Abstract
Introduction
Endothelial cell dysfunction is associated with cardiovascular disease and vasculogenic erectile dysfunction (ED). Measured via Peripheral Artery Tonometry (PAT), endothelial dysfunction in the penis is an independent predictor of future cardiovascular events.
Aim
Determine whether measurement of endothelial dysfunction differentiates men with vasculogenic ED identified by duplex ultrasound from those without.
Methods
A total of 142 men were retrospectively assessed using patient history, penile duplex ultrasonography (US) and PAT (EndoPAT 2000). ED was self reported and identified on history. Vasculogenic ED was identified in men who exhibited a peak systolic velocity (PSV) of ≤25 cm/s obtained 15 minutes following vasodilator injection. The reactive hyperemia index (RHI), a measurement of endothelial dysfunction in medium/small arteries and the Augmentation Index (AI), a measurement of arterial stiffness, were recorded via PAT.
Results
Penile duplex US separated men into those with ED (n=111) and without (n=31). The cohort with ED had a PSV of 21±1 cm/s (left cavernous artery) and 22±1 cm/s (Right). The control group without ED had values of 39±2 cm/s (Left) and 39±2 (Right). Given the potential for altered endothelial function in diabetes mellitus, we confirmed that hemoglobin A1c, urinary microalbumin, and vibration pulse threshold were not different in men with vasculogenic ED and those without. RHI in patients with ED (1.85±0.06) was significantly decreased compared to controls (2.15±0.2) (p<0.05). The AI was unchanged when examined in isolation, and when standardized to heart rate.
Conclusions
Measurement of endothelial function with EndoPAT differentiates men with vasculogenic ED from those without. RHI could be used as a non-invasive surrogate in the assessment of vasculogenic ED and to identify those patients with higher cardiovascular risk.
Keywords: Peripheral artery tonometry, erectile dysfunction, endothelium, reactive hyperemia, augmentation index, Doppler Ultrasound
INTRODUCTION
Vasculogenic erectile dysfunction (ED) is associated with arterial insufficiency and endothelial dysfunction 1,2. Studies suggest that early onset ED should be heralded as a marker of cardiovascular disease, one of the leading causes of death in the United States, prompting investigations into risk factors 1,2. The penis can be perceived as a specialized vascular bed magnifying the microscopic effects occurring within the cardiovascular system. Indeed, a study determined that patients with angiography-proven coronary artery disease had ED in 65% of cases 3. The goal of the current study was to determine whether measurement of endothelial dysfunction via peripheral arterial tonometry (PAT) predicted vasculogenic ED as identified by Doppler ultrasonography (US) following pharmacologic stimulation.
Penile erection is a complex hemodynamic process with nitric oxide (NO) released from the endothelium and parasympathetic nerve endings playing an important role. The resultant smooth muscle relaxation allows increased inflow of blood, venous occlusion of outflow, and a subsequent erection 4. Impaired endothelial function due to decreased release of NO is related to ED 5–7. Indeed, previous studies have suggested that patients with ED have significantly impaired endothelial function suggestive of generalized vascular, and focal endothelial, damage 2,7,8. A novel device, the EndoPAT (Itamar Medical), was recently developed to monitor arterial volume changes via finger plethysmography 9. The EndoPAT device, approved in 2003 for assessment of endothelial dysfunction, correlates with coronary microvascular function in patients with early atherosclerosis, while also predicting cardiovascular events and disease 10–12. Patients who are obese 13, smoke 14,15, and have diabetes mellitus (DM) 16 have all been found to have endothelial dysfunction. As such, there is growing evidence that endothelial dysfunction could be considered a harbinger of cardiovascular risk, and thus, vasculogenic ED.
Recently, several studies examined the relationship between endothelial activity and ED 7,17–19. Yavuzgil et al. 7 identified 36 patients with vasculogenic ED who exhibited decreased brachial artery flow-mediated dilation (FMD) suggestive of endothelial dysfunction. Vardi and colleagues 19 examined 40 patients with ED and 19 controls by comparing the blood flow and endothelial function of their systemic (i.e. forearm) and penile vasculatures. While forearm measurements were similar between the ED and control groups, penile blood flow and penile endothelial function were decreased in men with ED 19. The authors theorized that penile endothelial dysfunction could be manifested without the need for significant peripheral disease 19. A recent study by Mehta et al. 18 reviewed 194 men with generalized ED and 98 men with post-prostatectomy ED. The EndoPAT system was utilized; however, scores were not significantly different among the groups 18. Unfortunately, the cohorts had self-reported ED that was not proven with a Doppler US and included a significant number of patients with DM and hypogonadism; both of which can significantly affect the amount of endothelial dysfunction 16,20.
