Summary
Bordetella species cause respiratory infections in mammals. Their master regulatory system BvgAS controls expression of at least three distinct phenotypic phases in response to environmental cues. The Bvg+ phase is necessary and sufficient for respiratory infection while the Bvg− phase is required for survival ex vivo. We obtained large colony variants (LCVs) from the lungs of mice infected with B. bronchiseptica strain RBX9, which contains an in-frame deletion mutation in fhaB, encoding filamentous hemagglutinin. RBX9 also yielded LCVs when switched from Bvg− phase conditions to Bvg+ phase conditions in vitro. We determined that LCVs are composed of both Bvg+ and Bvg− phase bacteria and that they result from defective bvgAS positive autoregulation. The LCV phenotype was linked to the presence of a divergent promoter 5′ to bvgAS, suggesting a previously undescribed mechanism of transcriptional interference that, in this case, leads to feedback-based bistability (FBM). Our results also indicate that a small proportion of RBX9 bacteria modulates to the Bvg− phase in vivo. In addition to providing insight into transcriptional interference and FBM, our data provide an example of an in-frame deletion mutation exerting a ‘polar’ effect on nearby genes.
Keywords: phase variation, Bordetella, bistability, BvgAS, transcriptional interference
Introduction
The genus Bordetella includes Gram-negative bacteria that cause respiratory infections. Bordetella pertussis and Bordetella parapertussishu are strictly human-specific pathogens that cause whooping cough, an acute disease that has experienced a recent resurgence despite widespread vaccination (Mattoo and Cherry, 2005; Campos-Outcalt, 2005; Marconi et al., 2012). Phylogenetic analyses indicate that B. pertussis and B. parapertussishu have recently evolved from Bordetella bronchiseptica, which infects a wide range of mammals and can also survive naturally for long periods of time outside the host (Gerlach et al., 2001; Diavatopoulos et al., 2005; Gross et al., 2010). Although the factors that determine host specificity remain unknown, the presence and regulation of virulence factor-encoding genes is highly conserved between these three sub-species (Tejada et al., 1996; Cummings et al., 2006).
Filamentous hemagglutinin (FHA), encoded by the fhaB gene, is a well-characterized virulence factor of Bordetella and is a primary component of acellular pertussis vaccines (Domenighini et al., 1990; Sato and Sato, 1999; Mattoo and Cherry, 2005). A prototypical member of the Two Partner Secretion family of proteins, FHA is a large, surface-exposed protein that is produced and secreted at a high levels during growth in vitro (Domenighini et al., 1990; Coutte et al., 2001; Mazar and Cotter, 2006). In B. bronchiseptica, FHA mediates adherence to a wide range of cell lines and is required for colonization of the lower respiratory tract in both rats and mice (Cotter et al., 1998; Mattoo et al., 2000; Inatsuka et al., 2005). Although FHA was first characterized as an adhesin, it has subsequently been reported to perform several other important functions. For example, exposure of lipopolysaccharide and IFN-γ-stimulated macrophages to purified B. pertussis FHA suppresses interleukin-12 (IL-12) production, and macrophages treated with FHA exhibit higher rates of apoptosis compared to untreated controls (McGuirk and Mills, 2000; Abramson et al., 2001). FHA-deficient B. bronchiseptica causes an infection that is hyperinflammatory compared to infection caused by wild-type bacteria and is characterized by increased influx of interleukin-17 (IL-17)-positive neutrophils, macrophages, and CD4+ Tcells, suggesting that FHA plays an immunomodulatory role in vivo (Inatsuka et al., 2005; Henderson et al., 2012). Additionally, there is strong evidence that FHA interacts with another important virulence factor, adenylate cyclase toxin ACT (Zaretzky et al., 2002; Perez Vidakovics et al., 2006).
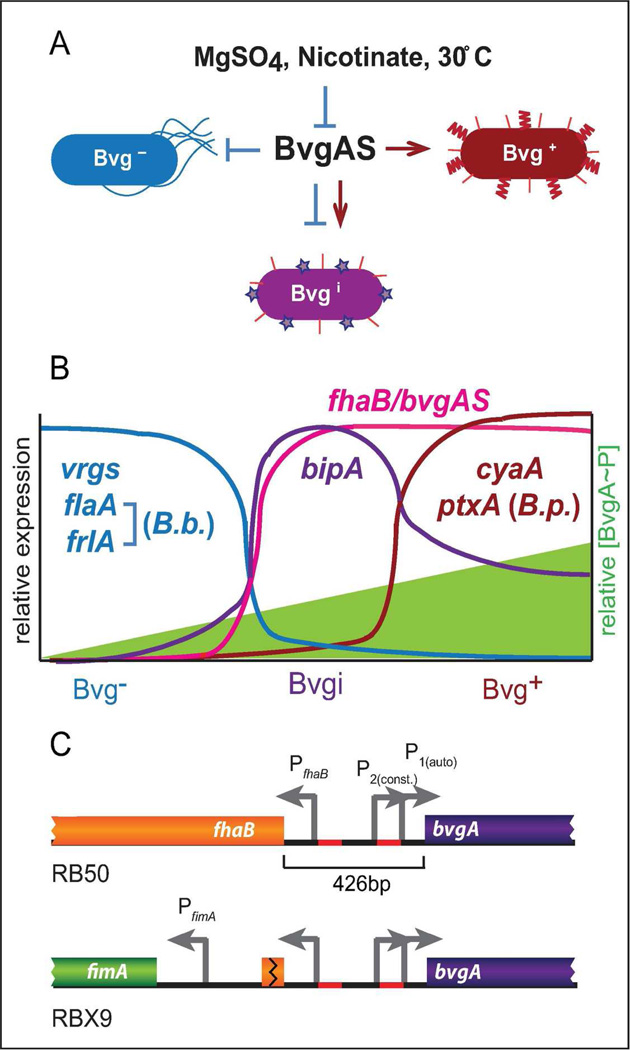
In Bordetella, the master regulator that controls the expression of all known virulence factor-encoding genes is called BvgAS (Aricò et al., 1989). A two-component sensory transduction system, BvgAS controls at least three distinct phenotypic phases (Bvg+, Bvgi, and Bvg−) by altering gene expression patterns in response to environmental stimuli (Figure 1A) (Cotter and Jones, 2003). The Bvg+ phase is induced during standard laboratory growth conditions at 37°C on Bordet Gengou (BG) blood agar or in Stainer-Scholte broth. Bvg+ phase bacteria are non-motile and form small, dome-shaped, hemolytic colonies on BG blood agar. Bacteria can be induced (or modulated) to the Bvg− phase in the laboratory by growth at room temperature or by supplementing media with millimolar concentrations of chemical modulators such as MgSO4 or nicotinic acid. Bacteria in the Bvg− phase are motile (B. bronchiseptica only) and form large, flat, non-hemolytic colonies. The Bvgi phase can be induced in the laboratory with intermediate concentrations of chemical modulators and these bacteria form colonies that appear phenotypically intermediate compared to Bvg− and Bvg+ phase colonies. Each phenotypic phase is defined by a unique pattern of gene expression (Figure 1B) (Cotter and Miller, 1997; Cotter and Jones, 2003; Cummings et al., 2006). For example, bacteria in the Bvg+ phase are characterized by maximal expression of virulence-activated genes (vags) such as fhaB, cyaA-E (encoding adenylate cyclase toxin ACT), ptxA-E (encoding pertussis toxin in B. pertussis), and bvgAS itself (which is positively autoregulated). In contrast, Bvg− phase bacteria maximally express virulence-repressed genes (vrgs) including those required for motility (i.e., flaA, encoding flagellin and frlAB, the Bordetella flhDC homolog) and chemotaxis (B. bronchiseptica only) but do not express vags. The vags fall into two classes: those expressed in the Bvgi phase and the Bvg+ phase, and those expressed maximally only in the Bvg+ phase. Additionally, some genes, such as bipA, (encoding Bvg-intermediate phase protein A) are expressed maximally only in the Bvgi phase (Deora et al., 2001; Williams et al., 2005). Thus, Bvgi phase bacteria are characterized by maximal expression of bipA, bvgAS, and fhaB, and minimal expression of vrgs, cyaA, and ptxA (B. pertussis only) (Figure 1B) (Cotter and Miller, 1997; Deora et al., 2001; Stockbauer et al., 2001; Williams et al., 2005; Cummings et al., 2006).
