Abstract
Methylphenidate (MPH) remains an important therapy for Attention-Deficit Hyperactivity Disorder but aspects of its pharmacology remain unclear. In the present study, we used a regimen of MPH (8 mg/kg daily X 14 days) in C57BL/6J mice to determine whether establishing locomotor sensitization to MPH influenced the acquisition and the dose-response function of MPH in a classic drug discrimination procedure. MPH-sensitized mice (SENS group) demonstrated enhanced locomotor activity to the 8 mg/kg exposure dose as well as a 2mg/kg dose prior to discrimination training. However, the SENS mice did not acquire discrimination of either a low dose (2mg/kg) or a higher dose (4mg/kg) of MPH any more rapidly than the CTRL mice. Further, during generalization testing, the dose-response functions for the SENS and CTRL mice were identical. Therefore, we did not find that prior exposure to MPH, which produced a sensitized locomotor response, facilitated MPH discrimination.
Keywords: sensitization, drug discrimination, methylphenidate, mouse
INTRODUCTION
Methylphenidate (MPH) continues to be an important pharmacotherapeutic option for treating Attention-Deficit Hyperactivity Disorder (Biederman and Faraone, 2005; Biederman and Spencer, 2002). Unfortunately, people also divert MPH to non-medical uses (Darredeau et al., 2007; Kroutil et al., 2006; Novak et al., 2007), especially by high school (McCabe et al., 2004a, b) and college (Godfrey, 2009; McCabe et al., 2006; Teter et al., 2003) students. Concern regarding prescription drug abuse in general, and MPH in particular, has prompted the continued study of MPH in humans and rodents under a variety of situations and conditions [e.g. (Bell et al., 2011; Brookshire and Jones, 2012; Griffin et al., 2012a; Hammerness et al., 2012; Jones and Dafny, 2013; Patrick et al., 2007)]. Moreover, recent reports indicate that despite behavioral effects similar to psychostimulants such as cocaine, MPH has distinct effects on monoaminergic transmission, which appear to be unique among the variety of drugs that target the dopamine transporter (Calipari et al., 2012; Ferris et al., 2012). Therefore, there is still a great deal to learn about this drug that has been in widespread clinical use for many decades.
In humans, MPH produces positive subjective effects (Heil et al., 2002; Kollins et al., 2009; Kollins et al., 2001; Patrick et al., 2007; Stoops et al., 2005a), which may serve as discriminative stimuli. Direct evidence that MPH produces a discriminative stimulus comes from several drug discrimination studies in humans (Duke et al., 2011; Lile et al., 2006; Stoops et al., 2005b), rats (Overton and Shen, 1988; Perkins et al., 1991) and mice (Griffin et al., 2012a; McGovern et al., 2011). A number of reports also describe the ability of MPH to at least partially substitute for other psychostimulants such as cocaine (Bondareva et al., 2002; Li et al., 2006; Rush et al., 2002; Schweri et al., 2002), amphetamine (Bondareva et al., 2002; Czoty et al., 2004) and methamphetamine (Desai et al., 2010; Sevak et al., 2009).
It has been appreciated for many years that prior experience with a psychoactive drug can influence subsequent responses to that drug, indicating that the underlying neurobiology has been adapted due to the previous exposure. Depending on the circumstances, the adapted response can be characterized as either tolerance or sensitization (Becker et al., 2013). Sensitization may be the most commonly studied neuroadaptation for psychostimulants because it appears to play an important role in addiction (Leyton, 2007; Robinson and Berridge, 2001, 1993; Vezina and Leyton, 2009). In experimental settings, psychostimulant sensitization is commonly demonstrated as increased locomotor activity following repeated exposures to the drug. Several reports indicate that repeated exposure to MPH induces locomotor sensitization in rodents (Askenasy et al., 2007; Yang et al., 2011; Yang et al., 2007).
With operant drug discrimination tasks, the influence of previous drug experience on discriminability has been most commonly studied with regard to effects of the training dose on the discriminative stimulus response function, and a comprehensive review has recently been published (Stolerman et al., 2011). Though there are exceptions, higher training doses generally lead to faster acquisition of the discriminative behavior compared to lower training doses, and discriminative stimulus response functions are shifted rightward with higher doses (Stolerman et al., 2011). Interestingly, early work with LSD found that establishing discrimination with 80 μg/kg and then substituting a lower dose (10 μg/kg) during training sessions significantly improved discriminability of the lower dose during subsequent testing, compared to tests conducted prior to the substitution (83% vs 30% responding, respectively, on the drug paired lever) (Greenberg et al., 1975). These findings are consistent with the development of sensitization to the discriminative stimulus effects of the drug.
In contrast, the impact of drug exposure outside the context of the discrimination task has been less commonly studied, although examples can be found. For example, exposure outside of the training context produces tolerance to the ethanol discriminative stimulus (Becker et al., 2004; Crissman et al., 2004) and the morphine discriminative stimulus (Sannerud and Young, 1987; Young et al., 1996). An early study demonstrated that pre-exposure to scopolamine reduced the time to acquire discrimination in an avoidance task but, interestingly, produced a rightward shift in the generalization function (McKim, 1976). Lastly, it was reported that pre-exposure to the psychostimulant methamphetamine produced a significant leftward shift in the discriminative stimulus response function for methamphetamine, indicating that low doses were more easily discriminated when rats were pre-exposed to the psychostimulant (Suzuki et al., 2004). Further, despite the leftward shift in the stimulus response function, no differences were noted on the acquisition of the discrimination task with the methamphetamine pre-exposure (Suzuki et al., 2004). These studies indicate that drug exposure separate from the training context can influence the discriminative stimulus control of reinforced behavior, though the effects may vary by drug and exposure procedure.
Our previous study demonstrated that mice could readily learn to discriminate doses of MPH equal to or greater than 4 mg/kg, but not lower doses (McGovern et al., 2011). Interestingly, although low doses of MPH (<5mg/kg) do not overtly increase locomotion (Griffin et al., 2010; 2012a; Williard et al., 2007), low doses can support the development of place preference (Griffin et al., 2012a) and reduce ethanol consumption (Griffin et al., 2010). Additionally, these low doses interact pharmacologically with ethanol to augment locomotion and discrimination (Griffin et al., 2010; 2012a). Work from other laboratories indicates that MPH (<5 mg/kg) produces significant changes in monoamine concentrations (Balcioglu et al., 2009; Berridge et al., 2006; Koda et al., 2010; Kuczenski and Segal, 1997). Collectively, these studies indicate that low doses of MPH are pharmacologically active, and it is possible that previous exposure to MPH may influence the acquisition of behaviors dependent upon the recognition of the discriminative stimulus effects of MPH. In the present study, we hypothesized that that pre-exposure to a locomotor sensitizing regimen of MPH would enhance discrimination of low doses of MPH.
