Abstract
To understand the impact of clinically relevant radiation therapy (RT) on tumor immune gene expression and to utilize the changes that occur during treatment to improve cancer treatment outcome, we examined how immune response genes are modulated in prostate cancer cells of varying p53 status. LNCaP (p53 wild-type), PC3 (p53 null) and DU145 (p53 mutant) cells received a 10 Gy single dose or 1 Gy × 10 multifractionated radiation dose to simulate hypofractionated and conventionally fractionated prostate radiotherapy. Total RNA was isolated 24 h after multi-fractionated radiation treatment and single-dose treatments and subjected to microarray analysis and later validated by RT-PCR. RT-PCR was utilized to identify total-dose inflection points for significantly upregulated genes in response to multifractionated radiation therapy. Radiation-induced damage-associated molecular pattern molecules (DAMPs) and cytokine analyses were performed using bioluminescence and ELISA. Multifractionated doses activated immune response genes more robustly than single-dose treatment, with a relatively larger number of immune genes upregulated in PC3 compared to DU145 and LNCaP cells. The inflection point of multifractionated radiation-induced immune genes in PC3 cells was observed in the range of 8–10 Gy total radiation dose. Although both multifractionated and single-dose radiation-induced proinflammatory DAMPs and positively modulated the cytokine environment, the changes were of higher magnitude with multifractionated therapy. The findings of this study together with the gene expression data suggest that cells subjected to multifractionated radiation treatment would promote productive immune cell–tumor cell interactions.
INTRODUCTION
Ionizing radiation is a standard modality of treatment for many solid tumors, with the goal of eliminating tumor cells through extensive DNA damage leading to growth arrest, apoptosis and clonogenic death (1). However, the high frequency of malignancies in immune-compromised patients supports a crucial role of the immune system in controlling tumorigenesis (2). Recent studies have emerged highlighting the importance of the immune response elicited by tumoricidal effects of radiation therapy (RT). The immune system can participate in antitumor mechanisms by eliminating transformed and premalignant cells, often observed in viral-induced cancers, which are mostly dependent on immune response stimulators such as stress or necrosis or those induced by radiation exposure (3). It has been demonstrated that melanoma mouse models release tumor antigens upon tumor cell death in response to the direct effects of radiotherapy on the tumor tissue. Antigen-presenting cells prime effector cells in the lymph nodes that travel to the tumor site and trigger malignant cell lysis (4).
Ionizing radiation triggers the release of various inflammatory cytokines, causing an overall antitumor effect on the tumor cell stroma (5). It is believed that inflammatory cytokines released from both cancer cells and non-cancer cells form a radiation-induced bystander/abscopal response, in which signals are released from irradiated cancer cells to neighboring normal cells (bystander) or to distant tumor cells (abscopal) and aid immunomodulatory response. These events are often caused by release of cytokines such as IL-6, IL-8, TGF-β1 and TNF-α, among others (6). Other studies indicate that CD8+ T cells play a role in orchestrating radiation-related therapeutic effects, when comparing tumor growth in immunocompetent versus T-cell-deficient mice (7).
Radiotherapy has the ability to make dendritic cells (DCs) capable of producing lymphocyte responses involving adaptive antitumor immune attack by taking up tumor antigens, consequently presenting them to effector T cells and thereby inhibiting tumor growth (8, 9). Moreover, recent studies have shown the use of radiotherapy in combination with Th1 cell therapies can enhance the production of cytotoxic-T-lymphocytes specific for the tumor malignancy, thereby actively participating in the regression of such cancers (10). Thus, radiation therapy can increase the T-cell response for antitumor effects, suggesting that radiation therapy has a direct link to the induction of immune modulation genes that participate in the overall immunological cascade to elicit a robust immunogenic tumor cell death (11).
Previous studies from our group demonstrated that the PC3 prostate carcinoma cell line showed a significant upregulation of immune-related genes after multifractionated treatment (12), suggesting that radiation therapy has the potential to elicit an immune response that will initiate a cascade of events leading to immunogenic antitumor effects. Corroborating evidence based on gene expression studies in prostate and breast cancers exhibited a distinctive upregulation of interferon-related genes after multifractionated therapy when compared to single-dose treatment (13). The current study was undertaken to identify the immuno-regulatory role of such differentially expressed genes in prostate cancer cells that were subjected to different radiation exposure schemes such as multifractionated or single dose. Further, multifractionated and single-dose mediated immune gene modulation in these prostate cancer cells was evaluated by analyzing the damage-associated molecular pattern molecules (DAMPs) such as HMGB1 along with evaluation of cytokines at the protein level. Overall, the data presented here suggest that although both single dose and multifractionated dose altered DAMPs and cytokine levels, in general the effect was of greater magnitude with multifractionated treatment.
MATERIALS AND METHODS
Cell Lines and Radiation Treatments
PC3, DU145 and LNCaP human prostate carcinoma cells were obtained from the American Type Culture Collection (ATCC®, Rockville, MD). PC3 cells are null for p53 function and DU145 harbors homozygous p53 mutation (14), whereas LNCaP cells contain intact p53 function (15). Cells were maintained and grown according to ATCC recommendations.
Radiation Treatment
Cells were irradiated in a PANTAK high frequency X-ray generator (Precision X-ray Inc., North Bedford, CT), operated at 300 kV and 10 MA. The dose rate was 1.6 Gy per min and this protocol was used for all experiments.
Sample Collection for Microarray Analysis
Cells were plated into T75 cm2 flasks (1–1.5 × 106 for single-dose treatment and 0.8–1 × 106 for fractionated treatment). After 24 h, cells were exposed to a total of 10 Gy, administered either as a single fraction or as a 1 Gy × 10 multifractionated treatment. These non-isoeffective doses were selected to simulate clinical hypofractionated and conventionally fractionated radiotherapy regimens. For the multifractionated protocol, cells were exposed to 1 Gy, twice a day, at 6 h intervals for 5 consecutive days. The cells were approximately 90% confluent at the time of harvesting. For both protocols, radiation-induced changes were analyzed at 24 h after the final dose of radiation. Separate controls were maintained for single-dose and multifractionated treatment protocols.
Inflection Point after Multifractionated Radiation Treatment
To investigate the number of 1 Gy fractions required to elicit a cellular adaptation response, PC3 and DU145 cells were plated into T75 cm2 flasks (1.5–2 × 105) and exposed to 1 Gy, twice a day, for a total of 1–10 fractions. RNA was collected 24 h after each of the indicated number of fractions (Fig. 1). Each fraction was compared to its own control collected at the same time.
