Abstract
Background and aims
Potassium channels, KV1.3 and KCa3.1, have been suggested to control T-cell activation, proliferation, and cytokine production and may thus constitute targets for anti-inflammatory therapy. Ulcerative colitis (UC) is a chronic inflammatory bowel disease characterized by excessive T-cell infiltration and cytokine production. It is unknown if KV1.3 and KCa3.1 in the inflamed mucosa are markers of active UC. We hypothesized that KV1.3 and KCa3.1 correlate with disease activity and cytokine production in patients with UC.
Methods
Mucosal biopsies were collected from patients with active UC (n = 33) and controls (n = 15). Protein and mRNA expression of KV1.3 and KCa3.1, immune cell markers, and pro-inflammatory cytokines were determined by quantitative-real-time-polymerase-chain-reaction (qPCR) and immunofluorescence, and correlated with clinical parameters of inflammation. In-vitro cytokine production was measured in human CD3+ T-cells after pharmacological blockade of KV1.3 and KCa3.1.
Results
Active UC KV1.3 mRNA expression was increased 5-fold compared to controls. Immunofluorescence analyses revealed that KV1.3 protein was present in inflamed mucosa in 57% of CD4+ and 23% of CD8+ T-cells. KV1.3 was virtually absent on infiltrating macrophages. KV1.3 mRNA expression correlated significantly with mRNA expression of pro-inflammatory cytokines TNF-α (R2 = 0.61) and IL-17A (R2 = 0.51), the mayo endoscopic subscore (R2 = 0.13), and histological inflammation (R2 = 0.23). In-vitro blockade of T-cell KV1.3 and KCa3.1 decreased production of IFN-γ, TNF-α, and IL-17A.
Conclusions
High levels of KV1.3 in CD4 and CD8 positive T-cells infiltrates are associated with production of pro-inflammatory IL-17A and TNF-α in active UC. KV1.3 may serve as a marker of disease activity and pharmacological blockade might constitute a novel immunosuppressive strategy.
Keywords: Novel treatment strategy, Colitis ulcerosa, KCNN4, KCNA3, Interleukins, KCa3.1
1. Introduction
Ulcerative colitis (UC) is a relapsing chronic inflammatory bowel disease (IBD) characterized by bloody diarrhea. It impairs quality of life and can lead to life-threatening complications, e.g. toxic megacolon.1 The present treatment options, such as corticosteroids, salicylates, and anti-TNF-α reagents, are unsatisfactory for many patients and there is a clear need to identify novel molecular targets for alternative treatment.
The etiology of UC is still under debate. Several hypotheses on the cause of IBD such as allergic disposition, hygiene conditions, infections, and nutrition have been presented.2–4 It has also been speculated that the inflammation could be of heritable5,6 and of autoimmune origin although the nature of this autoimmunity remains elusive.7 After onset, mucosal inflammation is likely maintained by an abnormal activity of cytotoxic T-cells (TC, CD8+) and T helper cells (TH, CD4+),8,9 and the concomitant cytotoxicity and excessive secretion of pro-inflammatory cytokines such as tumor necrosis factor α (TNF-α) and interleukin-17A (IL-17A), respectively.10–12 The role of IFN-γ in UC is still ambiguous; some studies report an increased IFN-γ production when compared to controls while others do not find an increase.13,14 It seems as if the immunological balance between TH1, TH2 and TH17 responses is disturbed, and that an atypical TH2 response occurs involving non-classical natural killer T cells.13,15–18
Ion channels in the T cell membrane play pivotal roles in T cell functions by maintaining intracellular Ca2+-homeostasis, cytokine production, and clonal expansion after T-cell receptor (TCR) activation.19 Of particular importance are the voltagegated potassium channel KV1.3 (encoded by the KCNA3 gene) and the calcium-activated potassium channel KCa3.1 (encoded by the KCNN4 gene). Constitutively expressed KV1.3 channels stabilize the membrane potential, thus keeping the electrical driving force for sustained Ca2+ influx through calcium release activated channels (CRAC) after TCR activation.20–22 This longlasting increase in cytosolic Ca2+ triggers T-cell proliferation and cytokine production.19,23 Increasing evidence also supports that KV1.3 channels play a role in autoimmune diseases such as type-1-diabetes, rheumatoid arthritis, multiple sclerosis, and glomerulonephritis.24–28 Effector memory T-cells (TEM)(CD8+/ CCR7− and CD4+/CCR7−), a subset of terminally differentiated T cells, express high levels of KV1.3 channels while activated TCM and naïve T-cells primarily express and use the calciumactivated KCa3.1 channel to regulate their Ca2+ signaling.23 In the light of these findings, small molecule blockers of KV1.3 and KCa3.1 may be of therapeutic utility in inflammatory and autoimmune diseases.23,29 KCa3.1 channels are generally upregulated in T cells following activation but are also found in macrophages and colonic crypts in which they are involved in the water and NaCl secretion to the intestinal lumen.23,26,30,31 Whether Kv1.3 and KCa3.1 channels are also pathomechanistically linked to the inflammation in UC is not known and most data about T cell potassium channels are from in-vitro, or animal studies.
Therefore, our hypotheses were that (1) high expression of KV1.3 and KCa3.1 in T cell infiltrates in the inflamed mucosa of UC patients correlated with disease activity and the synthesis of the pro-inflammatory cytokines TNF-α, IFN-γ, and IL-17A, and (2) that in vitro blockade of KV1.3 and KCa3.1 on human CD3+ T cells decreased production of TNF-α, IFN-γ, and IL-17A.
2. Materials and methods
2.1. Study design and patients
The study was a cross-sectional case–control study with follow-up. We included patients older than 18 years with established active UC. The control group consisted of patients scheduled for endoscopy at outpatient clinics with no inflammatory bowel disease (cancrophobia or control after previous polyp removal). Exclusion criteria were: (1) active treatment with anticoagulants, non-steroidal anti-inflammatory drugs (NSAID), or acetylsalicylic acid (ASA); (2) pregnancy or lactation; (3) stool cultures positive for pathogens; (4) contraindicated endoscopy. In the control group diarrhea was an exclusion criterion. Participants were included at Odense University Hospital and Hospital of South-West Jutland, Esbjerg, Denmark, after verbal and written informed consent. The study was approved by the Danish Ethics Committee (permit no. S-20110007) and the Danish Data Protection Agency (permit no. 2008-58-0035).
2.2. Collection of specimens
Blood sampling (C-reactive protein (CRP), leucocyte count (LEU)), fecal calprotectin, stool cultures, and endoscopy were performed the same day. Fecal samples were collected prior to endoscopy. Biopsies were obtained from rectal mucosa during endoscopic examination of healthy individuals and from patients with first-time attack or relapse of UC. Biopsies were immediately stored in RNAlater® (Ambion, Austin, TX, USA).
2.3. Scoring of inflammation and Mayo Score
Participants were scored according to the Mayo Score including the Mayo endoscopic subscore (0 none, 1 mild, 2 moderate, 3 severe).32 Histological inflammation of the biopsies was scored according to Morson and Dawson’s UC score for inflammation (0 none, 1 mild, 2 moderate, 3 severe) by a gastrointestinal pathologist.33
2.4. RNA preparation, DNase digestion, cDNA synthesis and quantitative Real Time PCR
RNA was isolated from biopsies using TRIZOL reagent® (Invitrogen, United Kingdom). The RNase-Free DNase Set (Qiagen, Germany) was used for DNase digestion. Complementary DNA (cDNA) was synthesized using iScript cDNA Synthesis Kit (Bio-Rad, CA, USA) and quantitative real-time PCR (qPCR) was performed according to the MIQE guidelines34 (except from using only one reference gene) using SYBR Green Supermix (Bio-Rad, CA, USA) on a Stratagene MX3000P qPCR instrument (Agilent Technologies, Santa Clara, CA, USA). All primers were obtained from Sigma-Aldrich (St. Louis, MO, USA); efficiencies were 90–105% and reactions were run in duplicates.
Data exclusion criteria: Cq-value (formerly known as Ct-value) of GAPDH above 25 (indicating low amounts or bad quality of cDNA/mRNA); Cq-values in a double determination differed by more than 2 Cq-values (inconclusive data); non exponential amplification slopes (false-positive, incomplete reaction), or if melting curves of the product did not show one product of expected size. Expression levels are presented as a percentage of GAPDH (%GAPDH). No significant difference was found in GAPDH expression between UC and controls (data not shown).