METHODS
Subjects
C57BL/6J mice (n=20) were obtained from Jackson Laboratories (Bar Harbor, ME) at 7 weeks of age. Animals were singly housed on a 12-h reverse light cycle (lights on at 20.00h, lights off at 08.00h), with free access to water, and allowed to acclimate to home cages for ~2 weeks prior to behavioral testing. Following this acclimation period, mice were maintained at 85- 90% of their free feeding body weight, except as noted below during the sensitization procedure. These experiments were approved by the Institutional Animal Care and Use Committee (IACUC) at the Medical University of South Carolina and conducted according to the NIH Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80-23, Revised 1996).
Locomotor Activity Apparatus
Locomotor activity was assessed using a Digiscan Animal Activity Monitor system, model RXYZCM(8) TAO (Accuscan Instruments, Columbus, OH) that has been described in several publications (Griffin et al., 2012a; Griffin and Middaugh, 2006; Griffin et al., 2012b; Griffin et al., 2010)
Drug Discrimination Apparatus
Drug discrimination was assessed in operant chambers controlled by MedPC software that were enclosed in sound and light attenuating cabinets (MedAssociates Inc., St Albans, VT) as described in several drug discrimination studies (Griffin et al., 2012a; Griffin et al., 2012b; McGovern et al., 2011). In this study, behavior was reinforced with a 5 second presentation of 0.01 cc of a 15% sucrose solution by a dipper located between two levers in each chamber. The MPH reinforced lever (right or left) was counterbalanced across the subjects in both groups.
Sensitization Induction and Testing Procedures
After mice were trained to press levers for sucrose reinforcement (see below), the sensitization regimen began, with group assignment counterbalanced for lever pressing rate during the final session of training. During this phase, mice resumed ad libitum feeding. Mice were treated once daily with either 8 mg/kg MPH (SENS group) or vehicle (CTRL group) for 14 days. This dose is near maximal for increasing locomotor activity in mice (Williard et al., 2007). MPH injections were given and the mice were returned to their home cage, however on Day 1, Day 8 and Day 14, mice were immediately placed into the locomotor activity monitor for 60 minutes. Following a 2 day washout period with no treatments given, mice were again challenged with MPH (2mg/kg) or vehicle in the locomotor activity monitor (60 minute sessions). For this re-challenge, all mice were treated with both MPH and vehicle using a Latin-square design over a 2 day period. Mice resumed drug discrimination training after a 2 week wash-out period.
Drug Discrimination Procedures
The procedures used for MPH discrimination have been described previously (Griffin et al., 2011, 2012a). Briefly, to establish responding for the sucrose reinforce, a shaping procedure began with a FR1 schedule (e.g. 1 lever press per reinforcer) that increased gradually over sessions to reach a final schedule of FR15, which was used for the remainder of the study. After training, mice entered the sensitization induction and testing phase of the study (described above) before resuming discrimination training, beginning first with 2 mg/kg methylphenidate and then increasing to 4 mg/kg. For this study, 15 min sessions occurred once per day, with injections given 15 min before the session. MPH or vehicle was administered according to a semi-randomized schedule that ensured no more than 2 consecutive days of MPH or vehicle and an equal number of exposures to each over a 2 week period. For successful discrimination, the first criterion was that mice make ≥80% of responses on the injection appropriate lever prior to the first reinforcement (called FFR: First Fixed Ratio) over at least 3 consecutive sessions. Additionally, mice were required to make ≥85% of total responses on the injection-appropriate lever during 3 consecutive sessions. Upon meeting these criteria, mice were eligible for MPH discrimination testing. Discrimination tests lasted 2 minutes and were conducted under extinction conditions. All other procedures were the same as during the training sessions. After every generalization test, mice resumed training and were required to meet discrimination criteria during at least 3 consecutive training sessions before another discrimination test session was conducted. For the dose substitution curves, mice were tested in ascending order of doses and twice at each dose, with the exception that MPH doses greater than the training doses were tested only once.
Drugs
Methylphenidate•HCl (Sigma-Aldrich, Inc) was used as the racemic mixture (i.e. dl-MPH) by dissolution in 0.9% saline, and administered i.p. in a volume of 0.01ml/g body weight.
Data Analysis
Comparison of group means was made using Student’s T-Test and between-group comparisons with multiple groups was made using Analysis of Variance (ANOVA), with repeated-measures as appropriate. Post hoc analysis was conducted using Bonferroni’s corrected Pairwise Comparisons. Evaluation of counted data was done using Chi Square analysis. For all analyses, significance levels were set at p< 0.05
RESULTS
Induction of locomotor sensitization by methylphenidate
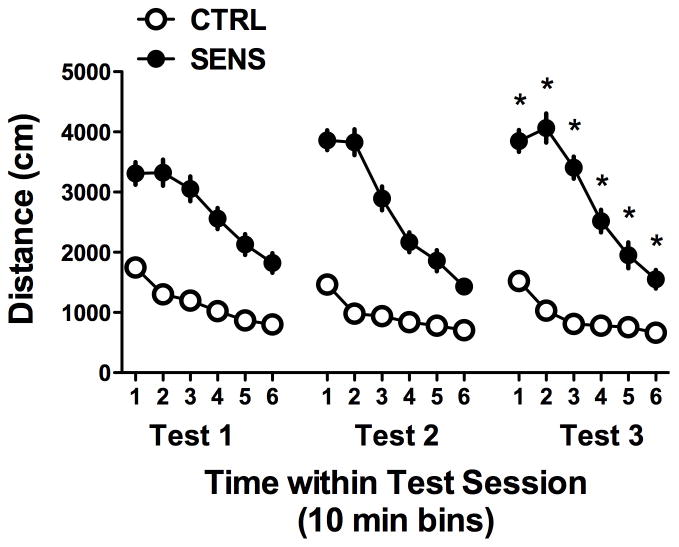
After all the mice were trained to press a lever for sucrose reinforcement, the sensitization phase of the study began. These results are summarized in Figure 1. As can be seen, in the CTRL group the dose of methylphenidate (MPH) used for the induction of locomotor sensitization (8 mg/kg) produced a large increase in distance traveled in the SENS group compared to vehicle (0 mg/kg). During the third session, the SENS group showed a slight increase in total distance traveled compared to previous sessions. These observations were supported by a 2(Group) X 3(Session) X 6(Time) repeated-measures ANOVA where Session and Time within session were treated as within-subjects repeated measures (RM). The 3-way interaction was significant [F (10,180) = 11.17, p<0.001] as were all three possible 2 way interactions (all F’s >5 and all p’s <0.01).
Figure 1.