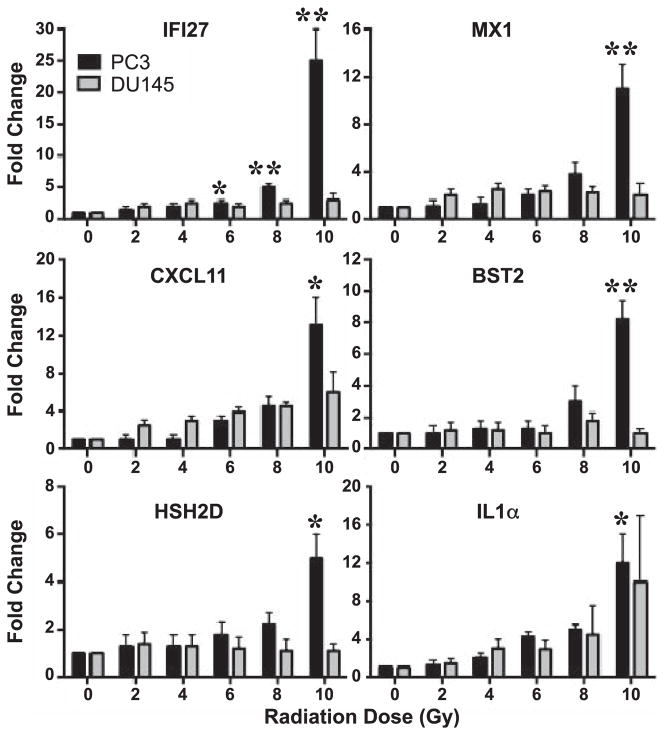
FIG. 1.
Inflection point kinetics of immune genes in multifractionated treated PC3 and DU145 cells as assessed by real-time RT-PCR. PC3 and DU145 cells were exposed to 1–10 Gy of radiation delivered as 1 Gy multifractionated treatment. Fold change in IFI27, MX1, CXCL11, BST2, HSH2D and IL1A expression 24 h after cells received 1–10 Gy of radiation was determined by RT-PCR. Data shown are fold change (AV ± SEM) of 3 biologically distinct experiments. *P < 0.05, **P < 0.01.
Cytokine Study
Cells were plated into T75 cm2 flasks (1.5–2 × 105) and after 24 h, cells were exposed to 10 Gy in multifractionated doses of 1 Gy, twice a day, for 5 days (1 Gy × 10), with 6 h between each dose. For the single dose a total of 10 Gy was administered (10 Gy × 1) during the last day of the multifractionated radiation treatment. For both single and multifractionated radiation-treated cells, culture supernatant was collected 48 and 72 h after the final radiation dose, and cells were counted using a Countess® Automated Cell Counter (cat. no. C10227, Invitrogen™ Life Technologies Inc., Grand Island, NY). Separate controls were maintained for single-dose and fractionated radiation treatment protocols.
Microarray Analysis
Microarray analysis was performed in all three cell lines using RNA isolated from three separate experiments. Cells were pelleted at 24 h after the final dose of the single or multifractionated radiation treatment and stored in liquid nitrogen. Total RNA, including small RNAs, was isolated using miRNeasy Mini Kit (cat. no. 217004, QIAGEN, Germantown, MD). The concentration and purity of total RNA was measured by spectrophotometry at OD260/280 and the quality of the total RNA sample was assessed using an Agilent Bioanalyzer with the RNA6000 Nano Lab Chip (Agilent Technologies Inc., Santa Clara, CA). The microarray analysis was done using CodeLink™ Whole Genome Bioarrays representing 55,000 probes (Applied Microarrays Inc., Tempe, AZ). CodeLink Expression Analysis software 5.0 (Applied Microarrays Inc.) was used to process the scanned images from arrays (gridding and feature intensity) and the data generated for each feature on the array were analyzed with GeneSpring® software (Agilent Technologies). Raw intensity data for each gene on every array were normalized to the median intensity of the raw values from such arrays. Data for all arrays were filtered for intensity values that were above background in at least two of any set of three replicates for any condition within each radiation-treated protocol. Unsupervised hierarchical clustering of all probes present (significantly above background detection levels) in at least one condition (control or radiation treatment) from all three replicates indicated that expression profiles clustered by biological replicate treatments, rather than by any technical condition of the experimental design. To ensure that genes were measured reliably, ANOVA was used to compare the means of each condition (n = 3). Cutoff ratios were greater than 2.0 and less than 0.5 with a P value <0.05 relative to the respective control group were selected for this study.
Real-Time RT-PCR
Immune response genes that exhibited significant differential expression patterns were further confirmed and analyzed for inflection point by RT-PCR using Taqman® Custom Express Plate (cat. no. 4413264, Applied Biosystems, Foster City, CA) and ABI PRISM® 7500 Sequence Detection System instrument equipped with the Sequence Detection Software (SDS) version 1.4. RNA (2 μg) were reverse transcribed to synthesize single-stranded cDNA using the High-Capacity cDNA Reverse Transcription Kit (cat. no. 4368814, Applied Biosystems). The threshold cycle (Ct) of the endogenous control (18S) was used to normalize target gene expression (ΔCt). The relative change in gene expression (ΔΔCt) was used for comparison of the gene expression in irradiated samples versus nonirradiated control.
Ingenuity® Pathway Analysis (IPA)
The functional significance of differentially expressed mRNAs (twofold change and P < 0.05) after single-dose and multifractionated radiation treatment was evaluated using IPA software (version 8.7–3203, Ingenuity Systems, Redwood City, CA). Data sets were uploaded into the IPA, then mapped to the functional networks available in the Ingenuity Pathway Knowledge Base and ranked by score as described previously (16).
Analysis of Radiation-Induced Damage-Associated Molecular Pattern Molecules
Prostate cancer cell lines (DU145, PC3, LNCaP) were mock irradiated (0 Gy), 1 Gy irradiated in 10 fractions or 10 Gy single-dose irradiated. Cell culture supernatants were collected after 48 and 72 h were analyzed for ATP by bioluminescence (Sigma-Aldrich LLC, St. Louis, MO) and high-mobility group box 1 (HMGB1) protein content by ELISA (IBL International, Hamburg, Germany), according to the manufacturer’s instructions.
Radiation-Induced Modulation of Tumor Cell Cytokine Production
Tumor cells (DU145, PC3, LNCaP) were mock irradiated (0 Gy), 1 Gy irradiated in 10 fractions or 10 Gy single-dose irradiated. Supernatants were analyzed after 48 and 72 h for GM-CSF, IFN-γ, IL-12p70, IL1β, IL-2, IL-6, IL-8 and TNF-α by 9-plex using the electrochemiluminescence detection system (Meso Scale Discovery®, Rockville, MD). The detection limit for all cytokines was 2.4–10,000 pg/ml.
Statistical Analysis
Tests of significance are reported as P values, derived from Student’s t test using a two-tailed distribution and calculated at 95% confidence using GraphPad Prism 4.0 for Macintosh (GraphPad Software Inc., La Jolla, CA).
Due to the specificity and sensitivity of the electrochemiluminescence assay utilized for the quantitation of cytokines, changes greater than or equal to twofold vs. control (0 Gy) were considered relevant.