2.5. Immunohistochemistry and immunofluorescence
Immunohistochemical (IHC) and immunofluorescent (IF) stainings were performed on the same paraffin-embedded biopsies cut in 5 µm serial sections as used for the pathological assessment. Tissue-Tek® Tissue-Clear® was used to deparaffinize the slides and then hydrated through an ethanol gradient from 99.9% ethanol to mQ water. After hydration the slides were put in TBS (Tris-Buffered Saline) with 1.5% H2O2. Heat-Induced Epitope Retrieval (HIER) was used to enhance the antibody/antigen binding capability. All antibodies were tested using three different buffers (Citrate (Dako #S2031), Tris-EGTA (TEG), and TRS (Dako #S1699) and in serial dilutions to find the best HIER buffer and the optimum concentration of the antibodies. Antibodies were checked for specificity with IHC before proceeding to IF stainings. We used the following antibodies: CD3 (AB Serotec, #MCA1477), CD4 (Thermo Scientific, #MA5–12259), CD8 (AB Serotec, #MCA1817), Macrophage/L1 molecule (MAC) (AB Serotec, #MCA874G), KV1.3 (Novus Biologicals, #NBP1–19415) and KCa3.1 (Sigma-Aldrich, #AV35098). Primary antibodies were identified using the DAKO Envision™+ Kit (DAKO, Glostrup, Denmark). DAB+ (DAKO) was used as substrate-chromogen system. IHC slides were counterstained with Hematoxyline. IF stainings were performed accordingly using secondary antibodies combined with fluorochromes: Alexa Fluor 488, Alexa Flour 568, and 4’-6-Diamidino-2-phenylindole dihydrochloride (DAPI) for nuclei.
We used the FLoid® Cell Imaging Station (Life Technologies Europe, Nærum, Denmark.) to measure fluorescent signals. Three pictures of each patient were taken on the basis of the nuclei staining (blue) and afterwards the fluorescent signals were obtained (blue for nuclei, red for cell markers, and green for potassium channels). Light intensity and contrast were adjusted for better analyses of signals from T-cell infiltrates. Each fluorescent picture (red, green or blue) was analyzed individually and then quantified with the CellProfiler software (Broad Institute, Boston, MA, USA)35 for co-localization of fluorescent signal. This was done automated and blinded.
2.6. Proliferation and cytokine assays
CD3+ T cells were isolated from peripheral blood of a healthy volunteer with RosetteSep (StemCell Technologies, Vancouver, BC, Canada), washed and seeded at 8×104 cells per well into flat bottom 96-well plates in RPMI-1640 culture medium. KV1.3 blockers: PAP-1 (concentrations: 100 nM, 250 nM, 1 µM) and ShK-L5 (concentrations: 1 nM, 10 nM),23,36 KCa3.1 blocker: Senicapoc (concentrations: 100 nM, 250 nM, 1 µM),37,38 or compound combinations were added at different concentrations and the cells then stimulated with 10 nM PMA + 175 nM ionomycin for 48 h. [3H]-Thymidine (1 µCi per well) was added for the last 8 h. Plates were then frozen and later harvested onto glass fiber filters and radioactivity measured in a scintillation counter. Amounts of secreted cytokines were determined by removing 50 µl/well of supernatant from plates after 40 h of incubation (before the [3H]-thymidine pulse) and frozen at −80 °C pending analysis. A cytokine panel (IL-1β, IL-2, IL-4, IL-10, IL-12, IL-17A, IL-17E/IL-25, IL-17F, IL-22, IFN-γ, and TNF-α) was then analyzed with a Millipore Milliplex magnetic bead human Th17 cytokine/chemokine kit and a Luminex 200™ reader according to the manufacturer’s instructions. PAP-1 and Senicapoc were synthesized in the Wulff laboratory at the University of California, Davis. ShK-L5 was a generous gift from Michael Pennington at Peptides International (Louisville, KY).
2.7. Follow-up data
UC patients’ records were studied to identify “days to relapse” after the date of inclusion. We defined a relapse as symptoms compatible with active UC and combined with alterations in treatment by the patient’s gastroenterologist. Not all patients had endoscopies performed at relapse. We also studied the “days to relapse after initial remission was achieved”.
2.8. Statistics
For comparison of datasets we used the unpaired Student’s t-test or if applicable the non-parametric Mann–Whitney test. Results are presented as Mean ± SEM. To assess differences between two or more groups one or two-way ANOVA followed by the Tukey-post hoc test was used and presented with 95% confidence interval [CI]. All correlations were made using linear regression. The significance level was set as p < 0.05. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
3. Results
3.1. Analyses of mRNA expression of T-cell potassium channels, immune cell markers, and cytokines
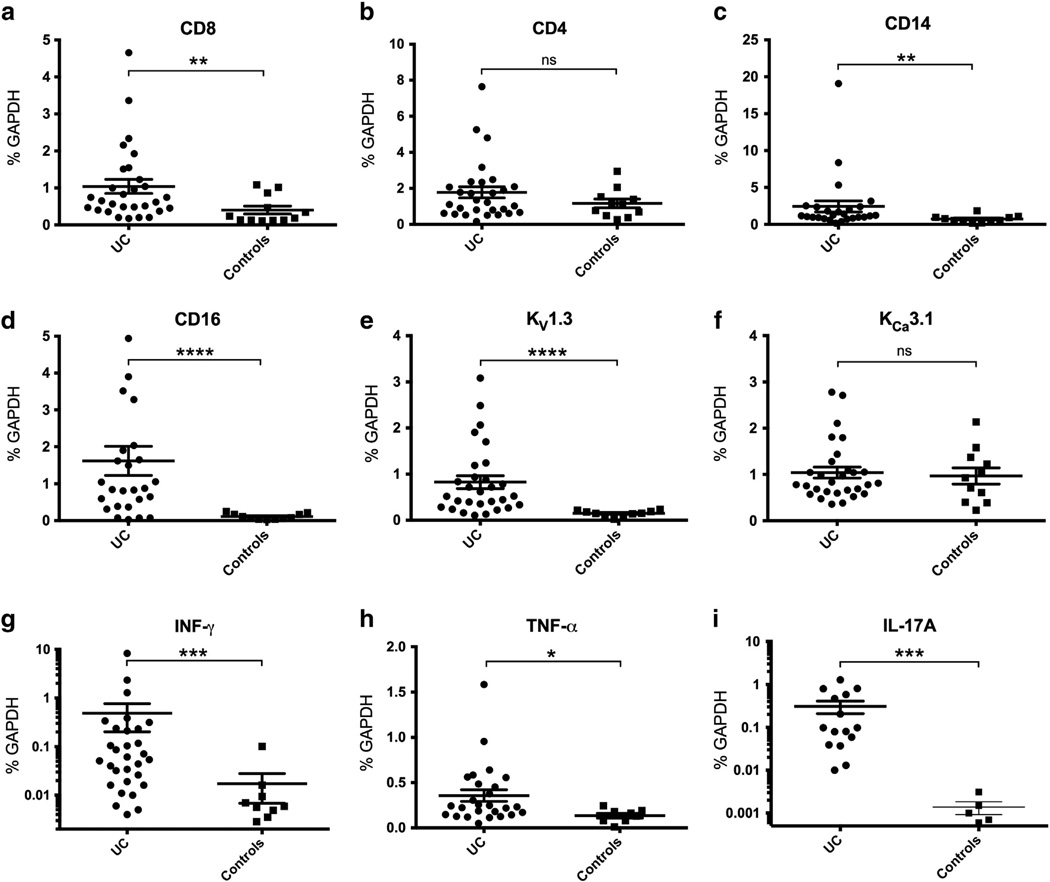
We included 33 UC patients and 15 healthy controls (Table 1) and performed qPCR on mRNA extracts from mucosal biopsies. Primer sequences are shown in Table 2. First, we examined the expression of CD8 (TC) and CD4 (TH) and found that in UC patients the expression of CD8 was clearly 2.5-fold higher than in controls (p < 0.01, Fig. 1a). In contrast, UC patients did not show higher expression of CD4 (p = 0.20; Fig. 1b). In the UC group we found a 3-fold increase in mRNA-expression of CD14, a marker of monocytes (p < 0.01; Fig. 1c) and a 14-fold increase of CD16, a marker of stimulated monocytes, phagocytic macrophages, and natural killer cells (p < 0.01; Fig. 1d).
Table 1.
