Abstract
Background
Poor CD4 lymphocyte recovery on antiretroviral therapy (ART) is associated with reduced function of the thymus. Palifermin (keratinocyte growth factor), by providing support to the thymic epithelium, promotes lymphopoiesis in animal models of bone marrow transplantation and graft-versus-host disease.
Methods
In AIDS Clinical Trials Group A5212, a randomized, double-blind, placebo-controlled study, 99 HIV-infected patients on ART with plasma HIV-1 RNA levels ≤200 copies/mL for ≥6 months and CD4 lymphocyte counts <200 cells/mm3 were randomized 1:1:1:1 to receive once daily intravenous administrations of placebo or 20, 40, or 60 µg/kg of palifermin on 3 consecutive days.
Results
The median change in CD4+ T-cell count from baseline to week 12 was not significantly different between the placebo arm [15 (-16, 23) cells/mm3] and the 20-μg/kg dose [11 (2, 32) cells/mm3], the 40-μg/kg dose [12 (−2, 25) cells/mm3], or the 60-μg/kg dose arm [8 (−13, 35) cells/mm3] of palifermin. No significant changes were observed in thymus size or in the number of naïve T cells or recent thymic emigrants.
Conclusions
Palifermin in the doses studied was not effective in improving thymic function and did not raise CD4 lymphocyte counts in HIV-infected patients with low CD4 cell counts despite virologically effective ART.
Keywords: keratinocyte growth factor, palifermin, HIV, thymus, CD4 T lymphocytes
INTRODUCTION
Poor recovery of CD4+ T-lymphocyte counts despite maximum suppression of HIV-1 replication with antiretroviral therapy (ART) places a patient at an increased risk of clinical disease progression. Multiple studies have found an association between lower increases in CD4+ T-cell counts and the rate of AIDS and non-AIDS-related clinical events, as well as death.1-9 These studies all support the importance of the CD4+ cell count in clinical prognosis and the potential value of therapies to enhance greater production of these cells when cell synthesis is inadequate with ART alone.
The thymus is the source of new naïve T lymphocytes. Uncontrolled HIV infection is associated with reduced thymopoiesis.10,11 The thymus is infected by HIV, and it is presumed that the architecture of the thymus is disrupted, with loss of the thymic epithelial space.12 Improvement in stromal cell function and the cytokine milieu resulting from ART contributes to enhanced thymic output.13 During treatment with ART, the production of recent thymic emigrants, as measured by T-cell receptor excision circle (TREC), and naïve T cells increases.10,11 The degree to which TREC levels and thymic size increase with ART is associated with the recovery of CD4+ cell counts.14,15 Thus, improvement in thymic function, although often incomplete, plays an important role in immune reconstitution from the treatment of HIV infection.
Because poor CD4+ cell recovery on ART is associated with lower thymic activity, therapeutic maneuvers to enhance thymic stromal support of thymopoiesis may prove beneficial. Keratinocyte growth factor (KGF) is a member of the fibroblast growth factor (FGF) family of polypeptides that are ligands for cell surface receptors with tyrosine kinase activity involved in embryonic development and differentiation.16 KGF (or FGF-7) is produced by mesenchymal cells and acts in a paracrine fashion on epithelial cells that express a splice variant of the FGFR2 receptor (FGFR2IIIb).17 The KGFR2IIIb receptor is expressed on thymic epithelial cells (TECs).17 The development of T cells in the thymus is dependent on support from the thymic epithelium.18 Mice deficient in KGFR2IIIb have poor TEC development, decreased thymic cellularity, and impaired thymopoiesis.17
KGF has proliferative and antiapoptotic effects on various epithelial cells expressing the KGFR2IIIb receptor.19-21 It is produced by thymic fibroblasts and other mesenchymal cells,16,17 as well as thymic lymphocytes.22,23 When administered to mice undergoing bone marrow transplantation (BMT) or experimental graft-versus-host disease (GVHD), KGF improves thymic function.22,24 In a rhesus macaque autologous transplant model, KGF treatment also resulted in the preservation of thymic architecture and increased naïve T-cell numbers in the peripheral lymph nodes compared with controls.25 Thus, KGF in short courses of treatment promotes T lymphopoiesis in the BMT and GVHD animal models.
Because of these observations, in AIDS Clinical Trials Group (ACTG) protocol A5212 we evaluated KGF's potential to enhance CD4+ T-cell recovery in HIV-infected subjects with CD4+ T-cell counts less than 200 cells/mm3 despite effective viral suppression on ART. The form of KGF used was palifermin (recombinant human KGF), an N-terminal truncated version of the naturally occurring KGF that has similar biologic activity to the endogenous protein but has increased stability.
