Abstract
The epigenetic impact of curcumin in stroke and neurodegenerative disorders is curiosity-arousing. It is derived from Curcuma longa (spice), possesses anti-oxidative, anti-inflammatory, anti-lipidemic, neuro-protective and recently shown to exhibit epigenetic modulatory properties. Epigenetic studies include DNA methylation, histone modifications and RNA-based mechanisms which regulate gene expression without altering nucleotide sequences. Curcumin has been shown to affect cancer by altering epigenetic changes but its role as an epigenetic agent in cerebral stroke has not been much explored. Although curcumin possesses remarkable medicinal properties, the bioavailability of curcumin has limited its success in epigenetic studies and clinical trials. The present review is therefore designed to look into epigenetic mechanisms that could be induced with curcumin during stroke, along with its molecular designing with different moieties that may increase its bioavailability. Curcumin has been shown to be encapsulated in exosomes, nano-vesicles (<200 nm), thereby showing its therapeutic effects in brain diseases. Curcumin delivered through nanoparticles has been shown to be neuroregenerative but the use of nanoparticles in brain has limitations. Hence, curcumin-encapsulated exosomes along with curcumin-primed exosomes (exosomes released by curcumin-treated cells) are much needed to be explored to broadly look into their use as a novel therapy for stroke.
Keywords: Exosomes, DNA methylation, Histone modifications, microRNA, Oxidative stress
Introduction
Cerebral stroke or “brain attack” is caused by interruption of blood supply to the brain which leads to the loss of brain functions (Langhorne et al. 2013). Cerebral stroke can be classified into ischemic (blockage of blood supply) or hemorrhagic (bursting of blood vessels). Thrombolytic agents, anti-platelet drugs, and neuro-surgery are the only available options for the treatment of stroke (Adams, Jr. et al. 2007); however the protective therapy against cerebral stroke is yet to be discovered. Dietary components have found to exert immense impacts on normal functioning of the brain (Alamy and Bengelloun, 2012; Bedi, 2003; Gomez-Pinilla, 2008) and contribute to the prevention of a series of brain diseases including stroke (Psaltopoulou et al. 2013). Reports indicate that dietary components not only evoke genetic, but also epigenetic components to compensate stroke, or stroke-like pathologies (Gallou-Kabani et al. 2007; Kalani et al. 2013a). In that regard the potential of curcumin, which also exhibits genetic and epigenetic influences, cannot be ignored. Curcumin is derived from the roots of Curcumin longa and due to remarkable medicinal properties; curcumin (diferuloylmethane) is termed as yellow gold. Curcumin treatment provides vascular protective effects in persons at risk for stroke (Ovbiagele, 2008). The stroke preventive properties of curcumin can be attributed to: 1) neuro-protection via free radical scavenging, inhibiting nitric oxide synthase and lipid peroxidation (Strimpakos and Sharma, 2008); 2) anti-inflammatory property by suppressing the production of IL-1, IL-8 and TNF-α (Strimpakos and Sharma, 2008); 3) anti-lipidemic property by lowering cholesterol and boosting up HDL (Soni and Kuttan, 1992) and; 4) anti-aggregation property by inhibiting platelet aggregation and inducing platelet aggregation factor (Strimpakos and Sharma, 2008). The ability of curcumin to cross blood-brain-barrier (BBB) also favors its selection over other therapeutic agents/molecules during cerebral stroke (Mishra and Palanivelu, 2008; Tsai et al. 2011). In addition, curcumin appears to have potential to inhibit amyloid beta oligomers and fibrils formation in mice (Yang et al. 2005). The therapeutic efficacy of curcumin in middle cerebral artery occlusion (MCAO) models of rat and mice has also been explored (Lapchak et al. 2011; Shukla et al. 2008; Tyagi et al. 2012; Zhao et al. 2010). Studies suggest that curcumin overcomes cerebral ischemia by its neuro-protective and anti-oxidative properties (Strimpakos and Sharma, 2008; Tyagi et al. 2012). Besides exhibiting anti-inflammtory, anti-lipidemic, and anti-oxidative properties, curcumin also induces signs of epigenetic changes (Chiu et al. 2013; Hardy and Tollefsbol, 2011; Martin et al. 2013; Teiten et al. 2013); however the epigenetic influence of curcumin on stroke epigenetics is needed to be explored. In this present review, we propose that curcumin affect molecular processes such as DNA methylation, histone modification, nucleosome remodeling, and small noncoding RNAs (ncRNAs) (e.g., miRNAs) that modulate gene expression and impart an important role in amelioration of stroke pathogenesis. Since, curcumin possesses potential therapeutic effects and therefore, it has been recommended for clinical trials to prevent / treat brain disease, including stroke (Goel and Aggarwal, 2010; Ovbiagele, 2008; Perry and Howes, 2011). Phase I clinical trials on curcumin were not successful due to its low bioavailability (Anand et al. 2007). The factors that limit curcumin bioavailability include; poor absorption, quick metabolism, and rapid systemic elimination. However, recent studies suggest that curcumin-encapsulated exosomes are more stable, highly soluble, highly concentrated in the blood and possess therapeutic potentials (Sun et al. 2010; Zhuang et al. 2011). Exosomes are the nano-vesicles (<200 nm) derived from the fusion of multivesicular body to the plasma membrane and found in the extracellular body fluids (serum, plasma, saliva, urine, breast milk, brancheo-alveoli lavage) including culture conditioned media (Kalani et al. 2014; Thery et al. 2002; Thery et al. 2006). These nano-units have been employed for the treatment of stroke in rat (Xin et al. 2013). Targeted delivery of curcumin-encapsulated exosomes to the brain through intra-nasal routes has been shown to be effective for brain inflammatory diseases (Zhuang et al. 2011). Interestingly, preliminary studies from our lab explored that curcumin-primed exosomes (CUR-EXO), derived from culture conditioned media of mouse brain endothelial cells (MBEC) treated with curcumin, might equally benefit since these units alleviate tight junction proteins and endothelial cell layer permeability in MBECs (unpublished data). These results concomitantly show the therapeutic aspects in CUR-primed, or CUR-encapsulated exosomes and provide a promising area to explore their potentials to recover cerebral ischemic stroke probably by amelioration of epigenetic and molecular events.