In summary, the goal of the current study was to determine whether measurement of endothelial dysfunction with EndoPAT could differentiate men with vasculogenic ED (proven using US with a PSV<25 cm/s) from those without. Also, we assess the possible use of EndoPAT as a non-invasive, surrogate technique in the assessment of vasculogenic ED.
METHODS
With the approval and oversight of the Institutional Review Board, the charts of 142 men from a large academic urology clinic were retrospectively reviewed. Patients seeking treatment at a large tertiary referral center for self-reported ED were included in the study. A thorough sexual and medical history was performed, followed by a detailed physical examination. Exclusion criteria for participation in the study included patients currently on PDE5 inhibitors, beta-blockers, those with active infections or chronic inflammatory diseases (i.e. Crohn’s disease) and those being treated with testosterone supplementation for hypogonadism.
In all cases, peripheral artery tonometry (PAT; EndoPAT 2000) was conducted prior to ultrasonography (US) in order to avoid the influence of pharmacological vasodilation on PAT recordings. Consisting of two finger-mounted probes, the pulsatile arterial volume changes were measured via a pressure transducer with the data subsequently filtered, amplified, and transferred to a computer.
After first obtaining informed consent, the procedure involved the patient being placed in the supine position with a blood pressure cuff secured on the upper arm. The room was quiet, temperature controlled, and dimly lit for comfort. PAT probes were stationed on the index finger of each hand. After a 3–5 minute lead-in equilibration time to evaluate baseline parameters, the blood pressure cuff was inflated to a level sufficient enough to inhibit arterial pulsations as recorded by the PAT probe (average value ~200–220 mmHg). The cuff was inflated to occlusion pressures for 5 minutes to allow adequate end-tissue hypoxia. Following this, the cuff was released and values recorded for the following 3–5 minutes (the hyperemic period). The values during this time constituted the Reactive Hyperemia Index (RHI). RHI values were normalized to the control arm (the arm without the blood pressure cuff) to compensate for possible systemic changes. As a measurement of endothelial dysfunction in medium/small arteries, the RHI values were interpreted as those <2 exhibiting endothelial dysfunction and those >2 being normal 21. Arterial stiffness was also obtained via PAT through measurement of the augmentation index (AI). AI was calculated automatically from analysis of the PAT waveform and functions as a measure of medium/large arterial wall elasticity 22. Normal arterial stiffness is defined by an AI of −30 to −10%, increased arterial stiffness from −10% to +10% and abnormal arterial stiffness > 10% 21.
Following assessment of PAT, examination of the vibration perception threshold for peripheral diabetic and/or sensory neuropathies was conducted using a Bio-Thesiometer (BioMedical Instrument Company, Newbury, Ohio). The probe was placed against the skin and the patient was instructed to notify the physician when first feelings of vibration were felt as the Bio-Thesiometer was slowly increased from zero to a maximum relative amplitude of 50. Six standardized contact points were examined including the right and left sides of the following: the index finger, thigh and penile shaft. Each of these six spots was checked three times with an average taken of the results to yield a final reading.
To assess the penile vasculature and determine whether vasculogenic compromise was present, Duplex Doppler US (10 megaHertz, Hitachi HiVision 6500 linear probe) was performed with B-mode, color flow imaging and spectral doppler analysis. Intracavernous injection and visual-aid stimulation was provided. Real-time, high resolution US of the cavernous arteries was performed at baseline and again at five and fifteen minutes post-injection of 10–20 μg prostaglandin E1, similar to other investigators 23,24. Normal penile vasculature was represented by a peak systolic velocity (PSV) of blood flow of >25 cm/s during the pharmacologically-induced erection. ED was defined as a PSV of ≤25 cm/s (in either cavernosal artery) 15 minutes following injection of the vasodilator as previously described 23. The lowest PSV of either artery was used in determining vasculogenic arterial compromise. A cut-point of ≤25 cm/second has previously been shown to have a sensitivity of 100% and a specificity of 86% with an overall accuracy of 91.7% 25.
No occurrences of priapism were observed. All EndoPAT, Biothesiometry and Duplex Doppler US procedures were each performed by a single operator; thus minimizing possible variability. The contribution of a psychogenic component to ED was assessed via direct questioning, detailed documentation of the patient’s history and by use of a quantitative test (i.e. PSV) to measure vasculogenic status. In cases where the patients self-reported ED but had normal PSVs, the values were used as controls.
Statistical data analysis was performed using Microsoft Excel and SPSS to calculate Student’s t-test and ANOVAs with p values <0.05 considered significant. Two-sample equal variance was assumed. All values are expressed as the average ± standard error of the mean.