Locomotor sensitization to methylphenidate (MPH) in C57BL/6J mice (n=10 per group). Locomotor activity was assessed 3 times during the course of a 14 day sensitization procedure: Test 1 occurred on Day 1, Test 2 occurred on Day 8 and Test 3 occurred on Day 14. The 8mg/kg dose of MPH clearly increased activity compared to vehicle during all 3 test sessions. Further, although the effect was small, the sensitized (SENS) mice demonstrated increased locomotion to the 8mg/kg dose by the third test (*p<0.05) compared to Test 1 and 2. Data are mean + SEM.
The data were further analyzed using 2-way ANOVAs within groups. Within the SENS group, the 3(Session) X 6(Time) RM ANOVA detected a significant interaction [F (10,90) = 10.99, p<0.001). Post hoc analysis of these data indicated that, within the SENS group, the distance traveled during Test 3 was greater than during Session 2 (p=0.018). On the other hand, the same analysis of the data from the CTRL group did not reveal a significant interaction [F(10,90) = 1.71, p = 0.09] although it did detect significant main effects of Session [F (2,18) = 5.736, p<0.02) and Time [F (5,45)=86, p<0.001]. Together, these analyses indicate that locomotor activity increased significantly as a function of repeated exposure to 8 mg/kg of methylphenidate, indicating sensitization to the locomotor activating effects of MPH.
Challenge with 2 mg/kg MPH
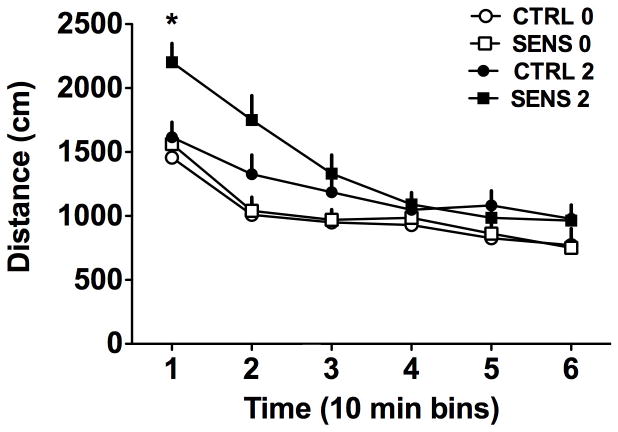
Following the induction phase, all mice were challenged with 0 and 2 mg/kg MPH, using a Latin-square design. The data from this experiment are summarized in Figure 2. The data show that MPH at a dose one quarter of that used in the previous phase of the study still increased distance traveled above that of the vehicle challenge. In the CTRL group, there was only a slight overall increase compared to vehicle, while the increase in distance traveled for the SENS group was greater. These data were analyzed using a 2(Group) X 2(Dose) X 6(Time) ANOVA with Dose and Time serving as repeated measures. This analysis found a significant 3-way interaction [F(5,90)=4.75, p<0.001]. The Dose X Time and Group X Time interactions were significant [both F’s >6 and p’s<0. 001] but the Group X Dose interaction was not [F(1,18)=1.56, NS].
Figure 2.
Evidence of locomotor sensitization after a 2 mg/kg MPH challenge (n=10 per group). The sensitized mice (SENS group) showed a larger response (*p<0.05) to this dose than did the non-sensitized mice (CTRL) mice, consistent with the development of sensitization to MPH. Data are mean + SEM.
The 3-way interaction was further evaluated according to Time using separate 2(Group) X 2(Dose) ANOVAs, where the Dose factor served as a repeated measure during each time bin. The analysis of data from the first time bin detected a significant interaction [F (1,18) = 6.58, p < 0.02]. Post-hoc analysis found that the SENS group traveled further after the 2mg/kg challenge than after vehicle challenge (p<0.001) as well traveling a greater distance than the CTRL mice challenged with 2mg/kg (p<0.01). Within the CTRL group, MPH-treated mice did not increase the distance traveled more than those treated with vehicle (p>0.2) at the first time point. At the second time point (i.e. the 20 min bin), the Group x Dose interaction approached significance [F (1,18) = 3.294, p=0.086] but the Group factor was not significant [F(1,18) = 1.73, NS]. At this time point, only the effect of Dose was significant [F(1,18) = 22, p<0.001]. For the remaining RM ANOVAs conducted for time points 3 through 6, only the Dose effect remained significant (all F’s >4) but none of the Group effects or interactions were significant (all F’s <1.5, NS). These analyses indicate that mice in the SENS group responded more to the low challenge dose of 2 mg/kg MPH than the CTRL group. The results of this challenge experiment further support the prior observation that repeated exposure to MPH produced locomotor sensitization in the SENS group.
MPH Discrimination
Following the sensitization procedure, mice resumed discrimination training. Initially, mice began training to discriminate 2 mg/kg MPH from vehicle. After 29 sessions of this procedure, the active dose was changed to 4 mg/kg MPH for an additional 20 sessions of acquisition training. Consistent with our previous work (McGovern et al., 2011), the 2mg/kg MPH dose did not engender reliable discrimination in either group during this evaluation period but once the dose was increased to 4 mg/kg the mice did demonstrate reliable discrimination.
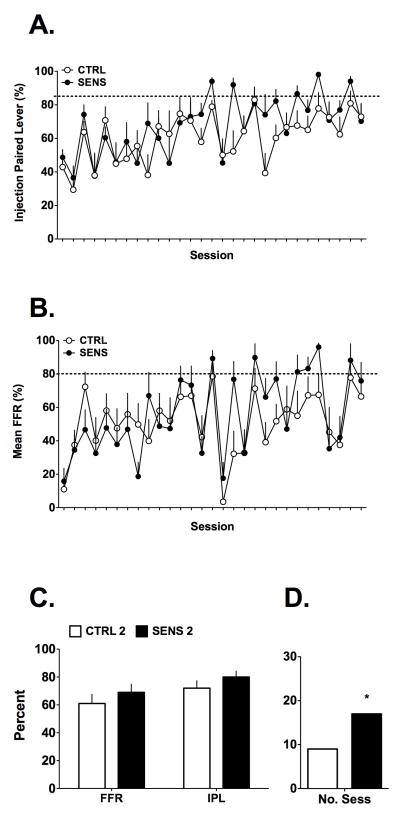
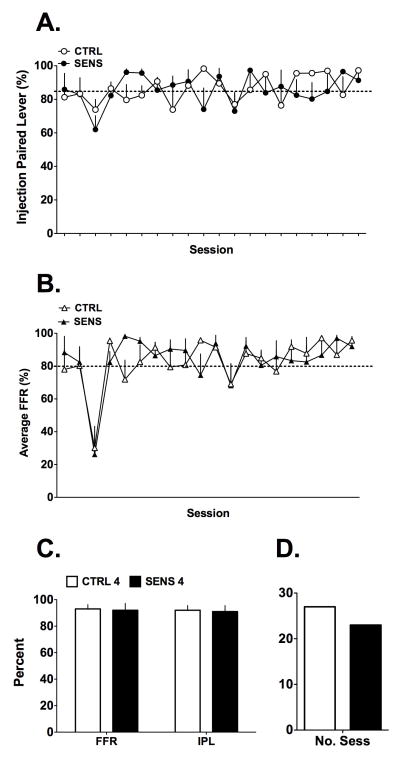
Responding for the 2 mg/kg and 4 mg/kg MPH doses are summarized in Figures 3 and 4, respectively, for all of the training sessions. The data in Figure 3A,B show that, in general, the 2 groups of mice responded similarly across the training sessions on the injection-paired lever (IPL; either for vehicle or 2 mg/kg of MPH) in terms of percent total responding on the IPL or for completing the FFR. Overall, the ability of the mice to discriminate the MPH injection from the vehicle injection increased with time but still did not consistently reach criteria regardless of MPH exposure history. These observations were supported by a 2(Group) X 29(Session) ANOVA on the %IPL data, with Session as a repeated measure, and no significant interaction was found [F(28, 504) = 1.24, NS], only a significant main effect of time [F(28, 504) = 5.21, p<0.001], consistent with the overall increase in %IPL as the sessions progressed. After the training dose was increased to 4 mg/kg, both groups of mice began responding consistently at criterion performance levels and, again, no influence of prior sensitization emerged for IPL responding (Figure 4A,B). These data were similarly analyzed as for the lower dose and only a significant main effect of Session was noted [F(28, 504) = 2.77, p<0.001], with no significant interaction [F(28, 504) = 1.48, NS]. Collectively, these data do not support the hypothesis that prior experience with MPH enhanced acquisition of discriminative stimulus control of reinforced behavior.