Palifermin when administered parenterally to humans has linear pharmacokinetics, with an elimination half-life of 4.5 to 6 hours.26 Epithelial cell proliferation in the buccal mucosa, as measured by Ki67 staining, persists for 48 hours.26 In the BMT mouse experiments, once daily administration of KGF over 3 days resulted in increased thymic cellularity 2 to 4 weeks later compared with control animals, and progressively greater increases in lymphocyte counts in the spleen and lymph nodes at 1, 2, and 3 months.22 Clinical studies in patients with hematologic cancers found that two 3-day cycles of daily administration of 60 μg/kg of palifermin, the first before the start of conditioning and the second after the autologous hematopoietic stem cell transplantation, was effective at preventing oral mucositis.27 Another study in patients with metastatic colon cancer found 40 μg/kg of palifermin given for 3 consecutive days before each of two 28-day cycles of chemotherapy to be similarly effective.28 Based on the animal model data and this clinical experience, and being cognizant of the potential toxicities and the theoretical risk of the induction of neoplasms, we elected to study a single 3-day cycle of consecutive daily doses up to 60 μg/kg of palifermin in this first exploratory investigation in humans. The primary outcome measure was change in the absolute CD4+ T-cell count, but naïve T cells were also measured, and an intensive assessment of T-cell subset markers of recent thymic emigration and cell maturation, turnover, and survival was done on a random subset of study participants.
METHODS
Study Design
ACTG A5212 was a randomized, double-blind, placebo-controlled study. HIV-1-infected men and women aged ≥18 years on ART with plasma HIV-1 RNA levels ≤200 copies/mL for at least 6 months prior to study entry and CD4+ lymphocyte counts <200 cells/mm3 at screening were eligible to enroll in the study. Potential study participants were excluded if they were pregnant or breastfeeding, had evidence of active pancreatitis or malignancy, or had used androgens or immune-modulating medications within 30 days prior to study entry. Study participants were randomized 1:1:1:1 to receive three daily intravenous administrations of placebo or 20, 40, or 60 μg/kg palifermin. Participants were then followed on study for 24 weeks after the completion of the palifermin/placebo treatment.
The protocol was approved by the institutional review board at each site. Written informed consent was obtained from potential participants.
Evaluation of Participants and Follow-up
After the screening and baseline evaluations, study participants were clinically assessed on the 2nd and 3rd days and at weeks 1, 2, 4, 8, 12, and 24. Concomitant medications and adverse events were recorded at these visits. Laboratory safety tests (hematology, serum chemistries, and liver function tests) were performed during screening, on days 1 (baseline) and 3, and at weeks 1, 2, 4, 8, 12, and 24. Plasma levels of HIV-1 RNA were measured in real time at a single ACTG laboratory (Johns Hopkins University) using the Amplicor HIV-1 Monitor test (version 1.5; Roche Diagnostics, Indianapolis, IN) at screening, baseline, and weeks 4, 12, and 24. Blood samples for CD4+ and CD8+ T-lymphocyte counts and other lymphocyte subsets were drawn at screening, 14 days before study entry (pre-entry), at study entry (pre-dosing on day 1), at day 3, and at weeks 1, 2, 4, 8, 12, and 24. Computed tomography (CT) scans of the thymus were performed at baseline and week 12.
Study medication was permanently discontinued in the event of pregnancy, the discontinuation of ART, the use of prohibited concomitant medications, or any grade 3 or 4 adverse event judged to be definitely, probably, or possibly related to study medication.
Immunologic Evaluations
The primary endpoint of the study was the change in CD4+ T-lymphocyte count between baseline (mean of pre-entry and entry values) and week 12. The CD4+ T-cell count was also measured at day 3 and weeks 1, 2, 4, 8, and 24.
Naïve T cells were measured at day 3 and weeks 1, 2, 4, 8, 12, and 24. More extensive T-cell subset measurements were done in a random sample of participants at weeks 4, 8, 12, and 24 and at earlier time points in a smaller sample. Peripheral blood mononuclear cells (PBMCs) were frozen and shipped to Dr. Sekaly's laboratory in liquid nitrogen dry-shippers. The cells were transferred to a liquid nitrogen tank upon receipt. Multiparameter flow cytometric evaluation was performed on the cryopreserved PBMCs to define recent thymic emigrants and naïve, central memory, and effector memory CD4 and CD8 subsets. These CD4 and CD8 subsets were also evaluated for markers defining cell turnover (Ki67) and survival (BCL2). Briefly, cryopreserved PBMCs were thawed and washed in PBS. Cell viability ranged between 74% and 100%; samples with viability of less than 60% did not undergo further analysis. Immunophenotyping was performed with the following antibody panel: anti-CD3 AmCyan, anti-CD4 Pacific Blue anti-CD8 PerCP Cy5.5, anti-CD45RA APC anti-CCR7 PeCy7 anti-CD27 APC Alexa 750, anti-CD31 PE anti-BCL2 FITC, and anti-Ki67 FITC. Following antibody staining, cells were fixed with 2% paraformaldehyde and kept at 4°C until data acquisition. Analysis was performed on a BD LSRII flow cytometer (BD Biosciences, San Jose, CA) and analyzed with Diva software (BD Biosciences). Dead cells were excluded by forward and side scatter. Positive and negative populations were identified by utilizing isotypes (Ki67 and BCL2) or FMO (all other antibodies). Data were reported for all subsets as absolute number and percent positive.
In a small subset of participants, plasma samples collected from EDTA-containing tubes at study entry and weeks 4, 12, and 24 were analyzed for levels of interleukin-7 (IL-7) (HS750, R&D Systems, Minneapolis, MN) according to the manufacturer's protocol.
