2011;336:809–815. doi: 10.1124/jpet.110.176610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EJ. Intranasal abuse of prescribed methylphenidate. Journal of the American Academy of Child and Adolescent Psychiatry. 1998;37:1242–1243. doi: 10.1097/00004583-199812000-00003. [DOI] [PubMed] [Google Scholar]
- Gautschi OP, Zellweger R. Necrotising myositis after intravenous methylphenidate (Ritalin) injection. Emerg Med J. 2006;23:739. doi: 10.1136/emj.2006.035386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerasimov MR, Franceschi M, Volkow ND, Gifford A, Gatley SJ, Marsteller D, Molina PE, Dewey SL. Comparison between intraperitoneal and oral methylphenidate administration: A microdialysis and locomotor activity study. The Journal of pharmacology and experimental therapeutics. 2000;295:51–57. [PubMed] [Google Scholar]
- Gill KE, Pierre PJ, Daunais J, Bennett AJ, Martelle S, Gage HD, Swanson JM, Nader MA, Porrino LJ. Chronic treatment with extended release methylphenidate does not alter dopamine systems or increase vulnerability for cocaine self-administration: a study in nonhuman primates. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012;37:2555–2565. doi: 10.1038/npp.2012.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd YL, Ungerstedt U. In vivo neurochemical profile of dopamine uptake inhibitors and releasers in rat caudate-putamen. European journal of pharmacology. 1989;166:251–260. doi: 10.1016/0014-2999(89)90066-6. [DOI] [PubMed] [Google Scholar]
- Jaffe SL. Intranasal abuse of prescribed methylphenidate by an alcohol and drug abusing adolescent with ADHD. Journal of the American Academy of Child and Adolescent Psychiatry. 1991;30:773–775. [PubMed] [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national survey results on drug use, 1975-2011: Volume II, College students and adults ages 19-50. Institute for Social Research, The University of Michigan; Ann Arbor: 2012. [Google Scholar]
- Kaye S, Darke S. The diversion and misuse of pharmaceutical stimulants: what do we know and why should we care? Addiction. 2012;107:467–477. doi: 10.1111/j.1360-0443.2011.03720.x. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Archives of general psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura O, Wee S, Specio SE, Koob GF, Pulvirenti L. Escalation of methamphetamine self-administration in rats: a dose-effect function. Psychopharmacology (Berl) 2006;186:48–53. doi: 10.1007/s00213-006-0353-z. [DOI] [PubMed] [Google Scholar]
- Kolar D, Keller A, Golfinopoulos M, Cumyn L, Syer C, Hechtman L. Treatment of adults with attention-deficit/hyperactivity disorder. Neuropsychiatr Dis Treat. 2008;4:389–403. doi: 10.2147/ndt.s6985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollins SH, MacDonald EK, Rush CR. Assessing the abuse potential of methylphenidate in nonhuman and human subjects: a review. Pharmacology, biochemistry, and behavior. 2001;68:611–627. doi: 10.1016/s0091-3057(01)00464-6. [DOI] [PubMed] [Google Scholar]
- Krasnova IN, Justinova Z, Ladenheim B, Jayanthi S, McCoy MT, Barnes C, Warner JE, Goldberg SR, Cadet JL. Methamphetamine self-administration is associated with persistent biochemical alterations in striatal and cortical dopaminergic terminals in the rat. PLoS One. 2010;5:e8790. doi: 10.1371/journal.pone.0008790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Exposure of adolescent rats to oral methylphenidate: preferential effects on extracellular norepinephrine and absence of sensitization and cross-sensitization to methamphetamine. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:7264–7271. doi: 10.1523/JNEUROSCI.22-16-07264.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert NM, Hartsough CS. Prospective study of tobacco smoking and substance dependencies among samples of ADHD and non-ADHD participants. Journal of learning disabilities. 1998;31:533–544. doi: 10.1177/002221949803100603. [DOI] [PubMed] [Google Scholar]
- Levine B, Caplan YH, Kauffman G. Fatality resulting from methylphenidate overdose. Journal of analytical toxicology. 1986;10:209–210. doi: 10.1093/jat/10.5.209. [DOI] [PubMed] [Google Scholar]
- Mannuzza S, Klein RG, Truong NL, Moulton JL, 3rd, Roizen ER, Howell KH, Castellanos FX. Age of methylphenidate treatment initiation in children with ADHD and later substance abuse: prospective follow-up into adulthood. The American journal of psychiatry. 2008;165:604–609. doi: 10.1176/appi.ajp.2008.07091465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusich JA, Beckmann JS, Gipson CD, Bardo MT. Methylphenidate as a reinforcer for rats: contingent delivery and intake escalation. Exp Clin Psychopharmacol. 2010;18:257–266. doi: 10.1037/a0019814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massello W, 3rd, Carpenter DA. A fatality due to the intranasal abuse of methylphenidate (Ritalin). Journal of forensic sciences. 1999;44:220–221. [PubMed] [Google Scholar]
