Abstract
Background
Nicaragua was the first developing nation to implement routine immunization with the pentavalent rotavirus vaccine (RV5). In this RV5-immunized population, understanding infectious etiologies of childhood diarrhea is necessary to direct diarrhea treatment and prevention efforts.
Methods
We followed a population-based sample of children less than 5 years in León, Nicaragua for diarrhea episodes through household visits. Information was obtained on RV5 history and sociodemographics. Stool samples collected during diarrhea episodes and among healthy children underwent laboratory analysis for viral, bacterial, and parasitic enteropathogens. Detection frequency and incidence of each enteropathogen was calculated.
Results
The 826 children in the cohort experienced 677 diarrhea episodes during 607.5 child-years of exposure time (1.1 episodes per child-year). At least one enteropathogen was detected among 61.1% of the 337 diarrheal stools collected. The most common enteropathogens among diarrheal stools were: norovirus (20.4%), sapovirus (16.6%), enteropathogenic Escherichia coli (EPEC, 11.3%), Entamoeba histolytica/dispar (8.3%), Giardia lamblia (8.0%), and enterotoxigenic E.coli (ETEC, 7.7%), with rotavirus detected among 5.3% of diarrheal stools. EPEC and ETEC were frequently detected among stools from healthy children. Among children with diarrhea, norovirus was more commonly detected among younger children (< 2 years) and G. lamblia was more commonly detected among older children (2-4 years). The mean age of rotavirus detection was 34.6 months.
Conclusions
In this Central American community following RV5 introduction, rotavirus was not commonly detected among children with diarrhea. Prevention and appropriate management of norovirus and sapovirus should be considered to further reduce the burden of diarrheal disease.
Keywords: Childhood, Community, Diarrhea, Nicaragua, Rotavirus vaccine
INTRODUCTION
Diarrheal diseases cause one in ten child deaths worldwide and are also associated with substantial morbidity [1-3]; the burden of diarrheal disease is highest in developing countries. To inform efforts to reduce this burden, it is necessary to identify common etiologies of childhood diarrhea. Prior community-based studies of diarrhea etiologies in developing countries have reported enterotoxigenic Escherichia coli (ETEC), enteropathogenic E.coli (EPEC), rotavirus, Shigella spp., Campylobacter jejuni, and intestinal parasites (Entamoeba histolytica, Giardia lamblia) to be commonly detected among children with diarrhea [4-7]. Other enteric viruses, such as the caliciviruses, norovirus and sapovirus, are increasingly recognized as important causes of childhood diarrhea in health care settings [8,9], but their role at the population level is poorly understood. A recent study of causes of diarrhea among children presenting to health facilities in sub-Saharan African and Asia prior to rotavirus vaccine introduction found that across sites, the majority of moderate to severe diarrhea was attributed to rotavirus, Cryptosporidium, ETEC, and Shigella [10].
Among all enteropathogens, rotavirus is widely recognized as the leading cause of moderate to severe diarrhea in children [11]. For this reason, many nations have added the rotavirus vaccine to their national immunization schedules. In 2006, Nicaragua became the first developing nation to implement universal infant immunization with the pentavalent rotavirus vaccine (RV5, Rotateq®, Merck, Whitehouse Station, NJ, USA). Nicaraguan infants are offered the vaccine at the age of 2, 4, and 6 months through the country’s Expanded Program on Immunization (EPI).Two studies conducted in Nicaraguan hospitals after the vaccine’s introduction found RV5 effectiveness was 58% and 76% against severe rotavirus diarrhea among children eligible to have received RV5 (2007 to 2008 [12], 2007 to 2009 [13]). In agreement with these studies, the incidence of diarrhea visits to health facilities had a modest decrease during the dry “rotavirus seasons” following the vaccine’s introduction and the prevalence of rotavirus diarrhea in primary care clinics declined from 14% to 4% [14, 15].
The goal of this study was to examine the infectious causes of childhood diarrhea at the community level in Nicaragua following RV5 introduction. Secondary objectives included examining the age distribution and seasonality of these enteropathogens. This study was conducted in the community, instead of in health facilities, since the majority of diarrhea episodes are not treated in the health care setting [16]. Elucidating the new distribution of diarrhea etiologies in the community may help direct diarrhea treatment protocols and diarrhea prevention efforts, including the development of future vaccine candidates.
MATERIALS AND METHODS
Setting
Nicaragua, a Central American nation with an estimated 2010 population of 5.8 million people, is among the lowest-income countries in Latin America [17]. This study was performed in Nicaragua’s second largest city, the municipality of León (estimated 2010 population: 192,628).
In Central America, there are two annual peaks in diarrhea incidence, one during the dry season and the other during the rainy season [18]; prior to RV5 introduction, the dry season peak in diarrhea incidence had been primarily attributed to rotavirus infection [19,20]. Based on official rainfall data in 2010, the rainy season lasted from May to November in León [21].
Study Design
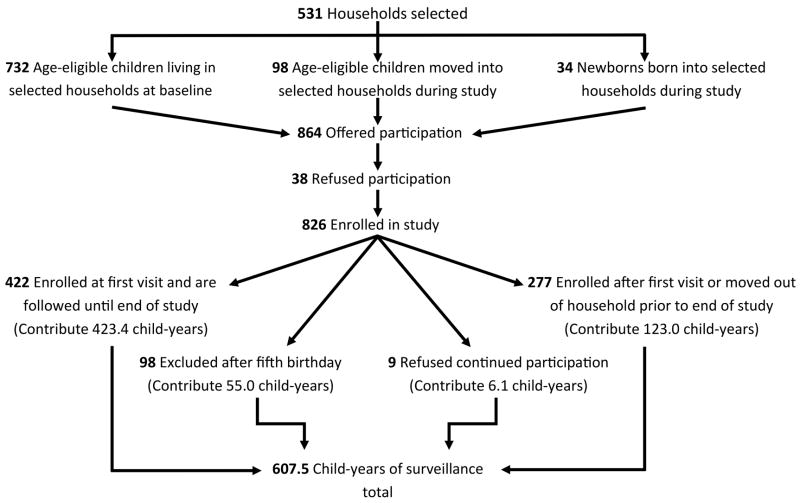
We followed a population-based sample of children from the Health and Demographic Surveillance Site-León (HDSS-León) for diarrhea episodes between January 25, 2010 and January 24, 2011. HDSS-León performs demographic surveillance of 10,994 households located in 50 out of 208 randomly selected geographical clusters in León [22,23]. As previously described [24], from these geographical clusters, HDSS-Leon provided a simple random sample of 531 households. An “open cohort” design was used; children were excluded from the study after their fifth birthday or after a move out of a selected household, while newborns or new children encountered in a selected household were offered enrollment.