Hence, the present review suggests the possible epigenetic mechanisms induced with curcumin along with a short discussion on molecular designing to enhance its bioavailability and impacts of curcumin encapsulated/curcumin-primed exosomes on stroke therapy.
Epigenetic impact of curcumin
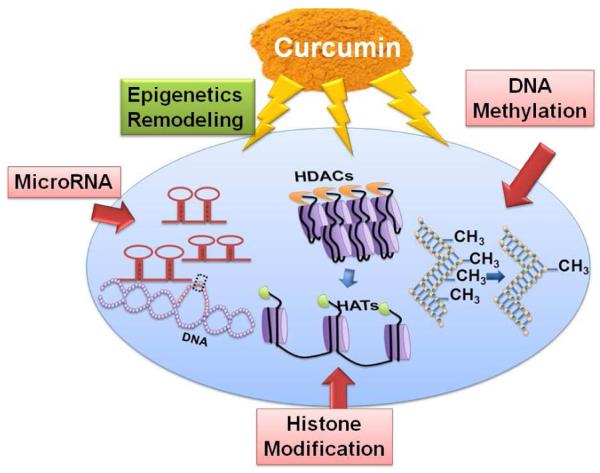
Fu et al. have suggested that curcumin may exert its biological activities through epigenetic modulation, even at lower concentrations (Fu and Kurzrock, 2010). Epigenetic mechanisms regulate functional gene environment by regulating gene expressions without altering gene sequence or structure. The normal genetic expressions are under the control of various mechanisms such as: DNA methylation, histone modifications, non-coding small RNA (micro RNA, miR), and RNA editing (Kalani et al. 2013b; Qureshi and Mehler, 2010b; Qureshi and Mehler, 2012). These associated mechanisms may play immense role in normal physiological functions of the brain. Curcumin as an epigenetic agent can be used for stroke protection and therapeutics by reversing erroneous epigenetic mechanisms or inducing/controlling normal epigenetic mechanisms.
A: Role of curcumin in DNA methylation
DNA methylation is the transfer of methyl (-CH3) group from an activated donor (s-adenosylmethionine, SAM) to the cytosine residue of the specific region of the gene by the enzyme DNA methyltransferases (DNMTs) (Qureshi and Mehler, 2010a). DNMTs play a profound role in DNA methylation and classified mainly as DNMT-1 (maintains methylation) and DNMT-3a, 3b (de-novo methylation). Studies suggest that abnormal methylation is linked to various diseases such as: cancer, atherosclerosis, auto-immunity and obesity (Novik et al. 2002). These regulators are the pivotal part of folate cycle, connected to homocysteine (Hcy) metabolism pathway (Kalani et al. 2013b). The potentials of curcumin as an epigenetic agent have been explored by molecular docking studies done for the interaction of curcumin with DNMT-1. This study suggests that curcumin covalently blocks the catalytic thiolate of DNMT1 with an IC50 of 30 nM after 72 h of its treatment and it leads to an inhibitory effect on DNA methylation (Liu et al. 2009). One of the studies reported the use of curcumin at 5 μM concentration reverses promoter CpG methylation of Neurog1, which is a cancer marker and known to be highly methylated in cancer (Shu et al. 2011). The high methylation interrupted the expression of Neurog1 in human prostate cancer LNCaP cells. These studies suggested the potential role of curcumin occurs by DNMT inhibition. Curcuminoids, which are stable derivatives of curcumin, are prepared for the formulation of different drugs and formed by adding different chemical groups to the curcumin to make it more soluble. Among different curcuminoids, demethoxycurcumin and bis- demethoxycurcumin have been reported to influence epigenetic properties (Liu et al. 2011). Curcuminoids have also found to induce hypomethylation of miR203 promoter in bladder cancer cell (Saini et al. 2011). Although much studies report curcumin in cancer epigenetics by mitigating DNA methylation errors, the reports are lacking that explore and prove epigenetic impact of curcumin in stroke epigenetics by improving the DNA methylation. Hence, epigenetic impact of curcumin in stroke can further be explored by directing the research towards cerebral stroke area (Fig.1).
Fig. 1.
Curcumin-mediated epigenetic mechanisms involving DNA methylation, histone modifications and microRNA based epigenetic processes.
B: Role of curcumin in histone modification
Histone modification is another important mechanism that regulates epigenetic events. Apoptotic and necrotic processes affect chromatin integrity and collectively progress neuronal injury in stroke. Histone modifications turn on or repress transcriptional processes that control gene regulations. Histone proteins (H2A, H2B, H3, and H4) along with linker DNA (H1) form a complex around which the DNA wraps to form nucleosome which is the basic unit of chromatin. Histone acetyltrasferases (HATs) and histone deacetylases (HDACs) are the enzymes which control histone modifications by activation or repression of transcriptional machinery and chromatin remodeling (Kalani et al. 2013b). Histone modification machinery also affects chromatin structurally and functionally by different processes such as; acetylation, methylation, phosphorylation, ubiquitination, and adenosine diphosphate ribosylation. Curcumin along with Trichostatin A, suberoylanilide hydroxamic acid, sodium butyrate, sodium 4-phenylbutyrate, valproic acid, and resveratrol have been reported as potential HDAC inhibitors (Kalani et al. 2013b; Qureshi and Mehler, 2011).