RESULTS
Multifractionated Radiation Treatment Activates Immune Response Genes More Robustly than Single-Dose Radiation Treatment with a Relatively High Number of Genes in PC3 Followed by LNCaP Cells
Current radiation therapy clinical protocols encompass a wide range of radiation doses, delivered as single dose or in multiple fractions of variable size (17). There is limited understanding about the mechanism of induction of immunogenicity in tumors irradiated with low-dose or high-dose radiotherapy. This includes the impact of radiation dose as well as fractionation schedules on stimulating immunity, on immune effector function and survival. This current study builds on previous work from our laboratory (12, 16, 18) and is designed to more fully understand the immune gene induction cascade in tumor cells, in response to 10 Gy single dose or 10 Gy given in 1 Gy fractions (multifractions) in three prostate cancer cell lines, PC3, DU145 and LNCaP.
PC3 Cells
Multifractionated radiation treatment showed greater than twofold changes in 31 immune-related genes in PC3 cells 24 h after the cells were irradiated (Table 1). Out of these 31 immune-related genes, seven genes were upregulated more than 10–34-fold. These include IFI27 (34-fold), MX1 (23-fold), BST2 (22-fold), IFITM1 (18-fold), IFIT1 (12-fold), OASL (12-fold) and CXCL11 (11-fold) (Table 1). These seven highly expressed genes in response to multifractionated treatment are mainly involved in sensing viral/ pathogen infection to trigger an immune response (19–23). No immune-related genes were downregulated in PC3 cells treated with multifractionated radiation treatment. In response to 10 Gy single radiation exposure, five immune genes were also upregulated to two- to threefold (Table 1). Out of these 5, two genes, IFITMI and OASL (19, 20), were upregulated in multifractionated treatment. The remaining three genes, S100A9, FABP4 and HLA-DMA, are involved in inflammatory secretions by T cells or macrophages and peptide exchanges (24–26) and were unique for single-dose treatment. One gene, PDL-1 (CD274), which was down-regulated by twofold, is an inhibitor of T-cell receptor signaling (27). To better evaluate the immune response gene expression changes specific for each radiation protocol, the specific genes were mapped to the functional networks in the IPA database and ranked by score. Only the networks with a score of 10 or more are shown (Table 4). Out of the three cell lines, in PC3 cells fractionated radiation treatment had a greater number of networks in comparison to single-dose radiation treatment. These findings demonstrate that for PC3 cells, 1 Gy multifractionated radiation treatment is able to trigger a high incidence of activation of immune genes, potentially eliciting a more robust immune response.
TABLE 1.
List of Immune Genes Modulated Greater than Twofold in PC3 Cells 24 h after Single-Dose or Multifractionated Treatment
| 10 Gy (single dose)
|
1 Gy × 10 (multifractionated)
|
|
|---|---|---|
| Up | Down | Up |
| OASL*,§ | PDL1 | IFI27 |
| IFITM1*,¶ | MX1 | |
| S100A9 | BST2 | |
| FABP4 | IFITM1* | |
| HLA-DMA | IFIT1 | |
| OASL* | ||
| CXCL11 | ||
| IFIT2 | ||
| HSH2D | ||
| IFIT3 | ||
| DDX58 | ||
| IL1A | ||
| IFI6 | ||
| ISGF3G | ||
| PTX3 | ||
| ISG15 | ||
| CCL5 | ||
| CCL20 | ||
| CXCL5 | ||
| IRF7 | ||
| IL23A | ||
| IL32 | ||
| LY96 | ||
| IFI35 | ||
| CTSS | ||
| RELB | ||
| APOL3 | ||
| MX2 | ||
| TAPBPL | ||
| ZFP36 | ||
| PSMB9 | ||
Denotes genes that are commonly upregulated in both single-dose and multifractionated treatment.
Upregulated in both PC3 and LNCaP treated with multifractionated radiation and DU145 treated with single dose radiation.
Upregulated in PC3 treated with multifractionated radiation but downregulated in LNCaP treated with single-dose radiation.
TABLE 4.
Networks and Associated Functional Categories Identified by IPA for PC3, DU 145 and LNCaP Cells 24 h after Exposure to 10 Gy of Radiation Administered as a Single-Dose or Multifractionated Regimen (1 Gy × 10)
| Cell type | Treatment (24 h) | Score | Functions (number of genes in the category implicated) |
|---|---|---|---|
| PC3 | Single dose | 18 | Connective tissue disorders, inflammatory disease, skeletal and muscular disorders (6) |
| Multifractionated | 57 | Infectious disease, dermatological diseases and conditions, antimicrobial response (21) | |
| 17 | Hematological system development and function, tissue morphology, inflammatory response (8) | ||
| 10 | Cell-to-cell signaling and interaction, metabolic disease, infectious disease (5) | ||
| DU145 | Single dose | 21 | Cellular development, hematological system development and function, hematopoiesis (7) |
| Multifractionated | 12 | Infectious disease, antimicrobial response, inflammatory response (4) | |
| LNCaP | Single dose | 23 | Cellular development, cellular growth and proliferation, hematological system development and function (8) |
| Multifractionated | 27 | Cellular development, hematological system development and function, hematopoiesis (10) | |
| 15 | Infectious disease, carbohydrate metabolism, lipid metabolism (6) |
Notes. IPA score refers to statistical significance, with all immune response genes with at least a twofold change and P < 0.05, which are mapped to the functional networks available in the Ingenuity Pathway Knowledge Base. Focus molecules indicate the number of genes that could be mapped to molecules out of a possible 35 molecules in each network.
DU145 Cells
Multifractionated radiation treatment of DU145 cells resulted in greater than twofold upregulation in four genes (Table 2) that include IL29, HSH2D and IFI16 (28, 29), which are important for antitumor T-cell activation. Whereas PDL1, an inhibitor of T-cell receptor signaling (27), was upregulated, which indicated that both activation as well as inactivation of immune response exist in multifractionated radiation treatment. Interestingly, PDL1 was downregulated to single-dose radiation treatment in PC3 cells. Single-dose radiation treatment of DU145 cells caused a greater than twofold increase in seven genes, three of which were unique to single-dose treatment compared to multifractionated treatment. These include OASL, AZU1 and APOBEC3G (Table 2). OASL and AZU1 are directly involved in immune activation (20), whereas APOBEC3G promotes DNA repair (30). IFIT1 and IFIT3 are robust activators of immune response (31), whereas HDAC5 regulates the expression of oncoproteins of T-cell leukemia virus (32). Together, in DU145 cell type, IPA findings (Table 4) indicated that 10 Gy single-dose treatment had a response similar to an immunological regulation of hematological function and hematopoiesis; whereas multi-fractionated treatment response was similar to an antimicrobial and inflammatory response.
TABLE 2.