Diarrhea was defined as an increase in stool frequency to at least 3 stools per 24-hour period or as a substantial change in stool consistency (bloody, very loose, watery) following at least three diarrhea-free days. Stool samples were requested from children for each diarrhea episode that occurred during the study.
To better understand the role of each enteric agent in causing disease, stool samples were also collected from healthy children enrolled in the study. A list of children who had provided any diarrhea sample was maintained and healthy controls were selected for every other child on the list in an alternating manner. If a child had provided more than one diarrhea sample during the study and a healthy control had already been selected for that child for a previous diarrhea episode, then that child was skipped on the list. The healthy controls did not have any diarrhea episodes in the previous 2 months, and were of the same sex, age group (<12 months, 12-23 months, 24-59 months), and lived in the same neighborhood (Subtiava, Perla Maria, or La Mantica) as the child with diarrhea.
Field Work and Study Instruments
Trained female field workers, who lived in the same community as the participants, performed household visits to each of the sampled households every 14 days during the study period. The interview was conducted with the mother or with the child’s caretaker, if the mother was unavailable. The study instrument administered included clinical characteristics of any diarrhea episodes, the child’s characteristics (age, sex, maternal education, breastfeeding history, RV5 history taken from the immunization card), and household characteristics (water source, sanitation system, floor type). Quality control of the interviews and data collection was performed by the field supervisor with systematic and random evaluations. Informed consent was requested of a parent or legal guardian of each participant. The study was approved by the Institutional Review Boards of the National Autonomous University of Nicaragua, León (UNAN-León) and the University of North Carolina at Chapel Hill.
Laboratory Methods
Stool specimens were obtained in a sterile plastic container or from the child’s soiled diaper. Mothers or caretakers were instructed to contact the field worker directly in the community or via cell phone for sample collection. Samples were transported within two hours from the household to the Microbiology Department of the UNAN-León at 4°C. Bacterial and parasite screening was performed using fresh specimens upon arrival to the laboratory. A 10% (wt/vol) suspension of stool was prepared using phosphate-buffered saline (pH=7.2); 2 aliquots were frozen at -20°C for later viral testing.
Bacterial Screening
Samples were cultured on deoxycholate citrate and XLD agar for the selection of Shigella and Salmonella spp., and on MacConkey and MacConkey Sorbitol agar for the selection of E. coli, incubated overnight at 37°C, and identified based on morphology and conventional biochemical tests [25]. Isolation of Camyplobacter spp. was performed on Campylobacter blood-free selective agar under microaerobic conditions at 42°C. For E. coli-positive plates, a 10 μL loop of colonies were removed and stored at -20°C for later pathotype analysis.
Detection of diarrheagenic E.coli pathotypes
Positive E. coli cultures were assayed by multiplex PCR for the following pathotypes: enteropathogenic E. coli [EPEC], enterotoxigenic E. coli [ETEC], enteroaggregative E. coli [EAEC], enteroinvasive E. coli [EIEC], and enterohemorrhagic E. coli [EHEC] as described by Vilchez [26].
Parasite Screening
Wet preparations of fresh stools using saline and iodine solution were examined by direct microscopy for the presence of intestinal parasites. Acid-fast staining as described by Garcia [27] was performed for Cryptosporidium spp. detection.
Rotavirus and Adenovirus Screening
Stored stool specimens were tested for rotavirus and enteric adenoviruses (serotypes 40 and 41) by direct enzyme immunoassays using commercially available kits (Oxoid, Cambridge, UK). The results were read visually and confirmed by absorbance measurements.
Rotavirus Genotying
Viral RNA was extracted from stool suspensions by use of a commercial kit (High Pure Viral Nucleic Acid Kit, Roche, Basel, Switzerland). A total of 60 μL of purified viral RNA obtained was stored at -20°C for later reverse transcription (RT) and PCR analysis. RT was carried out as described previously [8]. G and P genotyping was performed for rotavirus-positive samples also as described previously [28]. In brief, VP7 and VP4 genes were amplified in separate tubes using generic primers [28,29]. VP7 genotypes (G1, G2, G3, G4, G8, G9 and G10) and VP4 genotypes (P[4], P[6], P[8], P[9] and P[10]) were then investigated using generic and type-specific primers [29].
Norovirus screening and genotyping
The MagMax-96 Viral RNA Isolation Kit (Ambion, Foster City, CA, USA) was used for viral RNA extraction from 50 μl of clarified 10% stool suspension on an automated KingFisher magnetic particle processor (Thermo Fisher Scientific, Pittsburgh, PA, USA) according to the manufacturer’s instructions and eluted into 100 μL of elution buffer (10 mMTris pH 8.0 and 1 mM EDTA). As previously described [30], real-time PCR was performed to test for GI and GII noroviruses in a duplex format by using the AgPath-ID One-Step RT-PCR Kit (Applied Biosystems, Foster City, CA, USA) on a 7500 Real-Time PCR platform (Applied Biosystems). A sample was considered norovirus-positive if Ct values ≤ 36. [30]. For genotyping, viral RNA from real-time RT-PCR positive specimens was amplified using region C oligonucleotide primers [31] followed by sequencing of the RT-PCR products. Genotyping was performed as described using reference sequences used by CaliciNet [30].