Figure 1.
The Bordetella BvgAS system controls at least four different classes of genes and three different phenotypic phases in response to environmental stimuli. A, BvgAS is responsible for the Bvg+, Bvgi, and Bvg− phases and is repressed by chemical modulators or low temperature. B, The three phenotypic phases are defined by unique patterns of gene expression as indicated, and rely on the intracellular concentration of BvgA~P. C, The chromosomal organization of the fhaB and bvgAS loci in RB50 (top) and RBX9 (ΔfhaB, bottom).
Upon activation of the BvgAS phosphorelay in response to environmental signals, BvgS (the sensor kinase) autophosphorylates, becoming the substrate for BvgA (the response regulator). BvgA-phosphate (BvgA~P) binds DNA and activates or represses transcription (Boucher, Yang, Schmidt, et al., 2001; Boucher, Yang, and Stibitz, 2001; Cotter and Jones, 2003; Boucher et al., 2003; Jones et al., 2005; Williams et al., 2005). In vitro transcription and DNA binding experiments have identified both high and low affinity BvgA binding sites located at various positions relative to the transcription start sites of BvgAS-regulated genes (Steffen et al., 1996; Boucher, Yang, and Stibitz, 2001; Cotter and Jones, 2003; Jones et al., 2005; Williams et al., 2005). These data, together with a recent report describing a direct assessment of BvgA~P levels in B. pertussis cultures (Boulanger et al., 2013), support a model in which BvgA~P levels are extremely low under Bvg− phase conditions, moderate under Bvgi phase conditions, and high under Bvg+ phase conditions (Cotter and Jones, 2003; Boulanger et al., 2013). In addition to controlling distinct phenotypic phases in response to steady-state conditions, BvgAS can regulate gene expression in a temporal manner (Scarlato et al., 1991; Prugnola et al., 1995; Jones et al., 2005; Williams and Cotter, 2007). Because bvgAS is positively autoregulated, both the concentration of BvgA and the proportion that is phosphorylated increase when bacteria sense activating signals. Therefore, gene expression patterns change temporally as the total concentration of BvgA~P gradually increases when bacteria are switched from Bvg− to Bvg+ phase conditions (Scarlato et al., 1991; Prugnola et al., 1995; Jones et al., 2005; Williams and Cotter, 2007).
The bvgAS and fhaB genes are adjacent and transcribed divergently. Experiments with B. pertussis indicate that the 426 bp intergenic region contains at least three promoters (with at least two that control bvgAS, called P2 and P1) and multiple high-affinity BvgA binding sites (Scarlato et al., 1990; Roy and Falkow, 1991; Boucher, Yang, Schmidt, et al., 2001; Boucher, Yang, and Stibitz, 2001) (Figure 1C). In the Bvg− phase, bvgAS transcription is driven by the BvgAS-independent promoter P2 that is responsible for basal levels of BvgA (which likely remain unphosphorylated) (Scarlato et al., 1990; Roy and Falkow, 1991). When switched to the Bvg+ phase, BvgA becomes phosphorylated and activates fhaB and bvgAS via binding to high-affinity sites near PfhaB and P1 (Roy and Falkow, 1991; Zu et al., 1996). Once a relatively high concentration of BvgA~P is achieved, genes with low-affinity BvgA binding sites at their promoters, such as cyaA and ptxA in B. pertussis, are activated and the bacteria transition into the Bvg+ phase (Karimova et al., 1996; Jones et al., 2005; Williams and Cotter, 2007). The Bvg+ phase, and therefore high levels of BvgA~P, is maintained as long as bacteria sense a Bvg+ phase environment. Without bvgAS positive autoregulation, the ability of B. bronchiseptica to transition between and maintain each phenotypic phase is compromised (Williams and Cotter, 2007).
Data obtained thus far indicate that the Bvg+ phase is necessary and sufficient to cause respiratory infection, the Bvg− phase facilitates survival outside of the host, and BvgAS modulation to the Bvgi or Bvg− phase does not occur during infection (Cotter and Miller, 1994; Akerley et al., 1995; Tejada et al., 1998; Merkel et al., 1998; Vergara-Irigaray et al., 2005; Nicholson et al., 2012). For example, several groups have shown that Bvg+ phase-locked bacteria behave identically to wild-type bacteria in colonization, persistence, and contribution to lung pathology (Cotter and Miller, 1994; Akerley et al., 1995; Tejada et al., 1998; Nicholson et al., 2012). In contrast, Bvg− phase-locked bacteria cannot establish an infection and Bvgi phase-locked bacteria are severely limited in colonization and persistence (Cotter and Miller, 1994; Cotter and Miller, 1997; Tejada et al., 1998; Vergara-Irigaray et al., 2005; Nicholson et al., 2012). Additionally, we recently demonstrated that flaA is not expressed at a detectable level when mice are infected with the B. bronchiseptica wild-type strain RB50 (Byrd et al., 2013) and Akerley et. al. showed that production of flagella in the Bvg+ phase is detrimental to infection (Akerley et al., 1995). Although the natural signals that affect BvgAS activity and the role of modulation in nature remain unknown, all of these data suggest that wild-type Bordetella do not modulate to the Bvg− phase within the mammalian host.