Figure 3.
Acquisition of the operant discrimination task (n=10 per group) while maintained on the 2mg/kg training dose. A,B) Responding on the injection paired lever (IPL), after either vehicle or 2mg/kg MPH, increased with session number but was similar between the two groups over 29 sessions of training when examined as a percentage of total responding or as FFR. The dotted lines indicate criterion levels for the two measures. C) A comparison of FFR and IPL responding averaged over the last 3 days of this period. No significant differences were found. D) Over the last 3 days of the training period, the SENS mice as a group had more sessions (out of 30 possible) of criterion level performance (*p<0.05), but this was primarily due to 2 SENS mice that performed well. Data are mean + SEM except panel D, which are counts.
Figure 4.
Responding on the operant discrimination task after changing to the 4 mg/kg MPH training dose. A,B) Responding on the injection paired lever (IPL), after either vehicle or 4mg/kg MPH injection, was similar for the two groups over 20 sessions of training. The dotted lines indicate criterion levels for the two measures. C) A comparison of FFR and IPL responding averaged over the last 3 days. No significant differences were found and most mice met discrimination criteria. D) Over the last 3 days of the training period, the two groups of mice had similar numbers of sessions of criterion level performance out of 30 possible. Data are mean + SEM except panel D, which are counts.
Further examination of the data confirmed our initial evaluation: while maintained on the 2mg/kg training regimen, the SENS mice performed slightly better with regard to FFR responding (69% vs 61%) and total percent responding on the injection-paired lever (80% vs 72%) when compared to the CTRL mice. The data shown in Figure 3C are averaged from the last three days of training with 2 mg/kg MPH. Student’s t-tests on these data did not detect significant differences between CTRL and SENS mice [both t’s<1.3, df=18, NS] for the 2mg/kg dose. Further, it is clear that once the training dose was increased to 4 mg/kg, mice in both groups readily discriminated the active MPH dose from vehicle, easily meeting criteria for advancing to the discrimination testing phase (Figure 4C). Student’s t-tests on the 4 mg/kg FFR and total percent response data did not detect differences between CTRL and SENS mice [both t’s<1.3, df=18, NS]. These analyses using the traditional measures for acquisition of discrimination (FFR and percent responding on the drug paired lever) indicate that the 2 mg/kg training dose was inadequate for supporting MPH discrimination, compared to the 4 mg/kg dose.
Lastly, we investigated the performance of the two groups of mice by counting the number of sessions in which mice in either group met criteria for discrimination testing and the number of mice meeting criteria. These data came from the last 3 days of training on either the 2 or 4 mg/kg doses and are summarized in Figures 3D & 4D. The total number of sessions in which mice could meet criteria for discrimination testing was 30 per group across vehicle and MPH training sessions (n=10 mice for 3 sessions each). Interestingly, for the 2mg/kg data, we found that, as a group, the SENS mice had nearly double the number of sessions in which the mice met criteria (17 out of 30 sessions) compared to the CTRL group (9 out of 30 sessions). Chi square analysis on these data was significant [Χ2 = 4.34, df = 1, p < 0.05]. On the other hand, the same analysis for the analogous data with the 4 mg/kg training dose indicated no difference between CTRL (27 out of 30 sessions) and SENS (23 out of 30 sessions) groups [Χ2 = 1.92, df = 1, NS]. Additionally, when the numbers of mice were counted that consistently met criteria to be tested over each of the last 3 sessions for the 2 mg/kg dose, the CTRL group had zero mice and the SENS group had 2 mice. At the end of the 4 mg/kg training period, both groups had 7 mice consistently meeting criterion levels of responding. Chi square analysis on these data did not detect differences at either dose [both Χ2 < 2.2, df = 1, NS]. Although, as a group, the SENS mice had more sessions meeting performance criteria while training with 2 mg/kg MPH, this was largely due to only 2 out of the 10 mice in this group consistently meeting criteria for successful discrimination at the low dose.
MPH Discrimination Testing
At the conclusion of the acquisition phase, discrimination testing proceeded. Interestingly, 2 mice in the SENS group never met criteria for testing at any time and were excluded from further analysis. Additionally, 2 mice in the CTRL group were excluded from further analysis because their response rates declined to very low levels once discrimination testing began. Note that these 4 mice were included in all analyses described above, but are excluded from the analyses described below. Therefore, for the discrimination phase of the study, the group sizes were n=8 for both the CTRL and SENS groups. These data are summarized in Figure 5. During testing, mice of both groups demonstrated dose-dependent discrimination of methylphenidate. However, no differences were noted between the groups. A 2(Group) X 5(Dose) RM ANOVA found only a significant effect of Dose [F(3,42) = 13.52, p<0.001], but neither the main effect of Group nor the Dose x Group interaction were significant [both F’s<1, NS]. Total responses were analyzed in the same way and, again, there was only a significant main effect of Group, consistent with the lower responding for the 1 and 2 mg/kg doses [F(3,42) = 5.80, p < 0.002]. For total responses, there was no significant effects of Group and no significant interaction [both F’s<1, NS].
Figure 5.