Sapovirus screening and genotyping
Viral RNA extraction was performed as described above for norovirus. Real-time PCR was performed using the AgPath-ID One-Step RT-PCR Kit as described previously [32], using a 7500 Real-Time PCR System. A sample was considered sapovirus-positive if Ct values ≤ 40. For genotyping, viral RNA from real-time RT-PCR positive samples was amplified by a nested PCR as described previously [33]. Genotyping was performed using reference sapovirus sequences.
Statistical Analysis
The frequency of detection of each enteropathogen was calculated among the stool samples from children with diarrhea. Enteropathogen detection frequency was compared between stools collected from children with diarrhea and healthy control children using the Mantel-Haenszel test, with strata defined by sex, age group (0-11 months, 12-23 months, 24-59 months), and neighborhood (Subtiava, Perla Maria, La Mantica); for this analysis only, if a child provided more than one stool sample, only the first sample provided was included in the analysis. To examine potential bias in stool sample collection, we compared characteristics of sampled and non-sampled children with diarrhea using Pearson Chi-square tests. A child was classified as sampled if he or she provided at least one stool sample during the study. To compare enteropathogen detection frequency between different age groups of children, we used Fisher’s exact tests. Also for this comparison, if a child provided more than one stool sample, only the first sample provided was included in the analysis. For all comparisons, a p<0.05 was considered to be statistically significant.
The overall incidence rate of diarrhea during the study was estimated and expressed as numbers of diarrhea episodes divided by child-years of exposure. The exposure time was estimated as the number of days each child was followed during the study, divided by 365.2. Incidence rates and corresponding confidence intervals were calculated for diarrhea episodes with particular enteropathogens. For this analysis, multiple imputation was used to account for the 340 missing laboratory values from diarrhea episodes where the stool sample was not collected. Specifically, 10,000 imputed (i.e., complete) data sets were generated by randomly imputing enteropathogen lab results for each diarrhea episode with a missing stool sample. Missing lab results were imputed by Bernoulli random variables with means equal to the observed frequency of each enteropathogen among diarrheal episodes with available stool samples. Each imputed data set was analyzed using Poisson regression with offset equal to the log exposure time (in years) for each child; generalized estimating equations (GEE) were used to account for possible correlation between children in the same household. The resulting regression coefficient and standard error estimates from each imputed data set were combined using Rubin’s method [34].
To investigate seasonal patterns of infection, detection frequencies were calculated by month for the most common enteropathogens encountered among children with diarrhea. Statistical analyses were performed using Stata version 11.0 (College Station, TX, USA), SAS version 9.3 (Cary, NC, USA), and R version 3.0.1 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Participants
A total of 864 children were encountered in the selected households and offered participation in the study. The 826 children whose parent or guardian accepted participation contributed 607.5 child-years of exposure time (Figure 1). Almost all children (97.0%) had an indoor connection to the municipal water supply and 77.5% of participants’ mothers had received some secondary education (Table 1). Among those under two years of age at enrollment, 61.4% were currently breastfed. A total of 630 of 826 children were age-eligible to receive RV5; of these, 82.2% had received at least one dose.
Figure 1.
Study participation
Table 1.
Characteristics of enrolled children and their households (N=826)
| Characteristics | Total n = 826 |
|---|---|
| Sex, % male | 50.4% (416) |
| Age in months upon enrollment (mean, [SD]) | 28.9 [16.7] |
| Weight for age percentile upon enrollment <5th percentile* | 10.4% (86) |
| Breastfeeding upon enrollment† | 61.4% (215) |
| Rotavirus vaccine, at least one dose‡ | 82.2% (518) |
| Maternal education, received any secondary education | 77.5% (640) |
| Mother employed | 40.0% (330) |
| Indoor municipal water source | 97.0% (801) |
| Indoor toilet | 80.2% (662) |
| Cement or brick floor (vs. dirt floor) | 77.2% (638) |
WHO weight-for-age standards
for children under age 2 years only (N=350)
among children eligible by age to have received the vaccine (N=630)
Diarrhea Episodes
A total of 677 episodes of diarrhea were reported among 354 children, for an average of 1.1 episodes per child-year. Among the children with reported diarrhea episodes during the study period, 45.1% had 1 episode reported, 34.0% had 2 episodes reported, and 20.9% had 3 or more episodes reported. Diarrhea episodes were associated with a maximum of 4 stools per 24-hours on average (range 1 to 10). Vomiting was present among 25.7% of episodes, fever among 25.1%, and reports of blood in stool among 2.7%. Of all diarrhea episodes reported, 3.4% resulted in hospitalization.
Of the 677 episodes, 337 stool samples were collected from the community (49.8%) among a total of 223 children. Samples were collected an average of 3.1 days after the symptom onset of the diarrhea episode. The groups of sampled (N=223) and non-sampled (N=131) children with diarrhea were similar with regards to sex, age, rotavirus immunization, breastfeeding, nutritional status, household water source, maternal education and maternal employment. However, those with diarrhea in whom at least one stool sample was collected as compared to not collected were slightly more likely to live in a household with a toilet (85.1% vs. 79.4%, p=0.042) and were less likely to live in a household with a dirt floor (19.4% vs. 30.2%, p=0.001). In addition to stools collected from children with diarrhea, one healthy child was approached for every two children who provided any stool sample (N=223), with a total of 106 control stools collected.
Enteropathogen detection from stool samples
At least one enteropathogen was detected among 61.1% of the stool samples from children with diarrhea and among 41.5% of stools from healthy children. The most commonly detected enteropathogens among children with diarrhea were norovirus (20.4%), sapovirus (16.6%), EPEC (11.3%), E. histolytica/dispar (8.3%), G. lamblia (8.0%) and ETEC (7.7%), while rotavirus was detected among 5.3% (Table 2). Bacterial infections with Shigella, Campylobacter, and Salmonella spp. were uncommon (less than 1.0% each) among children in the study. We encountered a high percentage of co-infections; more than one enteropathogen was detected among 22.9% of diarrhea samples. Enteropathogens detected among stool samples from healthy children are also shown in Table 2. Differences in detection between diarrheal stools and stools from healthy children were statistically significant (p<0.05) for sapovirus, G. lamblia, and co-infections. EPEC was frequently isolated among stools from healthy children. While the frequency of ETEC isolation did not differ between diarrheal stools and stools from healthy children, ETEC with heat stable enterotoxin (ST) was only isolated in stools from children with diarrhea, and not in any stools from healthy children.