B. bronchiseptica strain RBX9, which contains an in-frame deletion mutation of fhaB (Figure 1C), has been used extensively to characterize the function of FHA in vitro and in vivo (Cotter et al., 1998; Irie et al., 2004; Inatsuka et al., 2005; Mazar and Cotter, 2006; Julio et al., 2009; Henderson et al., 2012). RBX9 is defective in adherence to multiple cell lines, is unable to autoaggregate in liquid culture, and causes hyperinflammation in the murine lung infection model (Cotter et al., 1998; Mattoo et al., 2000; Inatsuka et al., 2005; Julio et al., 2009; Henderson et al., 2012). We isolated large colony variants (LCVs) from mice infected with RBX9 and also by modulating RBX9 to the Bvg− phase in vitro. We determined that the LCVs were a product of transcriptional interference that influenced bvgAS and produced an unusual bistable phenotype. Despite evidence suggesting that Bordetella do not modulate during infection, the discovery of LCVs indicates that a subpopulation of RBX9 bacteria modulates in vivo.
Results
Isolation and characterization of LCVs
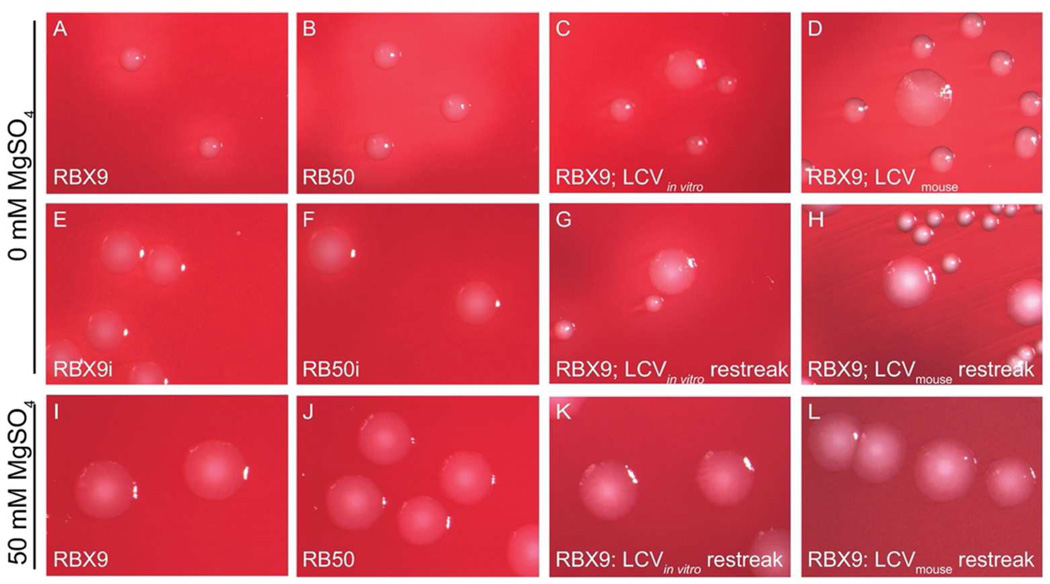
While comparing wild-type B. bronchiseptica strain RB50 with its ΔfhaB derivative RBX9 in a murine lung infection model, we noticed that at early time points post-inoculation (12 and 24 h), a small proportion (~1%) of cfus recovered from the lungs of RBX9-inoculated mice formed colonies on BG blood agar (Bvg+ phase conditions) that were larger, flatter, and less hemolytic than colonies typically formed by RBX9 and RB50 (Table 1) (Figure 2D). These Large Colony Variants (LCVs) were not recovered from RB50-infected mice. When LCVs were picked, diluted, and replated on BG blood agar, approximately 95% of the resulting colonies were phenotypically Bvg+ phase, and approximately 5% were LCVs (Table 1) (Figure 2H). When replated again, LCVs continued to yield 95% Bvg+ phase colonies and 5% LCVs. All phenotypically Bvg+ phase colonies yielded only phenotypically Bvg+ phase colonies after replating onto BG agar.
Figure 2.
RB50 and RBX9 colony morphology. Bacteria were plated on either BG agar or BG agar + 50mM MgSO4 and were imaged after 48h. A, RB50; B, RBX9; C, RBX9 LCV produced after in vitro modulation: D, RBX9 LCV recovered from mouse lung homogenate; E, RB50i (a Bvg-intermediate phase-locked strain in the RB50 background); F, RBX9i (a Bvg-intermediate phase-locked strain in the RBX9 background); G, RBX9 restreak of an LCV produced after modulation; H, RBX9 restreak of an LCV recovered from the mouse lung; I, RB50; J, RBX9; K, RBX9 restreak of an LCV produced after modulation; L, RBX9 restreak of an LCV recovered from the mouse lung.
Table 1.
LCV recovery frequency
| Condition | %a LCVs recovered on BG blood agar |
|---|---|
| Plating murine lung homogenate after 30 hours | 0.39 ± 0.13 |
| Replating any RBX9 Bvg− phase colony | 5.43 ± 0.98 |
| Replating an LCV from BG agar (derived in vitro) | 3.90 ± 0.80 |
| Replating an LCV from BG agar (derived in vivo) | 6.03 ± 1.26 |
| Replating any RBX9 Bvg+ phase colony | 0 |
Values are means ± standard errors for experiments performed at least in triplicate
We found serendipitously that LCVs were also induced in vitro under certain conditions. Specifically, when RBX9 was grown on BG blood agar + 50mM MgSO4 (Bvg− phase conditions) and then replated onto BG blood agar—effectively switching the bacteria from Bvg− to Bvg+ phase conditions—approximately 95% of the colonies were phenotypically Bvg+ phase and 5% were LCVs (Table 1) (Figure 2C). When these LCVs were picked, diluted, and replated onto BG blood agar, approximately 95% of colonies displayed the Bvg+ phase morphology and approximately 5% of colonies were LCVs (Table 1) (Figure 2G). Again, all phenotypically Bvg+ phase colonies yielded only phenotypically Bvg+ phase colonies after replating onto BG agar. When RBX9 was streaked onto BG agar supplemented with 50mM MgSO4, or passaged continuously under Bvg− phase conditions, all colonies displayed typical Bvg− phase morphology.
To determine if the generation of LCVs in RBX9 was due to the ΔfhaB mutation and not an unknown secondary mutation, we reconstructed strain RBX9 by allelic exchange. The newly constructed strain behaved identically to RBX9, producing LCVs following BvgAS modulation and generating a similar proportion of LCVs upon restreaking an LCV onto BG blood agar. Although LCVs appear morphologically similar to Bvgi phase colonies (Figure 2), the fact that restreaking LCVs yielded a heterogeneous population of morphologically different colonies indicates that the ΔfhaB mutation does not lock bacteria into one particular phenotypic phase (such as the Bvgi phase.)