Baseline characteristics of controls and patients with ulcerative colitis (UC) at inclusion. 5ASA = Mesalazine, GC = Glucocorticoids, IFX = Infliximab, AZA = Azathioprine.
| N | Mean age years | Male N (%) | Mayoscore Mean [CI] | 5ASA N (%) | GC N (%) | IFX N (%) | AZA N (%) | |
|---|---|---|---|---|---|---|---|---|
| Controls | 15 | 55 | 7 (47%) | 0 | 0 | 0 | 0 | 0 |
| UC | 33 | 44 | 13 (39%) | 8.3 [7.4–9.2] | 28 (85%) | 8 (24%) | 5 (15%) | 2 (1%) |
Table 2.
Primer specifications.
| Primer | Size (bp) | NCBI reference sequence | Sense | Anti-sense |
|---|---|---|---|---|
| GAPDH | 91 | NM 002046.3 | caccatcaaggctgagaacg | gccccacttgattttggagg |
| CD8 | 112 | NM 001145873.1 | gctggacttcgcctgtgatatc | acgtcttcggttcctgtggtt |
| CD4 | 74 | NM 000616.4 | cccttttaggcacttgcttctg | gcaccactttctttccctgagt |
| CD14 | 165 | NM 001174105.1 | gccgctgtgtaggaaagaag | ttcatcgtccagctcacaag |
| CD16 | 156 | NM_001127595.1 | gctccggatatctttggtga | agcaccctgtaccattgagg |
| KCa3.1 | 159 | NM_002250.2 | catcacattcctgaccatcg | acgtgcttctctgccttgtt |
| KV1.3 | 175 | NM_002232.3 | tctggtgggcagtggtaacc | ccttctgtctcccggtggta |
| IFN-γ | 236 | NM_000619.2 | tgaccagagcatccaaaaga | ctcttcgacctcgaaacagc |
| TNF-α | 151 | NM_000594.2 | tcttctcgaaccccgagtga | cctctgatggcaccaccag |
| IL-17A | 174 | NM_002190.2 | catccataaccggaataccaata | tagtccacgttcccatcagc |
Figure 1.
mRNA expression of cell markers, pro-inflammatory cytokines, and potassium channels in mucosal biopsies of UC patients and controls. Data from individual patients are also given as means ± SEM. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
KV1.3 was increased 5-fold in UC patient biopsies compared to the very low levels of controls (p < 0.01; Fig. 1e). In contrast, expression of KCa3.1 was not significantly different (ns; Fig. 1f).
UC patients exhibited a 28-fold increase in expression of IFN-γ, a 3-fold increase of TNF-α, and a 200-fold increase of IL-17A compared to the very low levels detected in controls (p < 0.01, p < 0.05, and p < 0.01, respectively; Fig. 1g, h, and i).
3.2. Correlations with clinical scores and blood samples
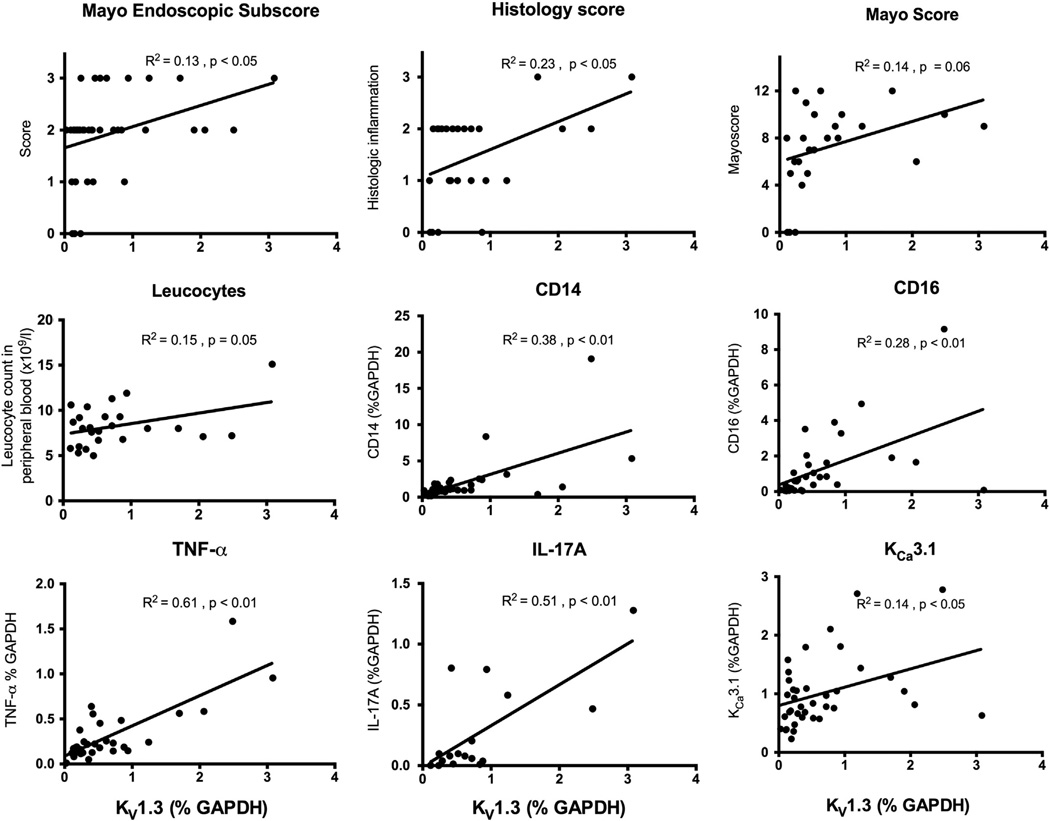
In keeping with the hypothesis that these genes are markers of disease activity we pooled all data from UC patients and controls and tested whether mRNA expression correlated positively with clinical scores (Mayo score, Mayo endoscopic subscore, and histology score) and laboratory test results (fecal calprotectin, LEU and CRP).
As shown in Fig. 2, mRNA expression of KV1.3 was found to correlate very well, and much better than IFN-γ, TNF-α and IL-17A, with the Mayo endoscopic subscore and the histology score. KV1.3 also showed borderline significant correlations with Mayo-score (p = 0.06) and LEU (p = 0.05; Fig. 2). The median level of calprotectin, LEU and CRP were 173.5 mg/kg, 8.0 × 109/l, and 2.0 mg/l, respectively. In contrast, KCa3.1 did not correlate with any of the clinical scores or laboratory findings (Table 3).
Figure 2.
Significant and borderline significant correlations of Kv1.3 mRNA expression (in percentage of GAPDH) with clinical scores, cell markers and cytokines. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
Table 3.
Correlations between mRNA expression of KV1.3 and KCa3.1 potassium channels in mucosal biopsies and clinical parameters. Statistical analyses were performed using linear regression.
| KV1.3 | R2 | p value | KCa3.1 | R2 | p value |
|---|---|---|---|---|---|
| Mayo-score | 0.142 | (*) 0.058 | Mayo-score | 0.004 | 0.773 |
| Mayo endoscopic subscore | 0.130 | * 0.024 | Mayo endoscopic subscore | 0.001 | 0.825 |
| Histology Score | 0.230 | * 0.015 | Histology score | 0.036 | 0.361 |
| Age | 0.007 | 0.622 | Age | 0.008 | 0.590 |
| Fecal Calprotectin (mg/kg) | <0.001 | 0.985 | Fecal calprotectin (mg/kg) | 0.015 | 0.723 |
| Leucocytes in PB (×109/l) | 0.153 | (*) 0.054 | Leucocytes in PB (×109/l) | 0.001 | 0.863 |
| C-reactive protein in PB (mg/l) | 0.020 | 0.495 | C-reactive protein in PB (mg/l) | 0.006 | 0.712 |
(PB = peripheral blood).
p < 0.05;
p = 0.05–0.1.
Subsequently, KV1.3 and KCa3.1 mRNA expression was correlated with the mRNA expression of CD8, CD4, CD14 and CD16, and pro-inflammatory cytokines: IFN-γ, TNF-α and IL-17A (Table 4). Expression of KV1.3 correlated significantly with the expression of CD14, CD16, TNF-α, and IL-17A. Similarly, KCa3.1 expression correlated with those of CD14, CD16 and TNF-α, but not with IL-17A. Additional correlations of CD4, CD8, CD14, CD16, IFN-γ, TNF-α, and IL-17A can be found in the supplement.
Table 4.