List of Immune Genes with Greater than Twofold Upregulation in DU145 Cells Treated with Single-Dose or Multifractionated Radiation
| 10 Gy (single dose) | 1 Gy × 10 (multifractionated) |
|---|---|
| CFB | IL29 |
| OASL*,§ | HSH2D |
| APOBEC3G | IFI16 |
| AZU1 | PDL1 |
| IFIT3 | |
| IFIT1 | |
| HDAC5 |
Denotes genes that are commonly upregulated in both single-dose and multifractionated treatment.
Upregulated in both PC3 and LNCaP treated with multifractionated radiation and DU145 treated with single-dose radiation. None were downregulated.
LNCaP Cells
Interestingly, functional p53 harboring LNCaP cells responded differently to single-dose treatment when compared to PC3 and DU145 cells, by downregulation of a greater number of immune-related genes. In response to multifractionated treatment, LNCaP cells increased expression of 12 genes (Table 3). Of notable, increased expression was observed in IFIT1, IFIT3, OASL, LY96, PLA2G4C, GBP3, CFB, EFNB1 and NM1 (19, 20, 33–37). One gene, FCGRT, which was downregulated by twofold, is involved in regulation of pharmacokinetics of therapeutic antibodies (38) and this may have implications particularly when combining a multifractionated treatment schedule with radio-immunotherapy. In response to the 10 Gy single-dose treatment, five genes were significantly downregulated and three genes were upregulated. It is clearly evident from IPA findings (Table 4) that 1 Gy multifractionated treatment has higher potency for eliciting a pathogenic immune response similar to immune regulation of hematopoiesis coupled with infectious states, and that infectious disease immune response was absent in single-dose treated LNCaP cells.
TABLE 3.
List of Immune Genes with Greater than Twofold Changes in LNCaP Cells Treated with Single-Dose or Multifractionated Radiation
| 10 Gy (single dose)
|
1 Gy × 10 (multifractionated)
|
||
|---|---|---|---|
| Up | Down | Up | Down |
| TRIM22 | PRG3 | CFB | FCGRT |
| C4B | CKLF | IFI27 | |
| TNFRSF14 | IFITM1¶ | OASL*,§ | |
| TCF7 | MAF | ||
| EXO1 | LY96 | ||
| NMI | |||
| PLA2G4C | |||
| GBP3 | |||
| IFIT3 | |||
| LGALS3BP | |||
| IFIT1 | |||
| EFNB1 | |||
Denotes genes that are commonly upregulated in both single-dose and multifractionated treatment.
Upregulated in both PC3 and LNCaP treated with multifractionated radiation and DU145 treated with single-dose radiation.
Upregulated in PC3 treated with multifractionated radiation but downregulated in LNCaP treated with single-dose radiation.
Inflection Point of Multifractionated Radiation Treatment-Induced Immune Genes in PC3 Cells were Observed in the Range of 8–10 Gy Total Dose of Radiation
Figure 1 shows the kinetics of induction of genes in response to 1 Gy fractionated treatment in PC3 and DU145 cells. Highly expressed genes from the microarray data were selected for inflection point analysis, and included IFI27, MX1, CXCL11, BST2, HSH2D and IL1α. In PC3 cells, IFI27, MX1, BST2 and HSH2D showed an increasing trend in gene expression at a total dose of 8 Gy and were significantly upregulated at a total of 10 Gy (Fig. 1), whereas CXCL11 and IL1α started to increase expression from 6 Gy total dose treatment. In DU145 cells, IFI27, MX1, BST2 and HSH2D failed to show any significant increase at any total radiation dose point (Fig. 1). Although CXCL11 and IL1α levels were increased at 10 Gy total dose, the increase was not statistically significant. Overall, these findings suggest that multifractionated treatment is effective in inducing critical immune function genes, particularly after adaptation at around 6–10 Gy total dose (>10 Gy was not analyzed in this study). Hence, this inflection point window could potentially be utilized for adjuvant immunotherapy agents.
Radiation Treatment Induces Proinflammatory DAMPs and Positively Modulates the Cytokine Environment
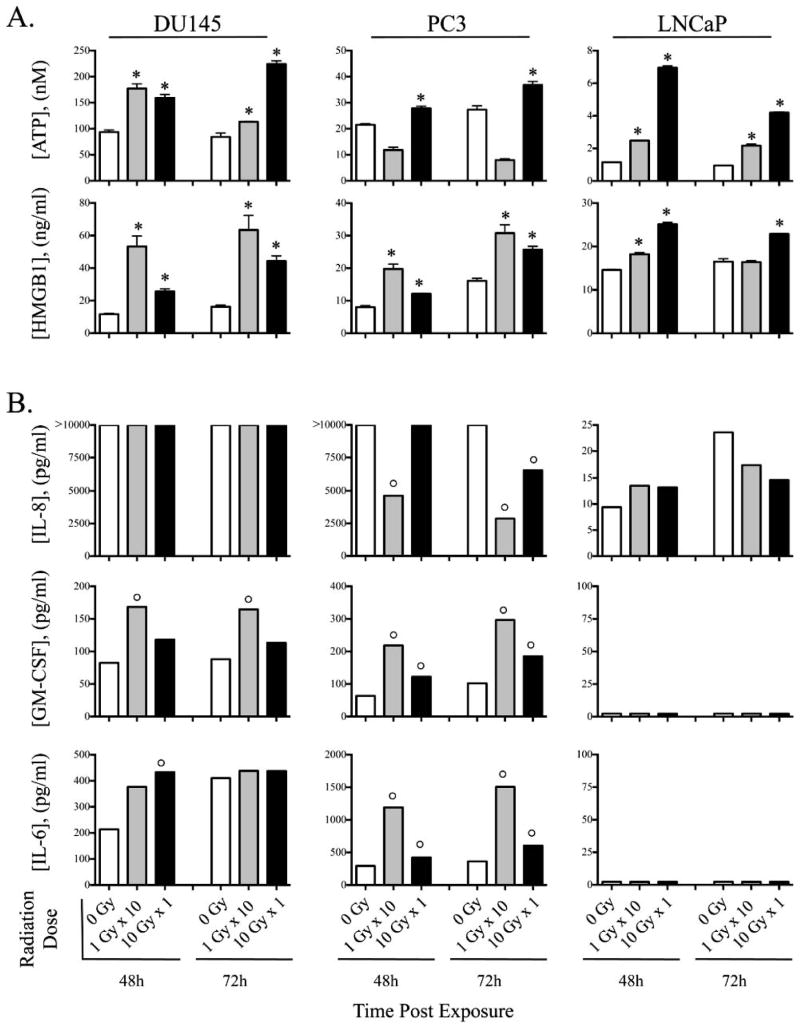
Tumor exposure to diverse sublethal doses of radiation has been demonstrated to induce immunogenic modulation in a wide variety of carcinoma types by altering the biology of surviving tumor cells to render them more susceptible to T-cell-mediated killing (39, 40). Having observed induction of genes that function to regulate immune activation, in this study it is pivotal to ascertain which immunogenic cell death events occur in response to single-dose or multi-fractionated treatment. The cardinal signs of immunogenic cell death include DAMPs, also know as danger-associated molecular pattern molecules, which can initiate and perpetuate immune responses. DAMPs include: 1. release of ATP, and 2. secretion of high-mobility group box 1 protein (HMGB1) (41). To examine the biologic significance of multifractionated vs. single-dose exposure observed as per gene expression analysis (Tables 1–3), we first sought to examine in vitro the effect of radiation as fractionated dose or single dose on the induction of cardinal DAMPs in human prostate carcinoma cell lines, DU145, PC3 and LNCaP. Cells were mock irradiated (0 Gy) or 10 Gy irradiated as either fractionated (1 Gy × 10, multi-fractionated) or single dose (10 Gy). Exposure of cells to 10 Gy single-dose treatment was sufficient to promote significant ATP release in all three prostate cell lines, while exposure to multifractionated treatment elicited significant ATP release in DU145 and LNCaP (Fig. 2A). Interestingly, exposure of DU145 and PC3 to multifractionated radiation treatment induced a greater level of HMGB1 than an equivalent dose delivered as a single fraction (Fig. 2A). These trends were seen at both 48 and 72 h after radiation exposure. Additionally the endogenous cytokine levels of 9 cytokines were analyzed after radiation exposure. No changes were