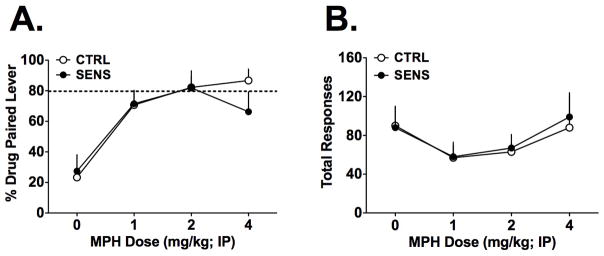
The discriminative stimulus dose-response function for CTRL and SENS mice (n=7 per group). After mice reached criterion performance on the 4 mg/kg training dose, they were challenged with several different doses of MPH. A) Although the mice demonstrated the expected partial generalization to doses lower than 4 mg/kg, there was no difference between the SENS and CTRL groups. B) Similarly, there were no differences between the groups on the total responding during the discrimination test sessions. Data are mean + SEM.
A higher dose of MPH (6mg/kg) was also tested once in each mouse. Total average responses were 99 ± 12 and 126 ± 15 for CTRL and SENS mice, respectively. The 6 mg/kg dose, however, generated most of this responding on the vehicle-paired lever since the percent responding on the drug-paired lever was only 4.93% ± 1.83 and 11.2% ± 4.61, for CTRL and SENS mice, respectively. Student’s t-tests on both of these data sets did not detect any differences [t< 1.2,df = 14, NS].
DISCUSSION
We found that daily exposure to 8 mg/kg methylphenidate (MPH) produces locomotor sensitization in B6 mice, consistent with other published work (Askenasy et al., 2007). Sensitization was also evident when the mice were re-challenged with a low dose of MPH (2mg/kg), the same dose subsequently used for initial training in the operant discrimination task, indicating that a dose that would ordinarily not increase activity had acquired new pharmacological relevance. However, this did not translate into more rapid acquisition of the discrimination behavior when the training dose was 2 mg/kg MPH. When the MPH dose was increased to 4 mg/kg, most of the mice in both groups ultimately acquired discrimination behavior, with no discernible differences in acquisition or in the discriminative stimulus response function. These results are interesting because they are counter to the expectation that previous experience with a drug should influence the acquisition or dose-response function for discrimination tasks.
While numerous studies have shown that higher doses within the training context lead to more rapid acquisition of drug discrimination behavior (Stolerman et al., 2011), at least one report indicates that prior exposure to a drug (scopolamine) before training reduces time to acquisition (McKim, 1976). However, the present results indicate that significant experience with high doses of MPH outside of training does not influence acquisition of MPH discrimination with a low dose. In support of the current findings, it is noteworthy that Suzuki et al. (2004) did not report any differences in acquisition in their study with methamphetamine.
On the other hand, some studies indicate the possibility that drug exposure outside of the training context influences the generalization curve after reliable discrimination is established. For instance, some studies have reported that once mice are trained in the discrimination task, drug exposure outside of the training context shifts the generalization curve to the right (Becker et al., 2004; Crissman et al., 2004; Sannerud and Young, 1987; Young et al., 1996), indicative of tolerance. Germane to the present study, two previous reports found that exposing subjects to the drug before discrimination training could shift the discriminative stimulus response function either to the right (McKim, 1976) or to the left (Suzuki et al., 2004). However, our data do not indicate that any shift occurred as the result of significant prior exposure to MPH.
Interest in low doses of MPH (e.g. 3 mg/kg or less) in behavioral pharmacology experiments is driven by clinical significance. Relatively low doses of MPH are used therapeutically in humans to treat ADHD (Kuczenski and Segal, 2005) and can significantly interact with ethanol in humans (Patrick et al., 2007) and in rodent models (Griffin et al., 2010, 2012a). Further, evidence indicates that low doses of MPH not only affect behavior but also increase extracellular levels of dopamine and norepinephrine in rodents in a regionally specific manner (Balcioglu et al., 2009; Berridge et al., 2006; Koda et al., 2010; Kuczenski and Segal, 1997, 2001).
The dissociation between significant pharmacological effects of low doses of MPH (e.g. 3 mg/kg or less) on some behaviors and the inability of these same doses to support acquisition of a classic drug discrimination task is interesting. Because MPH is used at relatively low doses in humans to improve attention (Biederman and Spencer, 2002) and improves performance of rodents in cognitive tasks (Berridge et al., 2006), it might be expected that the mice (whether sensitized or not) should be able to learn a challenging discrimination task quickly with a low dose (e.g. 2 mg/kg). However, that is not the case with the discrimination task we used. Evidence suggests that differential effects on monoamine transmission within cortical and subcortical regions could offer an explanation for this discrepancy. Reports indicate that low doses of MPH significantly increase extracellular dopamine and norepinephrine in the mouse and rat prefrontal cortex and, in contrast, cause relatively little change in striatal areas, even with repeated exposure (Berridge et al., 2006; Koda et al., 2010). Behaviorally, there is evidence from lesion studies that the prefrontal cortex is involved in the development of locomotor sensitization to MPH (Lee et al., 2008) and in discrimination tasks for alcohol (Hodge and Cox, 1998) and nicotine (Smith and Stolerman, 2009). Similarly, striatal regions have also been shown to be important for the discriminative stimulus control of reinforced behavior by alcohol (Besheer et al., 2003; Hodge and Alken, 1996; Hodge and Cox, 1998). Since low doses of MPH preferentially increase monoamines in frontal cortical areas but low doses are not associated with the acquisition of our discrimination task [present study and (McGovern et al., 2011)], it appears that significantly increasing extracellular monoamine levels in the prefrontal cortex may not be sufficient to drive acquisition of the discriminative stimulus control of behavior by MPH, even in sensitized mice. Thus, for the discriminative stimulus of MPH to gain control of reinforced behavior, the engagement of striatal areas that occurs at higher MPH doses (Koda et al., 2010) may be necessary. Of course, further testing is required to confirm this hypothesis.
There are some issues that deserve consideration in relation to the present studies. First, an argument can be made that training sessions should have simply continued using the 2 mg/kg training dose until mice demonstrated reliable discrimination. However, at the end of our training period with the low dose, mice in both groups were performing similarly. Thus, there was no strong evidence that sensitization to MPH influenced acquisition of the discrimination task to the low dose. Further, Suzuki and colleagues (2004) suggested that a leftward shift in the generalization curve for a psychostimulant could occur without apparent effects on acquisition. Therefore, we increased the training dose so that we could conduct generalization testing with mice reliably meeting discrimination criteria. Another consideration is that the pharmacological effects of 8 mg/kg MPH that the mice experienced during the sensitization phase may have simply been too different compared to those experienced during discrimination training, when 2 mg/kg MPH was administered to influence acquisition. Some evidence for this possibility was found in the generalization phase of the current study when 6 mg/kg MPH was tested and the mice nearly universally pressed on the vehicle-paired lever rather than the MPH-paired lever maintained on the 4mg/kg training dose.