Correlations between mRNA expression of KV1.3 and KCa3.1 potassium channels and pro-inflammatory cytokines and cell markers. Data are shown as Goodness of Fit (R2) and P value. Significant correlations are highlighted.
| GENE | KCa3.1 | IFN-γ | TNF-α | IL-17A | CD4 | CD8 | CD14 | CD16 |
|---|---|---|---|---|---|---|---|---|
| KV1.3 | R2= 0.14 p = 0.20 |
R2 = 0.02 p = 0.39 |
R2 = 0.61 p < 0.01 |
R2 = 0.51 p < 0.01 |
R2 = 0.07 p = 0.10 |
R2 = 0.08 p = 0.10 |
R2 = 0.38 p < 0.01 |
R2 = 0.28 p < 0.01 |
| KCa3.1 | R2 = 0.05 p = 0.19 |
R2 = 0.15 p = 0.03 |
R2 = 0.05 p = 0.39 |
R2 < 0.01 p = 0.69 |
R2 < .01 p = .89 |
R2 = 0.38 p < 0.01 |
R2 = 0.31 p < 0.01 |
UC patients were stratified according to treatment, receiving either 5ASA or 5ASA+ one of the following immunosuppressants: glucocorticoids, infliximab, and azathioprine. However, we did not find significant differences or significant correlations in gene expression levels (data not shown).
In keeping with the idea that KV1.3 and KCa3.1 mRNA expression in the individual UC patient could be indicative of relapse probability and thus of prognostic value, we correlated mRNA levels of channels at inclusion with “days to relapse” (n = 10). Here, we found no correlation with KV1.3 (R2 = 0.11, p = 0.35). However, there was a trend towards a negative correlation with KCa3.1 (R2 = 0.34, p = 0.08). Examining the “days to relapse after initial remission was achieved” (n = 9), we found no correlations with either KV1.3 (R2 = 0.21, p = 0.22) or KCa3.1 (R2 = 0.26, p = 0.16).
3.3. Immunostainings
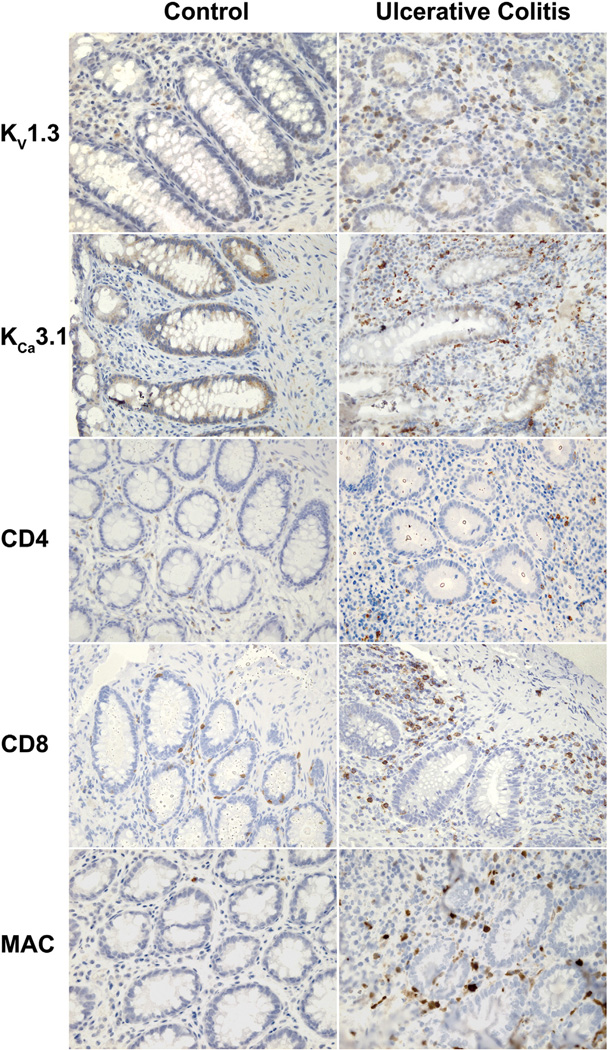
To identify the cell types in the colonic mucosa expressing KV1.3 and/or KCa3.1 channels, we performed immunohistochemical (IHC) and immunofluorescent stainings (IF) (Figs. 3 and 4). IHC revealed the presence of KV1.3 protein only in infiltrating cells in the inflamed mucosa but not in the non-inflamed samples from controls (Fig. 3). In contrast, KCa3.1 protein was found in both colonic crypts of UC and controls as described previously30,39 and in infiltrating cells present in the inflamed mucosa. In the inflamed mucosa these cells were positive for CD4, CD8, or MAC identifying them as TH, TC, or macrophages, respectively. CD4+, CD8+, or MAC+ cells were only present at normal low levels in controls. In the standard IHC stainings, we did not quantitate the cells and ion channels further as this was done in IF.
Figure 3.
Immunohistochemical stainings visualizing the potassium channels and cell types in the normal mucosa of controls and the inflamed mucosa of patients with active ulcerative colitis. Note that the crypts in UC are more irregular and contain less mucus. KV1.3+ cells are located in the interstitial tissue around crypts in UC patients. Crypts in both patient groups are positive for KCa3.1 and immunoreactivity is also detected in cell infiltrates in UC. Both CD4+ and CD8+ cells clearly infiltrate the tissue in UC. Infiltration of macrophages (MAC) is also evident in the inflamed mucosa of UC.
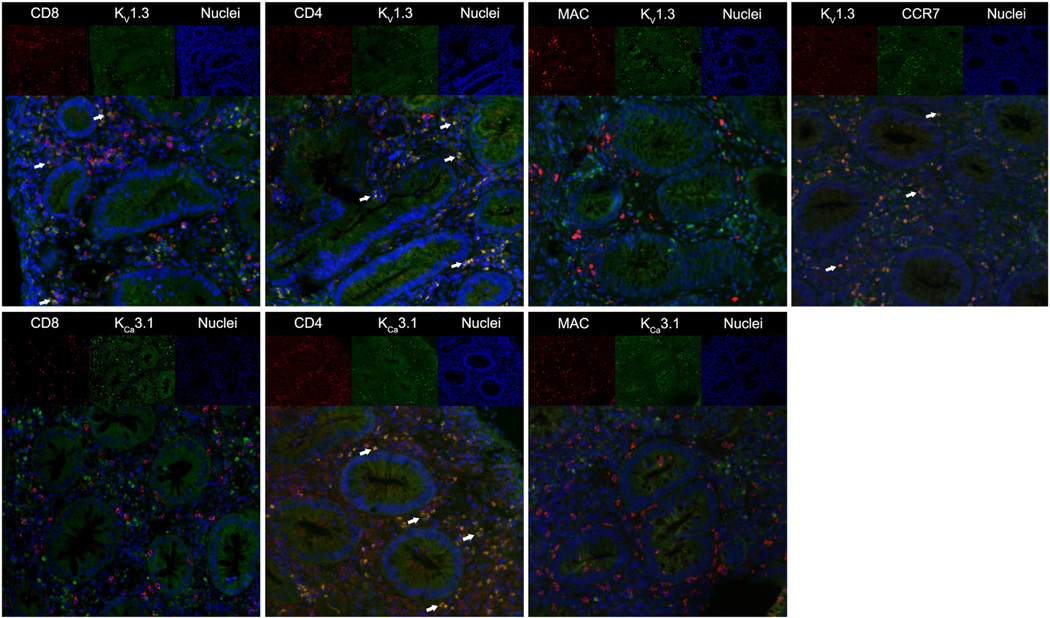
Figure 4.
Immunofluorescent stainings of potassium channels, cell markers, and chemokine-receptor-7 (CCR7). In the upper part of each picture the individual fluorochromes are shown. The lower part shows the merged pictures visualizing co-localized immunofluorescence as a yellow signal. Arrows indicates co-localized immunofluorescence. The top row displays immunostainings of KV1.3 and in the lower row KCa3.1.