Recent report demonstrated the expression patterns of HDAC isoforms during experimental ischemia stroke. This study described HDAC-3 and HDAC-6 as potential mediators of the neurotoxicity during ischemia stroke and suggested specific therapeutic approach according to HDAC subtype (Chen et al. 2012). Inhibition of zinc dependent histone deacetylases is also reported to protect neurons, axons and associated glia cells from oxygen and glucose deprivation (Baltan et al. 2013). The beneficial effect of HDAC inhibitors as a therapeutic strategy was also proved by Beltan et al. (Baltan et al. 2013). The study showed that HDAC inhibition mechanism perpetuates through targeting mitochondrial energy regulation and excitotoxicity in ischemic white matter injury (Baltan et al. 2011). Collectively, earlier reports indicate that HDAC inhibition alleviates stroke-related pathology including functional and behavior recovery. The associated mechanisms could be up-regulation of extracellular glutamate clearance, inhibition of p53-mediated cell death and maintenance of mitochondrial integrity (Baltan et al. 2013; Kim et al. 2007). The potential of curcumin as HDAC inhibitor was also proven by molecular docking assay carried out for the human HDAC-8 enzyme in order to predict inhibition activity and the 3D poses of inhibitor–enzyme complexes (Bora-Tatar et al. 2009). The study revealed that curcumin possesses HDAC inhibition potential with an IC50 of 115 μM. Hypoacetylation of histone 3 and 4, at lysine residue, was done by curcumin in TREM-1 promotor region that modulate TREM-1 gene expression (Yuan et al. 2012). Chen et al. reported down-regulation of HDAC-1, HDAC-3, HDAC-8 protein and up-regulation of histone H4 expression regulates Raji cell proliferation and apoptosis (Chen et al. 2007). Besides acting on HDAC, curcumin also inhibit HAT. Zhu et al. (2014) have determined that curcumin alleviates neuropathic pain by inhibiting p300/CREB-binding protein (CBP) of histone acetyltransferase (HAT) activity and regulating the expression of BDNF as well as cox-2 in rat. Kang et al. (2006) have shown that HAT inhibition by curcumin enhanced Caspase-3-dependent Glioma Cell Death and Neurogenesis of Neural Progenitor Cells. These studies established that curcumin can control acetylation/deacetylation machinery and these processes can alter chromatin structure to control gene expressions. Hence, curcumin being potential HDAC inhibitor can be used to study stroke-induced epigenetic mechanisms by regulating important machinery (acetylation / deacetylation) in stroke pathogenesis.
C: Curcumin and microRNA
Non-protein coding sequences of DNA that were earlier thought as junk DNA, transcribe non-coding RNAs (nc RNAs). Micro RNA (miR) is one of the classes of ncRNA that offer tremendous potential in unrevealing mechanisms underlying stroke pathogenesis (Qureshi and Mehler, 2012). Not only in disease conditions, studies also reflect change of microRNA expression profile in response to therapy (Williams et al. 2009). Mature miRs are derived from stem loop pre-miRNA, possess 20-22 nucleotide length, and regulate gene expression by binding to the 3′-UTR region of their corresponding messenger RNAs (mRNA). Micro RNA along with their target genes are involved in endothelial dysfunction, neurovascular integrity, neural differentiation, pro-apoptosis/anti-apoptosis, matrix remodeling, inflammation, angiogenesis and regenerative processes (Barringhaus and Zamore, 2009; Chen et al. 2011; Jovanovic and Hengartner, 2006; Le et al. 2009; Loyer et al. 2014; Sonkoly and Pivarcsi, 2009; Suarez and Sessa, 2009; Tan et al. 2011; Wu and Murashov, 2013a; Wu and Murashov, 2013b). Although the role of micro RNAs in stroke is still in infancy, the reports indicate their significant contribution in stroke development and unexpectedly stable nature in describing their potential use as diagnostic and prognostic markers (Qureshi and Mehler, 2010c; Rink and Khanna, 2011). Polymorphism study by Jeon et al. described the association of miR-146a, miR-149, miR-196a2, and miR-499 with cerebral ischemia stroke and silent brain infarction risk (Jeon et al. 2013). Likewise, the study by Zhu et al. described the correlation of miR-124 with neuronal death during ischemia/Reperfusion by regulating Ku 70 expression (Zhu et al. 2014). Khanna et al. described the loss of miR29b with neuronal cell death during stroke (Khanna et al. 2013), and Pandi et al. showed down-regulation of miR-29c in de-repression of DNMT 3a during ischemic brain damage (Pandi et al. 2013). The role of miR140 was studied as a candidate molecule involved in regenerative processes in post-ischemic brain as it was found to be up-regulated and tissue repair processes after 3 h of middle cerebral artery occlusion (MCAO) (Nicolas et al. 2008). Apart from that, the significant detection of micro RNA in peripheral blood after 24 h of MCAO (rno-miR-19b, rno-miR-290, and rno-miR-292-5p), and 48 h of MCAO (rno-miR-352, rno-miR-26b, rno-miR-26a, rno-miR-20a, rno-miR17, rno-miR-140,rno-miR-92, rno-miR214, rno-miR-15b, and rno-miR-328) suggest their potential as future biomarkers in stroke (Jeyaseelan et al. 2008).
The role of curcumin in regulation of MicroRNA during cerebral storke has not been studied much. However, curcumin has been studied as neuro-protective agent by regulating Akt/Nrf2, and Nrf2-HO-1 pathways (Wu et al. 2013; Yang et al. 2009). The associations of microRNAs in these mechanisms would further help to elaborate the potential impact of curcumin and its therapeutic efficacy. Moreover, mechanisms-induced with therapeutic microRNA, generated with curcumin treatment, can also be studied for different epigenetic processes such as; DNA methylation, histone modification, sumoylation, phosphorylaion, and ADP ribosylation.
Curcumin for stroke prevention
Curcumin possess multiple pharmacological properties (anti-inflammatory, anti-thrombotic, and anti-oxidative) and these properties further add on to its anti-ischemic property. The anti-ischemic effect of curcumin is believed to be contributed by its free radical scavenging activity which is unique upon having phenolic and diketonic groups present in its structure. However, other natural anti-oxidants lack the presence of two groups together and possess either of these. The neuroprotective effect of curcumin is well documented over different neurotoxicants; such as Hcy (Kalani et al. 2013a). These protective effects not only rescue the metabolite alterations but also improve brain edema, Evans Blue leakage and infarct size during ischemic brain injury (stroke) (Tyagi et al. 2012). The beneficiary effect of curcumin is also reported to be executed by lowering lipid peroxidation, when administered orally or intraperitoneally (Ghoneim et al. 2002; Thiyagarajan and Sharma, 2004). Hence, the major advantages that lay with curcumin treatment have been explored as its non-toxic effect (even at high doses), ability to cross BBB in aged mice and gerbils, and its cerebro-protective behavior (Thiyagarajan and Sharma, 2004; Tyagi et al. 2012; Wang et al. 2005).