RESULTS
A total of 142 patients who underwent both Doppler US and EndoPAT testing for self-reported ED were reviewed for this study. In this context, self-reported ED was defined as any patient who sought advice and treatment for erectile dysfunction. The diagnosis of ED was confirmed during clinic visit where the treating physician gathered all available data including a focused history and physical examination. Information obtained from the sexual health inventory for men and/or the quantitative androgen deficiency in the aging male (ADAM) questionnaire were also used in the diagnosis but the raw data were unavailable. Penile Doppler US was used to document the presence of vasculogenic ED that was defined as a PSV <25 cm/s in either cavernosal artery 15 minutes following injection of a vasodilator. Based on these US results, vasculogenic ED was identified in 111 men, while 31 men had completely normal results and were thus classified as controls (Table 1A). The men with vasculogenic ED had a PSV of 20.5±0.8 cm/s (left cavernous artery) and 22.1±1.2 cm/s (right) (Table 1A). Men classified as not having vasculogenic ED (i.e. controls) had PSVs of 39.2±2.1 cm/s (left) and 39.5±2.5 (right) (Table 1A). These differences were statistically significant (p<0.05). End diastolic velocities (EDV) were similar between controls (left=4.8±2.1 cm/s, right=4.5±1.5 cm/s) and men with ED (left=4.3±0.5 cm/s, right=4.4±0.5 cm/s). Arterial diameter, as recorded by US, was not different between the controls (left=1.7±0.05 mm, right=1.7±0.1 mm) and those with ED (left=1.5±0.04 mm, right=1.5±1.0 mm)(Table 1A).
Table 1A.
Doppler US Characteristics:
| Control (n=31) | Erectile Dysfunction (n=111) | |
|---|---|---|
|
| ||
| Peak Systolic Velocity: | ||
| Left | 39.2±2.1 cm/s | 20.5±0.8 cm/s* |
| Right | 39.5±2.5 cm/s | 22.1±1.2 cm/s* |
|
| ||
| End Diastolic Velocity: | ||
| Left | 4.8±2.1 cm/s | 4.3±0.5 cm/s |
| Right | 4.5±1.5 cm/s | 4.4±0.5 cm/s |
|
| ||
| Arterial Diameter: | ||
| Left | 1.7±0.05 mm | 1.5±0.04 mm |
| Right | 1.7±0.1 mm | 1.5±1.0 mm |
With regards to our patient population, the majority of men were Caucasian (controls=57%, ED=69%). Men with ED were significantly younger than controls without ED (p<0.05); however, regression analysis found the EndoPAT-calculated RHI did not correlate to age (R2=0.007613; data not shown). Patient weight and the calculated body mass index (BMI) were similar (Table 1B). Calculations were based on recorded heights and weights obtained immediately prior to EndoPAT and Doppler US testing. Previous history of vascular risk factors including alcohol consumption, smoking, and DM, were also similar between the groups (Table 1B).
Table 1B.
Patient demographics:
| Control | Erectile Dysfunction (PSV<25 cm/s) | |
|---|---|---|
|
| ||
| Patients (n) | 31 | 111 |
|
| ||
| Race: | ||
| Caucasian | 20 | 77 |
| African-American | 5 | 14 |
| Other | 6 | 20 |
|
| ||
| Age (years) | 46.8±3.2 | 53.1±1.3 * |
|
| ||
| Weight (lbs) | 192.6±6.2 | 207.2±4.7 |
|
| ||
| BMI (kg/m2) | 27.6±1.0 | 29.3±0.58 |
|
| ||
| Alcohol History | 19 (61%) | 57 (51%) |
|
| ||
| Smoking History | 9 (29%) | 43 (38%) |
|
| ||
| Diabetes Mellitus | 3 (10%) | 11 (9%) |
Laboratory investigations revealed no differences in levels of serum testosterone, free testosterone, estradiol, SHBG, and somatomedin-C (Table 2). Triglycerides were significantly different between the ED and control groups (p<0.05); however, total cholesterol, LDL and HDL cholesterol values were all similar (Table 2). Given the potential for altered endothelial function in DM, we confirmed hemoglobin A1c and urinary microalbumin were not different between the groups (Table 2).
Table 2.