- Merikangas KR, He JP, Burstein M, Swanson SA, Avenevoli S, Cui L, Benjet C, Georgiades K, Swendsen J. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication--Adolescent Supplement (NCS-A). Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49:980–989. doi: 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton WA, Stockton GG. Methylphenidate Abuse and Psychiatric Side Effects. Primary care companion to the Journal of clinical psychiatry. 2000;2:159–164. doi: 10.4088/pcc.v02n0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olfson M, Marcus SC, Weissman MM, Jensen PS. National trends in the use of psychotropic medications by children. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41:514–521. doi: 10.1097/00004583-200205000-00008. [DOI] [PubMed] [Google Scholar]
- Parran TV, Jr., Jasinski DR. Intravenous methylphenidate abuse. Prototype for prescription drug abuse. Archives of internal medicine. 1991;151:781–783. [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237:1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- SAMHSA . The NSDUH Report: Nonmedical Use of Adderall among Full-Time College Students. Office of Applied Studies; Rockville, MD: 2009. Substance Abuse and Mental Health Services Administration. (2009) [Google Scholar]
- Schenk S, Izenwasser S. Pretreatment with methylphenidate sensitizes rats to the reinforcing effects of cocaine. Pharmacology, biochemistry, and behavior. 2002;72:651–657. doi: 10.1016/s0091-3057(02)00735-9. [DOI] [PubMed] [Google Scholar]
- Schweri MM, Skolnick P, Rafferty MF, Rice KC, Janowsky AJ, Paul SM. [3H]Threo-(+/−)-methylphenidate binding to 3,4-dihydroxyphenylethylamine uptake sites in corpus striatum: correlation with the stimulant properties of ritalinic acid esters. Journal of neurochemistry. 1985;45:1062–1070. doi: 10.1111/j.1471-4159.1985.tb05524.x. [DOI] [PubMed] [Google Scholar]
- Spencer T, Biederman J, Mick E. Attention-deficit/hyperactivity disorder: diagnosis, lifespan, comorbidities, and neurobiology. J Pediatr Psychol. 2007;32:631–642. doi: 10.1093/jpepsy/jsm005. [DOI] [PubMed] [Google Scholar]
- Stefanski R, Ladenheim B, Lee SH, Cadet JL, Goldberg SR. Neuroadaptations in the dopaminergic system after active self-administration but not passive administration of methamphetamine. Eur J Pharmacol. 1999;371:123–135. doi: 10.1016/s0014-2999(99)00094-1. [DOI] [PubMed] [Google Scholar]
- Sweeney CT, Sembower MA, Ertischek MD, Shiffman S, Schnoll SH. Nonmedical use of prescription ADHD stimulants and preexisting patterns of drug abuse. J Addict Dis. 2013;32:1–10. doi: 10.1080/10550887.2012.759858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teter CJ, McCabe SE, Boyd CJ, Guthrie SK. Illicit methylphenidate use in an undergraduate student sample: prevalence and risk factors. Pharmacotherapy. 2003;23:609–617. doi: 10.1592/phco.23.5.609.34187. [DOI] [PubMed] [Google Scholar]
- Teter CJ, McCabe SE, LaGrange K, Cranford JA, Boyd CJ. Illicit use of specific prescription stimulants among college students: prevalence, motives, and routes of administration. Pharmacotherapy. 2006;2:1501–1510. doi: 10.1592/phco.26.10.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos PK, Michaelides M, Benveniste H, Wang GJ, Volkow ND. Effects of chronic oral methylphenidate on cocaine self-administration and striatal dopamine D2 receptors in rodents. Pharmacology, biochemistry, and behavior. 2007;87:426–433. doi: 10.1016/j.pbb.2007.05.020. [DOI] [PubMed] [Google Scholar]
- Weyandt LL, Janusis G, Wilson KG, Verdi G, Paquin G, Lopes J, Varejao M, Dussault C. Nonmedical prescription stimulant use among a sample of college students: relationship with pscyhological variables. J Atten Disord. 2009;13:284–296. doi: 10.1177/1087054709342212. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Dodson W. A clinical perspective of attention-deficit/hyperactivity disorder into adulthood. J Clin Psychiatry. 2004;65:1301–1313. doi: 10.4088/jcp.v65n1003. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Biederman J, Spencer TJ, Prince J. Pharmacotherapy of adult attention deficit/hyperactivity disorder: a review. Journal of clinical psychopharmacology. 1995;15:270–279. doi: 10.1097/00004714-199508000-00006. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Adler LA, Adams J, Sgambati S, Rotrosen J, Sawtelle R, Utzinger L, Fusillo S. Misue and diversion of stimulants prescribed for ADHD: a systematic review of the literature. J Am Acad Child Adolesc Psychiatry. 2008;47:21–31. doi: 10.1097/chi.0b013e31815a56f1. [DOI] [PubMed] [Google Scholar]
- Zito JM, Safer DJ, dosReis S, Gardner JF, Boles M, Lynch F. Trends in the prescribing of psychotropic medications to preschoolers. JAMA : the journal of the American Medical Association. 2000;283:1025–1030. doi: 10.1001/jama.283.8.1025. [DOI] [PubMed] [Google Scholar]