LCVs are composed of both Bvg− and Bvg+ phase bacteria
To better understand the properties of LCVs, we investigated specific gene expression patterns within the bacteria that composed them. The fact that LCVs are hemolytic suggests that cyaA, a Bvg+ phase gene, is expressed because ACT is responsible for hemolysis on BG blood agar (Bellalou et al., 1990). Additionally, electron micrographs of negatively stained LCVs revealed the presence of numerous flagella, which are only produced in the Bvg− phase (data not shown). To determine if bacteria within LCVs were motile, we grew bacteria on Stainer-Scholte plates with 0.3% agar (Bvg+ phase conditions). LCVs stab-inoculated into this agar produced a zone of migration that was smaller than that produced by Bvg− phase-locked bacteria, but larger than Bvg+ and Bvgi phase bacteria, which do not produce a zone of migration (data not shown), suggesting that at least some bacteria within LCVs are motile. Together, our observations indicate that both cyaA and flaA are expressed within each LCV. However, previous studies have shown that expression of vags, such as cya, and vrgs, such as flaA, is mutually exclusive (Cummings et al., 2006). Therefore, we hypothesized that LCVs are composed of at least two phenotypically distinct populations of bacteria: a population in the Bvg+ phase and a population in the Bvg− phase.
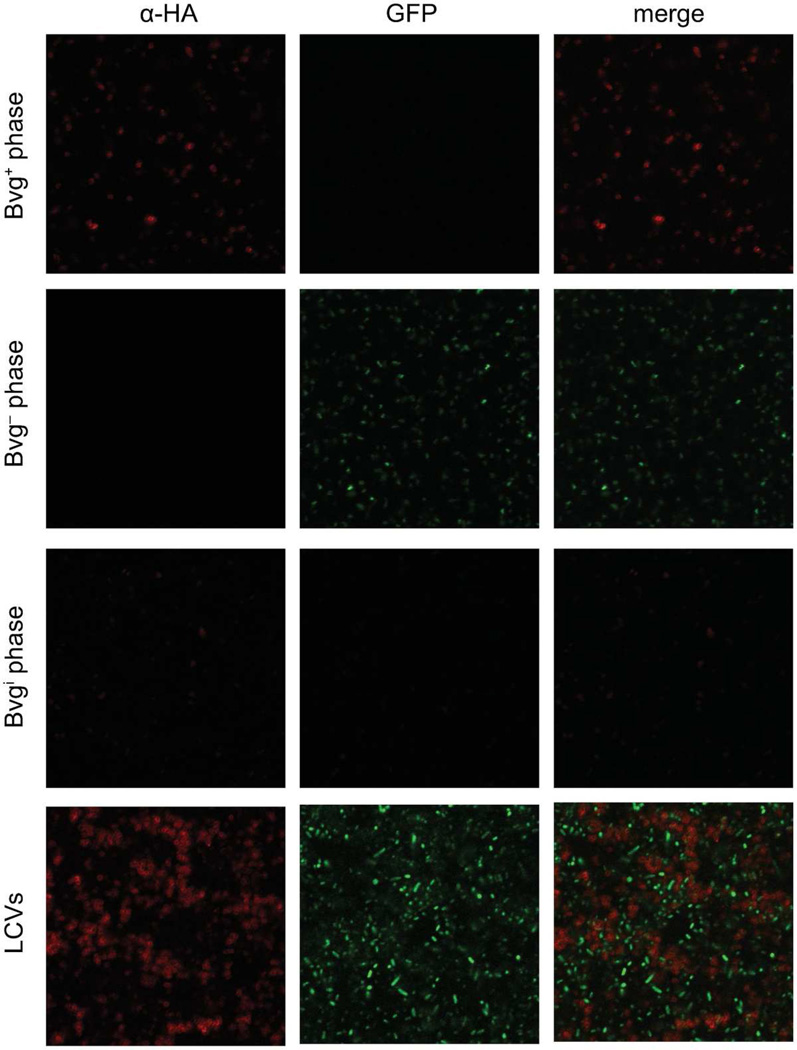
To determine the phenotypes of individual bacteria present in each LCV, we created RBX9BatB-HAfla-gfp, a strain that contains two unique tags that permit the distinction between Bvg+ and Bvg− phase bacteria. RBX9BatB-HAflaA-gfp contains an HA epitope-encoding sequence in batB (encoding the Bvg+ phase surface-exposed protein BatB) as well as gfp driven by the flaA promoter at a neutral site in the chromosome. The batB gene is expressed maximally in the Bvg+ phase and minimally in the Bvgi and Bvg− phases (Williams et al., 2008). The flaA gene, as described previously, is a typical vrg and contains a strong promoter that is active only in the Bvg− phase (Akerley et al., 1992; Cotter and Jones, 2003). Therefore, Bvg+ phase bacteria should produce a surface-exposed HA-tagged BatB protein and be GFP− and Bvg− phase bacteria should be GFP+ and lack a surface-exposed HA epitope.
We used Alexa-Fluor 594-conjugated antibodies to indirectly recognize HA epitopes so that BatB-producing bacteria displayed red fluorescence. Bacteria expressing fla-gfp produced GFP and displayed green fluorescence. When Bvg+ phase colonies of RBX9BatB-HAfla-gfp were stained with anti-HA and an Alexa-Fluor 594-conjugated secondary antibody, only red-fluorescing bacteria were observed and no green fluorescence was detected (Figure 3). When Bvg− phase colonies were stained, only green-fluorescing bacteria were observed and no red fluorescence was detected. When Bvgi phase colonies were stained, a small proportion of cells displayed red or green fluorescence, but the majority of cells were not fluorescent and no bacteria displayed both red and green fluorescence (Figure 3). In contrast, when LCVs from RBX9BatB-HAfla-gfp were stained, approximately half of the bacteria fluoresced red and approximately half fluoresced green (Figure 3). No co-localization of red and green fluorescence from either LCVs or Bvgi phase colonies was observed, confirming that the expression of vags and vrgs is mutually exclusive under these conditions. These data demonstrate that LCVs are composed of both Bvg+ and Bvg− phase bacteria and are not a homogeneous population of Bvgi phase bacteria.
Figure 3.
Detection of Bvg+ (α-HA, red) and Bvg− phase (flaA-gfp, green) bacteria in typical RBX9 colonies and LCVs. RBX9BatBN-HAflaA-gfp was grown on BG blood agar (Bvg+ phase conditions), BG blood agar + 50 mM MgSO4 (Bvg− phase conditions), or BG blood agar + 6 mM MgSO4 (Bvgi phase conditions). Several colonies of each phenotype were combined and stained with mouse monoclonal α-HA IgG followed by an Alexa Fluor 594-conjugated goat anti-mouse IgG secondary antibody. Fluorescence was detected using a Zeiss LSM 710 confocal microscope.