Laboratory Values:
| Control (n=31) | Erectile Dysfunction (n=111) | |
|---|---|---|
| Total Testosterone (ng/dL) | 518.5±66.8 | 445.4±24.5 |
| Free Testosterone (ng/dL) | 10.6±2.0 | 8.3±0.6 |
| Estradiol (ng/dL) | 19.1±3.3 | 20.6±1.6 |
| Sex Hormone Binding Globulin (SHBG; nmol/L) | 37.9±4.7 | 43.0±2.2 |
| Somatomedin-C (IGF, mg/dL) | 186.6±18.7 | 171.5±7.4 |
| Triglycerides (mg/dL) | 222.1±40.4 | 141.5±12.8* |
| Total Cholesterol (mg/dL) | 186.3±15.7 | 175±6.3 |
| LDL Cholesterol (mg/dL) | 109±14.8 | 99.8±5.3 |
| HDL Cholesterol (mg/dL) | 39.3±3.3 | 47.7±2.3 |
| HgA1c (%) | 5.7±0.3 | 6.2±0.2 |
| Urinary microalbumin (mg/dL) | 0.7±0.2 | 2.4±1.0 |
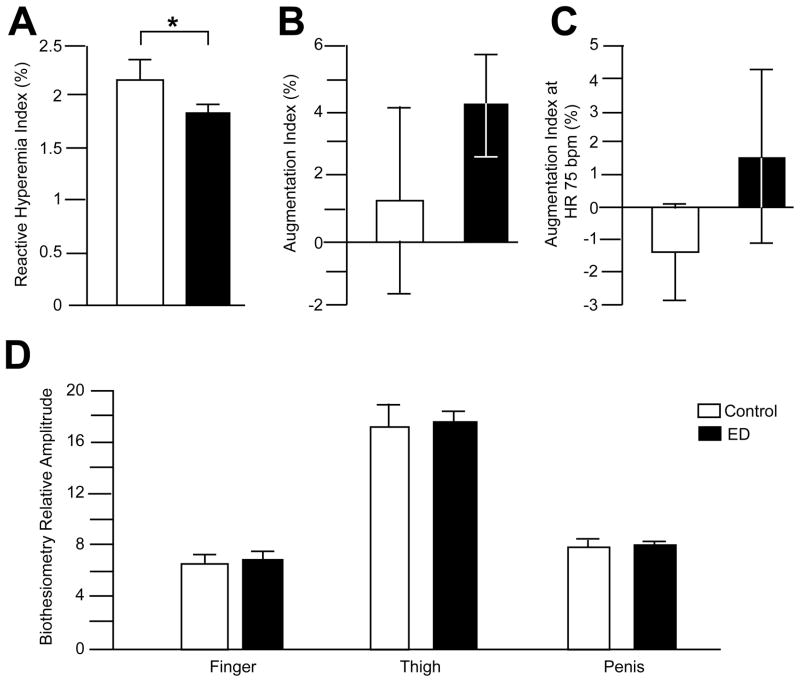
On assessment of PAT, endothelial dysfunction was monitored via the EndoPAT calculated RHI. The arterial stiffness was measured with the AI as well as with the AI standardized to a heart rate of 75 beats per minute (AI75). Lower AI values reflect better arterial elasticity and can increase with age. Those patients with ED had a RHI of 1.85±0.06%, while those without ED had an increased RHI of 2.15±0.18% that was significantly different (p<0.05) (Figure 1A). These RHI findings suggest endothelial dysfunction is present in a greater degree in men with vasculogenic ED (determined by PSV) than controls. No differences were observed in the raw AI scores (Figure 1B; ED=4.19±1.6%; control=1.24±2.86%) or when they were standardized to heart rate (Figure 1C; ED=1.48±2.64%; control=−1.4±1.42%).
Figure 1. Endothelial dysfunction, as measured by EndoPAT, is present in men with vasculogenic ED.
The EndoPAT-derived reactive hyperemia index (RHI), a measure of endothelial dysfunction, was significantly (p<0.05) decreased in patients with vasculogenic ED (black bars) compared to controls (hollow bars; Panel A). The augmentation index, or AI (a measure of arterial stiffness), was not different when measured in isolation (Panel B) or when standardized to a heart rate of 75 beats per minute (Panel C). Given that early changes in diabetic neuropathy result in changes in endothelial function 26, examination of the vibration perception threshold using a biothesiometer was conducted. The relative amplitudes of the vibration threshold at the fingers, thigh and penile shaft were not significantly different between controls (hollow bars) and those men with vasculogenic ED (black bars) (Panel D).
Examination of the vibration perception threshold using a biothesiometer was conducted, given that early changes in diabetic neuropathy could result in changes in endothelial function 26. The relative amplitudes of the vibration threshold measured at the fingers (ED=6.79±0.46; control=6.64±0.57), thigh (ED=17.44±0.92; control=17.26±1.58) and penile shaft (ED=7.53±0.48; control=7.65±0.91) were not significantly different amongst those men with vasculogenic ED and those without (Figure 1D). A separate analysis of the biothesiometry data did not identify any differences between those patients with and without DM (data not shown).