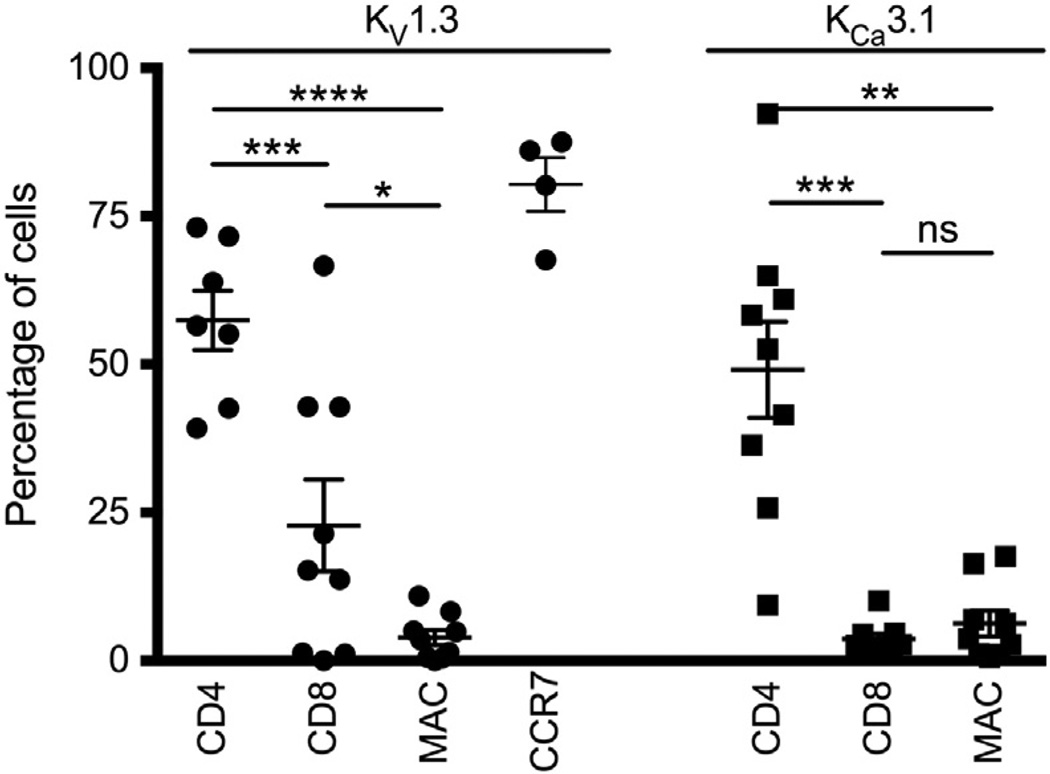
Immunofluorescent cell markers were studied for colocalization with KV1.3 and KCa3.1 in the UC patients (Fig. 4 for representative images and Fig. 5 for quantification) and we found that KV1.3 co-localized with CD4 in 57% [CI: 45–70] of cells and with 23% [CI: 5–41] of CD8 cells. Moreover, we found that 80% [CI: 66–95] of the KV1.3+ cells were chemokine receptor 7 (CCR7) positive (Fig. 5). KV1.3 immunofluorescence was only detectable in 4% [CI: 1–7]of the macrophages. KCa3.1 co-localized with CD4 in 49% [CI: 31–68] of the cells and to a minor extent with 4% [CI: 2–6] of CD8. In macrophages, we found a similar weak co-localization in 6% of the cells [CI: 1–11]. As for controls, immunostainings were also performed, but there were only very few infiltrating T cells. Therefore, we preferred to not compare the relative colocalization between UC and control samples.
Figure 5.
The left side of the graph shows the percentage of KV1.3 immunofluorescence that co-localize with CD8, CD4, macrophages and Chemokine Receptor 7 (CCR7) after analyses with the Cell Profiler software. The right side shows the percentage of KCa3.1 immunofluorescence co-localizing with CD4, CD8 and macrophages. Error bars show mean ± SEM.
This analysis demonstrates that TH cells (CD4+) showed more abundant expression of both KV1.3 and KCa3.1 proteins compared to TC cells (CD8+) and macrophages (Fig. 5).
3.4. Cytokine production and cell proliferation assay
To study if KV1.3 and KCa3.1 play a role in T cell cytokine production, we stimulated CD3+ T cells with PMA+ ionomycin to drive a strong, Ca2+-dependent T cell activation.40 Successful stimulation was verified by incorporation of [3H]-thymidine (Fig. 6), which is a measure of proliferation.
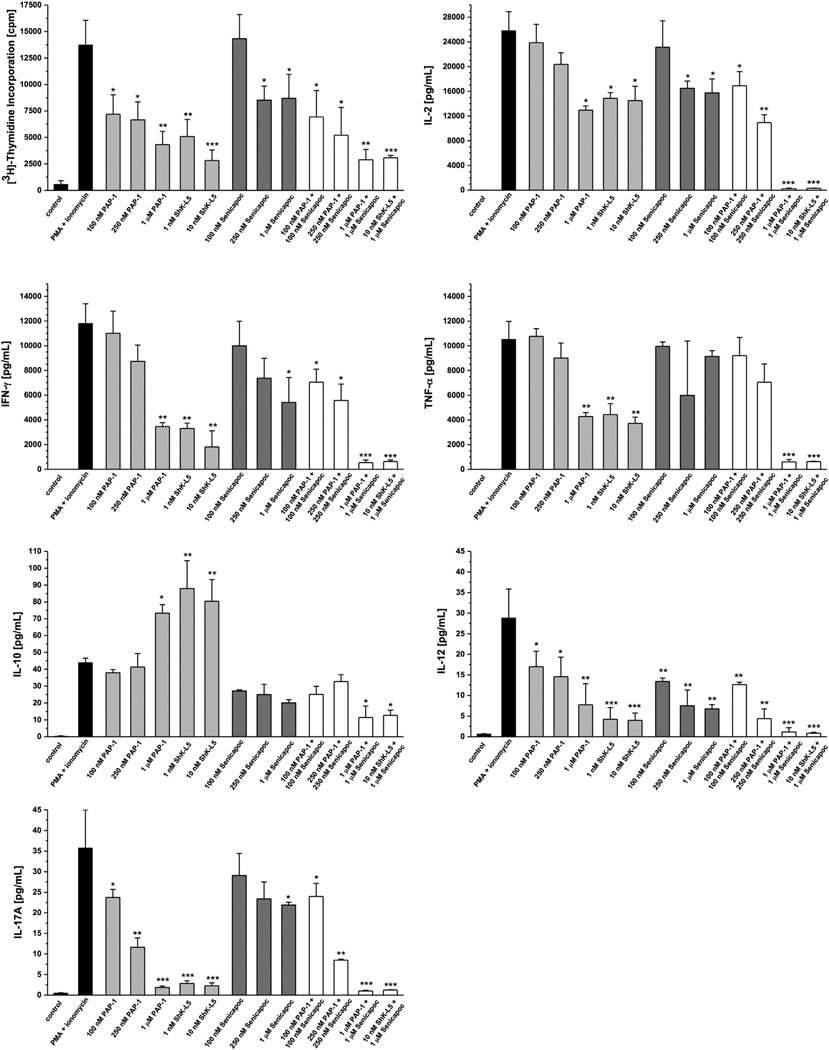
Figure 6.
Effect of KV1.3 and KCa3.1 blockage in PMA (phorbol-12-myristate-13-acetate) + Ionomycin-stimulated human CD3+ T cells. First figure shows [3H]-thymidine incorporation, which is a measure of cell proliferation. Additional figures visualize production of inflammatory cytokines. PAP-1 and ShK-L5 are KV1.3 blockers; Senicapoc is a KCa3.1 blocker. The blockers were tested at different concentrations, and in the right-hand side of each graph combinations of blockers are presented. Individually, the blockers decrease the level of cell proliferation and cytokine secretion and when used in combination the effect is even more prominent.
Blockade of the KV1.3 channel with 1 µM PAP-1 revealed a decrease in cell proliferation and a decrease in production of inflammatory cytokines: IL-2, IFN-γ, TNF-α, IL-12, and IL17-A. In contrast, the anti-inflammatory cytokine IL-10 was increased. Using another KV1.3 blocker, ShK-L5, similar results were found (Fig. 6).
Blockade of KCa3.1 with 1 µM Senicapoc showed decreased production of IL-2, IFN-γ, IL-12, and IL-17A, but not of TNF-α and IL-10. Using the KV1.3 and KCa3.1 blockers in combination (at concentrations of 1 µM), the secretion of all the above-mentioned cytokines and proliferation were suppressed almost to the same levels of non-stimulated cells. The cytokines IL-1β, IL-4, IL-17E, IL-17 F and IL-22 were below detection limit.
4. Discussion
The main outcome of our study is that mRNA expression and protein expression of KV1.3 in infiltrating TH and TC in the inflamed mucosa are characteristic features of active ulcerative colitis. Moreover, we found that pharmacological blockade of KV1.3 and KCa3.1 in isolated human T cells, in vitro, led to decreased production of pro-inflammatory cytokines, including TNF-α, IFN-γ, and IL-17A. Furthermore, increased mRNA expression of KV1.3, in vivo, was linked to increased expression of the inflammatory cytokines TNF-α and to IL-17A.