Thymus CT scans
Thymus size was measured at study entry and at week 12 by CT scan of the chest, as previously described.29 Briefly, noncontrast CT scans were performed with contiguous 5-mm-thick sections from the sternal notch to the xiphoid. A central radiologist, blinded to the subject clinical data and without knowledge of prior patient scores, scored the CT scan from 0 to 5 (0— no thymic tissue, only fat; 1—barely recognizable thymic tissue; 2—minimal thymic tissue; 3— more obvious thymic tissue; 4—moderate amount of thymic tissue; 5—thymic mass of concern for thymoma).
Statistical Methods
The primary endpoint was defined as the change from baseline to study week 12 in absolute CD4+ lymphocyte counts. Baseline value was defined as the arithmetic mean of pre-entry and entry absolute CD4 cells counts. In the primary analysis (intent-to-treat analysis), missing values at week 12 were handled as follows: a CD4 count evaluation obtained after starting study treatment and closest in time to week 12 (using the earlier evaluation if necessary to break a tie) was used in place of the missing week 12 evaluation. If no such evaluation existed, then the worst rank was assigned to the study participant.
The primary analysis consisted of three pairwise comparisons between each of the palifermin arms and the placebo arm. A two-sample Wilcoxon rank sum test was used to test the null hypothesis of no palifermin treatment effect against the one-sided alternative that a study participant receiving palifermin was more likely to have a higher primary endpoint than a participant receiving placebo, adjusted by the Hochberg multiplicity procedure with a family-wise one-sided type I error set at 0.05. The statistical analysis for continuous secondary endpoints employed two-sided nonparametric exploratory tests, unadjusted for multiple comparisons, with nominal 5% type I errors. Several imputation methods were used to handle missing observations: using observed data only, using last-post-baseline-value carried forward, and assigning the worst rank to missing data.
An as-treated analysis of the change in CD4 T-cell count was also done, as well as an analysis that stratified by duration of ART (greater versus less than or equal to the median duration of ART in the study population).
Spearman's correlation coefficient was used to evaluate the association between change in thymus size on CT scan and change in CD4+ T-cell counts from study entry and week 12.
Safety endpoints were defined as the number/proportion of study participants who experienced grade ≥3 signs/symptoms or laboratory abnormalities. Pairwise comparisons of each palifermin arm with the placebo arm were made with Fisher's exact test.
RESULTS
Study Population
Between February 2007 and April 2008, 99 study participants were enrolled at 22 clinical sites (Table 1). Twenty-five participants were randomized to placebo; 25 to receive 20 μg/kg; 25 to receive 40 μg/kg; and 24 to receive 60 μg/kg of palifermin. No participants were excluded from the analysis. The median age was 49 years. Most study participants were male (91%). The median CD4 T-lymphocyte count was 153 cells/mm3 (placebo=147, 20 μg/kg=155, 40 μg/kg=156, 60 μg/kg=152 cells/mm3). The median CD8 T-cell count was 665 cells/mm3 (placebo=638, 20 μg/kg=615, 40 μg/kg=716, 60 μg/kg=761 cells/mm3).
Table 1.
Baseline Characteristics: Demographic/ Enrollment Information/ Baseline CD4 Counts/ Percentages and HIV-1 RNA copies/mL
| Characteristic | Placebo (N=25) | 20 μg/kg (N=25) | 40 μg/kg (N=25) | 60 μg/kg (N=24) | Total (N=99) |
|---|---|---|---|---|---|
| Age, y Mean (s.d.) | 48 (8) | 50 (9) | 47 (10) | 51 (9) | 49 (9) |
| Sex, no. (%) | |||||
| Male | 22 (88%) | 23 (92%) | 22 (88%) | 23 (96%) | 90 (91%) |
| Female | 3 (12%) | 2 (8%) | 3 (12%) | 1 (4%) | 9 (9%) |
| Race/ethnicity, no. (%)* | |||||
| White non-Hispanic | 15 (60%) | 13 (52%) | 12 (50%) | 12 (50%) | 52 (53%) |
| Black non-Hispanic | 6 (24%) | 9 (36%) | 4 (17%) | 6 (25%) | 25 (26%) |
| Hispanic (regardless of race) | 3 (12%) | 3 (12%) | 8 (33%) | 6 (25%) | 20 (20%) |
| Asian, Pacific Islander | 1 (4%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (1%) |
| CD4+ cell count, cells/mm3 Median (Q1-Q3) | 147 (110-180) | 155 (126-194) | 156 (116-197) | 152 (112-192) | 153 (116-194) |
| CD4+ cell percentages of all lymphocytes Median (Q1-Q3) | 12 (9-16) | 13 (12-16) | 11 (9-17) | 10 (8-16) | 12 (9-16) |
| HIV-1 RNA, copies/mL, no. (%)* | |||||
| ≥50 | 3 (12%) | 3 (12%) | 1 (4%) | 0 (0%) | 7 (7%) |
| <50 | 22 (88%) | 22 (88%) | 23 (96%) | 24 (100%) | 91 (93%) |
One subject declined to report race/ethnicity and one subject missed entry HIV-1 RNA evaluation.