Curcumin Bioavailability
Although curcumin demonstrated efficient and safe in nature, its limited bioavailability continues to be a major concern. Due to its rapid metabolism and elimination, low levels are found in serum and tissue irrespective of the route of administration. Low solubility in water probably reduces its effective action in the target protein site. Different attempts were tried to enhance its bioavailability including; modulation of the administering route, medium of curcumin administration, structural medications and encapsulation in exosomes.
A: Structural modifications of curcumin
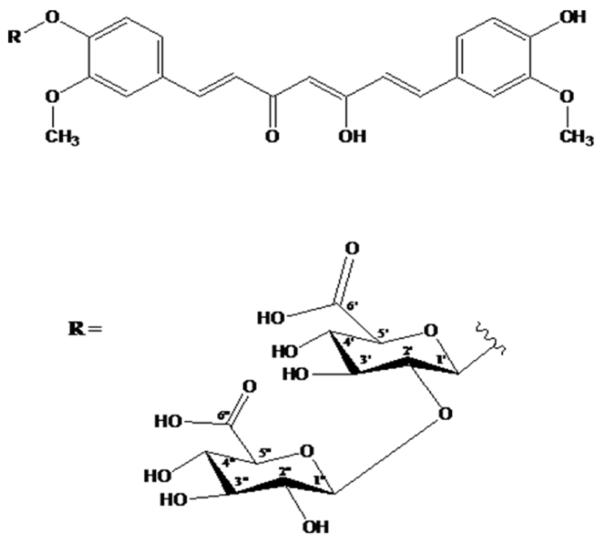
Recently, attempts utilizing structural modifications of curcumin are largely explored to prepare new formulations in order to enhance in vitro and in vivo efficacies of curcumin. Previously, we have reported protective potential of tetrahydrocurcumin (THC) in amelioration of cerebral ischemia-stroke (Tyagi et al. 2012). THC is an active metabolite of curcumin and is more soluble and stable. Therefore, we speculate that curcumin solubility and stability is increased with the insertion of one or two moieties of glucuronic acid at the -OH group which has been presented in Fig.2. Earlier reports also found increase in solubility of curcumin with similar molecular insertion (Anand et al. 2007). Hence, the addition of glucose to curcumin would enhance its pharmacokinetic properties and candidature as a potential drug molecule.
Fig. 2.
Structure of curcumin (top) and glucuronic acid (bottom). Increase in curcumin solubility and stability with addition of glucuronic acid at R group of curcumin.
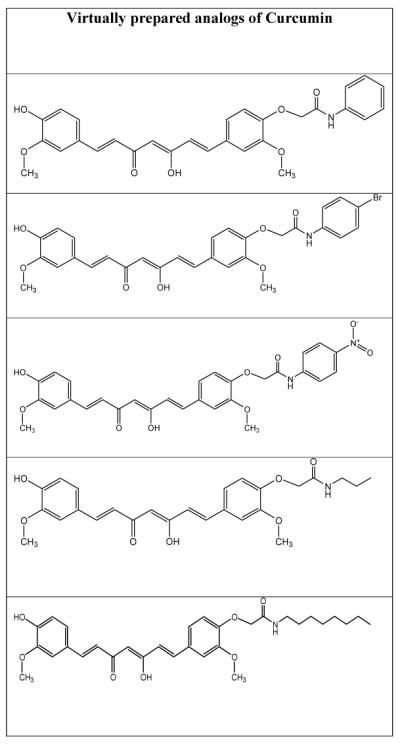
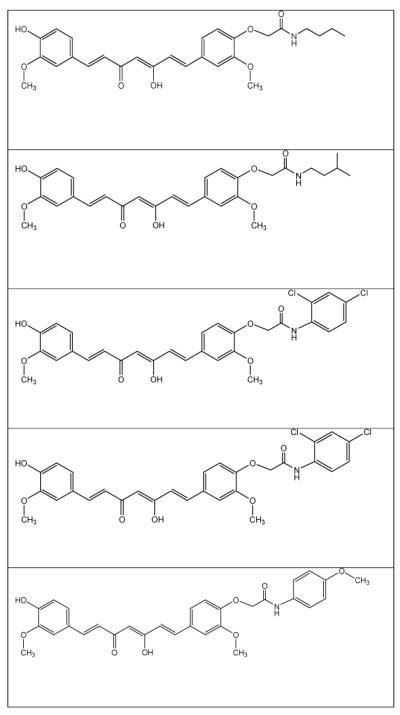
It has been stated that modification at the -OH group of curcumin has tendency to enhance its activity for Alzheimer’s disease (Fang et al. 2014). In the view of recent reports (Fang et al. 2014), some new analogs of curcumin have been virtually designed and the perception has been made that these analogs may find the same or better activity than the parental molecule. Table-1 represents virtually prepared analogs of curcumin; however further QSAR and molecular docking work could confirm their potentials for stoke or stroke-like pathologies.
Table-1.
Virtually prepared analogs of curcumin
|
|
Exosomes and Curcumin
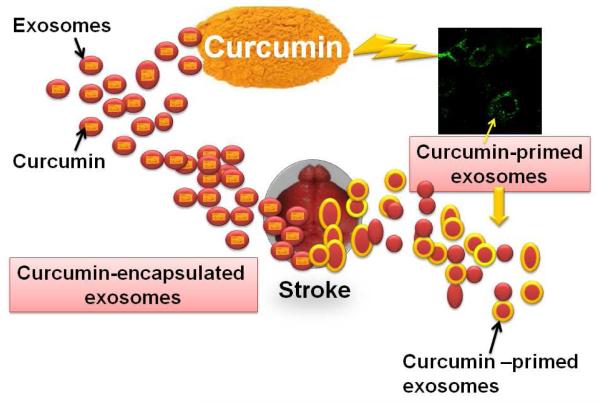
A recent report by Tiwari et al (2014) has shown nanoparticle mediated delivery of curcumin to be neuroregenerative which strengthens the therapeutic link towards stroke therapy. Xin et al. describes the potential of exosomes in rat MCAO model (Xin et al. 2013). The investigators derived exosomes from mesenchymal stromal cells (MSC) and used these nano-units against MCAO injury through tail vein injection. They find functional improvements; neurite remodeling, neurogenesis and angiogenesis post MCAO with MSC-exosome treatment which suggests therapeutic efficacy of exosomes. Although exosomes have been implicated in stroke therapeutics, encapsulation of curcumin in exosomes further enhances its protective effects. Investigators have found that curcumin-encapsulated exosomes are highly concentrated in the blood with increased solubility and stability (Sun et al. 2010; Zhuang et al. 2011). Authors confirmed that delivery of curcumin-encapsulated exosomes is beneficial for inflammatory diseases since the approach has no significant side effects (Sun et al. 2010). Interestingly, another report suggests targeted delivery of curcumin-encapsulated exosomes to the brain through nasal route as a promising, non-invasive and novel therapeutic approach for treating brain inflammatory diseases (Zhuang et al. 2011). We have also observed that curcumin-primed exosomes have potential to recover junction proteins and permeability of endothelial cells (fig. 3). These novel therapeutic options should be tried in order to alleviate stroke pathology.