To our knowledge, we provide the first evidence that T-cell KV1.3 potassium channels could serve as a marker of disease activity and as a potential treatment target in active UC in vivo.
KV1.3 contributes to T-cell function by setting and keeping the membrane potential and thus maintains the driving force for calcium influx after T-cell stimulation.19 Moreover, KV1.3 channels are highly expressed in effector memory T-cells (TEM) in autoimmune diseases and are believed to contribute to the pathology of multiple sclerosis, type-1 diabetes and rheumatoid arthritis by sustaining the activity of CCR7−TEM cells.24–28,41 Although UC is not considered a pure T cell disease, but also may be related to barrier problems etc., our study still suggests that KV1.3 channels play a role in the inflammation of patients with active UC. Here, we report that KV1.3 is present on T-cell infiltrates of inflamed mucosa and particularly in most TH (80%) and to a lesser extent in TC (23%) cells in UC. However, the majority of the Kv1.3+ cells are CCR7+. This suggests that KV1.3 is a feature of general T-cell infiltration and activity of T cells in active UC, but unlike in inflammatory infiltrates in MS brain,24 we do not have evidence that the Kv1.3+ cells are classical CCR7− TEM cells.
Considering the channel as a molecular marker of disease activity, high KV1.3 expression correlates better with the degree of inflammation than the expression of TNF-α and IL-17A, which are known mediators of the inflammatory response in both UC and CD.11,12,42,43 Interestingly, up-regulation of IL-17A mRNA expression has been associated with treatment success in infliximab treatment of active UC.44 However, IL-17A’s pathomechanistic roles are still unclear since IL-17A secretion by activated T-cells has been suggested to be pro-inflammatory10,11 – by recruiting other immune cells – as well as protective in UC as IL17-deficient T-cells produce more TH1 cytokines in a model of T-cell driven colitis.45
Interestingly, KV1.3 mRNA expression distinguishes more clearly between UC and controls than the classical markers of TH (CD4+) and TC (CD8+) but equally well as a marker of stimulated monocytes, phagocytic macrophages, and natural killer cells (CD16). Importantly, KV1.3 expression correlated positively with the clinical parameters and non-molecular markers of disease activity thus further supporting the idea that this channel is a strong molecular marker of UC activity.
Mechanistically, our T cells stimulation assay revealed that KV1.3 and KCa3.1 are indeed involved in cell proliferation and in the secretion of inflammatory cytokines. In other studies, KV1.3 channel inhibition or knockout in mice subjected to experimental autoimmune encephalomyelitis has also been associated with decreased production of pro-inflammatory cytokines, IFN-γ and IL-17A.27 Additionally, pharmacological inhibition of KV1.3 in rats by PAP-1 ameliorated oxazolone-induced allergic contact dermatitis in vivo and decreased production of IFN-γ and IL17-A.46
In our study, we also demonstrated that KV1.3 correlated strongly with expression levels of TNF-α and IL-17A suggesting a link between the function of the channels and the syntheses of these two cytokines in particularly TH (CD4+) cells. But in keeping with the presence of the channel in a subset of TC (CD8+) cells we cannot exclude that the channel may also drive cytotoxic functions of TC (CD8+) cells. While CD8-mRNA expression was significantly increased almost 3-fold, surprisingly, the 1.5-fold difference in mRNA expression of CD4 between the two groups did not reach statistical significance despite the fact that both CD4+ and CD8+ T cells infiltrated the mucosa of the UC patients in the present study and of those of previous studies.9,47
Moreover, our study showed increased mRNA expressions of IFN-γ, TNF-α, and IL-17A in these UC patients compared to controls, which suggests both TH1 and TH17 responses. However, it is also well established that an atypical TH2 response is involved in the pathogenesis of UC in contrast to CD, which has been suggested to be TH1 driven with IFN-γ as the major cytokine.13,15,16,18,48 Our results do not allow us to distinguish between a TH1 and a TH2 response.
The precise mechanism, by which KV1.3 channels contribute to the functions of T-cells in the inflamed mucosa of UC patients, remains unclear. However, KV1.3 probably stabilizes a negative membrane potential that in turn maintains the electrical driving force for calcium influx through store-operated calcium channels in the activated TH and TC as reported previously.21,22,49–51 KV1.3 mRNA expression also correlated with CD14 and CD16 expression. This suggests that high expression of KV1.3 in T-cells, but not in the macrophages themselves, is involved in the recruitment of macrophages into the mucosa presumably by its above mentioned control of TNF-α synthesis in T-cells. Together, the above findings support that KV1.3 modulation is affecting mechanisms up-stream of the synthesis of these inflammatory cytokines.
Regarding the other T-cell potassium channel KCa3.1, a steep up-regulation of the channel in activated T-cells (from very low basal expression levels in naïve unstimulated T-cells) is found in in vitro experimentation and is suggested to drive re-stimulated T-cell function, expansion, and migration by having a strong positive feedback on calcium influx and thus maintaining elevated intracellular calcium levels.19,52–54 Here, we found that KCa3.1 mRNA expression did not differentiate between UC and controls. This is apparently due to the constitutive expression of the channels in colonic crypts in both UC and control group, which has been described previously39,55 thus masking potential differences in T-cell expression levels between the two. Moreover, a down-regulation of KCa3.1 functions and unchanged mRNA expression has been reported to occur in crypts of UC patients.30 Together, these circumstances may explain the lack of correlation of KCa3.1 mRNA expression with clinical parameters of UC. Nonetheless, expression levels correlated positively with the expression of TNF-α, CD14 and CD16 suggesting that KCa3.1 is involved in the recruitment of monocytes, macrophages, and possibly natural killer cells to the inflammation site. Despite the fact that we did not find a difference between controls and UC patients, studies investigating the role of KCa3.1 in two mouse models and a rat model of UC showed that inhibition of KCa3.1 led to decreased inflammation, thus suggesting KCa3.1 as a potential pharmacological target.56,57
Although KV1.3 mRNA expression correlated well with disease activity in our UC cohort, we did not find significant correlations between either of the two potassium channels and “days to relapse” from the inclusion date or after remission was achieved. This indicates that in this small patient cohort and the relatively short follow-up period (range: 4 to 26 months) the observed expression levels of the channel are not indicative of relapse probability. Nonetheless, we observed a trend (p = 0.08) towards a negative correlation of KCa3.1 expression and “days to relapse”, which could indicate that high expression of KCa3.1 increases relapse probability. However, longer follow-up intervals and more patient data are needed to clarify whether channel expression levels are of potential prognostic value.
In conclusion, the present study identified, for the first time, KV1.3 in T-cell infiltrates in active UC as a novel molecular marker of disease activity and more interestingly, showed that KV1.3 and to some extent also KCa3.1 play a role in the production of inflammatory cytokines and thus may serve as a new pharmacological target upstream of TNF-α and IL17-A for the treatment of IBD.
Supplementary Material
Acknowledgment
We would like to thank the clinical staff of Odense University Hospital, Hospital of South-West Jutland Esbjerg, and Institute of Molecular Medicine at the University of Southern Denmark, Odense. The study received funding from the Regional Research Foundation of Southern Denmark (to JK, TK, and RK), the National Institute of Health (RO1 GM076063 to HW), the Fondo de Investigación Sanitaria (Red HERACLES RD12/ 0042/0014 to RK), the Danish Colitis–Crohn Society, Institute of Regional Health Science, the Karola Jørgensen’s Research Foundation, the Edith and Vagn Hedegaard Jensens’s Foundation, the Jens Lysholdts Eftf. Ltd, the Johannes M. Klein and Spouse’s Foundation, the A.P. Møller Foundation for the Advancements in Medical Science, the A.J. Andersen and Spouse’s Foundation, and the Foundation of CEO Jacob Madsen and his spouse Olga Madsen (to LKH). DL received a studentship from the Vibeke Binder and Povl Riis’s Foundation.
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.crohns.2014.04.003.
Footnotes
Conference presentation: Part of this data was presented at the 8th Congress of ECCO, Vienna, Austria, February 2013.
Conflict of interest
None.
Statement of authorship: LKH, TPH, TK, JK, HW, and RK designed the study. LKH, HW, TK, JK, and RK analyzed and interpreted the data. LKH, DL, LSM, HW, and MR conducted experiments. LKH, LK, TK, and JK collected human biopsies. TP performed all pathology assessments. LKH, HW, and RK drafted the manuscript. All authors took active part in revising the manuscript and all authors read and approved the final manuscript.