All study participants (n=99) were on a potent ART regimen defined as a combination of three or more antiretroviral drugs, with the combination of ritonavir and another protease inhibitor counted as one antiretroviral agent during the whole study. At study entry, 26 participants were receiving a nonnucleoside reverse-transcriptase inhibitor and 73 participants were receiving a protease inhibitor. All study participants were receiving nucleoside reverse-transcriptase inhibitors, specific agents evenly distributed across arms.
Of the 98 study participants with entry plasma HIV-1 RNA evaluations, 93% had values below 50 copies/mL. The values for the 7 participants with quantifiable plasma HIV-1 RNA levels were 52, 77, 81, 84, 88, 97, and 69,440 copies/mL respectively. Of the 95 study participants with entry hepatitis C virus (HCV) RNA evaluations, 12 (13%) had a positive HCV RNA result.
No substantial imbalances across treatment arms in these baseline variables were apparent.
Ninety-seven (out of 99) study participants received the full dose of palifermin or placebo on each of the 3 consecutive dates. The other two participants (one in the 40-μg/kg arm, the other in the 60-μg/kg arm) received one-third dose of the study medication (by pharmacy error) on each of 3 consecutive days.
CD4+ T-Lymphocyte Counts
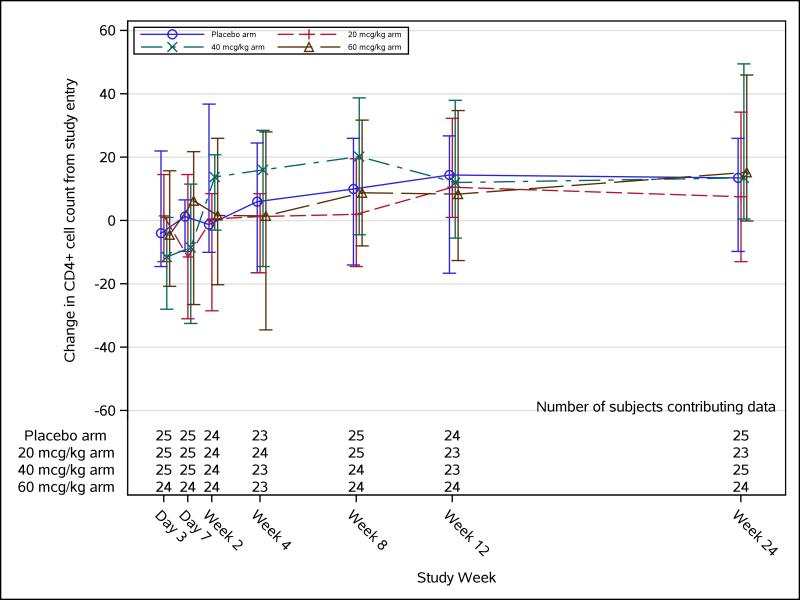
Ninety-four study participants had week 12 CD4+ T-cell count determined. For the 5 study participants (placebo=1, 20 μg/kg=2, 40 μg/kg=2, 60 μg/kg=0) who did not have week 12 CD4+ T-cell count, their week 8 CD4+ T-cell counts were used instead. The median (Q1, Q3) change in absolute CD4 T-cell count from baseline to week 12 was not significantly different between the placebo arm [15 (−16, 23) cells/mm3] and either the 20-μg/kg palifermin dose arm [11 (2, 32) cells/mm3, P=0.20], the 40-μg/kg palifermin dose arm [12 (−2, 25) cells/mm3, P=0.31], or the 60-μg/kg palifermin dose arm [8 (−13, 35) cells/mm3, P=0.37]. The result was the same when the change in CD4 T-cell count was analyzed as-treated and when stratified by duration of ART. As noted above, during the course of the study, two study participants received the wrong dose of study medication, and for the as-treated analysis, their results were assigned to the arms matching the actual doses received. There were also no statistically significant differences compared with the placebo arm in CD4 T-cell count changes from baseline to day 3 or weeks 1, 2, 4, 8, 12, or 24 (observed data only; Figure 1). There was a suggestion of a positive palifermin dose effect on change in CD4 T-cell count from baseline to week 24 using observed data only (P=0.07), using the last-post-baseline value carried forward to impute missing data (P=0.08) and using the worst rank to impute missing data (P=0.06).
FIGURE 1. CD4+ cell count change over time.
Longitudinal CD4+ cell count change trajectory with median (Q1, Q3) for each treatment arm. Medians are represented by square, triangle, diamond, and star for their corresponding dose arms.