Table 2.
Enteropathogens detected from children with diarrhea and healthy children (N=443)
| Enteropathogen | Diarrhea (N=337) | Healthy Children (N=106) | p-value* |
|---|---|---|---|
| Viral | |||
| Norovirus | 20.4% (68/333)† | 13.2% (14/106) | 0.33 |
| Sapovirus | 16.6% (56/337) | 1.9% (2/106) | 0.0022 |
| Rotavirus | 5.3% (18/337) | 0.9% (1/106) | 0.11 |
| Adenovirus | 1.8% (6/337) | 2.8% (3/106) | 0.93 |
| Bacterial | |||
| EPEC‡ | 11.3% (38/337) | 14.2% (15/106) | 0.35 |
| ETEC | 7.7% (26/337) | 6.6% (7/106) | 0.38 |
| EAEC | 3.6% (12/337) | 4.7% (5/106) | 0.21 |
| EHEC | 3.0% (10/337) | 0.9% (1/106) | 0.29 |
| Salmonella enteritidis | 0.3% (1/337) | 0.0% (0/106) | 0.48 |
| Shigella flexneri | 0.3% (1/337) | 0.0% (0/106) | 0.61 |
| Campylobacter spp. | 0.3% (1/337) | 0.0% (0/106) | 0.46 |
| Parasitic | |||
| Entamoeba histolytica/dispar | 8.3% (28/337) | 3.8% (4/106) | 0.13 |
| Giardia lamblia | 8.0% (27/337) | 1.9% (2/106) | 0.0104 |
| Cryptosporidium | 1.2% (4/337) | 0.9% (1/106) | 0.48 |
| Co-Infections | 22.8% (77/337) | 10.4% (11/106) | 0.0042 |
For comparison of cases and controls using Mantel-Haenszel statistic.
Four specimens were not analyzed for norovirus.
EPEC=enteropathogenic E.coli; ETEC=enterotoxigenic E.coli; EAEC=enteroaggregative E.coli; EHEC=enterohemorrhagic E.coli
The frequency of detection of enteropathogens among children with diarrhea by age group is shown in Table 3. Norovirus was the most common enteropathogen detected among all age groups. The frequency of norovirus detection was higher in the children under 24 months of age as compared to older children (p=0.022), while the detection frequency of G. lamblia was higher in children 24 months of age and older as compared to younger children (p=0.013). The mean age of those with rotavirus diarrhea was 34.6 months. Overall incidence rates of enteropathogens detected among children with diarrhea are shown in Table 4.
Table 3.
Detection frequency of enteropathogens among children with diarrhea by age group (N=337 samples)
| Enteropathogens* | Age group
|
p-value† | ||
|---|---|---|---|---|
| 0-11 months | 12-23 months | 24-59 months | ||
| Norovirus | 34.8% (16/46) | 22.6% (23/102) | 15.7% (29/185) | 0.038 |
| Sapovirus | 10.9% (5/46) | 19.4% (20/103) | 16.6% (31/187) | 0.52 |
| Rotavirus | 2.1% (1/47) | 2.9% (3/103) | 7.5% (14/187) | 0.50 |
| Adenovirus | 2.1% (1/47) | 1.0% (1/103) | 2.1% (4/187) | 1.00 |
| EPEC‡ | 10.6% (5/47) | 14.6% (15/103) | 9.6% (18/187) | 0.41 |
| ETEC | 4.3% (2/47) | 8.7% (9/103) | 8.0% (15/187) | 1.00 |
| EAEC | 8.5% (4/47) | 3.9% (4/103) | 2.1% (4/187) | 0.097 |
| EHEC | 2.1% (1/47) | 3.9% (4/103) | 2.7% (5/187) | 1.00 |
| Entamoeba histolytica/ dispar | 8.5% (4/47) | 5.8% (6/103) | 9.6% (18/187) | 0.35 |
| Giardia lamblia | 4.3% (2/47) | 2.9% (3/103) | 11.8% (22/187) | 0.035 |
| Cryptosporidium | 2.1% (1/47) | 1.9% (2/103) | 0.5% (1/187) | 1.00 |
| Co-infections | 23.4% (11/47) | 19.4% (20/103) | 24.6% (46/187) | 0.26 |
Salmonella, Shigella, and Campylobacter spp. each with one infection among children during study and are not included on this table.
For comparison between age groups using Fisher’s exact testing. p=0.022 for comparison of norovirus prevalence between <2 years of age and 2-5 years of age, p=0.013 for comparison of Giardia lamblia prevalence between <2 years of age and 2-5 years of age.
EPEC=enteropathogenic E.coli; ETEC=enterotoxigenic E.coli; EAEC=enteroaggregative E.coli; EHEC=enterohemorrhagic E.coli
Table 4.
Incidence rates of diarrhea episodes by enteropathogen
| Enteropathogen | Incidence rate per 100 child-years | 95% Confidence intervals |
|---|---|---|
| Viral | ||
| Norovirus | 22.9 | (18.9, 27.8) |
| Sapovirus | 18.7 | (15.0, 23.3) |
| Rotavirus | 5.9 | (4.2, 8.2) |
| Adenovirus | 1.9 | (1.1, 3.4) |
| Bacterial* | ||
| EPEC† | 12.5 | (9.8, 15.8) |
| ETEC | 8.5 | (6.4, 11.3) |
| EAEC | 3.9 | (2.5, 6.1) |
| EHEC | 3.2 | (2.0, 5.2) |
| Parasitic | ||
| Entamoeba histolytica/dispar | 9.2 | (6.9, 12.2) |
| Giardia lamblia | 8.8 | (6.7, 11.7) |
| Cryptosporidium | 1.3 | (0.6, 2.6) |
| Co-Infections | 25.6 | (21.3, 30.9) |
Salmonella, Shigella, and Campylobacter spp. were isolated only one time each and are not included in table.