Fig. 3.
Image showing protective potential of curcumin-encapsulated exosomes (left) and curcumin-primed exosomes (right) during stroke. Curcumin-primed exosomes (labeled with PKH67) are shown as green dots in the image.
Conclusion and Future direction
Although curcumin possess anti-inflammatory, anti-oxidative, neuro-protective, and anti-cancer properties mediated through multiple intercellular/regulatory signaling mechanisms; very little is known about the effect of curcumin on epigenetic aspect during cerebral ischemia stroke. It has been suspected through earlier literature that curcumin possess epigenetic modulation properties and that’s how it affects epigenetic factors, such as HDAC, HAT, DNMTs, and miRNAs. Exploring epigenetic properties of curcumin in neuroprotection potentiates its use in stroke therapeutics. Nonetheless the question that still exists is whether the protective mechanism of curcumin is epigenetically regulated or it has only potential impact? If the epigenetic aspect of curcumin to rescue ischemia stroke becomes clearer, more exciting results can come and give direction for the protective efficacy and therapy. To cope with decreased bioavailability issue, discovery of the novel lead molecule might hopefully bring advancement in the safe and effective treatment of stroke. However, further, QSAR and docking guided lead optimization is under progress, which will assist in elucidating the precise mechanism of action. Alongside, exciting results on the use of curcumin-encapsulated / curcumin-derived exosomes pave the way to the future novel therapeutics in cerebral stroke where drug target is still a challenge.
Acknowledgement
This work was supported by National Institutes of Health grants HL107640-NT.
Footnotes
Conflict of Interest: The authors declared no conflict of interest.
References
- Adams HP, Jr., del ZG, Alberts MJ, Bhatt DL, Brass L, Furlan A, Grubb RL, Higashida RT, Jauch EC, Kidwell C, Lyden PD, Morgenstern LB, Qureshi AI, Rosenwasser RH, Scott PA, Wijdicks EF. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: The American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Circulation. 2007;115:e478–e534. doi: 10.1161/CIRCULATIONAHA.107.181486. [DOI] [PubMed] [Google Scholar]
- Alamy M, Bengelloun WA. Malnutrition and brain development: an analysis of the effects of inadequate diet during different stages of life in rat. Neurosci Biobehav Rev. 2012;36:1463–1480. doi: 10.1016/j.neubiorev.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- Baltan S, Morrison RS, Murphy SP. Novel protective effects of histone deacetylase inhibition on stroke and white matter ischemic injury. Neurotherapeutics. 2013;10:798–807. doi: 10.1007/s13311-013-0201-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltan S, Murphy SP, Danilov CA, Bachleda A, Morrison RS. Histone deacetylase inhibitors preserve white matter structure and function during ischemia by conserving ATP and reducing excitotoxicity. J Neurosci. 2011;31:3990–3999. doi: 10.1523/JNEUROSCI.5379-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barringhaus KG, Zamore PD. MicroRNAs: regulating a change of heart. Circulation. 2009;119:2217–2224. doi: 10.1161/CIRCULATIONAHA.107.715839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedi KS. Nutritional effects on neuron numbers. Nutr Neurosci. 2003;6:141–152. doi: 10.1080/1028415031000098549. [DOI] [PubMed] [Google Scholar]
- Bora-Tatar G, Dayangac-Erden D, Demir AS, Dalkara S, Yelekci K, Erdem-Yurter H. Molecular modifications on carboxylic acid derivatives as potent histone deacetylase inhibitors: Activity and docking studies. Bioorg Med Chem. 2009;17:5219–5228. doi: 10.1016/j.bmc.2009.05.042. [DOI] [PubMed] [Google Scholar]
- Chen KC, Wang YS, Hu CY, Chang WC, Liao YC, Dai CY, Juo SH. OxLDL up-regulates microRNA-29b, leading to epigenetic modifications of MMP-2/MMP-9 genes: a novel mechanism for cardiovascular diseases. FASEB J. 2011;25:1718–1728. doi: 10.1096/fj.10-174904. [DOI] [PubMed] [Google Scholar]
- Chen Y, Shu W, Chen W, Wu Q, Liu H, Cui G. Curcumin, both histone deacetylase and p300/CBP-specific inhibitor, represses the activity of nuclear factor kappa B and Notch 1 in Raji cells. Basic Clin Pharmacol Toxicol. 2007;101:427–433. doi: 10.1111/j.1742-7843.2007.00142.x. [DOI] [PubMed] [Google Scholar]
- Chen YT, Zang XF, Pan J, Zhu XL, Chen F, Chen ZB, Xu Y. Expression patterns of histone deacetylases in experimental stroke and potential targets for neuroprotection. Clin Exp Pharmacol Physiol. 2012;39:751–758. doi: 10.1111/j.1440-1681.2012.05729.x. [DOI] [PubMed] [Google Scholar]
- Chiu S, Terpstra KJ, Bureau Y, Hou J, Raheb H, Cernvosky Z, Badmeav V, Copen J, Husni M, Woodbury-Farina M. Liposomal-formulated curcumin [Lipocurc] targeting HDAC (histone deacetylase) prevents apoptosis and improves motor deficits in Park 7 (DJ-1)-knockout rat model of Parkinson’s disease: implications for epigenetics-based nanotechnology-driven drug platform. J Complement Integr Med. 2013:10. doi: 10.1515/jcim-2013-0020. [DOI] [PubMed] [Google Scholar]
- Fang L, Gou S, Liu X, Cao F, Cheng L. Design, synthesis and anti-Alzheimer properties of dimethylaminomethyl-substituted curcumin derivatives. Bioorg Med Chem Lett. 2014;24:40–43. doi: 10.1016/j.bmcl.2013.12.011. [DOI] [PubMed] [Google Scholar]