Lymphocyte Subset and Plasma Interleukin-7 Measures
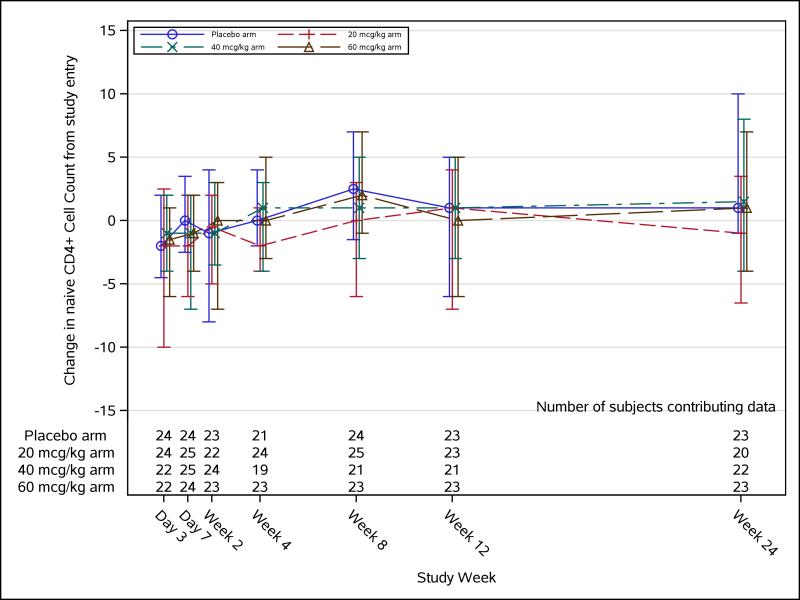
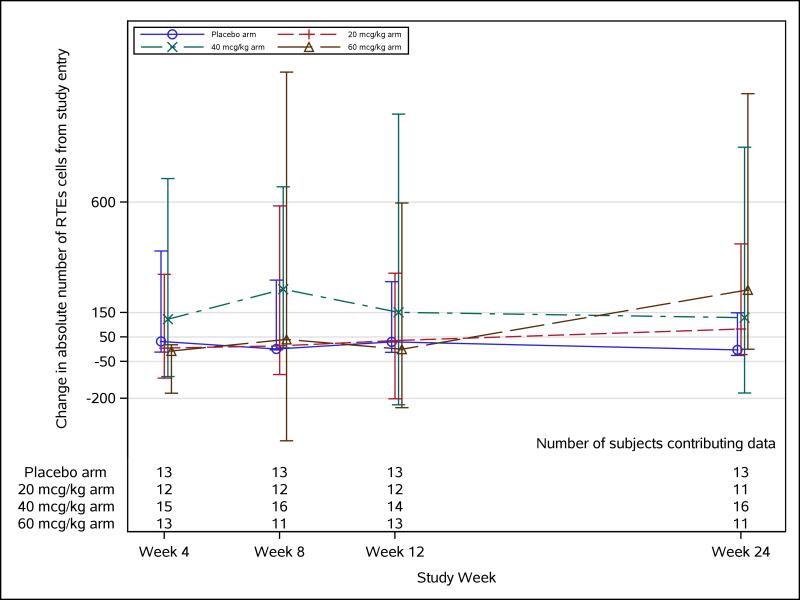
Flow cytometry phenotypic assaying was conducted on a total of 57 study participants, 15 in the placebo group, and 12, 17, and 13, respectively, in the 20-, 40-, and 60-μg/kg palifermin dose groups. No significant changes were observed in naïve CD4 T cells (Figure 2), recent thymic emigrant naïve CD4 T cells (Figure 3), or any other lymphocyte subset measured in any palifermin dose arm compared with placebo at any time-point. Plasma IL-7 levels measured in a small subset of study participants did not change significantly from baseline to weeks 4, 12, or 24, compared with placebo (data not shown).
FIGURE 2. Naïve CD4+ cell count change over time.
Longitudinal naïve CD4+ cell count change trajectory with median (Q1, Q3) for each treatment arm. Medians are represented by square, triangle, diamond, and star for their corresponding dose arms.
FIGURE 3. Change in absolute number of recent thymic emigrants (RTEs) in naïve CD4+ cells over time.
Longitudinal absolute number of RTE cells change trajectory with median (Q1, Q3) for each treatment arm. Medians are represented by square, triangle, diamond, and star for their corresponding dose arms.
Thymus CT Scan
Eighty-eight study participants had thymus CT scans at study entry and at week 12. Forty-nine (56%) had unchanged thymus, 21 (24%) had a one-size-larger thymus at week 12, 15 (17%) had a one-size-smaller thymus, 2 (2%) had a two-sizes-smaller thymus, and 1 (1%) had a two-sizes-larger thymus. There were no significant differences in the change in thymus size as measured on CT scan in any of the active treatment arms, or in the treatment arms combined, compared with placebo.
Safety
No study participant discontinued the study medication during the 3 days of daily administration because of adverse effects of treatment. There were no significant differences in the occurrence of new adverse events of grade 3 or higher (as classified by the NIAID criteria) in any of the active dose arms compared with the placebo arm. Grade 3 or higher adverse events in the palifermin arms that were thought to be possibly or definitely related to study treatment included heart failure, abdominal pain, elevated lipase (different patient), and allergic reaction. In the placebo group, no adverse events deemed to be at least possibly related to study treatment occurred.
Two study participants treated with palifermin developed neoplasms (one laryngeal cancer, the other basal cell skin cancer). No deaths or new episodes of opportunistic infections occurred.