Lastly, it is worth considering that a different discrimination task may have yielded a different outcome. For example, the prior work of McKim (1976) with scopolamine used a task that required rats to avoid a potent shock (0.5 mA), which is quite relevant to the test subject and therefore quickly learned. The demonstration that pre-exposure to scopolamine enhanced acquisition of this task suggests that drug exposure outside the training context could exert an influence on the discriminative stimulus control of reinforced behavior when the reinforcer (e.g. shock avoidance) has immediate salience to the test subject. Alternatively, rather than using a procedure that relies on negative reinforcement, another strategy might be to use the discriminative stimulus of MPH as an occasion setter predicting when a discrete cue signals delivery (or not) of a reinforcer such as sucrose. Such Pavlovian conditioning procedures can be rapidly trained and have been demonstrated for drugs like alcohol (Besheer et al., 2012) and nicotine (Besheer et al., 2004; Palmatier et al., 2004). Strategies like these may prove useful in future studies that examine the influence of drug exposure outside of the training context on the discriminative stimulus control of reinforced behavior by abused drugs.
In conclusion, the data presented here indicate that a locomotor sensitizing regimen of MPH in B6 mice does not enhance the acquisition of an MPH discrimination task, nor does it result in a left-shift of the discrimination response function. The disconnect between MPH locomotor sensitization and discrimination in a classic operant task may be related to the differential pharmacological effects on monoaminergic neurotransmission between cortical and subcortical brain regions, and the role they have in supporting discriminative stimulus control of behavior.
Acknowledgments
This work was supported by NIH grants K12 GM081265 and UL1 RR029882.
References
- Askenasy EP, Taber KH, Yang PB, Dafny N. Methylphenidate (Ritalin): behavioral studies in the rat. Int J Neurosci. 2007;117:757–794. doi: 10.1080/00207450600910176. [DOI] [PubMed] [Google Scholar]
- Balcioglu A, Ren JQ, McCarthy D, Spencer TJ, Biederman J, Bhide PG. Plasma and brain concentrations of oral therapeutic doses of methylphenidate and their impact on brain monoamine content in mice. Neuropharmacology. 2009;57:687–693. doi: 10.1016/j.neuropharm.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker HC, Crissman AM, Studders S, Kelley BM, Middaugh LD. Differential neurosensitivity to the discriminative stimulus properties of ethanol in C57BL/6J and C3H/He mice. Alcohol Clin Exp Res. 2004;28:712–719. doi: 10.1097/01.alc.0000125351.09265.f0. [DOI] [PubMed] [Google Scholar]
- Becker H, Griffin W, Lopez M. Neuroadaptive Changes that Result from Chronic Drug Exposure. In: Miller P, editor. Biological Research on Addiction: Comprehensive Addictive Behaviors and Disorders. Vol. 2. Academic Press; 2013. pp. 169–179. [Google Scholar]
- Bell GH, Griffin WC, 3rd, Patrick KS. Oral and transdermal DL-methylphenidate-ethanol interactions in C57BL/6J mice: potentiation of locomotor activity with oral delivery. Pharmacol Biochem Behav. 2011;100:264–270. doi: 10.1016/j.pbb.2011.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Devilbiss DM, Andrzejewski ME, Arnsten AF, Kelley AE, Schmeichel B, et al. Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biol Psychiatry. 2006;60:1111–1120. doi: 10.1016/j.biopsych.2006.04.022. [DOI] [PubMed] [Google Scholar]
- Besheer J, Cox AA, Hodge CW. Coregulation of ethanol discrimination by the nucleus accumbens and amygdala. Alcohol Clin Exp Res. 2003;27:450–456. doi: 10.1097/01.ALC.0000057036.64169.C1. [DOI] [PubMed] [Google Scholar]
- Besheer J, Palmatier MI, Metschke DM, Bevins RA. Nicotine as a signal for the presence or absence of sucrose reward: a Pavlovian drug appetitive conditioning preparation in rats. Psychopharmacology (Berl) 2004;172:108–117. doi: 10.1007/s00213-003-1621-9. [DOI] [PubMed] [Google Scholar]
- Besheer J, Fisher KR, Durant B. Assessment of the interoceptive effects of alcohol in rats using short-term training procedures. Alcohol. 2012;46:747–755. doi: 10.1016/j.alcohol.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J, Faraone SV. Attention-deficit hyperactivity disorder. Lancet. 2005;366:237–248. doi: 10.1016/S0140-6736(05)66915-2. [DOI] [PubMed] [Google Scholar]
- Biederman J, Spencer T. Methylphenidate in treatment of adults with Attention-Deficit/Hyperactivity Disorder. J Atten Dis. 2002;6(Suppl 1):S101–107. doi: 10.1177/070674370200601s12. [DOI] [PubMed] [Google Scholar]
- Bondareva TS, Young R, Glennon RA. Central stimulants as discriminative stimuli. Asymmetric generalization between (−)ephedrine and S(+)methamphetamine. Pharmacol Biochem Behav. 2002;74:157–162. doi: 10.1016/s0091-3057(02)00963-2. [DOI] [PubMed] [Google Scholar]
- Brookshire BR, Jones SR. Chronic methylphenidate administration in mice produces depressive-like behaviors and altered responses to fluoxetine. Synapse. 2012;66:844–847. doi: 10.1002/syn.21569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Ferris MJ, Melchior JR, Bermejo K, Salahpour A, Roberts DC, et al. Methylphenidate and cocaine self-administration produce distinct dopamine terminal alterations. Addict Biol. 2012 doi: 10.1111/j.1369-1600.2012.00456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crissman AM, Studders SL, Becker HC. Tolerance to the discriminative stimulus effects of ethanol following chronic inhalation exposure to ethanol in C57BL/6J mice. Behav Pharmacol. 2004;15:569–575. doi: 10.1097/00008877-200412000-00005. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Makriyannis A, Bergman J. Methamphetamine discrimination and in vivo microdialysis in squirrel monkeys. Psychopharmacology (Berl) 2004;175:170–178. doi: 10.1007/s00213-004-1798-6. [DOI] [PubMed] [Google Scholar]
- Darredeau C, Barrett SP, Jardin B, Pihl RO. Patterns and predictors of medication compliance, diversion, and misuse in adult prescribed methylphenidate users. Hum Psychopharmacol. 2007;22:529–536. doi: 10.1002/hup.883. [DOI] [PubMed] [Google Scholar]