The ΔfhaB mutation in RBX9, but not lack of FHA protein, is responsible for the LCV phenotype
To determine if the generation of LCVs was due to lack of FHA protein production or the specific genetic architecture created by the ΔfhaB mutation in RBX9, we first determined if other fhaB mutants yielded LCVs. Strain RB50ΔPfhaB contains a deletion mutation of the fhaB promoter, strain RB50ΔSPfhaB contains a deletion mutation in fhaB such that the extended signal peptide of FHA is missing, and strain RB50ΔβhelixfhaB contains a large deletion mutation in the region of fhaB encoding the β-helix structure (Figure S1). These strains were analyzed for FHA production by western blot, and either produced no FHA protein (RB50ΔPfhaB and RB50ΔSPfhaB) or a severely truncated FHA protein (RB50Δβ-helix) (data not shown). We grew these strains under Bvg− phase conditions and plated single colonies onto Bvg+ phase conditions to determine if they would produce LCVs similar to RBX9. No LCVs were observed, suggesting that a lack of wild-type FHA protein is not sufficient to produce the LCV phenotype.
To investigate the contribution of the genetic architecture created by the fhaB deletion to the LCV phenotype, we created RB50::pBam, a strain that produces FHA and contains an altered fhaB-bvgAS locus (Figure S1). RB50::pBam was created by integrating pBam, a suicide plasmid containing the fhaB-bvgAS intergenic region, into the RB50 chromosome. When integrated in the chromosome, the genetic architecture 5’ to bvgAS is similar in that fhaB coding sequences are replaced with non-native sequences (those from the suicide plasmid, in this case). By contrast with RBX9, however, the complete, intact fhaB gene, including its promoter region, is present in RB50::pBam – it is located 5’ (relative to bvgAS) to the integrated plasmid sequences. After modulation, RB50::pBam produced LCVs similar to RBX9. These data suggest that the LCV phenotype can be produced by altering the genetic architecture 5′ to the fhaB-bvgAS intergenic region and is independent of FHA production.
The LCV phenotype results from a defect in bvgAS positive autoregulation
In addition to activating all of the known virulence factor-encoding genes in Bordetella, BvgAS activates bvgAS expression through positive autoregulation. Williams et al. demonstrated that positive autoregulation is required for the precise transition between and maintenance of the Bvg+, Bvgi, and Bvg− phases (Williams and Cotter, 2007). Three observations suggested that LCVs resulted from defective bvgAS autoregulation in RBX9. First, the mutations that cause LCVs are genetically linked (immediately 5′) to bvgAS; second, LCVs consist of bacteria in least two separate BvgAS-controlled phenotypic phases; and third, LCVs were induced in vitro following a switch from Bvg− to Bvg+ phase growth conditions.
We hypothesized that when RBX9 (or RB50::pBam) bacteria are switched from Bvg− phase conditions to Bvg+ phase conditions, most, like all wild-type bacteria, are able to activate transcription at the bvgAS P1 promoter, leading to increased BvgAS levels and resulting in the transition to and maintenance of the Bvg+ phase. According to our hypothesis, however, a small subset of RBX9 and RB50::pBam bacteria are unable to activate bvgAS transcription, possibly due to insufficient levels of BvgA and/or BvgS, and these bacteria are therefore “trapped” in the Bvg− phase. We hypothesized further that although some descendants of these “Bvg− phase-trapped” bacteria will be able to activate bvgAS transcription and hence “escape” to the Bvg+ phase, many will remain Bvg− phase-trapped and thus a substantial Bvg− phase population will be maintained in the LCV. An alternate hypothesis is that LCVs arise from spontaneous or transient shifting of bacteria between the Bvg+ and Bvg− phases, which would also result in a mixture of Bvg+ and Bvg− phase bacteria within a single colony.
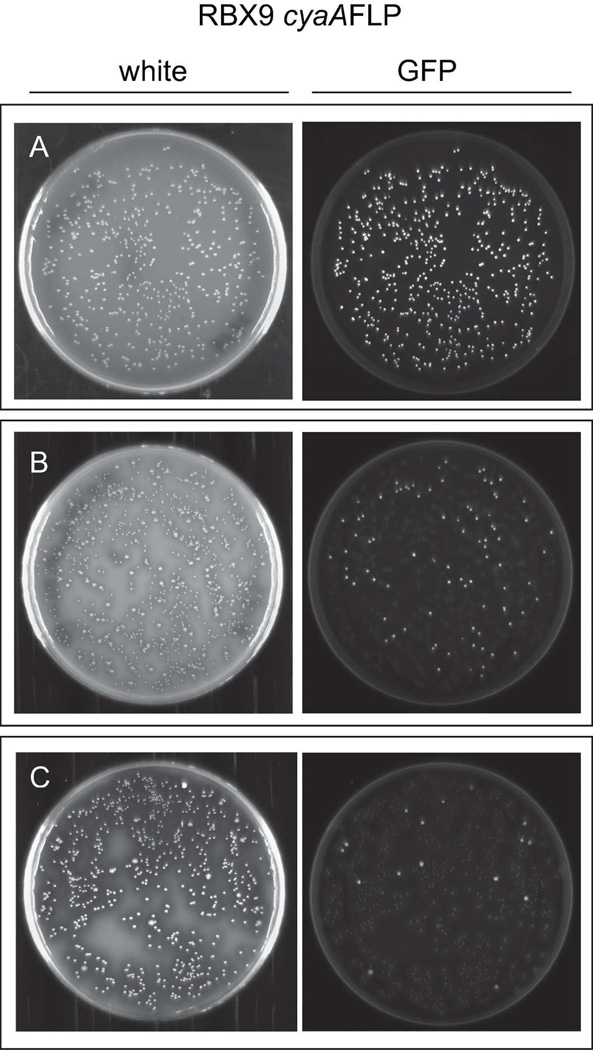
To determine if LCVs contain Bvg− phase-trapped bacteria, we used the recombinase-based reporter system pGFLIP, which creates a permanent genetic change in response to gene activation (Byrd et al., 2013). In this system, a promoter of interest drives expression of the site-specific recombinase-encoding gene flp, which when activated, results in recombination between Flp recombinase target (FRT) sites that flank gfp and the kanamycin (Km) resistance gene nptII. Therefore, any activation (even transient, low-level expression) of flp results in a permanent loss of Km resistance and GFP fluorescence. This system targets the reporter construct to the neutral attTn7 site 3’ to glmS. We created strain RBX9cyaAFLP by mating the plasmid pGFLIP-PcyaA, in which the B. bronchiseptica cyaA promoter drives flp expression, into RBX9. The cyaA gene is exclusively controlled by BvgAS, is highly expressed in the Bvg+ phase, and is expressed minimally in the Bvg− phase (Cotter and Jones, 2003). In RBX9cyaAFLP, bacteria that have never expressed cyaA should remain GFP+ and Kmr, whereas bacteria that have expressed cyaA should convert to GFP− and Kms. If gfp is lost due to PcyaA activation, all descendent cells will also be GFP− and Kms.