DISCUSSION
ED is common, with 52% of men between the ages of 40–70 years old affected 27. Currently, the gold standard in diagnosing vascular ED is duplex Doppler US in conjunction with pharmacologic stimulation. EndoPAT, a novel device for detecting alterations in numerous parameters of the vasculature, including endothelial dysfunction, can predict cardiovascular events and disease 10–12. Given that endothelial dysfunction is systemic, and that cardiovascular disease and ED are linked, it is tempting to speculate that the EndoPAT device could be used in a non-invasive manner to detect those with current, or impending vasculogenic ED.
Recently, four main studies examined the importance of monitoring endothelial dysfunction in the setting of ED 7,17–19. Yavuzgil and colleagues 7 conducted the first of these studies in 2005. The authors employed an established technique used to monitor endothelial function, brachial artery FMD. Indeed, FMD was useful in establishing the relationship between ED and endothelial dysfunction 28. In Yavuzgil et al. 7, patients with risk factors (i.e. hypertension, obesity, smoking, DM) for cardiovascular disease who had ED, were compared to potent patients with similar risk factors 7. The FMD in patients with ED was worse compared to those patients without ED 7. This suggested that the endothelial dysfunction seen in patients with ED could be due to a generalized vascular condition rather than a disorder solely of the penis 7. However, one of the primary drawbacks of the study was that the duration of DM in the ED group was significantly longer. Furthermore, the authors did not measure insulin sensitivity or glucose tolerance in order to ascertain what systemic effects the DM had on their patients. This is important since other investigators established that endothelial function is impaired in diabetic patients 29. In a study by DeAngelis et al. 29, 30 patients with both ED and Type 2 DM were compared to 30 potent patients with only DM. A significant relationship between HbA1c and erectile function was identified.
A study by Vardi et al. was one of the first to provide evidence of focal impairment in the endothelial function of the penis in men with ED 19. In this study, 59 subjects were classified according to their IIEF-ED domain scores, and those with ED (n=40) were compared to those without (n=19). Patient age was significantly different with older patients in the ED group. Penile endothelial function was obtained using a specialized veno-occlusive pneumatic cuff that was placed at the base of the penis and maintained for 5 minutes. While forearm blood flow was similar between the groups, penile blood flow was lower in the ED group compared with controls. Penile endothelial function was higher in controls than the ED group. Taken together, the results suggest poor vascular flow and focal penile endothelial dysfunction contributing to ED 19.
Aversa and colleagues 17 first examined the EndoPAT device with respect to ED in 2011. The authors investigated EndoPAT-derived RHI and AI values in men with non-specific, generalized, self-reported ED. Specifically, they subjected 40 patients with ED and 30 without to penile duplex US and PAT testing with the EndoPAT device. Interestingly, the authors employed a PSV cut-off of 35 cm/s to divide the patients into vascular and non-vascular origins for their ED. No differences were identified in the RHI values between the groups; however, arterial stiffness, as calculated by the AI, was higher in men with ED compared with controls 17. Considered an independent predictor of CV risk 30, AI was postulated by the authors to be a more reliable marker of early endothelial damage than RHI 17. Unfortunately, by using a cut-off of 35 cm/s for PSV, the authors may have unintentionally skewed their results. Indeed, while a PSV of >35 cm/s is indicative of normal arterial sufficiency, a PSV below 25 cm/s is diagnostic of arterial insufficiency as the cause of ED 31,32. Intermediate values are typically categorized as non-specific 31,32. Furthermore, the finding that AI correlated to endothelial dysfunction is unusual given that previous studies did not suggest this relationship 33.
The most recent study on the use of EndoPAT in ED was by Mehta et al. 18. No differences between men with general ED and those with post-prostatectomy ED were identified. The authors included SHIM-5 scores, assessment of medical co-morbidities, and EndoPAT scores, while excluding men with pre-operative ED 18. Once the groups were defined, significantly more men with DM and hypogonadism were found within the ED group – both conditions that affect endothelial function 16,20. Furthermore, testosterone levels were not discussed, and the method of treatment for those patients with hypogonadism was not mentioned. Given that there is an inverse relationship between testosterone levels with vascular risk factors and metabolic syndrome and/or Type 2 DM 34, the findings by Mehta et al. 18 become particularly difficult to interpret. Indeed, knowledge of patient testosterone levels would be particularly informative given that the presence of testosterone improves and protects endothelial function 35,36. Further work is necessary to determine how testosterone influences endothelial function via effects on EndoPAT results. Another concern regarding the work of Mehta et al. 18 is that it relied on the self-reporting of ED. There were no investigations done to determine if ED was actually vasculogenic or not. Lastly, another potential confounder was that men on PDE5 inhibitors were not excluded. The use of these medications is known to improve endothelial cell function 34 and thus their possible use by patients being tested with EndoPAT masks any potential relevant findings.