- Fu S, Kurzrock R. Development of curcumin as an epigenetic agent. Cancer. 2010;116:4670–4676. doi: 10.1002/cncr.25414. [DOI] [PubMed] [Google Scholar]
- Gallou-Kabani C, Vige A, Gross MS, Junien C. Nutri-epigenomics: lifelong remodelling of our epigenomes by nutritional and metabolic factors and beyond. Clin Chem Lab Med. 2007;45:321–327. doi: 10.1515/CCLM.2007.081. [DOI] [PubMed] [Google Scholar]
- Ghoneim AI, Abdel-Naim AB, Khalifa AE, El-Denshary ES. Protective effects of curcumin against ischaemia/reperfusion insult in rat forebrain. Pharmacol Res. 2002;46:273–279. doi: 10.1016/s1043-6618(02)00123-8. [DOI] [PubMed] [Google Scholar]
- Goel A, Aggarwal BB. Curcumin, the golden spice from Indian saffron, is a chemosensitizer and radiosensitizer for tumors and chemoprotector and radioprotector for normal organs. Nutr Cancer. 2010;62:919–930. doi: 10.1080/01635581.2010.509835. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F. Brain foods: the effects of nutrients on brain function. Nat Rev Neurosci. 2008;9:568–578. doi: 10.1038/nrn2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy TM, Tollefsbol TO. Epigenetic diet: impact on the epigenome and cancer. Epigenomics. 2011;3:503–518. doi: 10.2217/epi.11.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon YJ, Kim OJ, Kim SY, Oh SH, Oh D, Kim OJ, Shin BS, Kim NK. Association of the miR-146a, miR-149, miR-196a2, and miR-499 polymorphisms with ischemic stroke and silent brain infarction risk. Arterioscler Thromb Vasc Biol. 2013;33:420–430. doi: 10.1161/ATVBAHA.112.300251. [DOI] [PubMed] [Google Scholar]
- Jeyaseelan K, Lim KY, Armugam A. MicroRNA expression in the blood and brain of rats subjected to transient focal ischemia by middle cerebral artery occlusion. Stroke. 2008;39:959–966. doi: 10.1161/STROKEAHA.107.500736. [DOI] [PubMed] [Google Scholar]
- Jovanovic M. miRNAs and apoptosis: RNAs to die for. Oncogene. 2006;25:6176–6187. doi: 10.1038/sj.onc.1209912. [DOI] [PubMed] [Google Scholar]
- Kalani A, Kamat PK, Givvimani S, Brown K, Metreveli N, Tyagi SC, Tyagi N. Nutri-epigenetics Ameliorates Blood-Brain Barrier Damage and Neurodegeneration in Hyperhomocysteinemia: Role of Folic Acid. J Mol Neurosci. 2013a doi: 10.1007/s12031-013-0122-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalani A, Kamat PK, Tyagi SC, Tyagi N. Synergy of homocysteine, MicroRNA, and epigenetics: a novel therapeutic approach for stroke. Mol Neurobiol. 2013b;48:157–168. doi: 10.1007/s12035-013-8421-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalani A, Tyagi A, Tyagi N. Exosomes: Mediators of Neurodegeneration, Neuroprotection and Therapeutics. Mol Neurobiol. 2014;49:590–600. doi: 10.1007/s12035-013-8544-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SK, Cha SH, Jeon HG. Curcumin-induced histone hypoacetylation enhances caspase-3-dependent glioma cell death and neurogenesis of neural progenitor cells. Stem Cells Dev. 2006;15:165–74. doi: 10.1089/scd.2006.15.165. [DOI] [PubMed] [Google Scholar]
- Khanna S, Rink C, Ghoorkhanian R, Gnyawali S, Heigel M, Wijesinghe DS, Chalfant CE, Chan YC, Banerjee J, Huang Y, Roy S, Sen CK. Loss of miR-29b following acute ischemic stroke contributes to neural cell death and infarct size. J Cereb Blood Flow Metab. 2013;33:1197–1206. doi: 10.1038/jcbfm.2013.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Rowe M, Ren M, Hong JS, Chen PS, Chuang DM. Histone deacetylase inhibitors exhibit anti-inflammatory and neuroprotective effects in a rat permanent ischemic model of stroke: multiple mechanisms of action. J Pharmacol Exp Ther. 2007;321:892–901. doi: 10.1124/jpet.107.120188. [DOI] [PubMed] [Google Scholar]
- Langhorne P, Fearon P, Ronning OM, Kaste M, Palomaki H, Vemmos K, Kalra L, Indredavik B, Blomstrand C, Rodgers H, Dennis MS, Al-Shahi SR. Stroke unit care benefits patients with intracerebral hemorrhage: systematic review and meta-analysis. Stroke. 2013;44:3044–3049. doi: 10.1161/STROKEAHA.113.001564. [DOI] [PubMed] [Google Scholar]
- Lapchak PA, Schubert DR, Maher PA. Delayed treatment with a novel neurotrophic compound reduces behavioral deficits in rabbit ischemic stroke. J Neurochem. 2011;116:122–131. doi: 10.1111/j.1471-4159.2010.07090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le MT, Xie H, Zhou B, Chia PH, Rizk P, Um M, Udolph G, Yang H, Lim B, Lodish HF. MicroRNA-125b promotes neuronal differentiation in human cells by repressing multiple targets. Mol Cell Biol. 2009;29:5290–5305. doi: 10.1128/MCB.01694-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YL, Yang HP, Gong L, Tang CL, Wang HJ. Hypomethylation effects of curcumin, demethoxycurcumin and bisdemethoxycurcumin on WIF-1 promoter in non-small cell lung cancer cell lines. Mol Med Rep. 2011;4:675–679. doi: 10.3892/mmr.2011.473. [DOI] [PubMed] [Google Scholar]
- Liu Z, Xie Z, Jones W, Pavlovicz RE, Liu S, Yu J, Li PK, Lin J, Fuchs JR, Marcucci G, Li C, Chan KK. Curcumin is a potent DNA hypomethylation agent. Bioorg Med Chem Lett. 2009;19:706–709. doi: 10.1016/j.bmcl.2008.12.041. [DOI] [PubMed] [Google Scholar]