- Desai RI, Paronis CA, Martin J, Desai R, Bergman J. Monoaminergic psychomotor stimulants: discriminative-stimulus effects and dopamine efflux. J Pharmacol Exp Ther. 2010 doi: 10.1124/jpet.110.165746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke AN, Bigelow GE, Lanier RK, Strain EC. Discriminative stimulus effects of tramadol in humans. J Pharmacol Exp Ther. 2011;338:255–262. doi: 10.1124/jpet.111.181131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris MJ, Calipari ES, Mateo Y, Melchior JR, Roberts DC, Jones SR. Cocaine self-administration produces pharmacodynamic tolerance: differential effects on the potency of dopamine transporter blockers, releasers, and methylphenidate. Neuropsychopharmacology. 2012;37:1708–1716. doi: 10.1038/npp.2012.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey J. Safety of therapeutic methylphenidate in adults: a systematic review of the evidence. J Psychopharmacol. 2009;23:194–205. doi: 10.1177/0269881108089809. [DOI] [PubMed] [Google Scholar]
- Greenberg I, Kuhn DM, Appel JB. Behaviorally induced sensitivity to the discriminable properties of LSD. Psychopharmacologia. 1975;43:229–232. doi: 10.1007/BF00429255. [DOI] [PubMed] [Google Scholar]
- Griffin WC, 3rd, Middaugh LD. The influence of sex on extracellular dopamine and locomotor activity in C57BL/6J mice before and after acute cocaine challenge. Synapse. 2006;59:74–81. doi: 10.1002/syn.20218. [DOI] [PubMed] [Google Scholar]
- Griffin WC, 3rd, Novak AJ, Middaugh LD, Patrick KS. The interactive effects of methylphenidate and ethanol on ethanol consumption and locomotor activity in mice. Pharmacol Biochem Behav. 2010 doi: 10.1016/j.pbb.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC, 3rd, McGovern RW, Bell GH, Randall PK, Middaugh LD, Patrick KS. Interactive effects of methylphenidate and alcohol on discrimination, conditioned place preference and motor coordination in C57BL/6J mice. Psychopharmacol. 2012a;225:613–625. doi: 10.1007/s00213-012-2849-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC, 3rd, Nguyen SA, Deleon CP, Middaugh LD. Effects of vigabatrin, an irreversible GABA transaminase inhibitor, on ethanol reinforcement and ethanol discriminative stimuli in mice. Behav Pharm. 2012b;23:178–190. doi: 10.1097/FBP.0b013e3283512c56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerness P, Petty C, Faraone SV, Biederman J. Do Stimulants Reduce the Risk for Alcohol and Substance Use in Youth With ADHD? A Secondary Analysis of a Prospective, 24-Month Open-Label Study of Osmotic-Release Methylphenidate. J Atten Disord. 2012 doi: 10.1177/1087054712468051. [DOI] [PubMed] [Google Scholar]
- Heil SH, Holmes HW, Bickel WK, Higgins ST, Badger GJ, Laws HF, et al. Comparison of the subjective, physiological, and psychomotor effects of atomoxetine and methylphenidate in light drug users. Drug Alcohol Depend. 2002;67:149–156. doi: 10.1016/s0376-8716(02)00053-4. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Alken AS. Discriminative stimulus function of ethanol: role of GABAA receptors in the nucleus accumbens. Alcohol Clin Exp Res. 1996;20:1221–1228. doi: 10.1111/j.1530-0277.1996.tb01116.x. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Cox AA. The discriminative stimulus effects of ethanol are mediated by NMDA and GABA(A) receptors in specific limbic brain regions. Psychopharmacology. 1998;139:95–107. doi: 10.1007/s002130050694. [DOI] [PubMed] [Google Scholar]
- Jones Z, Dafny N. Dose response effect of methylphenidate on ventral tegmental area neurons and animal behavior. Brain Res Bull. 2013;96:86–92. doi: 10.1016/j.brainresbull.2013.03.004. [DOI] [PubMed] [Google Scholar]
- Koda K, Ago Y, Cong Y, Kita Y, Takuma K, Matsuda T. Effects of acute and chronic administration of atomoxetine and methylphenidate on extracellular levels of noradrenaline, dopamine and serotonin in the prefrontal cortex and striatum of mice. J Neurochem. 2010;114:259–270. doi: 10.1111/j.1471-4159.2010.06750.x. [DOI] [PubMed] [Google Scholar]
- Kollins SH, MacDonald EK, Rush CR. Assessing the abuse potential of methylphenidate in nonhuman and human subjects: a review. Pharmacol Biochem Behav. 2001;68:611–627. doi: 10.1016/s0091-3057(01)00464-6. [DOI] [PubMed] [Google Scholar]
- Kollins SH, English J, Robinson R, Hallyburton M, Chrisman AK. Reinforcing and subjective effects of methylphenidate in adults with and without attention deficit hyperactivity disorder (ADHD) Psychopharmacology (Berl) 2009;204:73–83. doi: 10.1007/s00213-008-1439-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroutil LA, Van Brunt DL, Herman-Stahl MA, Heller DC, Bray RM, Penne MA. Nonmedical use of prescription stimulants in the United States. Drug Alcohol Depend. 2006;84:135–143. doi: 10.1016/j.drugalcdep.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Effects of methylphenidate on extracellular dopamine, serotonin, and norepinephrine: comparison with amphetamine. J Neurochem. 1997;68:2032–2037. doi: 10.1046/j.1471-4159.1997.68052032.x. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Locomotor effects of acute and repeated threshold doses of amphetamine and methylphenidate: relative roles of dopamine and norepinephrine. J Pharmacol Exp Ther. 2001;296:876–883. [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Stimulant actions in rodents: implications for attention-deficit/hyperactivity disorder treatment and potential substance abuse. Biol Psychiatry. 2005;57:1391–1396. doi: 10.1016/j.biopsych.2004.12.036. [DOI] [PubMed] [Google Scholar]
- Lee MJ, Swann AC, Dafny N. Methylphenidate sensitization is prevented by prefrontal cortex lesion. Brain Res Bull. 2008;76:131–140. doi: 10.1016/j.brainresbull.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Leyton M. Conditioned and sensitized responses to stimulant drugs in humans. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1601–1613. doi: 10.1016/j.pnpbp.2007.08.027. [DOI] [PubMed] [Google Scholar]
- Li SM, Campbell BL, Katz JL. Interactions of cocaine with dopamine uptake inhibitors or dopamine releasers in rats discriminating cocaine. J Pharmacol Exp Ther. 2006;317:1088–1096. doi: 10.1124/jpet.105.100594. [DOI] [PubMed] [Google Scholar]
- Lile JA, Stoops WW, Durell TM, Glaser PE, Rush CR. Discriminative-stimulus, self-reported, performance, and cardiovascular effects of atomoxetine in methylphenidate-trained humans. Exp Clin Psychopharmacol. 2006;14:136–147. doi: 10.1037/1064-1297.14.2.136. [DOI] [PubMed] [Google Scholar]