In the present study however, we took several steps to ensure that our EndoPAT measurements were clinically valid through measures that were not employed in previous studies examining the role of the EndoPAT device in ED 17,18. First, we analyzed men with Doppler US to confirm the presence of vasculogenic ED (defined as a PSV<25 cm/s). Furthermore, given that patients with DM or metabolic syndrome have altered endothelial dysfunction, we ensured that our populations were similar with regards to HgA1c, urinary microalbumin, and biothesiometry. Since hypogonadism and its treatment can affect endothelial function, testosterone levels were recorded and were similar between the groups. Moreover, we examined not only the RHI, but also the AI and AI75, the latter of which should not be altered by endothelial dysfunction. Furthermore, we ensured that the populations were similar for variables that may modify endothelial function such as obesity and smoking 13–16. Through these efforts, we obtained similar populations with ED and determined that EndoPAT-obtained RHI was significantly decreased in those men with vasculogenic ED, therefore suggesting endothelial dysfunction in this population. Several weaknesses of this study include the fact that it was retrospective in nature, relied on self-reported ED and failed to exclude blood pressure medications like ACE-inhibitors and diuretic. Furthermore, PSV controls were conducted in patients with self-reported ED. A more accurate measurement would have included a comparison to healthy men without both cardiovascular disease and erectile dysfunction. Lastly, IIEF scores were not available. IIEF scores would have allowed a more comprehensive classification of the ED (beyond the PSV) while and permitted comparisons between the IIEF and EndoPAT.
In summary, the measurement of endothelial function (RHI) with EndoPAT differentiates men with vasculogenic ED from those without. EndoPAT-derived RHI could thus be used as a non-invasive surrogate in the assessment of vasculogenic ED. Furthermore, given that previous studies in the cardiovascular literature have found the EndoPAT test to be significantly correlated with the status of the coronary circulation 37, and that impairment of the hyperemic response was related to coronary artery dysfunction 11,38, the technology is well positioned to function as an indicator for the requirement of more specialized assessment by a cardiologist–especially in the presence of erectile dysfunction.
Abbreviations
- AI
Augmentation index
- ED
erectile dysfunction
- NO
nitric oxide
- PAT
peripheral artery tonometry
- PSV
peak systolic velocity
- RHI
reactive hyperemia index
- US
ultrasonography
References
- 1.Thompson IM, Tangen CM, Goodman PJ, Probstfield JL, Moinpour CM, Coltman CA. Erectile dysfunction and subsequent cardiovascular disease. JAMA: the journal of the American Medical Association. 2005;294(23):2996–3002. doi: 10.1001/jama.294.23.2996. [DOI] [PubMed] [Google Scholar]
- 2.Speel TG, van Langen H, Meuleman EJ. The risk of coronary heart disease in men with erectile dysfunction. European urology. 2003;44(3):366–370. doi: 10.1016/s0302-2838(03)00304-x. discussion 370–361. [DOI] [PubMed] [Google Scholar]
- 3.Solomon H, Man JW, Wierzbicki AS, Jackson G. Relation of erectile dysfunction to angiographic coronary artery disease. The American journal of cardiology. 2003;91(2):230–231. doi: 10.1016/s0002-9149(02)03113-2. [DOI] [PubMed] [Google Scholar]
- 4.Kovac JR, Mak SK, Garcia MM, Lue TF. A pathophysiology-based approach to the management of early priapism. Asian journal of andrology. 2013;15(1):20–26. doi: 10.1038/aja.2012.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sullivan ME, Thompson CS, Dashwood MR, Khan MA, Jeremy JY, Morgan RJ, et al. Nitric oxide and penile erection: is erectile dysfunction another manifestation of vascular disease? Cardiovascular research. 1999;43(3):658–665. doi: 10.1016/s0008-6363(99)00135-2. [DOI] [PubMed] [Google Scholar]
- 6.Saenz de Tejada I, Goldstein I, Azadzoi K, Krane RJ, Cohen RA. Impaired neurogenic and endothelium-mediated relaxation of penile smooth muscle from diabetic men with impotence. The New England journal of medicine. 1989;320(16):1025–1030. doi: 10.1056/NEJM198904203201601. [DOI] [PubMed] [Google Scholar]