Contributor Information
Lars Koch Hansen, Email: larskochhansen@dadlnet.dk.
Linda Sevelsted-Møller, Email: lmoeller@health.sdu.dk.
Maj Rabjerg, Email: missrabjerg@gmail.com.
Dorte Larsen, Email: larsen.dorte@gmail.com.
Tine Plato Hansen, Email: tine.hansen@rsyd.dk.
Lone Klinge, Email: klinge@dadlnet.dk.
Heike Wulff, Email: hwulff@ucdavis.edu.
Torben Knudsen, Email: torben.knudsen@rsyd.dk.
Jens Kjeldsen, Email: jens.kjeldsen@rsyd.dk.
Ralf Köhler, Email: rkohler.iacs@aragon.es.
References
- 1.Sheth SG, LaMont JT. Toxic megacolon. Lancet. 1998;351(9101):509–513. doi: 10.1016/S0140-6736(97)10475-5. [DOI] [PubMed] [Google Scholar]
- 2.Neuman MG, Nanau RM. Inflammatory bowel disease: role of diet, microbiota, life style. Transl Res. 2012;160(1):29–44. doi: 10.1016/j.trsl.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Lopez-Serrano P, Perez-Calle JL, Perez-Fernandez MT, Fernandez-Font JM, Boixeda de Miguel D, Fernandez-Rodriguez CM. Environmental risk factors in inflammatory bowel diseases. Investigating the hygiene hypothesis: a Spanish case-control study. Scand J Gastroenterol. 2010;45(12):1464–1471. doi: 10.3109/00365521.2010.510575. [DOI] [PubMed] [Google Scholar]
- 4.Porter CK, Tribble DR, Aliaga PA, Halvorson HA, Riddle MS. Infectious gastroenteritis and risk of developing inflammatory bowel disease. Gastroenterology. 2008;135(3):781–786. doi: 10.1053/j.gastro.2008.05.081. [DOI] [PubMed] [Google Scholar]
- 5.Halfvarson J, Bodin L, Tysk C, Lindberg E, Jarnerot G. Inflammatory bowel disease in a Swedish twin cohort: a long-term follow-up of concordance and clinical characteristics. Gastroenterology. 2003;124(7):1767–1773. doi: 10.1016/s0016-5085(03)00385-8. [DOI] [PubMed] [Google Scholar]
- 6.Orholm M, Binder V, Sorensen TI, Rasmussen LP, Kyvik KO. Concordance of inflammatory bowel disease among Danish twins. Resultsof a nationwide study. Scand J Gastroenterol. 2000;35(10):1075–1081. doi: 10.1080/003655200451207. [DOI] [PubMed] [Google Scholar]
- 7.Wen Z, Fiocchi C. Inflammatory bowel disease: autoimmune or immune-mediated pathogenesis? Clin Dev Immunol. 2004;11(3–4):195–204. doi: 10.1080/17402520400004201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel DD, Lee DM, Kolbinger F, Antoni C. Effect of IL-17A blockade with secukinumab in autoimmune diseases. Ann Rheum Dis. 2013;72(Suppl 2):i116–i123. doi: 10.1136/annrheumdis-2012-202371. [DOI] [PubMed] [Google Scholar]
- 9.Holmen N, Lundgren A, Lundin S, Bergin AM, Rudin A, Sjovall H, et al. Functional CD4 + CD25high regulatory T cells are enriched in the colonic mucosa of patients with active ulcerative colitis and increase with disease activity. Inflamm Bowel Dis. 2006;12(6):447–456. doi: 10.1097/00054725-200606000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Schoenborn JR, Wilson CB. Regulation of interferon-gamma during innate and adaptive immune responses. Adv Immunol. 2007;96:41–101. doi: 10.1016/S0065-2776(07)96002-2. [DOI] [PubMed] [Google Scholar]
- 11.Feghali CA, Wright TM. Cytokines in acute and chronic inflammation. Front Biosci. 1997;2:d12–d26. doi: 10.2741/a171. [DOI] [PubMed] [Google Scholar]
- 12.Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28(4):454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuss IJ, Neurath M, Boirivant M, Klein JS, de la Motte C, Strong SA, et al. Disparate CD4+ lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. Crohn’s disease LP cells manifest increased secretion of IFN-gamma, whereas ulcerative colitis LP cells manifest increased secretion of IL-5. J Immunol. 1996;157(3):1261–1270. [PubMed] [Google Scholar]
- 14.Tsukada Y, Nakamura T, Iimura M, Iizuka BE, Hayashi N. Cytokine profile in colonic mucosa of ulcerative colitis correlates with disease activity and response to granulocytapheresis. Am J Gastroenterol. 2002;97(11):2820–2828. doi: 10.1111/j.1572-0241.2002.07029.x. [DOI] [PubMed] [Google Scholar]
- 15.Ordas I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ. Ulcerative colitis. Lancet. 2012;380(9853):1606–1619. doi: 10.1016/S0140-6736(12)60150-0. [DOI] [PubMed] [Google Scholar]
- 16.Danese S, Fiocchi C. Ulcerative colitis. N Engl J Med. 2011;365(18):1713–1725. doi: 10.1056/NEJMra1102942. [DOI] [PubMed] [Google Scholar]
- 17.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 18.Fuss IJ, Heller F, Boirivant M, Leon F, Yoshida M, Fichtner-Feigl S, et al. Nonclassical CD1d–restricted NK T cells that produce IL-13 characterize an atypical Th2 response in ulcerative colitis. J Clin Invest. 2004;113(10):1490–1497. doi: 10.1172/JCI19836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cahalan MD, Chandy KG. The functional network of ion channels in T lymphocytes. Immunol Rev. 2009;231(1):59–87. doi: 10.1111/j.1600-065X.2009.00816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feske S, Prakriya M, Rao A, Lewis RS. A severe defect in CRAC Ca2+ channel activation and altered K + channel gating in T cells from immunodeficient patients. J Exp Med. 2005;202(5):651–662. doi: 10.1084/jem.20050687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaw PJ, Qu B, Hoth M, Feske S. Molecular regulation of CRAC channels and their role in lymphocyte function. Cell Mol Life Sci. 2013;70(15):2637–2656. doi: 10.1007/s00018-012-1175-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oh-hora M, Rao A. Calcium signaling in lymphocytes. Curr Opin Immunol. 2008;20(3):250–258. doi: 10.1016/j.coi.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chi V, Pennington MW, Norton RS, Tarcha EJ, Londono LM, Sims-Fahey B, et al. Development of a sea anemone toxin as an immunomodulator for therapy of autoimmune diseases. Toxicon. 2012;59(4):529–546. doi: 10.1016/j.toxicon.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rus H, Pardo CA, Hu L, Darrah E, Cudrici C, Niculescu T, et al. The voltage-gated potassium channel Kv1.3 is highly expressed on inflammatory infiltrates in multiple sclerosis brain. Proc Natl Acad Sci U S A. 2005;102(31):11094–1109. doi: 10.1073/pnas.0501770102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wulff H, Calabresi PA, Allie R, Yun S, Pennington M, Beeton C, et al. The voltage-gated Kv1.3K(+) channel in effector memory T cells as new target for MS. J Clin Invest. 2003;111(11):1703–1713. doi: 10.1172/JCI16921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beeton C, Wulff H, Standifer NE, Azam P, Mullen KM, Pennington MW, et al. Kv1.3 channels are a therapeutic target for T cell-mediated autoimmune diseases. Proc Natl Acad Sci U S A. 2006;103(46):17414–17419. doi: 10.1073/pnas.0605136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gocke AR, Lebson LA, Grishkan IV, Hu L, Nguyen HM, Whartenby KA, et al. Kv1.3 deletion biases T cells toward an immunoreg-ulatory phenotype and renders mice resistant to autoimmune encephalomyelitis. J Immunol. 2012;188(12):5877–5886. doi: 10.4049/jimmunol.1103095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hyodo T, Oda T, Kikuchi Y, Higashi K, Kushiyama T, Yamamoto K, et al. Voltage-gated potassium channel Kv1.3 blocker as a potential treatment for rat anti-glomerular basement membrane glomerulonephritis. Am J Physiol Renal Physiol. 2010;299(6):F1258–F1269. doi: 10.1152/ajprenal.00374.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wulff H, Pennington M. Targeting effector memory T-cells with Kv1.3 blockers. Curr Opin Drug Discov Devel. 2007;10(4):438–445. [PubMed] [Google Scholar]