Previously, we demonstrated that RB50cyaAFLP bacteria remain GFP+ under Bvg− phase conditions with Km selection and that they reach 100% resolution (GFP− cfu/total cfu) when grown under Bvg+ phase conditions (Byrd et al., 2013). When RBX9cyaAFLP was plated under Bvg− phase conditions with Km selection, each colony was morphologically identical and fluorescent, indicating that cyaA had not been activated to a level required for sufficient flp expression to lead to recombination between FRT sites (Figure 4A). In contrast, when a colony of RBX9cyaAFLP that was grown under Bvg− phase conditions was plated and grown under Bvg+ phase conditions, approximately 15% of the colonies were LCVs and approximately 80% of those LCVs were GFP+ (Figure 4B). None of the Bvg+ phase colonies were GFP+. When a GFP+ LCV was replated and grown under Bvg+ phase conditions, approximately 5% of the colonies were LCVs and approximately 80% of those were GFP+ (Figure 4C). We serially replated GFP+ LCVs eight times and in all cases, additional GFP+ LCVs were generated (data not shown). These data indicate that a significant proportion of bacteria within a GFP+ LCV had never activated cyaA and had therefore failed to switch to Bvg+ phase in response to a change in conditions; i.e., they were Bvg− phase-trapped. Moreover, our data suggest that all LCVs arise from a Bvg− phase-trapped bacterium and that upon subsequent multiplication, most descendants have “escaped” to the Bvg+ phase but a small proportion remain trapped in the Bvg− phase. These data do not support a model in which LCVs consist of bacteria that transiently fluctuate between Bvg+ and Bvg− phase, because if this was true, LCVs would not be GFP+.
Figure 4.
LCVs from the strain RBX9PcyaAFLP are GFP+, indicating that cyaA has not been activated in a substantial proportion of these colonies. A, RBX9PcyaAFLP on BG blood agar + 50mM MgsO4 (Bvg− phase conditions); B, a GFP+ colony from A plated onto BG blood agar (Bvg+ phase conditions); C, a GFP+ LCV from B plated onto BG blood agar (Bvg+ phase conditions). Colonies were visualized after 48h of growth.
Approximately 20% of the LCV colonies were GFP−, indicating that PcyaA-flp expression was sufficient to mediate recombination in the bacterium that founded the LCV or in its early descendants. Although this result appears inconsistent with our model, we have observed previously that when RB50cyaAFLP is grown under Bvg− phase conditions (when cyaA expression is minimal) and without Km selection, PcyaA-flp is expressed sufficiently in approximately 15% of bacteria such that they convert to GFP− and KmS (Byrd et al., 2013). These data indicate that the cyaA promoter activity under Bvg− phase conditions is near the threshold level required for flp expression and subsequent recombination. Therefore, GFP− LCVs are most likely due to the activity level of the cyaA promoter under Bvg− phase conditions and not due to bacteria switching to the Bvg+ phase and then back to the Bvg− phase. Nonetheless, our data indicate that in approximately 80% of LCVs, there are a substantial number of bacteria that appear to have a defect in bvgAS positive autoregulation, leading to the observed Bvg− phase-trapped population.
Sequences upstream of the fhaB-bvgAS intergenic region affect the efficiency of bvgAS activation in the Bvg− phase
Our data indicate that bvgAS autoregulation is defective in RBX9 and specifically, that LCVs are composed of a subpopulation of Bvg− phase-trapped bacteria. As stated in the introduction, bvgAS expression is controlled primarily by two promoters. Studies with B. pertussis have shown that under Bvg− phase conditions, P2 is transcribed at a low basal level (Scarlato et al., 1990; Roy and Falkow, 1991). This level of transcription results in BvgS levels that are sufficient to respond to Bvg+ phase conditions by autophosphorylating and mediating phosphorylation of BvgA. The resulting BvgA~P levels are sufficient to bind at the bvgAS P1 promoter, recruit RNAP, and activate transcription (Zu et al., 1996). (Although similar transcriptional analyses have not been conducted with B. bronchiseptica, the nucleotide sequence of the fhaB-bvgAS intergenic region in B. bronchiseptica is %91.1% identical and most of those differences are located in regions that, based on B. pertussis studies, are not bound by either BvgAS or RNAP.) We considered two hypotheses: 1) the level of transcription from P2 in RBX9 is lower than in RB50 such that in some bacteria the levels of BvgAS are too low to activate transcription at P1 in response to Bvg+ phase conditions, and 2) transcription activation at P1 by BvgA~P is somehow defective in RBX9 compared to RB50. Our data suggest that defective autoregulation in RBX9 is due to the lack of native sequences or presence of non-native sequences 5′ to the fhaB-bvgAS intergenic region.
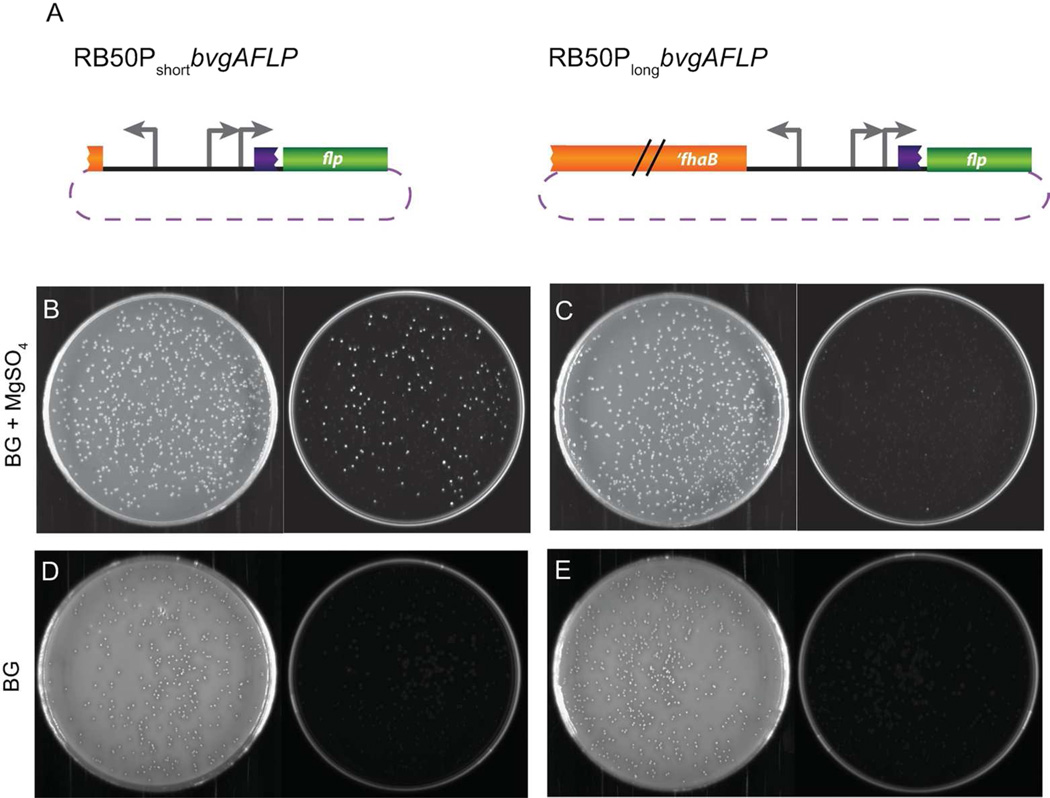
To test our hypotheses, we constructed RB50PshortbvgAFLP and RB50PlongbvgAFLP, where RB50PshortbvgAFLP contains only the fhaB-bvgAS intergenic region driving flp and RB50PlongbvgAFLP contains this region plus an additional 1200 bp of fhaB sequences driving flp (Figure 5A). These strains were constructed under Bvg− phase conditions in the presence of Km. To investigate P2 expression, we determined the percent resolution (the percentage of cfu that had activated flp) of each strain grown under Bvg− phase conditions by counting the ratio of GFP− cfu to total cfu when one colony was plated from Bvg− phase conditions with Km selection to Bvg− phase conditions without Km selection. The average resolution under Bvg− phase conditions in RB50PshortbvgAFLP was 68% whereas the average resolution in RB50PlongbvgAFLP was 97%. Plates are shown from one representative experiment (Figure 5B, C). Our data indicate that the per-cell activation of RB50PshortbvgAFLP is lower than the per-cell activation of RB50PlongbvgAFLP under Bvg− phase conditions, and demonstrate that either a lack of native fhaB sequences or the presence of non-native sequences has a direct effect on the efficiency of bvgAS transcription from the P2 promoter in the Bvg− phase.