- 7.Yavuzgil O, Altay B, Zoghi M, Gurgun C, Kayikcioglu M, Kultursay H. Endothelial function in patients with vasculogenic erectile dysfunction. International journal of cardiology. 2005;103(1):19–26. doi: 10.1016/j.ijcard.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Deyoung L, Chung E, Kovac JR, Romano W, Brock GB. Daily use of sildenafil improves endothelial function in men with type 2 diabetes. Journal of andrology. 2012;33(2):176–180. doi: 10.2164/jandrol.111.013367. [DOI] [PubMed] [Google Scholar]
- 9.Flammer AJ, Anderson T, Celermajer DS, Creager MA, Deanfield J, Ganz P, et al. The assessment of endothelial function: from research into clinical practice. Circulation. 2012;126(6):753–767. doi: 10.1161/CIRCULATIONAHA.112.093245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rubinshtein R, Kuvin JT, Soffler M, Lennon RJ, Lavi S, Nelson RE, et al. Assessment of endothelial function by non-invasive peripheral arterial tonometry predicts late cardiovascular adverse events. European heart journal. 2010;31(9):1142–1148. doi: 10.1093/eurheartj/ehq010. [DOI] [PubMed] [Google Scholar]
- 11.Bonetti PO, Pumper GM, Higano ST, Holmes DR, Jr, Kuvin JT, Lerman A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. Journal of the American College of Cardiology. 2004;44(11):2137–2141. doi: 10.1016/j.jacc.2004.08.062. [DOI] [PubMed] [Google Scholar]
- 12.Brunner H, Cockcroft JR, Deanfield J, Donald A, Ferrannini E, Halcox J, et al. Endothelial function and dysfunction. Part II: Association with cardiovascular risk factors and diseases. A statement by the Working Group on Endothelins and Endothelial Factors of the European Society of Hypertension. Journal of hypertension. 2005;23(2):233–246. doi: 10.1097/00004872-200502000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G, Baron AD. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. The Journal of clinical investigation. 1996;97(11):2601–2610. doi: 10.1172/JCI118709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Celermajer DS, Sorensen KE, Georgakopoulos D, Bull C, Thomas O, Robinson J, et al. Cigarette smoking is associated with dose-related and potentially reversible impairment of endothelium-dependent dilation in healthy young adults. Circulation. 1993;88(5 Pt 1):2149–2155. doi: 10.1161/01.cir.88.5.2149. [DOI] [PubMed] [Google Scholar]
- 15.Zeiher AM, Schachinger V, Minners J. Long-term cigarette smoking impairs endothelium-dependent coronary arterial vasodilator function. Circulation. 1995;92(5):1094–1100. doi: 10.1161/01.cir.92.5.1094. [DOI] [PubMed] [Google Scholar]
- 16.Makimattila S, Virkamaki A, Groop PH, Cockcroft J, Utriainen T, Fagerudd J, et al. Chronic hyperglycemia impairs endothelial function and insulin sensitivity via different mechanisms in insulin-dependent diabetes mellitus. Circulation. 1996;94(6):1276–1282. doi: 10.1161/01.cir.94.6.1276. [DOI] [PubMed] [Google Scholar]
- 17.Aversa A, Francomano D, Bruzziches R, Pili M, Natali M, Spera G, et al. The application of digital pulse amplitude tonometry to the diagnostic investigation of endothelial dysfunction in men with erectile dysfunction. Andrologia. 2011;43(1):9–15. doi: 10.1111/j.1439-0272.2009.00998.x. [DOI] [PubMed] [Google Scholar]
- 18.Mehta A, Miner M, Sigman M. Assessment of EndoPAT scores in men with vasculogenic and non-vasculogenic erectile dysfunction. International journal of clinical practice. 2013;67(1):46–51. doi: 10.1111/ijcp.12011. [DOI] [PubMed] [Google Scholar]
- 19.Vardi Y, Dayan L, Apple B, Gruenwald I, Ofer Y, Jacob G. Penile and systemic endothelial function in men with and without erectile dysfunction. European urology. 2009;55(4):979–985. doi: 10.1016/j.eururo.2008.07.041. [DOI] [PubMed] [Google Scholar]
- 20.Fahed AC, Gholmieh JM, Azar ST. Connecting the lines between hypogonadism and atherosclerosis. International Journal of Endocrinology. 2012;2012(793953) doi: 10.1155/2012/793953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moerland M, Kales AJ, Schrier L, van Dongen MG, Bradnock D, Burggraaf J. Evaluation of the EndoPAT as a Tool to Assess Endothelial Function. International journal of vascular medicine. 2012;2012:904141. doi: 10.1155/2012/904141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nichols WW, Singh BM. Augmentation index as a measure of peripheral vascular disease state. Current opinion in cardiology. 2002;17(5):543–551. doi: 10.1097/00001573-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 23.Lue TF, Hricak H, Marich KW, Tanagho EA. Vasculogenic impotence evaluated by high-resolution ultrasonography and pulsed Doppler spectrum analysis. Radiology. 1985;155(3):777–781. doi: 10.1148/radiology.155.3.3890009. [DOI] [PubMed] [Google Scholar]