seen in the IL-8 expression from DU145 cells after radiation exposure. PC3 cells exposed to the 10 Gy multifractionated treatment significantly decreased the endogenous expression of IL-8 while exposure of these cells to 10 Gy single-dose radiation treatment failed to lower the expression of this cytokine at 48 h (Fig. 2B). At 72 h post exposure, both single-dose and multifractionated treatments reduced expression of IL-8 in PC3 cells. Multifractionated and single-dose treatment induced increased levels of GM-CSF in both DU145 and PC3 cells, however, the multi-fractionated treatment induced greater levels of GM-CSF than single-dose treatment (Fig. 2B). Similarly, IL-6 was induced in DU145 and PC3 lines after exposure to radiation, with multifractionated treatment in PC3 cells inducing much greater levels of IL-6 than an equivalent single dose (Fig. 2B). Similar results were seen at 48 and 72 h after irradiation. There were no differences seen postirradiation for these three prostate cell lines for the cytokines for IFNγ, IL-12p70, IL1β, IL-2 and TNF-α. Taken together, these results indicate that exposure to radiation causes proinflammatory DAMPs and positively modulates the cytokine environment, particularly downregulation of IL-8, which can lead to curtailing of the pro-immunogenic tumor growth events.
FIG. 2.
Radiation induces proinflammatory DAMPs and positively modulates the cytokine environment. Prostate carcinoma cells (DU145, PC3, LNCaP) were exposed to 10 Gy fractionated treatment (1 Gy × 10, multifractionated, gray bars) or 10 Gy single-dose treatment (10 Gy, single dose, black bars) or were mock irradiated (0 Gy, open bars). Culture supernatant was analyzed in triplicates after 48 and 72 h. Panel A: secreted DAMPs ATP and HMGB1, and panel B: modulation of tumor derived cytokines IL-8, GM-CSF and IL-6. *Denotes statistical significance vs. control (0 Gy). °Denotes marked modulation (≥twofold) relative controls (0 Gy).
DISCUSSION
We hypothesized that radiation exposure (either multi-fractionated or single dose) would modulate the tumor secretome in a manner that would potentially promote productive immune cell–tumor cell interactions. DAMPs are molecules that can initiate and perpetuate immune responses.
Gene expression studies clearly demonstrated that multi-fractionated treatment modulates genes directly involved in the activation of the immune system, similar to a response to a pathogenic infection irrespective of cell lines with different genotype. The following pivotal genes support the proposal that 1 Gy fractions are highly immunogenic in its effect: BST-2 functions not only as an effector of the interferon-induced antiviral response but also as a negative feedback regulator of interferon production by plasmacytoid dendritic cells (23); DDX58 functions as a pattern recognition receptor (42); ISG15 is a type I interferon (43); IFI6 is IFN-inducible protein that recognizes dsDNA (44); IL23A specifically acts on memory CD4+T cells (45) to enhance T-cell priming that stimulates the production of proinflammatory cytokines; HSH2D is an important target involved in the T-cell activation (28). IL29 is implicated in antiviral activity, anti-proliferative activity and in vivo antitumor activity (29); IFIT1, IFIT3 and OASL (19, 20) are genes that respond to viral infection to activate several components of immune system; LY96 (also called as MD2) is required for activating TLR4 signaling as a part of innate immune activation against bacterial infection (33); PLA2G4C is an enzyme induced in response to cytokine induction cascade as well as inflammation (34); guanylate-binding proteins (GBPs) belong to the family of large GTPases that are induced in response to interferons, specifically, hGBP-3 possess anti-influenza viral activity (35); CFB acts as a downstream effector of TLR signaling and plays a critical role in the pathogenesis of severe bacterial sepsis (36); Efnb1 proteins have been reported to regulate thymocyte development, peripheral T-cell differentiation and antiviral immune responses and are essential for interleukin-6 (IL-6) signaling (37) and NMI augments STAT-mediated transcription in response to IL6 and IFNγ cytokines (46).
The functions of the above genes, which were modulated predominantly in response to multifractionated treatment in this study, strongly indicate that 1 Gy dose fractions have the potency to induce a variety of immune regulatory genes in these tumor cells that will facilitate the recognition by the host immune machinery. It has been well documented that during total-body irradiation, doses in the range of 0.01–0.25 Gy fractions lead to immune stimulation that can produce high remission rates (47) in hematological malignancies, and therefore, the host immune system appears to play an important role in positive clinical outcomes.
The findings of the current study demonstrate the activation of immune response genes in tumor cells exposed to 1 Gy fractions, four times higher than low-dose total-body irradiation used for the treatment of chronic lymphocytic leukemia and non-Hodgkin lymphoma (0.1–0.25 Gy), suggests that solid tumors (unlike lymphoid malignancies) may need a higher threshold total dose to activate its own immunogenic environment. In such a scenario, multifractionated treatment can be effectively combined with immunotherapy to harness the immunogenic induction programs intratumorally, and may also produce an abscopal effect. The sequence of combination therapy with immune modulating agents can potentially be utilized at a cumulative tumor dose of 6–8 Gy in multifractionated treatment settings based on the observation of an inflection point in PC3 and DU145 cells.