DISCUSSION
This double-blind, randomized, placebo-controlled study showed that palifermin in the doses studied was not effective in raising CD4+ T-lymphocyte counts in HIV-infected patients with CD4+ T-cell counts less than 200 cells/mm3 despite providing virologically effective ART. There was no evidence that palifermin enhanced the production of new lymphocytes in the thymus in this situation, as assessed by assays measuring the number of naïve lymphocytes and recent thymic emigrants in the blood. Thymus size was not altered by treatment with palifermin, suggesting that thymus architecture was not restored.
Palifermin, in the doses used, proved to be safe and well tolerated. There were no significant differences between the palifermin-treated groups and the placebo-treated group in the incidence of serious adverse events. The adverse events associated with palifermin use were reversible and did not lead to discontinuation of dosing. No opportunistic infections or deaths occurred. Two neoplasms occurred in patients treated with palifermin, but because of the short duration of the study, the temporal relationship was unclear.
Palifermin is approved by the US Food and Drug Administration for patients with hematologic malignancies who are receiving myelotoxic therapy requiring hematopoietic stem cell support to decrease the incidence and duration of severe oral mucositis. Our results are consistent with a recently reported study of palifermin in patients undergoing T-cell replete, matched-donor allogeneic hematopoietic cell transplantation after myeloablative conditioning.30 In that randomized, placebo-controlled study, the peritransplant administration of palifermin in total doses up to 720 μg/kg did not improve absolute lymphocyte count recovery 30, 60, or 100 days after transplantation, despite having the previously demonstrated effect on decreasing mucositis. The results of both human studies differ from the findings in studies conducted in mouse and rhesus macaque BMT and mouse GVHD models, in which KGF improved thymic function and lymphocyte production. In the human BMT study, lymphocyte subsets were not measured, but our study did not reveal an effect on early lymphocyte formation as measured by recent thymic emigrants (CD31hi) and naïve (RA+CD27+CCR7+) lymphocytes.
It is conceivable that higher doses and/or longer administration of palifermin might be effective, but the human BMT study found that up to four repeated cycles of 3-day dosing, leading to total doses of 720 μg/kg, did not increase lymphocyte counts. Higher and longer dosing would risk higher rates of toxicity. It is also possible that the damage to the thymus in HIV-infected patients with CD4+ T-cell counts of less than 200 cells/mm3 is too advanced and the architecture of the thymic epithelium too disrupted with fibrous tissue replacement to respond to KGF.31 Perhaps patients with higher CD4 T-cell counts would respond better.
In prospective cohort studies, patients with good virologic responses from ART but inadequate CD4+ T-cell responses (immunologic “discordant” nonresponders) are at higher risk for clinical events.1-3,5-9 In particular, CD4+ T-cell counts of less than 200 cells/mm3 had prognostic importance, independent of viral load.3,5-9 In addition to raising CD4+ T-cell numbers, improving thymic function could serve to provide more naïve lymphocytes capable of responding to new antigens, whether in the form of HIV therapeutic vaccines, vaccines against other potential pathogens, or the pathogens themselves. Even after ART, patients with HIV infection, particularly those with low nadir CD4+ T-cell counts, have impaired responses to vaccines. KGF induces proliferation of endothelial cells.32
The thymus epithelium provides critical support to thymic function and the production of new lymphocytes. KGF is a key ligand of thymic epithelial cell receptors and promotes thymopoiesis through this interaction. Unfortunately, our study did not find a benefit of the administration of KGF to HIV-infected patients with advanced immune damage and poor CD4+ T-cell recovery from virologically effective ART. Further studies could evaluate KGF in HIV-infected patients with less advanced immunologic damage.
Attempts to boost bone marrow or thymus production of CD4+ T lymphocytes with granulocyte-macrophage colony-stimulating factor and human growth factor, respectively, have had modest success.33,34 Other approaches to enhance CD4+ T-cell recovery could include measures to promote lymphocyte expansion (IL-7, which also affects thymic activity) and techniques to reduce cell destruction by downregulating the HIV-associated immune activation that leads to lymphocyte apoptosis. Ultimately, larger randomized clinical trials with clinical endpoints will be required to demonstrate that any of these immune-based approaches to increasing CD4+ T-lymphocyte counts are clinically meaningful.
Acknowledgments
Funding sources: Grant to Jeffrey Jacobson from NIAID. See Appendix for information on funding sources for other authors.
Footnotes
Conflicts of interest: The authors of this manuscript declare no potential conflicts of interest.
Scientific presentations: none.