- 24.Kovac JR, Brock GB. Surgical outcomes and patient satisfaction after dermal, pericardial, and small intestinal submucosal grafting for Peyronie’s disease. The journal of sexual medicine. 2007;4(5):1500–1508. doi: 10.1111/j.1743-6109.2007.00453.x. [DOI] [PubMed] [Google Scholar]
- 25.Quam JP, King BF, James EM, Lewis RW, Brakke DM, Ilstrup DM, et al. Duplex and color Doppler sonographic evaluation of vasculogenic impotence. AJR American journal of roentgenology. 1989;153(6):1141–1147. doi: 10.2214/ajr.153.6.1141. [DOI] [PubMed] [Google Scholar]
- 26.Donaghue KC, Robinson J, McCredie R, Fung A, Silink M, Celermajer DS. Large vessel dysfunction in diabetic adolescents and its relationship to small vessel complications. Journal of Pediatric Endocrinology and Metabolism. 2011;10(6):593–598. doi: 10.1515/jpem.1997.10.6.593. [DOI] [PubMed] [Google Scholar]
- 27.Johannes CB, Araujo AB, Feldman HA, Derby CA, Kleinman KP, McKinlay JB. Incidence of erectile dysfunction in men 40 to 69 years old: longitudinal results from the Massachusetts male aging study. The Journal of urology. 2000;163(2):460–463. [PubMed] [Google Scholar]
- 28.Kaiser DR, Billups K, Mason C, Wetterling R, Lundberg JL, Bank AJ. Impaired brachial artery endothelium-dependent and -independent vasodilation in men with erectile dysfunction and no other clinical cardiovascular disease. Journal of the American College of Cardiology. 2004;43(2):179–184. doi: 10.1016/j.jacc.2003.07.042. [DOI] [PubMed] [Google Scholar]
- 29.De Angelis L, Marfella MA, Siniscalchi M, Marino L, Nappo F, Giugliano F, et al. Erectile and endothelial dysfunction in Type II diabetes: a possible link. Diabetologia. 2001;44(9):1155–1160. doi: 10.1007/s001250100616. [DOI] [PubMed] [Google Scholar]
- 30.Willum-Hansen T, Staessen JA, Torp-Pedersen C, Rasmussen S, Thijs L, Ibsen H, et al. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation. 2006;113(5):664–670. doi: 10.1161/CIRCULATIONAHA.105.579342. [DOI] [PubMed] [Google Scholar]
- 31.Aversa A, Sarteschi LM. The role of penile color-duplex ultrasound for the evaluation of erectile dysfunction. The journal of sexual medicine. 2007;4(5):1437–1447. doi: 10.1111/j.1743-6109.2007.00546.x. [DOI] [PubMed] [Google Scholar]
- 32.Patel U, Amin Z, Friedman E, Vale J, Kirby RW, Lees WR. Colour flow and spectral Doppler imaging after papaverine-induced penile erection in 220 impotent men: study of temporal patterns and the importance of repeated sampling, velocity asymmetry and vascular anomalies. Clinical radiology. 1993;48(1):18–24. doi: 10.1016/s0009-9260(05)80101-1. [DOI] [PubMed] [Google Scholar]
- 33.Peled N, Shitrit D, Fox BD, Shlomi D, Amital A, Bendayan D, et al. Peripheral arterial stiffness and endothelial dysfunction in idiopathic and scleroderma associated pulmonary arterial hypertension. The Journal of rheumatology. 2009;36(5):970–975. doi: 10.3899/jrheum.081088. [DOI] [PubMed] [Google Scholar]
- 34.Teloken PE, Mulhall JP. Impact of phosphodiesterase type 5 inhibitors on endothelial function. Reviews in urology. 2008;10(1):26–30. [PMC free article] [PubMed] [Google Scholar]
- 35.Makhsida N, Shah J, Yan G, Fisch H, Shabsigh R. Hypogonadism and metabolic syndrome: implications for testosterone therapy. The Journal of urology. 2005;174(3):827–834. doi: 10.1097/01.ju.0000169490.78443.59. [DOI] [PubMed] [Google Scholar]
- 36.Aversa A, Bruzziches R, Francomano D, Natali M, Gareri P, Spera G. Endothelial dysfunction and erectile dysfunction in the aging man. International journal of urology: official journal of the Japanese Urological Association. 2010;17(1):38–47. doi: 10.1111/j.1442-2042.2009.02426.x. [DOI] [PubMed] [Google Scholar]
- 37.Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation. 2007;115(10):1285–1295. doi: 10.1161/CIRCULATIONAHA.106.652859. [DOI] [PubMed] [Google Scholar]
- 38.Kuvin JT, Patel AR, Sliney KA, Pandian NG, Sheffy J, Schnall RP, et al. Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. American heart journal. 2003;146(1):168–174. doi: 10.1016/S0002-8703(03)00094-2. [DOI] [PubMed] [Google Scholar]