EPEC=enteropathogenic E.coli; ETEC=enterotoxigenic E.coli; EAEC=enteroaggregative E.coli; EHEC=enterohemorrhagic E.coli
The seasonality of the most commonly detected enteropathogens and rotavirus among the diarrheal stools are shown in Figure 2. Sapovirus detection peaked during the transition between dry and rainy season; a peak in norovirus detection followed early in the rainy season. Among the few cases of rotavirus detected, the majority (15/18) occurred during the rainy season. EPEC detection was highest during the dry season and then declined during the rest of the year. E. histolytica/dispar was more commonly detected in the rainy season.
Figure 2.
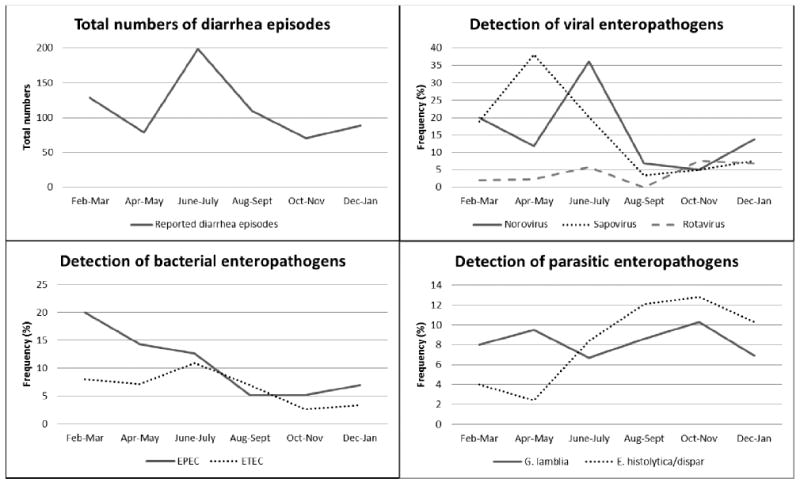
Total numbers of diarrhea episodes and enteropathogen detection frequency* by month†
*Frequency of detection (%) among all diarrheal stools collected.
†Rainy season occurred from May to November.
Detailed findings on norovirus, sapovirus, rotavirus, and G. lamblia are described below.
Norovirus
Among the 68 total norovirus diarrhea cases, a genogroup was identified in 63 cases: 23.8% (15/63) were GI, 71.4% (45/63) were GII, and 4.8% (3/63) were GI/GII co-infections. GI or GI/GII co-infections were only detected in stools collected from children with diarrhea, whereas GII was detected in both stools collected from children with diarrhea and healthy children.
Eight of the 68 children who experienced a norovirus diarrhea episode had another episode between one and ten months following the first norovirus diarrhea episode. In all but one case, the second norovirus diarrhea episode was caused by a norovirus of a different genogroup than the first episode.
Almost half (33, 48.5%) of the noroviruses cases were co-infected with another enteropathogen. The co-infections most commonly detected were with sapovirus (10), G. lamblia (8), ETEC (4), EPEC (4), and E. histolytica/dispar (4).
Sapovirus
Among the 56 total sapovirus diarrhea cases, a genogroup was identified in 22 cases: GI was identified in 40.9% (9/22), and GII was identified in 59.1% (13/22).
The majority of sapovirus cases (62.5%, 35/56) were co-infected with another enteropathogen, most commonly, norovirus, EPEC, or ETEC. Finally, diarrhea episodes caused by either norovirus or sapovirus were more likely to be accompanied by vomiting as compared to diarrhea episodes where no calicivirus was detected (39.3% vs. 21.4%, p=0.001).
Rotavirus
Further laboratory analysis of the 18 rotavirus infections among children with diarrhea showed that three were P[4]G9, two were P[10]G9, two were P[8]G3, one was P[8]G9, four were P[un-typable]G1, three were P[10]G[un-typable], two had both a G and P-type that were un-typable, and one was a mixed infection (P[4]G9, P[10]G9). Among the 18 children with rotavirus diarrhea, six had not been immunized against rotavirus, one was partially immunized, and 11 children had received all three RV5 doses.
G. lamblia
The majority of G. lamblia cases (70.4%, 19/27) were co-infected with another enteropathogen, most commonly norovirus, sapovirus, or EPEC.
DISCUSSION
We found caliciviruses (norovirus, sapovirus) to be the most common enteropathogens identified among children with diarrhea in this Central American community following RV5 introduction. Interestingly, we detected norovirus with the same frequency as in US children with diarrhea (21%) [35]. However, as compared to US children [36], we detected a higher frequency of sapovirus, which was more prevalent among symptomatic children as compared to healthy children. In contrast, caliciviruses were not identified as a significant contributor to childhood diarrhea burden in Africa and Asia [10]. This difference may be explained by our inclusion of mild cases of diarrhea, as our study was not based at health facilities, but instead captured diarrhea cases in the household. Also, despite Nicaragua’s economic status as a low-middle income country, high access to improved water sources and universal infant rotavirus immunization in this population may simulate industrialized countries in terms of enteropathogen transmission.
We also observed a low incidence rate of rotavirus diarrhea among children under two years of age (incidence rate=4.2 episodes per 100 child-years, frequency=2.7%). In comparison, a birth cohort followed in the same city prior to the rotavirus immunization program found a higher incidence rate of rotavirus diarrhea among children under two year of age (incidence rate=11.5 episodes per 100 child-years, frequency=12.4%), with the highest rotavirus incidence rate observed during the first year of life [37]. In our study, rotavirus was detected at a mean age of 34.6 months. As compared to the previous study, this finding suggests a delay in acquiring symptomatic rotavirus infection in the era of rotavirus immunization. Several developing countries have documented a lower effectiveness of the rotavirus vaccine after the first year of life [12, 38, 39] possibly due to diminished vaccine-elicited immune responses over time [40]. Even if the vaccine has a lower effectiveness in the second year of life, both an overall reduction in all age groups, and a possible delay in acquiring rotavirus diarrhea to an older, more “resilient” age may explain the decrease in diarrhea-related mortality in countries where universal rotavirus immunization programs have been implemented [41, 42]. Finally, we did not observe the traditional peak of rotavirus transmission during the dry season, as was observed prior to the immunization program [19, 20].