The gene expression data revealed that 10 Gy single-dose treatment was generally less pro-immunogenic than 1 Gy multifractionated treatment as very few genes were classified by IPA as being related to lymphocyte development or infectious disease, and also because overall multifractionated treatment modulated more immune genes than single-dose treatment. To interpret the functional impact of the changes observed it is important to keep in mind that: IFIT1 and IFIT3 are both implicated to function against viral infections (19); OASL functions in antiviral response (20); AZU1 serves as an important mediator during the initiation of the immune response (30); and APOBEC3G promotes cytidine deaminase-dependent DNA repair to render radiation resistance in lymphoma (48). In addition, IFN-inducible IFITM proteins (IFITM1, 2 and 3) inhibit the replication of various viruses including HIV-1 (21). Therefore, functions of the single-dose treatment gene signature support the fact that few genes are implicated in eliciting an immune response that could possibly be exploited with immunotherapy or vaccination to harness the tumor cell mediated immunogenic events.
The most notable inference from the microarray findings is the downregulation of PDL-1 in response to single-dose radiation treatment in PC3 cells (Table 1). Recently, we reported the downregulation of PDL-1 protein in both PC3 and DU145 cells treated with a 10 Gy single-dose treatment (49). At the RNA level in PC3 cells, this was similar to what we reported at the protein level in PC3 cells, however, we did not observe downregulation of PDL-1 in DU145. On the contrary, we found that DU145 cells showed upregulation of PDL-1 in response to multifractionated treatment (Table 2). These observations suggest that combining anti-PDL1 immunotherapy with single-dose treatment will be more effective than combining with multifractionated treatment.
The above gene expression findings were further tested to compare how certain cardinal immunogenic responses differed between multifractionated vs. single dose by functional assays. DAMPs have been shown to stimulate dendritic cells to facilitate the presentation of tumor antigens to the immune system (50). ATP binds purinergic PRX7 receptors on DCs, further supporting T-cell activation (7, 51). HMGB1, a nonhistone chromatin binding protein, promotes DC maturation through TLR-4 signaling (7). We found that irradiation of tumor cells induced these DAMPs (Fig. 2A). Also playing a role in DC function, GM-CSF and IL-6 have a well-defined role in the recruitment and maturation of DC (52, 53). We found that although both multifractionated and single-dose treatment induced elevated levels of GM-CSF, multifractionated treatment induced the greatest level of GM-CSF in PC3 but LNCaP had lower levels. Similar results were seen with radiation-induced secretion of IL-6 (Fig. 2B). Finally, a strong correlation has been observed between the metastatic phenotype of a cell and its IL-8 expression, suggesting a role for IL-8 in promoting the metastatic potential of tumor cells (54). We found that in PC3 cells, multifractionated treatment was more effective in reducing the levels of IL-8 (Fig. 2B). In summary, the analysis of DAMPs and cytokines together with the gene expression data suggest that cells subjected to multifractionated radiation treatment could indeed promote productive immune cell–tumor cell interactions.
Several preclinical studies have demonstrated that ionizing radiation induces pro-immunogenic and inflammatory changes in vivo and that the response varies with the size of the dose (55–57). In B16/OVA murine melanoma model, a 15 Gy single-dose treatment resulted in greater numbers of host immune cells infiltrating tumors than a 5 × 3 Gy fractionated-dose treatment (55). In the same B16/ OVA model radiation doses of 7.5 Gy/fraction gave the best tumor immune activation compared to a 15 Gy single-dose treatment (56). Similarly, in terms of fractionation our previous in vitro multifractionated radiation treatment studies have shown that even 1 Gy fractions differentially expressed immune response genes in prostate carcinoma cells (12). In an earlier study, DU145 xenografts revealed very different gene expression patterns compared to the DU145 cells irradiated in vitro (13). Radiation-induced gene expression changes in tumor cells can vary depending on whether the cells are grown in in vitro or in vivo settings suggesting the importance of tumor microenvironment and tumor-host interaction in radiation-induced gene expression changes.
Much work remains to be done and studies currently in progress include additional radiation fractionation schemes with a broader range of cell types including cells with normal and dysfunctional p53, as well as in vivo studies. In addition, investigations are underway of the induction of susceptibility to molecular targeted therapy after fractionated radiation treatment using the wide range of available molecular therapeutics. Exploiting the phenotype of cells surviving fractionated treatment offers great potential for combined modality therapy to both enhance tumor cell killing by immunological approaches and to reduce normal tissue toxicity. This approach could not only improve outcomes but provide additional benefit from the investment in molecular imaging, radiation therapy technology and drug development.
Acknowledgments
This study was supported by the NIH Intramural Research Program, National Cancer Institute, Center for Cancer Research. The authors would like to thank Momodou Jammeh for excellent technical assistance and Orieta Celiku, Radiation Oncology Branch of the NCI, for the help with statistical analysis.
References
- 1.Rupnow BA, Knox SJ. The role of radiation-induced apoptosis as a determinant of tumor responses to radiation therapy. Apoptosis. 1999;4:115–43. doi: 10.1023/a:1009675028784. [DOI] [PubMed] [Google Scholar]
- 2.Mueller N. Overview of the epidemiology of malignancy in immune deficiency. J Acquir Immune Defic Syndr. 1999;1:21(Suppl 1):S5–10. [PubMed] [Google Scholar]