- McCabe SE, Teter CJ, Boyd CJ, Guthrie SK. Prevalence and correlates of illicit methylphenidate use among 8th, 10th, and 12th grade students in the United States, 2001. J Adolesc Health. 2004a;35:501–504. doi: 10.1016/j.jadohealth.2004.02.004. [DOI] [PubMed] [Google Scholar]
- McCabe SE, Teter CJ, Boyd CJ, Guthrie SK. Prevalence and correlates of illicit methylphenidate use among 8th, 10th, and 12th grade students in the United States, 2001. J Adol Health. 2004b;35:501–504. doi: 10.1016/j.jadohealth.2004.02.004. [DOI] [PubMed] [Google Scholar]
- McCabe SE, Cranford JA, Morales M, Young A. Simultaneous and concurrent polydrug use of alcohol and prescription drugs: prevalence, correlates, and consequences. J Stud Alcohol. 2006;67:529–537. doi: 10.15288/jsa.2006.67.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGovern RW, Middaugh LD, Patrick KS, Griffin WC., 3rd The discriminative stimulus properties of methylphenidate in C57BL/6J mice. Behav Pharmacol. 2011;22:14–22. doi: 10.1097/FBP.0b013e3283423d92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKim WA. The effects of pre-exposure to scopolamine on subsequent drug state discrimination. Psychopharmacologia. 1976;47:153–155. doi: 10.1007/BF00735814. [DOI] [PubMed] [Google Scholar]
- Novak SP, Kroutil LA, Williams RL, Van Brunt DL. The nonmedical use of prescription ADHD medications: results from a national Internet panel. Subst Abuse Treat Prev Policy. 2007;2:32. doi: 10.1186/1747-597X-2-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overton DA, Shen CF. Comparison of four-drug discriminations in training compartments with four identical levers versus four different responses manipulanda. Pharmacol Biochem Behav. 1988;30:879–888. doi: 10.1016/0091-3057(88)90114-1. [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Peterson JL, Wilkinson JL, Bevins RA. Nicotine serves as a feature-positive modulator of Pavlovian appetitive conditioning in rats. Behav Pharmacol. 2004;15:183–194. [PubMed] [Google Scholar]
- Patrick KS, Straughn AB, Minhinnett RR, Yeatts SD, Herrin AE, DeVane CL, et al. Influence of ethanol and gender on methylphenidate pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2007;81:346–353. doi: 10.1038/sj.clpt.6100082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins AN, Eckerman DA, MacPhail RC. Discriminative stimulus properties of triadimefon: comparison with methylphenidate. Pharmacol Biochem Behav. 1991;40:757–761. doi: 10.1016/0091-3057(91)90081-c. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96:103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Rush CR, Kelly TH, Hays LR, Wooten AF. Discriminative-stimulus effects of modafinil in cocaine-trained humans. Drug Alcohol Depend. 2002;67:311–322. doi: 10.1016/s0376-8716(02)00082-0. [DOI] [PubMed] [Google Scholar]
- Sannerud CA, Young AM. Environmental modification of tolerance to morphine discriminative stimulus properties in rats. Psychopharmacology (Berl) 1987;93:59–68. doi: 10.1007/BF02439587. [DOI] [PubMed] [Google Scholar]
- Schweri MM, Deutsch HM, Massey AT, Holtzman SG. Biochemical and behavioral characterization of novel methylphenidate analogs. J Pharmacol Exp Ther. 2002;301:527–535. doi: 10.1124/jpet.301.2.527. [DOI] [PubMed] [Google Scholar]
- Sevak RJ, Stoops WW, Hays LR, Rush CR. Discriminative stimulus and subject-rated effects of methamphetamine, d-amphetamine, methylphenidate, and triazolam in methamphetamine-trained humans. J Pharmacol Exp Ther. 2009;328:1007–1018. doi: 10.1124/jpet.108.147124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JW, Stolerman IP. Recognising nicotine: the neurobiological basis of nicotine discrimination. Handb Exp Pharmacol. 2009:295–333. doi: 10.1007/978-3-540-69248-5_11. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Childs E, Ford MM, Grant KA. Role of training dose in drug discrimination: a review. Behav Pharmacol. 2011;22:415–429. doi: 10.1097/FBP.0b013e328349ab37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoops WW, Lile JA, Fillmore MT, Glaser PE, Rush CR. Reinforcing effects of methylphenidate: influence of dose and behavioral demands following drug administration. Psychopharmacology (Berl) 2005a;177:349–355. doi: 10.1007/s00213-004-1946-z. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Lile JA, Glaser PE, Rush CR. Discriminative stimulus and self-reported effects of methylphenidate, d-amphetamine, and triazolam in methylphenidate-trained humans. Exp Clin Psychopharmacol. 2005b;13:56–64. doi: 10.1037/1064-1297.13.1.56. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Fukuoka Y, Mori T, Miyatake M, Narita M. Behavioral sensitization to the discriminative stimulus effects of methamphetamine in rats. Eur J Pharmacol. 2004;498:157–161. doi: 10.1016/j.ejphar.2004.07.064. [DOI] [PubMed] [Google Scholar]
- Teter CJ, McCabe SE, Boyd CJ, Guthrie SK. Illicit methylphenidate use in an undergraduate student sample: prevalence and risk factors. Pharmacotherapy. 2003;23:609–617. doi: 10.1592/phco.23.5.609.34187. [DOI] [PubMed] [Google Scholar]
- Vezina P, Leyton M. Conditioned cues and the expression of stimulant sensitization in animals and humans. Neuropharmacology. 2009;56(Suppl 1):160–168. doi: 10.1016/j.neuropharm.2008.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williard RL, Middaugh LD, Zhu HJ, Patrick KS. Methylphenidate and its ethanol transesterification metabolite ethylphenidate: brain disposition, monoamine transporters and motor activity. Behav Pharmacol. 2007;18:39–51. doi: 10.1097/FBP.0b013e3280143226. [DOI] [PubMed] [Google Scholar]
- Yang PB, Swann AC, Dafny N. Chronic administration of methylphenidate produces neurophysiological and behavioral sensitization. Brain Res. 2007;1145:66–80. doi: 10.1016/j.brainres.2007.01.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang PB, Atkins KD, Dafny N. Behavioral sensitization and cross-sensitization between methylphenidate amphetamine, and 3,4-methylenedioxymethamphetamine (MDMA) in female SD rats. Eur J Pharmacol. 2011;661:72–85. doi: 10.1016/j.ejphar.2011.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young AM, McMullen WJ, Makhay MM, Goushaw PJ. Behavioral contingencies modulate tolerance to discriminative stimulus effects of morphine. Psychopharmacology (Berl) 1996;125:220–230. doi: 10.1007/BF02247332. [DOI] [PubMed] [Google Scholar]