REFERENCES
- 1.Grabar S, Le Moing V, Goujard C, et al. Clinical outcome of patients with HIV-1 infection according to immunologic and virologic response after 6 months of highly active antiretroviral therapy. Ann Intern Med. 2000;133:401–410. doi: 10.7326/0003-4819-133-6-200009190-00007. [DOI] [PubMed] [Google Scholar]
- 2.Piketty C, Weiss L, Thomas F, et al. Long-term clinical outcome of human immunodeficiency virus-infected patients with discordant immunologic and virologic responses to a protease inhibitor-containing regimen. J Infect Dis. 2001;183:1328–1335. doi: 10.1086/319861. doi:10.1086/319861. [DOI] [PubMed] [Google Scholar]
- 3.Dronda F, Moreno S, Moreno A, et al. Long-term outcomes among antiretroviral-naive human immunodeficiency virus-infected patients with small increases in CD4+ cell counts after successful virologic suppression. Clin Infect Dis. 2002;35:1005–1009. doi: 10.1086/342695. doi:10.1086/342695. [DOI] [PubMed] [Google Scholar]
- 4.Coakley EP, Samore MH, Gillis JM, et al. The values of quantitative serum HIV-1 RNA levels and CD4 cell counts for predicting survival time among HIV-positive individuals with CD4 counts of < or = 50 x 10(6) cells/l. AIDS. 2000;14:1147–1153. doi: 10.1097/00002030-200006160-00011. [DOI] [PubMed] [Google Scholar]
- 5.Anastos K, Barrón Y, Cohen MH, et al. The prognostic importance of changes in CD4+ cell count and HIV-1 RNA level in women after initiating highly active antiretroviral therapy. Ann Intern Med. 2004;140:256–264. doi: 10.7326/0003-4819-140-4-200402170-00007. [DOI] [PubMed] [Google Scholar]
- 6.Chêne G, Sterne JA, May M, et al. Prognostic importance of initial response in HIV-1 infected patients starting potent antiretroviral therapy: analysis of prospective studies. Lancet. 2003;362:679–686. doi: 10.1016/s0140-6736(03)14229-8. [DOI] [PubMed] [Google Scholar]
- 7.Lundgren JD, Mocroft A, Gatell JM, et al. EuroSIDA Study Group A clinically prognostic scoring system for patients receiving highly active antiretroviral therapy: results from the EuroSIDA study. J Infect Dis. 2002;185:178–187. doi: 10.1086/338267. doi:10.1086/338267. [DOI] [PubMed] [Google Scholar]
- 8.Ghani AC, de Wolf F, Ferguson NM, et al. Surrogate markers for disease progression in treated HIV infection. J Acquir Immune Defic Syndr. 2001;28:226–231. doi: 10.1097/00042560-200111010-00004. [DOI] [PubMed] [Google Scholar]
- 9.Lewden C, Raffi F, Cuzin L, et al. Factors associated with mortality in human immunodeficiency virus type 1-infected adults initiating protease inhibitor-containing therapy: role of education level and of early transaminase level elevation (APROCO-ANRS EP11 study). The Antiprotéases Cohorte Agence Nationale de Recherches sur le SIDA EP 11 study. J Infect Dis. 2002;186:710–714. doi: 10.1086/342047. doi:10.1086/342047. [DOI] [PubMed] [Google Scholar]
- 10.Douek DC, McFarland RD, Keiser PH, et al. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396:690–695. doi: 10.1038/25374. doi:10.1038/25374. [DOI] [PubMed] [Google Scholar]
- 11.Zhang L, Lewin SR, Markowitz M, et al. Measuring recent thymic emigrants in blood of normal and HIV-1-infected individuals before and after effective therapy. J Exp Med. 1999;190:725–732. doi: 10.1084/jem.190.5.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ye P, Kourtis AP, Kirschner DE. The effects of different HIV type 1 strains on human thymic function. AIDS Res Hum Retroviruses. 2002;18:1239–1251. doi: 10.1089/088922202320886280. doi:10.1089/088922202320886280. [DOI] [PubMed] [Google Scholar]
- 13.Ye P, Kourtis AP, Kirschner DE. Reconstitution of thymic function in HIV-1 patients treated with highly active antiretroviral therapy. Clin Immunol. 2003;106:95–105. doi: 10.1016/s1521-6616(02)00024-4. [DOI] [PubMed] [Google Scholar]
- 14.Franco JM, Rubio A, Martínez-Moya M, et al. T-cell repopulation and thymic volume in HIV-1-infected adult patients after highly active antiretroviral therapy. Blood. 2002;99:3702–3706. doi: 10.1182/blood.v99.10.3702. [DOI] [PubMed] [Google Scholar]
- 15.Kolte L, Dreves AM, Ersbøll AK, et al. Association between larger thymic size and higher thymic output in human immunodeficiency virus-infected patients receiving highly active antiretroviral therapy. J Infect Dis. 2002;185:1578–1585. doi: 10.1086/340418. doi:10.1086/340418. [DOI] [PubMed] [Google Scholar]
- 16.Rubin JS, Osada H, Finch PW, et al. Purification and characterization of a newly identified growth factor specific for epithelial cells. Proc Natl Acad Sci U S A. 1989;86:802–806. doi: 10.1073/pnas.86.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Revest JM, Suniara RK, Kerr K, et al. Development of the thymus requires signaling through the fibroblast growth factor receptor R2-IIIb. J Immunol. 2001;167:1954–1961. doi: 10.4049/jimmunol.167.4.1954. [DOI] [PubMed] [Google Scholar]