- 3.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–99. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerber SA, Sedlacek AL, Cron KR, Murphy SP, Frelinger JG, Lord EM. IFN-gamma mediates the antitumor effects of radiation therapy in a murine colon tumor. Am J Pathol. 2013;182:2345–54. doi: 10.1016/j.ajpath.2013.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chargari C, Clemenson C, Martins I, Perfettini JL, Deutsch E. Understanding the functions of tumor stroma in resistance to ionizing radiation: emerging targets for pharmacological modulation. Drug Resist Updat. 2013;16:10–21. doi: 10.1016/j.drup.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Prise KM, O’Sullivan JM. Radiation-induced bystander signalling in cancer therapy. Nat Rev Cancer. 2009;9:351–60. doi: 10.1038/nrc2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–9. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 8.Liao YP, Wang CC, Butterfield LH, Economou JS, Ribas A, Meng WS, et al. Ionizing radiation affects human MART-1 melanoma antigen processing and presentation by dendritic cells. J Immunol. 2004;173:2462–9. doi: 10.4049/jimmunol.173.4.2462. [DOI] [PubMed] [Google Scholar]
- 9.Hatfield P, Merrick A, Harrington K, Vile R, Bateman A, Selby P, et al. Radiation-induced cell death and dendritic cells: potential for cancer immunotherapy? Clin Oncol (R Coll Radiol) 2005;17:1–11. doi: 10.1016/j.clon.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 10.Takeshima T, Chamoto K, Wakita D, Ohkuri T, Togashi Y, Shirato H, et al. Local radiation therapy inhibits tumor growth through the generation of tumor-specific CTL: its potentiation by combination with Th1 cell therapy. Cancer Res. 2010;70:2697–706. doi: 10.1158/0008-5472.CAN-09-2982. [DOI] [PubMed] [Google Scholar]
- 11.Burnette B, Fu YX, Weichselbaum RR. The confluence of radiotherapy and immunotherapy. Front Oncol. 2012;2:143. doi: 10.3389/fonc.2012.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.John-Aryankalayil M, Palayoor ST, Cerna D, Simone CB, 2nd, Falduto MT, Magnuson SR, et al. Fractionated radiation therapy can induce a molecular profile for therapeutic targeting. Radiat Res. 2010;174:446–58. doi: 10.1667/RR2105.1. [DOI] [PubMed] [Google Scholar]
- 13.Tsai MH, Cook JA, Chandramouli GV, DeGraff W, Yan H, Zhao S, et al. Gene expression profiling of breast, prostate, and glioma cells following single versus fractionated doses of radiation. Cancer Res. 2007;67:3845–52. doi: 10.1158/0008-5472.CAN-06-4250. [DOI] [PubMed] [Google Scholar]
- 14.Isaacs WB, Carter BS, Ewing CM. Wild-type p53 suppresses growth of human prostate cancer cells containing mutant p53 alleles. Cancer Res. 1991;51:4716–20. [PubMed] [Google Scholar]
- 15.Liu C, Zhu Y, Lou W, Nadiminty N, Chen X, Zhou Q, et al. Functional p53 determines docetaxel sensitivity in prostate cancer cells. Prostate. 2013;73:418–27. doi: 10.1002/pros.22583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.John-Aryankalayil M, Palayoor ST, Makinde AY, Cerna D, Simone CB, 2nd, Falduto MT, et al. Fractionated radiation alters oncomir and tumor suppressor miRNAs in human prostate cancer cells. Radiat Res. 2012;178:105–17. doi: 10.1667/rr2703.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mantel F, Flentje M, Guckenberger M. Stereotactic body radiation therapy in the re-irradiation situation–a review. Radiat Oncol. 2013;8:7. doi: 10.1186/1748-717X-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simone CB, 2nd, John-Aryankalayil M, Palayoor ST, Makinde AY, Cerna D, Falduto MT, et al. mRNA Expression Profiles for Prostate Cancer following Fractionated Irradiation Are Influenced by p53 Status. Transl Oncol. 2013;6:573–85. doi: 10.1593/tlo.13241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diamond MS, Farzan M. The broad-spectrum antiviral functions of IFIT and IFITM proteins. Nat Rev Immunol. 2013;13:46–57. doi: 10.1038/nri3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marques J, Anwar J, Eskildsen-Larsen S, Rebouillat D, Paludan SR, Sen G, et al. The p59 oligoadenylate synthetase-like protein possesses antiviral activity that requires the C-terminal ubiquitin-like domain. J Gen Virol. 2008;89:2767–72. doi: 10.1099/vir.0.2008/003558-0. [DOI] [PubMed] [Google Scholar]
- 21.Chutiwitoonchai N, Hiyoshi M, Hiyoshi-Yoshidomi Y, Hashimoto M, Tokunaga K, Suzu S. Characteristics of IFITM, the newly identified IFN-inducible anti-HIV-1 family proteins. Microbes Infect. 2013;15:280–90. doi: 10.1016/j.micinf.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goetschy JF, Zeller H, Content J, Horisberger MA. Regulation of the interferon-inducible IFI-78K gene, the human equivalent of the murine Mx gene, by interferons, double-stranded RNA, certain cytokines, and viruses. J Virol. 1989;63:2616–22. doi: 10.1128/jvi.63.6.2616-2622.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tokarev A, Skasko M, Fitzpatrick K, Guatelli J. Antiviral activity of the interferon-induced cellular protein BST-2/tetherin. AIDS Res Hum Retroviruses. 2009;25:1197–210. doi: 10.1089/aid.2009.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roth J, Vogl T, Sorg C, Sunderkotter C. Phagocyte-specific S100 proteins: a novel group of proinflammatory molecules. Trends Immunol. 2003;24:155–8. doi: 10.1016/s1471-4906(03)00062-0. [DOI] [PubMed] [Google Scholar]
- 25.Furuhashi M, Fucho R, Gorgun CZ, Tuncman G, Cao H, Hotamisligil GS. Adipocyte/macrophage fatty acid-binding proteins contribute to metabolic deterioration through actions in both macrophages and adipocytes in mice. J Clin Invest. 2008;118:2640–50. doi: 10.1172/JCI34750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Painter CA, Negroni MP, Kellersberger KA, Zavala-Ruiz Z, Evans JE, Stern LJ. Conformational lability in the class II MHC 310 helix and adjacent extended strand dictate HLA-DM susceptibility and peptide exchange. Proc Natl Acad Sci U S A. 2011;108:19329–34. doi: 10.1073/pnas.1108074108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santos J, Gonzalez-Sanchez L, Villa-Morales M, Ors I, Lopez-Nieva P, Vaquero C, et al. The stromal gene encoding the CD274 antigen as a genetic modifier controlling survival of mice with gamma-radiation-induced T-cell lymphoblastic lymphomas. Oncogene. 2010;29:5265–73. doi: 10.1038/onc.2010.280. [DOI] [PubMed] [Google Scholar]
- 28.Greene TA, Powell P, Nzerem C, Shapiro MJ, Shapiro VS. Cloning and characterization of ALX, an adaptor downstream of CD28. J Biol Chem. 2003;278:45128–34. doi: 10.1074/jbc.M306283200. [DOI] [PubMed] [Google Scholar]
- 29.Uze G, Monneron D. IL-28 and IL-29: newcomers to the interferon family. Biochimie. 2007;89:729–34. doi: 10.1016/j.biochi.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 30.Soehnlein O, Lindbom L. Neutrophil-derived azurocidin alarms the immune system. J Leukoc Biol. 2009;85:344–51. doi: 10.1189/jlb.0808495. [DOI] [PubMed] [Google Scholar]