Figure 5.
Sequences upstream of bvgAS affect transcription efficiency under Bvg− phase conditions. A, Schematics of RB50PshortbvgAFLP and RB50PlongbvgAFLP showing the sequences 5′ to flp (not drawn to scale); B and C, strains were first grown on BG blood agar + 50 mM MgSO4 + Km and one colony was plated onto BG blood agar + 50 mM MgSO4 (Bvg− phase conditions) without Km selection; D and E, strains were grown on BG blood agar + 50 mM MgSO4 + Km selection and then one colony of each was plated onto BG blood agar (Bvg+ phase conditions) without selection. Representative white light (left) and fluorescent (right) images are shown for panels B, C, D, and E.
When a colony from each strain was grown on Bvg− phase conditions with Km selection and then plated onto Bvg+ phase conditions without Km selection, maximum resolution was achieved and there were no GFP+ colonies in either strain (Figure 5D, E). These data suggest that RBX9 does not have a defect in transcription activation at P1.
The LCV phenotype is caused by a (divergent) promoter in proximity to bvgAS
Based on our results, we hypothesized that sequences within fhaB (1–1200 nucleotides of the coding region) 5′ to the fhaB-bvgAS intergenic region effect the low level of transcription from P2 that occurs under Bvg− phase conditions. To test this hypothesis, we constructed a strain containing a deletion mutation from nt 8 to 1256 of fhaB (Figure S1, C). This mutant did not produce LCVs when switched from the Bvg− to the Bvg+ phase. We conclude that the lack of specific sequences within the first 1200bp of fhaB does not cause the LCV phenotype.
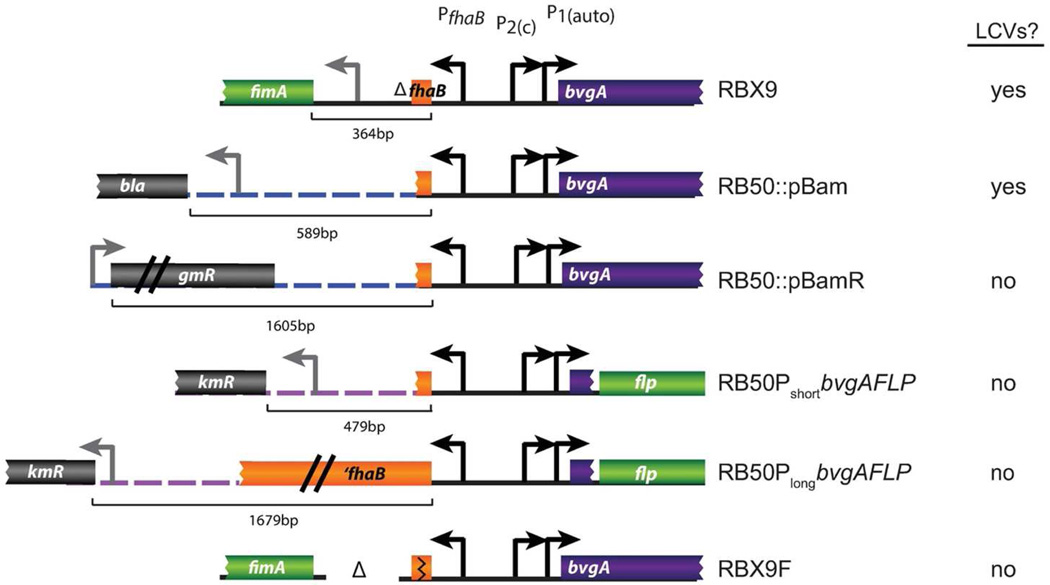
These data led us to closely reexamine the genetic architecture of each strain that produced LCVs (RBX9 and RB50::pBam) as well as the strains that showed a difference in bvgAS-flp reporter activation (RB50PshortbvgAFLP and RB50PlongbvgAFLP). A comparison of these strains revealed the presence of a divergent promoter 5′ to the fhaB-bvgAS intergenic region in RBX9, RB50::pBam, and RB50PshortbvgAFLP (Figure 6). In RBX9, the fimA promoter is very close to the fhaB-bvgAS intergenic region, in contrast to RB50 in which it is separated by the entire (>12kb) fhaB gene. In RB50::pBam, the bla promoter (driving expression of the ampicillin resistance gene on the plasmid) is adjacent to the fhaB-bvgAS intergenic region. In the strain RB50PbvgAshortFLP, the npt promoter (driving expression of the kanamycin resistance gene on the plasmid) is proximal to PbvgA-flp, whereas the RB50PbvgAlongFLP reporter is “buffered” from the same npt promoter by an additional 1200bp of fhaB (Figure 6). We hypothesized that the presence of a promoter 5′ to the bvgAS-fhaB intergenic region was interfering with bvgAS P2 transcription, possibly by sequestering RNA polymerase away from P2. To test the hypothesis that a nearby promoter could affect P2 transcription, we reversed the orientation of the insert in the plasmid pBam. In the resulting plasmid, pBamR, the bla promoter is no longer proximal to the bvgAS homology region. Instead, the closest promoter 5’ to bvgAS on the plasmid is the aaC1 promoter (driving expression of the gentamicin resistance gene), which is more than 1.5kb away (Figure 6). We created RB50::pBamR by integrating the pBamR plasmid into the RB50 chromosome. Modulating RB50::pBamR and plating bacteria onto Bvg+ phase conditions resulted in all colonies having the typical Bvg+ phase morphology, and no LCVs were observed. The LCV phenotype was therefore abolished by changing the sequences upstream of the bvgAS-fhaB region, presumably by increasing the distance between a promoter and bvgAS. Additionally, we deleted the intergenic region between fhaB and fimA (which includes the fimA promoter) in RBX9. The resulting strain RBX9F did not produce LCVs after modulation. These data strongly support a model in which a promoter upstream of bvgAS interferes with normal P2 transcription efficiency, resulting in some cells having an insufficient quantity of BvgAS to activate transcription at P1.