Among bacterial causes of diarrhea, diarrheagenic E. coli was commonly isolated among all age groups, but was also frequently isolated in stools from healthy children, as has been reported in prior developing world studies [26, 43, 44]. This high detection frequency among healthy children may be explained by heterogeneity within each E. coli pathotype; it is possible that only certain strains are truly diarrheagenic [45, 46]. For example, in the case of ETEC, the ST virulence-type was not detected in any stools from healthy children in our study. In addition, the high prevalence of diarrheagenic E. coli among healthy children may reflect the “endemic phenomena” of this group of bacteria in a highly exposed population [7,26]. Young children may be symptomatic during their first infection, but symptoms may decrease as immune responses develop in response to subsequent infections.
EHEC was found at an incidence rate of 3.6 cases per 100 child-years. While this incidence rate is relatively low, the association between EHEC and hemolytic-uremic syndrome raises concern about the potential serious sequelae of EHEC infection in this population. Finally, the unexpected lower incidence of the bacterial pathogens Shigella, Salmonella, and Campylobacter spp. may be due to annual variation in transmission rates, or may reflect the high prevalence of access to improved water, normal nutritional status, and breastfeeding among the children in the study, factors which provide protection against these infections [47-51].
G. lamblia was a commonly detected parasitic pathogen among children with diarrhea in the study, especially among those two years of age and older. Contrary to some prior studies [10, 52], we detected G. lamblia in a higher proportion of symptomatic episodes of diarrhea as compared to in healthy controls. Interestingly, in our study, G. lamblia was commonly detected together with a calicivirus. The role of G. lamblia in potentiating diarrhea symptoms in coinfection with caliciviruses warrants further investigation.
A strength of this study in comparison to prior community-based studies was its ability to detect caliciviruses (norovirus and sapovirus), using newly developed PCR-based laboratory techniques for their detection. However, we acknowledge several limitations. We did not analyze stools from children who experienced vomiting only without diarrhea, so our study may have underestimated the incidence of norovirus cases. Secondly, a temporary delay in the collection of control stools resulted in more control stool collection during the dry season. Finally, we acknowledge that in the laboratory analysis of Entamoeba spp., microscopy alone cannot distinguish E. histolytica, a pathogenic parasite, from E. dispar, a commensal parasite.
In conclusion, we found that rotavirus was not a common cause of childhood diarrhea in this developing world community setting following RV5 introduction. Instead, caliciviruses were most commonly detected among children with diarrhea. The high detection frequency of caliciviruses argues against the empiric use of antibiotics for the treatment of diarrhea in this setting. Future research should focus on effective prevention of these enteric viruses to reduce the remaining burden of childhood diarrhea in Nicaragua, and perhaps elsewhere in Latin America. In addition, on-going local surveillance of the causes of childhood diarrhea is needed to ensure the effectiveness of management guidelines and to guide future prevention strategies.
Acknowledgments
We would like to thank Claudia Cortez, Lesbia Medrano, Patricia Blandón, Yahoska Reyes, Marlon Meléndez, and the participating families for their contributions to this study. The technical assistance of Hannah Shirley (CDC) and Erica Lloyd (UNC) is greatly acknowledged.
This study was supported in part by a research grant from the Investigator-Initiated Studies Program of Merck Sharp & Dohme Corp. The opinions expressed in this paper are those of the authors and do not necessarily represent those of Merck Sharp & Dohme Corp.
This study received funding from the Merck Investigator-Initiated Studies Program (laboratory analysis) and the Thrasher Research Fund (field work). Dr. Becker-Dreps was supported by 5K01TW008401-04 from the Fogarty International Center at the National Institutes of Health. Dr. Bucardo received support for calicivirus research in Nicaragua from NETROPICA (05-N-2010).