- 18.Anderson G, Moore NC, Owen JJ, Jenkinson EJ. Cellular interactions in thymocyte development. Annu Rev Immunol. 1996;14:73–99. doi: 10.1146/annurev.immunol.14.1.73. doi:10.1146/annurev.immunol.14.1.73. [DOI] [PubMed] [Google Scholar]
- 19.Farrell CL, Bready JV, Rex KL, et al. Keratinocyte growth factor protects mice from chemotherapy and radiation-induced gastrointestinal injury and mortality. Cancer Res. 1998;58:933–939. [PubMed] [Google Scholar]
- 20.Rubin JS, Bottaro DP, Chedid M, et al. Keratinocyte growth factor as a cytokine that mediates mesenchymal-epithelial interaction. EXS. 1995;74:191–214. doi: 10.1007/978-3-0348-9070-0_10. [DOI] [PubMed] [Google Scholar]
- 21.Yi ES, Williams ST, Lee H, et al. Keratinocyte growth factor amelio7rates radiation- and bleomycin-induced lung injury and mortality. Am J Pathol. 1996;149:1963–1970. [PMC free article] [PubMed] [Google Scholar]
- 22.Min D, Taylor PA, Panoskaltsis-Mortari A, et al. Protection from thymic epithelial cell injury by keratinocyte growth factor: a new approach to improve thymic and peripheral T-cell reconstitution after bone marrow transplantation. Blood. 2002;99:4592–4600. doi: 10.1182/blood.v99.12.4592. [DOI] [PubMed] [Google Scholar]
- 23.Erickson M, Morkowski S, Lehar S, et al. Regulation of thymic epithelium by keratinocyte growth factor. Blood. 2002;100:3269–3278. doi: 10.1182/blood-2002-04-1036. [DOI] [PubMed] [Google Scholar]
- 24.Rossi S, Blazar BR, Farrell CL, et al. Keratinocyte growth factor preserves normal thymopoiesis and thymic microenvironment during experimental graft-versus-host disease. Blood. 2002;100:682–691. doi: 10.1182/blood.v100.2.682. [DOI] [PubMed] [Google Scholar]
- 25.Seggewiss R, Loré K, Guenaga FJ, et al. Keratinocyte growth factor augments immune reconstitution after autologous hematopoietic progenitor cell transplantation in rhesus macaques. Blood. 2007;110:441–449. doi: 10.1182/blood-2006-12-065623. doi:10.1182/blood-2006-12-065623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zia-Amirhosseini P, Salfi M, Leese P, et al. Pharmacokinetics, pharmacodynamics, and safety assessment of palifermin (rHuKGF) in healthy volunteers. Clin Pharmacol Ther. 2006;79:558–569. doi: 10.1016/j.clpt.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 27.Spielberger R, Stiff P, Bensinger W, et al. Palifermin for oral mucositis after intensive therapy for hematologic cancers. N Engl J Med. 2004;351:2590–2598. doi: 10.1056/NEJMoa040125. [DOI] [PubMed] [Google Scholar]
- 28.Rosen LS, Abdi E, Davis ID, et al. Palifermin reduces the incidence of oral mucositis in patients with metastatic colorectal cancer treated with fluorouracil-based chemotherapy. J Clin Oncol. 2006;24:5194–5200. doi: 10.1200/JCO.2005.04.1152. [DOI] [PubMed] [Google Scholar]
- 29.McCune JM, Loftus R, Schmidt DK, et al. High prevalence of thymic tissue in adults with human immunodeficiency virus-1 infection. J Clin Invest. 1998;101:2301–2308. doi: 10.1172/JCI2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rizwan R, Levine JE, DeFor T, et al. Peri-transplant palifermin use and lymphocyte recovery after T-cell replete, matched related allogeneic hematopoietic cell transplantation. Am J Hematol. 2011;86:879–882. doi: 10.1002/ajh.22136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeng M, Southern PJ, Reilly CS, et al. Lymphoid tissue damage in HIV-1 infection depletes naive T cells and limits T cell reconstitution after antiretroviral therapy. PLoS Pathog. 2012;8:e1002437. doi: 10.1371/journal.ppat.1002437. doi:10.1371/journal.ppat.1002437 (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lange CG, Lederman MM, Medvik K, et al. Nadir CD4+ T-cell count and numbers of CD28+CD4+ T-cells predict functional responses to immunizations in chronic HIV-1 infection. AIDS. 2003;17:2015–2023. doi: 10.1097/00002030-200309260-00002. [DOI] [PubMed] [Google Scholar]
- 33.Jacobson JM, Lederman MM, Spritzler J, et al. Granulocyte-macrophage colony-stimulating factor induces modest increases in plasma human immunodeficiency virus (HIV) type 1 RNA levels and CD4+ lymphocyte counts in patients with uncontrolled HIV infection. J Infect Dis. 2003;88:1804–1814. doi: 10.1086/379899. doi:10.1086/379899. [DOI] [PubMed] [Google Scholar]
- 34.Smith K, Zheng L, Bosch R, et al. Treatment with recombinant growth hormone is associated with modest improvement in CD4 lymphocyte reconstitution in HIV-infected persons on antiretroviral therapy: results of ACTG A5174. AIDS Res Hum Retroviruses. 2010;26:425–432. doi: 10.1089/aid.2009.0052. doi:10.1089/aid.2009.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]