- 31.Zhou X, Michal JJ, Zhang L, Ding B, Lunney JK, Liu B, et al. Interferon induced IFIT family genes in host antiviral defense. Int J Biol Sci. 2013;9:200–8. doi: 10.7150/ijbs.5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lodewick J, Sampaio C, Boxus M, Rinaldi AS, Coulonval K, Willems L, et al. Acetylation at lysine 346 controls the transforming activity of the HTLV-1 Tax oncoprotein in the Rat-1 fibroblast model. Retrovirology. 2013;10:75. doi: 10.1186/1742-4690-10-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller SI, Ernst RK, Bader MW. LPS, TLR4 and infectious disease diversity. Nat Rev Microbiol. 2005;3:36–46. doi: 10.1038/nrmicro1068. [DOI] [PubMed] [Google Scholar]
- 34.Lindbom J, Ljungman AG, Lindahl M, Tagesson C. Increased gene expression of novel cytosolic and secretory phospholipase A(2) types in human airway epithelial cells induced by tumor necrosis factor-alpha and IFN-gamma. J Interferon Cytokine Res. 2002 Sep;22(9):947–55. doi: 10.1089/10799900260286650. [DOI] [PubMed] [Google Scholar]
- 35.Nordmann A, Wixler L, Boergeling Y, Wixler V, Ludwig S. A new splice variant of the human guanylate-binding protein 3 mediates anti-influenza activity through inhibition of viral transcription and replication. FASEB. 2012;26:1290–300. doi: 10.1096/fj.11-189886. [DOI] [PubMed] [Google Scholar]
- 36.Zou L, Feng Y, Li Y, Zhang M, Chen C, Cai J, et al. Complement factor B is the downstream effector of TLRs and plays an important role in a mouse model of severe sepsis. J Immunol. 2013;191:5625–35. doi: 10.4049/jimmunol.1301903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luo H, Charpentier T, Wang X, Qi S, Han B, Wu T, et al. Efnb1 and Efnb2 proteins regulate thymocyte development, peripheral T cell differentiation, and antiviral immune responses and are essential for interleukin-6 (IL-6) signaling. J Biol Chem. 2011;286:41135–52. doi: 10.1074/jbc.M111.302596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Passot C, Azzopardi N, Renault S, Baroukh N, Arnoult C, Ohresser M, et al. Influence of FCGRT gene polymorphisms on pharmacokinetics of therapeutic antibodies. MAbs. 2013;5:614–9. doi: 10.4161/mabs.24815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chakraborty M, Abrams SI, Coleman CN, Camphausen K, Schlom J, Hodge JW. External beam radiation of tumors alters phenotype of tumor cells to render them susceptible to vaccine-mediated T-cell killing. Cancer Res. 2004;64:4328–37. doi: 10.1158/0008-5472.CAN-04-0073. [DOI] [PubMed] [Google Scholar]
- 40.Chakraborty M, Wansley EK, Carrasquillo JA, Yu S, Paik CH, Camphausen K, et al. The use of chelated radionuclide (samarium-153-ethylenediaminetetramethylenephosphonate) to modulate phenotype of tumor cells and enhance T cell-mediated killing. Clin Cancer Res. 2008;14:4241–9. doi: 10.1158/1078-0432.CCR-08-0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kepp O, Galluzzi L, Martins I, Schlemmer F, Adjemian S, Michaud M, et al. Molecular determinants of immunogenic cell death elicited by anticancer chemotherapy. Cancer Metastasis Rev. 2011;30:61–9. doi: 10.1007/s10555-011-9273-4. [DOI] [PubMed] [Google Scholar]
- 42.Hou F, Sun L, Zheng H, Skaug B, Jiang QX, Chen ZJ. MAVS forms functional prion-like aggregates to activate and propagate antiviral innate immune response. Cell. 2011;146:448–61. doi: 10.1016/j.cell.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matsumoto M, Tanaka N, Harada H, Kimura T, Yokochi T, Kitagawa M, et al. Activation of the transcription factor ISGF3 by interferon-gamma. Biol Chem. 1999;380:699–703. doi: 10.1515/BC.1999.087. [DOI] [PubMed] [Google Scholar]
- 44.Morrone SR, Wang T, Constantoulakis LM, Hooy RM, Delannoy MJ, Sohn J. Cooperative assembly of IFI16 filaments on dsDNA provides insights into host defense strategy. Proc Natl Acad Sci U S A. 2014;111:E62–71. doi: 10.1073/pnas.1313577111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kikly K, Liu L, Na S, Sedgwick JD. The IL-23/Th(17) axis: therapeutic targets for autoimmune inflammation. Curr Opin Immunol. 2006;18:670–5. doi: 10.1016/j.coi.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 46.Zhu M, John S, Berg M, Leonard WJ. Functional association of Nmi with Stat5 and Stat1 in IL-2- and IFNgamma-mediated signaling. Cell. 1999;96:121–30. doi: 10.1016/s0092-8674(00)80965-4. [DOI] [PubMed] [Google Scholar]
- 47.Safwat A. The immunobiology of low-dose total-body irradiation: more questions than answers. Radiat Res. 2000;153:599–604. doi: 10.1667/0033-7587(2000)153[0599:tioldt]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 48.Nowarski R, Wilner OI, Cheshin O, Shahar OD, Kenig E, Baraz L, et al. APOBEC3G enhances lymphoma cell radioresistance by promoting cytidine deaminase-dependent DNA repair. Blood. 2012;120:366–75. doi: 10.1182/blood-2012-01-402123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bernstein MB, Garnett CT, Zhang H, Velcich A, Wattenberg MM, Gameiro SR, et al. Radiation-induced modulation of costimulatory and coinhibitory T-cell signaling molecules on human prostate carcinoma cells promotes productive antitumor immune interactions. Cancer Biother Radiopharm. 2014;29:153–61. doi: 10.1089/cbr.2013.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Locher C, Conforti R, Aymeric L, Ma Y, Yamazaki T, Rusakiewicz S, et al. Desirable cell death during anticancer chemotherapy. Ann N Y Acad Sci. 2010;1209:99–108. doi: 10.1111/j.1749-6632.2010.05763.x. [DOI] [PubMed] [Google Scholar]
- 51.Martins I, Tesniere A, Kepp O, Michaud M, Schlemmer F, Senovilla L, et al. Chemotherapy induces ATP release from tumor cells. Cell Cycle. 2009;8:3723–8. doi: 10.4161/cc.8.22.10026. [DOI] [PubMed] [Google Scholar]
- 52.Shi Y, Liu CH, Roberts AI, Das J, Xu G, Ren G, et al. Granulocyte-macrophage colony-stimulating factor (GM-CSF) and T-cell responses: what we do and don’t know. Cell Res. 2006;16:126–33. doi: 10.1038/sj.cr.7310017. [DOI] [PubMed] [Google Scholar]
- 53.Frick JS, Grunebach F, Autenrieth IB. Immunomodulation by semi-mature dendritic cells: a novel role of Toll-like receptors and interleukin-6. Int J Med Microbiol. 2010;300:19–24. doi: 10.1016/j.ijmm.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 54.De Larco JE, Wuertz BR, Rosner KA, Erickson SA, Gamache DE, Manivel JC, et al. A potential role for interleukin-8 in the metastatic phenotype of breast carcinoma cells. Am J Pathol. 2001;158:639–46. doi: 10.1016/S0002-9440(10)64005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol. 2005;174:7516–23. doi: 10.4049/jimmunol.174.12.7516. [DOI] [PubMed] [Google Scholar]
- 56.Schaue D, Ratikan JA, Iwamoto KS, McBride WH. Maximizing tumor immunity with fractionated radiation. Int J Radiat Oncol Biol Phys. 2012;83:1306–10. doi: 10.1016/j.ijrobp.2011.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Natl Cancer Inst. 2013;105:256–65. doi: 10.1093/jnci/djs629. [DOI] [PMC free article] [PubMed] [Google Scholar]