References
- 1.Liu L, Johnson HL, Cousens S, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379(9832):2151–61. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- 2.Guerrant RL, Schorling JB, McAuliffe JF, de Souza MA. Diarrhea as a cause and an effect of malnutrition: diarrhea prevents catch-up growth and malnutrition increases diarrhea frequency and duration. Am J Trop Med Hyg. 1992;47(1, Pt 2):28–35. doi: 10.4269/ajtmh.1992.47.28. [DOI] [PubMed] [Google Scholar]
- 3.Niehaus MD, Moore SR, Patrick PD, et al. Early childhood diarrhea is associated with diminished cognitive function 4 to 7 years later in children in a northeast Brazilian shantytown. Am J Trop Med Hyg. 2002;66(5):590–593. doi: 10.4269/ajtmh.2002.66.590. [DOI] [PubMed] [Google Scholar]
- 4.Hasan KZ, Pathela P, Alam K, et al. Aetiology of diarrhoea in a birth cohort of children aged 0-2 years in rural Mirzapur, Bangladesh. J Health Popul Nutr. 2006;24(1):25–35. [PubMed] [Google Scholar]
- 5.Cravioto A, Reyes RE, Ortega R, Fernández G, Hernández R, López D. Prospective study of diarrhoeal disease in a cohort of rural Mexican children: incidence and isolated pathogens during the first two years of life. Epidemiol Infect. 1988;101(1):123–34. doi: 10.1017/s0950268800029289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Téllez A, Morales W, Rivera T, Meyer E, Leiva B, Linder E. Prevalence of intestinal parasites in the human population of León, Nicaragua. Acta Trop. 1997;66(3):119–25. doi: 10.1016/s0001-706x(97)00037-5. [DOI] [PubMed] [Google Scholar]
- 7.Paniagua M, Espinoza F, Ringman M, Reizenstein E, Svennerholm AM, Hallander H. Analysis of incidence of infection with enterotoxigenic Escherichia coli in a prospective cohort study of infant diarrhea in Nicaragua. J Clin Microbiol. 1997;35(6):1404–10. doi: 10.1128/jcm.35.6.1404-1410.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bucardo F, Nordgren J, Carlsson B, et al. Pediatric norovirus diarrhea in Nicaragua. J Clin Microbiol. 2008;46(8):2573–80. doi: 10.1128/JCM.00505-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel MM, Widdowson MA, Glass RI, Akazawa K, Vinjé J, Parashar UD. Systematic literature review of role of noroviruses in sporadic gastroenteritis. Emerg Infect Dis. 2008;14(8):1224–31. doi: 10.3201/eid1408.071114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kotloff KL, Nataro JP, Blackwelder WC, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. 2013;382(9888):209–22. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 11.Parashar UD, Burton A, Lanata C, et al. Global mortality associated with rotavirus disease among children in 2004. J Infect Dis. 2009;200(Suppl 1):S9–S15. doi: 10.1086/605025. [DOI] [PubMed] [Google Scholar]
- 12.Patel M, Pedreira C, De Oliveira LH, et al. Association between pentavalent rotavirus vaccine and severe rotavirus diarrhea among children in Nicaragua. JAMA. 2009;301(21):2243–51. doi: 10.1001/jama.2009.756. [DOI] [PubMed] [Google Scholar]
- 13.Mast TC, Khawaja S, Espinoza F, et al. Case-control study of the effectiveness of vaccination with pentavalent rotavirus vaccine in Nicaragua. Pediatr Infect Dis J. 2011;30(11):e209–15. doi: 10.1097/INF.0b013e31822a8527. [DOI] [PubMed] [Google Scholar]
- 14.Becker-Dreps S, Paniagua M, Dominik R, et al. Changes in childhood diarrhea incidence in Nicaragua following 3 years of universal infant rotavirus immunization. Pediatr Infect Dis J. 2011;30(3):243–7. doi: 10.1097/INF.0b013e3181f87ffe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Becker-Dreps S, Paniagua M, Zambrana LE, et al. Rotavirus prevalence in the primary care setting in Nicaragua after universal infant rotavirus immunization. Am J Trop Med Hyg. 2011;85(5):957–60. doi: 10.4269/ajtmh.2011.11-0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parashar UD, Hummelman EG, Bresee JS, Miller MA, Glass RI. Global illness and deaths caused by rotavirus disease in children. Emerg Infect Dis. 2003;9(5):565–72. doi: 10.3201/eid0905.020562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Bank. [December 21, 2013];Nicaragua at a glance. 3/17/13 Available at http://devdata.worldbank.org/AAG/nic_aag.pdf.
- 18.Nicaraguan Ministry of Health. Weekly Epidemiological Bulletings. [December 21, 2013]; Available at: http://www.minsa.gob.ni.
- 19.Levy K, Hubbard AE, Eisenberg JN. Seasonality of rotavirus disease in the tropics: a systematic review and meta-analysis. Int J Epidemiol. 2009;38(6):1487–96. doi: 10.1093/ije/dyn260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Oliveira LH, Danovaro-Holliday MC, Andrus JK, et al. Sentinel hospital surveillance for rotavirus in Latin American and Caribbean countries. J Infect Dis. 2009;200(Suppl 1):S131–9. doi: 10.1086/605060. [DOI] [PubMed] [Google Scholar]
- 21.Instituto Nicaragüense de Estudios Teritoriales (INETER) Dirección General de Meteorlogía, Resumen Meteoróligo Diario. Aeropuerto Godoy; León: 2010-2011. [Google Scholar]
- 22.Pena R, Perez W, Melendez M, Kallestal C, Persson LA. The Nicaraguan Health and Demographic Surveillance Site, HDSS-Leon: a platform for public health research. Scand J Public Health. 2008;36(3):318–25. doi: 10.1177/1403494807085357. [DOI] [PubMed] [Google Scholar]
- 23.Universidad Nacional Autónoma de Nicaragua, León, Centro de Investigación en Demografía y Salud (CIDS) [December 21, 2013]; Available at: http://www.cids.edu.ni/svds.html.