- Loyer X, Potteaux S, Vion AC, Guerin CL, Boulkroun S, Rautou PE, Ramkhelawon B, Esposito B, Dalloz M, Paul JL, Julia PL, Maccario J, Boulanger CM, Mallat Z, Tedgui A. Inhibition of microRNA-92a Prevents Endothelial Dysfunction and Atherosclerosis in Mice. Circ Res. 2013;14:434–43. doi: 10.1161/CIRCRESAHA.114.302213. [DOI] [PubMed] [Google Scholar]
- Martin SL, Hardy TM, Tollefsbol TO. Medicinal chemistry of the epigenetic diet and caloric restriction. Curr Med Chem. 2013;20:4050–4059. doi: 10.2174/09298673113209990189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra S, Palanivelu K. The effect of curcumin (turmeric) on Alzheimer’s disease: An overview. Ann Indian Acad Neurol. 2008;11:13–19. doi: 10.4103/0972-2327.40220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas FE, Pais H, Schwach F, Lindow M, Kauppinen S, Moulton V, Dalmay T. Experimental identification of microRNA-140 targets by silencing and overexpressing miR-140. RNA. 2008;14:2513–2520. doi: 10.1261/rna.1221108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novik KL, Nimmrich I, Genc B, Maier S, Piepenbrock C, Olek A, Beck S. Epigenomics: genome-wide study of methylation phenomena. Curr Issues Mol Biol. 2002;4:111–128. [PubMed] [Google Scholar]
- Ovbiagele B. Potential role of curcumin in stroke prevention. Expert Rev Neurother. 2008;8:1175–1176. doi: 10.1586/14737175.8.8.1175. [DOI] [PubMed] [Google Scholar]
- Pandi G, Nakka VP, Dharap A, Roopra A, Vemuganti R. MicroRNA miR-29c down-regulation leading to de-repression of its target DNA methyltransferase 3a promotes ischemic brain damage. PLoS One. 2013;8:e58039. doi: 10.1371/journal.pone.0058039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry E, Howes MJ. Medicinal plants and dementia therapy: herbal hopes for brain aging? CNS Neurosci Ther. 2011;17:683–698. doi: 10.1111/j.1755-5949.2010.00202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psaltopoulou T, Sergentanis TN, Panagiotakos DB, Sergentanis IN, Kosti R, Scarmeas N. Mediterranean diet, stroke, cognitive impairment, and depression: A meta-analysis. Ann Neurol. 2013;74:580–591. doi: 10.1002/ana.23944. [DOI] [PubMed] [Google Scholar]
- Qureshi IA, Mehler MF. Emerging role of epigenetics in stroke: part 1: DNA methylation and chromatin modifications. Arch Neurol. 2010a;67:1316–1322. doi: 10.1001/archneurol.2010.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi IA, Mehler MF. Emerging themes in epigenetics: implications for understanding and treating nervous system disorders. Epigenomics. 2010b;2:345–349. doi: 10.2217/epi.10.17. [DOI] [PubMed] [Google Scholar]
- Qureshi IA, Mehler MF. The emerging role of epigenetics in stroke: II. RNA regulatory circuitry. Arch Neurol. 2010c;67:1435–1441. doi: 10.1001/archneurol.2010.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi IA, Mehler MF. Chromatin-modifying agents for epigenetic reprogramming and endogenous neural stem cell-mediated repair in stroke. Transl Stroke Res. 2011;2:7–16. doi: 10.1007/s12975-010-0051-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi IA, Mehler MF. Emerging roles of non-coding RNAs in brain evolution, development, plasticity and disease. Nat Rev Neurosci. 2012;13:528–541. doi: 10.1038/nrn3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rink C, Khanna S. MicroRNA in ischemic stroke etiology and pathology. Physiol Genomics. 2011;43:521–528. doi: 10.1152/physiolgenomics.00158.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini S, Arora S, Majid S, Shahryari V, Chen Y, Deng G, Yamamura S, Ueno K, Dahiya R. Curcumin modulates microRNA-203-mediated regulation of the Src-Akt axis in bladder cancer. Cancer Prev Res (Phila) 2011;4:1698–1709. doi: 10.1158/1940-6207.CAPR-11-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu L, Khor TO, Lee JH, Boyanapalli SS, Huang Y, Wu TY, Saw CL, Cheung KL, Kong AN. Epigenetic CpG demethylation of the promoter and reactivation of the expression of Neurog1 by curcumin in prostate LNCaP cells. AAPS J. 2011;13:606–614. doi: 10.1208/s12248-011-9300-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla PK, Khanna VK, Ali MM, Khan MY, Srimal RC. Anti-ischemic effect of curcumin in rat brain. Neurochem Res. 2008;33:1036–1043. doi: 10.1007/s11064-007-9547-y. [DOI] [PubMed] [Google Scholar]
- Soni KB, Kuttan R. Effect of oral curcumin administration on serum peroxides and cholesterol levels in human volunteers. Indian J Physiol Pharmacol. 1992;36:273–275. [PubMed] [Google Scholar]
- Sonkoly E, Pivarcsi A. microRNAs in inflammation. Int Rev Immunol. 2009;28:535–561. doi: 10.3109/08830180903208303. [DOI] [PubMed] [Google Scholar]
- Strimpakos AS, Sharma RA. Curcumin: preventive and therapeutic properties in laboratory studies and clinical trials. Antioxid Redox Signal. 2008;10:511–545. doi: 10.1089/ars.2007.1769. [DOI] [PubMed] [Google Scholar]
- Suarez Y, Sessa WC. MicroRNAs as novel regulators of angiogenesis. Circ Res. 2009;104:442–454. doi: 10.1161/CIRCRESAHA.108.191270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Zhuang X, Xiang X, Liu Y, Zhang S, Liu C, Barnes S, Grizzle W, Miller D, Zhang HG. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther. 2010;18:1606–1614. doi: 10.1038/mt.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan JR, Koo YX, Kaur P, Liu F, Armugam A, Wong PT, Jeyaseelan K. microRNAs in stroke pathogenesis. Curr Mol Med. 2011;11:76–92. doi: 10.2174/156652411794859232. [DOI] [PubMed] [Google Scholar]