- 30.Al-Hazza A, Linley JE, Aziz Q, Maclennan KA, Hunter M, Sandle GI. Potential role of reduced basolateral potassium (IKCa3.1) channel expression in the pathogenesis of diarrhoea in ulcerative colitis. J Pathol. 2011 doi: 10.1002/path.2994. [DOI] [PubMed] [Google Scholar]
- 31.Izu LT, McCulle SL, Ferreri-Jacobia MT, Devor DC, Duffey ME. Vasoactive intestinal peptide-stimulated Cl- secretion: activation of cAMP-dependent K+ channels. J Membr Biol. 2002;186(3):145–157. doi: 10.1007/s00232-001-0145-7. [DOI] [PubMed] [Google Scholar]
- 32.Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317(26):1625–1629. doi: 10.1056/NEJM198712243172603. [DOI] [PubMed] [Google Scholar]
- 33.Morson BC, Dawson IMP. Gastrointestinal pathology. 2nd Edition. Oxford: Blackwell Scientific; 1979. pp. 551–562. [Google Scholar]
- 34.Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55(4):611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- 35.Carpenter AE, Jones TR, Lamprecht MR, Clarke C, Kang IH, Friman O, et al. Cell Profiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 2006;7(10):R100. doi: 10.1186/gb-2006-7-10-r100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmitz A, Sankaranarayanan A, Azam P, Schmidt-Lassen K, Homerick D, Hansel W, et al. Design of PAP-1, a selective small molecule Kv1.3 blocker, for the suppression of effector memory T cells in autoimmune diseases. Mol Pharmacol. 2005;68(5):1254–1270. doi: 10.1124/mol.105.015669. [DOI] [PubMed] [Google Scholar]
- 37.Ataga KI, Stocker J. Senicapoc (ICA-17043): a potential therapy for the prevention and treatment of hemolysis-associated complications in sickle cell anemia. Expert Opin Investig Drugs. 2009;18(2):231–239. doi: 10.1517/13543780802708011. [DOI] [PubMed] [Google Scholar]
- 38.Stocker JW, De Franceschi L, McNaughton-Smith GA, Corrocher R, Beuzard Y, Brugnara C. ICA-17043, a novel Gardos channel blocker, prevents sickled red blood cell dehydration in vitro and in vivo in SAD mice. Blood. 2003;101(6):2412–2418. doi: 10.1182/blood-2002-05-1433. [DOI] [PubMed] [Google Scholar]
- 39.Matos JE, Sausbier M, Beranek G, Sausbier U, Ruth P, Leipziger J. Role of cholinergic-activated KCa1.1 (BK), KCa3.1 (SK4) and KV7.1 (KCNQ1) channels in mouse colonic Cl-secretion. Acta Physiol. 2007;189(3):251–258. doi: 10.1111/j.1748-1716.2006.01646.x. [DOI] [PubMed] [Google Scholar]
- 40.Morgan AJ, Jacob R. Ionomycin enhances Ca2+ influx by stimulating store-regulated cation entry and not by a direct action at the plasma membrane. Biochem J. 1994;300(Pt 3):665–672. doi: 10.1042/bj3000665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Toldi G, Vasarhelyi B, Kaposi A, Meszaros G, Panczel P, Hosszufalusi N, et al. Lymphocyte activation in type 1 diabetes mellitus: the increased significance of Kv1.3 potassium channels. Immunol Lett. 2010;133(1):35–41. doi: 10.1016/j.imlet.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 42.Olsen T, Rismo R, Cui G, Goll R, Christiansen I, Florholmen J. TH1 and TH17 interactions in untreated inflamed mucosa of inflammatory bowel disease, and their potential to mediate the inflammation. Cytokine. 2011;56(3):633–640. doi: 10.1016/j.cyto.2011.08.036. [DOI] [PubMed] [Google Scholar]
- 43.Akazawa A, Sakaida I, Higaki S, Kubo Y, Uchida K, Okita K. Increased expression of tumor necrosis factor-alpha messenger RNA in the intestinal mucosa of inflammatory bowel disease, particularly in patients with disease in the inactive phase. J Gastroenterol. 2002;37(5):345–353. doi: 10.1007/s005350200048. [DOI] [PubMed] [Google Scholar]
- 44.Caprioli F, Bose F, Rossi RL, Petti L, Vigano C, Ciafardini C, et al. Reduction of CD68+ macrophages and decreased IL-17 expression in intestinal mucosa of patients with inflammatory bowel disease strongly correlate with endoscopic response and mucosal healing following infliximab therapy. Inflamm Bowel Dis. 2013;19(4):729–739. doi: 10.1097/MIB.0b013e318280292b. [DOI] [PubMed] [Google Scholar]
- 45.O’Connor W, Jr, Kamanaka M, Booth CJ, Town T, Nakae S, Iwakura Y, et al. A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nat Immunol. 2009;10(6):603–609. doi: 10.1038/ni.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Azam P, Sankaranarayanan A, Homerick D, Griffey S, Wulff H. Targeting effector memory T cells with the small molecule Kv1.3 blocker PAP-1 suppresses allergic contact dermatitis. J Invest Dermatol. 2007;127(6):1419–1429. doi: 10.1038/sj.jid.5700717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matsuzaki K, Hokari R, Kato S, Tsuzuki Y, Tanaka H, Kurihara C, et al. Differential expression of CCR5 and CRTH2 on infiltrated cells in colonic mucosa of patients with ulcerative colitis. J Gastroenterol Hepatol. 2003;18(9):1081–1088. doi: 10.1046/j.1440-1746.2003.03088.x. [DOI] [PubMed] [Google Scholar]
- 48.Heller F, Florian P, Bojarski C, Richter J, Christ M, Hillenbrand B, et al. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology. 2005;129(2):550–564. doi: 10.1016/j.gastro.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 49.Calloway N, Vig M, Kinet JP, Holowka D, Baird B. Molecular clustering of STIM1 with Orai1/CRACM1 at the plasma membrane depends dynamically on depletion of Ca2+ stores and on electrostatic interactions. Mol Biol Cell. 2009;20(1):389–399. doi: 10.1091/mbc.E07-11-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arimilli S, Sharma SK, Yammani R, Reid SD, Parks GD, Alexander-Miller MA. Pivotal advance: nonfunctional lung effectors exhibit decreased calcium mobilization associated with reduced expression of ORAI1. J Leukoc Biol. 2010;87(6):977–988. doi: 10.1189/jlb.0809575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lam J, Wulff H. The lymphocyte potassium channels Kv1.3 and KCa3.1 as targets for immunosuppression. Drug Dev Res. 2011;72(7):573–584. doi: 10.1002/ddr.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kuras Z, Yun YH, Chimote AA, Neumeier L, Conforti L. KCa3.1 and TRPM7 channels at the uropod regulate migration of activated human T cells. PLoS One. 2012;7(8):e43859. doi: 10.1371/journal.pone.0043859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ghanshani S, Wulff H, Miller MJ, Rohm H, Neben A, Gutman GA, et al. Up-regulation of the IKCa1 potassium channel during T-cell activation. Molecular mechanism and functional consequences. J Biol Chem. 2000;275(47):37137–37149. doi: 10.1074/jbc.M003941200. [DOI] [PubMed] [Google Scholar]
- 54.Wulff H, Castle NA. Therapeutic potential of KCa3.1 blockers: recent advances and promising trends. Expert review of clinical. Pharmacology. 2010;3(3):385–396. doi: 10.1586/ecp.10.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Warth R, Hamm K, Bleich M, Kunzelmann K, von Hahn T, Schreiber R, et al. Molecular and functional characterization of the small Ca(2+)-regulated K + channel (rSK4) of colonic crypts. Pflugers Arch. 1999;438(4):437–444. doi: 10.1007/s004249900059. [DOI] [PubMed] [Google Scholar]
- 56.Di L, Srivastava S, Zhdanova O, Ding Y, Li Z, Wulff H, et al. Inhibition of the K+ channel KCa3.1 ameliorates T cell-mediated colitis. Proc Natl Acad Sci U S A. 2010;107(4):1541–1546. doi: 10.1073/pnas.0910133107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Strobaek D, Brown DT, Jenkins DP, Chen YJ, Coleman N, Ando Y, et al. NS6180, a new K(Ca) 3.1 channel inhibitor prevents T-cell activation and inflammation in a rat model of inflammatory bowel disease. Br J Pharmacol. 2013;168(2):432–444. doi: 10.1111/j.1476-5381.2012.02143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.