- 24.Becker-Dreps S, Meléndez M, Liu L, et al. Community Diarrhea Incidence Before and After Rotavirus Vaccine Introduction in Nicaragua. Am J Trop Med Hyg. 2013;89(2):246–50. doi: 10.4269/ajtmh.13-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gillespie SH, Hawkey PM. Principles and Practice of Clinical Bacteriology. 2. Chichester, UK: John Wiley & Sons; 2006. [Google Scholar]
- 26.Vilchez S, Reyes D, Paniagua M, Bucardo F, Möllby R, Weintraub A. Prevalence of diarrhoeagenic Escherichia coli in children from León, Nicaragua. J Med Microbiol. 2009;58(Pt 5):630–7. doi: 10.1099/jmm.0.007369-0. [DOI] [PubMed] [Google Scholar]
- 27.Garcia LS, Bruckner DA, Brewer TC, Shimizu RY. Techniques for the recovery and identification of Cryptosporidium oocysts from stool specimens. J Clin Microbiol. 1983;18(1):185–90. doi: 10.1128/jcm.18.1.185-190.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iturriza-Gómara M, Kang G, Gray J. Rotavirus genotyping: keeping up with an evolving population of human rotaviruses. J Clin Virol. 2004;31(4):259–265. doi: 10.1016/j.jcv.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 29.Gentsch JR, Glass RI, Woods P, et al. Identification of group A rotavirus gene 4 types by polymerase chain reaction. J Clin Microbiol. 1992;30(6):1365–73. doi: 10.1128/jcm.30.6.1365-1373.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vega E, Barclay L, Gregoricus N, Williams K, Lee D, Vinjé J. Novel surveillance network for norovirus gastroenteritis outbreaks, United States. Emerg Infect Dis. 2011;17(8):1389–95. doi: 10.3201/eid1708.101837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kojima S, Kageyama T, Fukushi S, et al. Genogroup-specific PCR primers for detection of Norwalk-like viruses. J Virol Methods. 2002;100(1-2):107–14. doi: 10.1016/s0166-0934(01)00404-9. [DOI] [PubMed] [Google Scholar]
- 32.Oka T, Katayama K, Hansman GS, et al. Detection of human sapovirus by real-time reverse transcription-polymerase chain reaction. J Med Virol. 2006;78(10):1347–53. doi: 10.1002/jmv.20699. [DOI] [PubMed] [Google Scholar]
- 33.Okada M, Yamashita Y, Oseto M, Shinozaki K. The detection of human sapoviruses with universal and genogroup-specific primers. Arch Virol. 2006;151(12):2503–9. doi: 10.1007/s00705-006-0820-1. [DOI] [PubMed] [Google Scholar]
- 34.Rubin DB. Multiple Imputation for Nonresponse in Surveys. J Wiley & Sons; New York: 1987. [Google Scholar]
- 35.Payne DC, Vinjé J, Szilagyi PG, et al. Norovirus and medically attended gastroenteritis in U.S. children. N Engl J Med. 2013;368(12):1121–30. doi: 10.1056/NEJMsa1206589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chhabra P, Payne DC, Szilagyi PG, et al. Etiology of Viral Gastroenteritis Infections in Children ≤ 5 Years of Age in the United States, 2008-2009. J Infect Dis. 2013;208(5):790–800. doi: 10.1093/infdis/jit254. [DOI] [PubMed] [Google Scholar]
- 37.Espinoza F, Paniagua M, Hallander H, Svensson L, Strannegard O. Rotavirus infections in young Nicaraguan children. Pediatr Infect Dis J. 1997;16(6):564–71. doi: 10.1097/00006454-199706000-00006. [DOI] [PubMed] [Google Scholar]
- 38.de Palma O, Cruz L, Ramos H, et al. Effectiveness of rotavirus vaccination against childhood diarrhoea in El Salvador: case-control study. BMJ. 2010;340:c2825. doi: 10.1136/bmj.c2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Armah GE, Sow SO, Breiman RF, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;376(9741):606–14. doi: 10.1016/S0140-6736(10)60889-6. [DOI] [PubMed] [Google Scholar]
- 40.Patel M, Glass RI, Jiang B, Santosham M, Lopman B, Parashar U. A systematic review of anti-rotavirus serum IgA antibody titer as a potential correlate of rotavirus vaccine efficacy. J Infect Dis. 2013;208(2):284–94. doi: 10.1093/infdis/jit166. [DOI] [PubMed] [Google Scholar]
- 41.do Carmo GM, Yen C, Cortes J, et al. Decline in diarrhea mortality and admissions after routine childhood rotavirus immunization in Brazil: a time-series analysis. PLoS Med. 2011;8(4):e1001024. doi: 10.1371/journal.pmed.1001024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Richardson V, Hernandez-Pichardo J, Quintanar-Solares M, et al. Effect of rotavirus vaccination on death from childhood diarrhea in Mexico. N Engl J Med. 2010;362(4):299–305. doi: 10.1056/NEJMoa0905211. [DOI] [PubMed] [Google Scholar]
- 43.Wennerås C, Erling V. Prevalence of enterotoxigenic Escherichia coli-associated diarrhoea and carrier state in the developing world. J Health Popul Nutr. 2004;22(4):370–82. [PubMed] [Google Scholar]
- 44.Huilan S, Zhen LG, Mathan MM, et al. Etiology of acute diarrhoea among children in developing countries: a multicentre study in five countries. Bull World Health Organ. 1991;69(5):549–55. [PMC free article] [PubMed] [Google Scholar]
- 45.Nataro JP, Deng Y, Cookson S, et al. Heterogeneity of enteroaggregative Escherichia coli virulence demonstrated in volunteers. J Infect Dis. 1995;171:465–8. doi: 10.1093/infdis/171.2.465. [DOI] [PubMed] [Google Scholar]
- 46.Trabulsi LR, Keller R, Tardelli Gomes TA. Typical and atypical enteropathogenic Escherichia coli. Emerg Infect Dis. 2002;8:508–13. doi: 10.3201/eid0805.010385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chompook P, Todd J, Wheeler JG, von Seidlein L, Clemens J, Chaicumpa W. Risk factors for shigellosis in Thailand. Int J Infect Dis. 2006;10(6):425–33. doi: 10.1016/j.ijid.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 48.Fullerton KE, Ingram LA, Jones TF, et al. Sporadic campylobacter infection in infants: a population-based surveillance case-control study. Pediatr Infect Dis J. 2007;26(1):19–24. doi: 10.1097/01.inf.0000247137.43495.34. [DOI] [PubMed] [Google Scholar]
- 49.Kosek M, Yori PP, Pan WK, et al. Epidemiology of highly endemic multiply antibiotic-resistant shigellosis in children in the Peruvian Amazon. Pediatrics. 2008;122(3):e541–9. doi: 10.1542/peds.2008-0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brooks JT, Shapiro RL, Kumar L, et al. Epidemiology of sporadic bloody diarrhea in rural Western Kenya. Am J Trop Med Hyg. 2003;68(6):671–7. [PubMed] [Google Scholar]
- 51.Ahmed F, Clemens JD, Rao MR, Ansaruzzaman M, Haque E. Epidemiology of shigellosis among children exposed to cases of Shigella dysentery: a multivariate assessment. Am J Trop Med Hyg. 1997;56(3):258–64. doi: 10.4269/ajtmh.1997.56.258. [DOI] [PubMed] [Google Scholar]
- 52.Swierczewski BE, Odundo EA, Koech MC, et al. Surveillance for enteric pathogens in a case-control study of acute diarrhea in Western Kenya. Trans R Soc Trop Med Hyg. 2013;107(2):83–90. doi: 10.1093/trstmh/trs022. [DOI] [PubMed] [Google Scholar]