- Teiten MH, Dicato M, Diederich M. Curcumin as a regulator of epigenetic events. Mol Nutr Food Res. 2013;57:1619–1629. doi: 10.1002/mnfr.201300201. [DOI] [PubMed] [Google Scholar]
- Thery C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006 doi: 10.1002/0471143030.cb0322s30. Chapter 3:Unit. [DOI] [PubMed] [Google Scholar]
- Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- Thiyagarajan M, Sharma SS. Neuroprotective effect of curcumin in middle cerebral artery occlusion induced focal cerebral ischemia in rats. Life Sci. 2004;74:969–985. doi: 10.1016/j.lfs.2003.06.042. [DOI] [PubMed] [Google Scholar]
- Tiwari SK, Agarwal S, Seth B, Yadav A, Nair S, Bhatnagar P, Karmakar M, Kumari M, Chauhan LK, Patel DK, Srivastava V, Singh D, Gupta SK, Tripathi A, Chaturvedi RK, Gupta KC. Curcumin-loaded nanoparticles potently induce adult neurogenesis and reverse cognitive deficits in Alzheimer’s disease model via canonical Wnt/β-catenin pathway. ACS Nano. 2014;8:76–103. doi: 10.1021/nn405077y. [DOI] [PubMed] [Google Scholar]
- Tsai YM, Chien CF, Lin LC, Tsai TH. Curcumin and its nano-formulation: the kinetics of tissue distribution and blood-brain barrier penetration. Int J Pharm. 2011;416:331–338. doi: 10.1016/j.ijpharm.2011.06.030. [DOI] [PubMed] [Google Scholar]
- Tyagi N, Qipshidze N, Munjal C, Vacek JC, Metreveli N, Givvimani S, Tyagi SC. Tetrahydrocurcumin ameliorates homocysteinylated cytochrome-c mediated autophagy in hyperhomocysteinemia mice after cerebral ischemia. J Mol Neurosci. 2012;47:128–138. doi: 10.1007/s12031-011-9695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Sun AY, Simonyi A, Jensen MD, Shelat PB, Rottinghaus GE, MacDonald RS, Miller DK, Lubahn DE, Weisman GA, Sun GY. Neuroprotective mechanisms of curcumin against cerebral ischemia-induced neuronal apoptosis and behavioral deficits. J Neurosci Res. 2005;82:138–148. doi: 10.1002/jnr.20610. [DOI] [PubMed] [Google Scholar]
- Williams AE, Larner-Svensson H, Perry MM, Campbell GA, Herrick SE, Adcock IM, Erjefalt JS, Chung KF, Lindsay MA. MicroRNA expression profiling in mild asthmatic human airways and effect of corticosteroid therapy. PLoS One. 2009;4:e5889. doi: 10.1371/journal.pone.0005889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Murashov AK. MicroRNA-431 regulates axon regeneration in mature sensory neurons by targeting the Wnt antagonist Kremen1. Front Mol Neurosci. 2013a;6:35. doi: 10.3389/fnmol.2013.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Murashov AK. Molecular mechanisms of peripheral nerve regeneration: emerging roles of microRNAs. Front Physiol. 2013b;4:55. doi: 10.3389/fphys.2013.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Li Q, Wang X, Yu S, Li L, Wu X, Chen Y, Zhao J, Zhao Y. Neuroprotection by curcumin in ischemic brain injury involves the Akt/Nrf2 pathway. PLoS One. 2013;8:e59843. doi: 10.1371/journal.pone.0059843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin H, Li Y, Cui Y, Yang JJ, Zhang ZG, Chopp M. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J Cereb Blood Flow Metab. 2013;33:1711–1715. doi: 10.1038/jcbfm.2013.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Zhang X, Fan H, Liu Y. Curcumin upregulates transcription factor Nrf2, HO-1 expression and protects rat brains against focal ischemia. Brain Res. 2009;1282:133–141. doi: 10.1016/j.brainres.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Yang F, Lim GP, Begum AN, Ubeda OJ, Simmons MR, Ambegaokar SS, Chen PP, Kayed R, Glabe CG, Frautschy SA, Cole GM. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem. 2005;280:5892–5901. doi: 10.1074/jbc.M404751200. [DOI] [PubMed] [Google Scholar]
- Yuan Z, Syed MA, Panchal D, Rogers D, Joo M, Sadikot RT. Curcumin mediated epigenetic modulation inhibits TREM-1 expression in response to lipopolysaccharide. Int J Biochem Cell Biol. 2012;44:2032–2043. doi: 10.1016/j.biocel.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Zhao J, Yu S, Zheng W, Feng G, Luo G, Wang L, Zhao Y. Curcumin improves outcomes and attenuates focal cerebral ischemic injury via antiapoptotic mechanisms in rats. Neurochem Res. 2010;35:374–379. doi: 10.1007/s11064-009-0065-y. [DOI] [PubMed] [Google Scholar]
- Zhu F, Liu JL, Li JP, Xiao F, Zhang ZX, Zhang L. MicroRNA-124 (miR-124) Regulates Ku70 Expression and is Correlated with Neuronal Death Induced by Ischemia/Reperfusion. J Mol Neurosci. 52:148–55. doi: 10.1007/s12031-013-0155-9. [DOI] [PubMed] [Google Scholar]
- Zhu X, Li Q, Chang R, Yang D, Song Z, Guo Q, Huang C. Curcumin Alleviates Neuropathic Pain by Inhibiting p300/CBP Histone Acetyltransferase Activity-Regulated Expression of BDNF and Cox-2 in a Rat Model. PLoS One. 2014;9:e91303. doi: 10.1371/journal.pone.0091303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang X, Xiang X, Grizzle W, Sun D, Zhang S, Axtell RC, Ju S, Mu J, Zhang L, Steinman L, Miller D, Zhang HG. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol Ther. 2011;19:1769–1779. doi: 10.1038/mt.2011.164. [DOI] [PMC free article] [PubMed] [Google Scholar]