Figure 6.
The genetic architecture of the bvgAS-fhaB intergenic region of strains that do and do not produce LCVs. Strains that produce LCVs or demonstrate a defect in bvgAS-flp activation have divergent promoters 5′ to the bvgAS-fhaB intergenic region. Schematics for RB50PshortbvgAFLP and RB50PlongbvgAFLP represent sequences inserted at the attTn7 site. Dotted lines represent non-coding plasmid DNA. Sequence lengths from the ATG of fhaB to the nearest 5’ ATG are indicated.
Each Bvg− phase-trapped bacterium within an LCV initiates the formation of a new LCV and the proportion of Bvg− phase-trapped bacteria within an LCV decreases over time
Our data indicate that LCVs are founded by a single Bvg− phase-trapped bacterium and that each LCV harbors Bvg− phase-trapped bacteria that can propagate additional LCVs. However, it is unclear whether all Bvg− phase-trapped bacteria, or only a subset of these cells, yield LCVs upon replating. To address this question, we needed two pieces of information: the proportion of Bvg− phase-trapped bacteria within one LCV and the frequency of new LCV formation from the same parent colony when replated. We used RBX9cyaAFLP to evaluate the composition and LCV-forming capacity of single LCVs.
Plating a GFP+ LCV onto Bvg− phase conditions, in which cyaA expression is minimal, will minimize further Flp-mediated recombination due to cyaA activation during colony formation, permitting us to determine the proportion of GFP+ bacteria (and hence Bvg− phase-trapped) that existed within the original LCV at that time. Plating the same GFP+ LCV onto Bvg+ phase conditions allows us to determine the number of new LCVs generated from the subpopulation of Bvg− phase-trapped bacteria in the parent LCV. Comparing the frequency of newly generated LCVs under Bvg+ phase conditions to the frequency of GFP+ cfu under Bvg− phase conditions will reveal the proportion of Bvg− phase bacteria that form LCVs when replated.
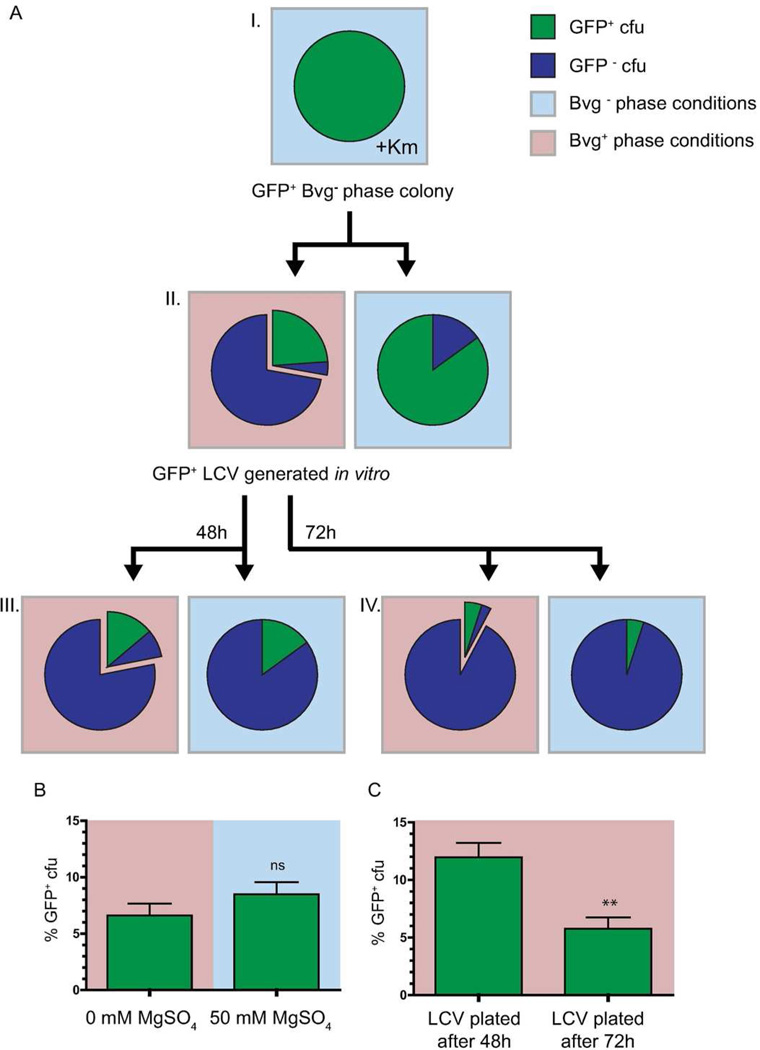
We first grew RBX9cyaAFLP on Bvg− phase conditions with Km selection to maintain the gfp and Kmr markers (Figure 7A I). Then we took single GFP+ (Bvg− phase) colonies and plated them onto Bvg+ and Bvg− phase conditions (Figure 7A II). GFP+ LCVs that were recovered from Bvg+ phase plates were then plated again onto Bvg+ and Bvg− phase conditions (Figure 7A III). The frequencies of GFP+ cfu, LCVs, and Bvg+ phase colonies from each plate were recorded, and the results from one representative experiment are shown in Figure 7A. When the GFP+ LCVs were plated onto Bvg+ and Bvg− phase conditions, there was no significant difference in the average number of GFP+ cfu under Bvg− phase conditions compared to the average number of GFP+ LCVs on the corresponding Bvg+ phase BG blood agar plate (Figure 7B). These data suggest that each Bvg− phase bacterium within an LCV is Bvg− phase-trapped and forms an LCV when replated onto Bvg+ phase conditions.
Figure 7.
A, Schematic of RBX9cyaAFLP experimental design, including a data set from one replicate. Each pie chart represents the population obtained from plating a single GFP+ colony from the previous plate (see text for details). Blue sectors in pie charts represent the frequency of GFP− cfu; Green sectors in pie charts represent the frequency of GFP+ cfu; offset regions of pie charts represent the frequency of LCVs; Frequencies were determined by counting at least 500 cfu per condition. B, Comparison of GFP+ cfu frequencies obtained from plating a single GFP+ LCV onto Bvg+ and Bvg− phase conditions. C, Comparison of GFP+ cfu frequencies obtained from plating a GFP+ LCV grown after 48h and 72h. Background color represents BvgAS conditions, where red is Bvg+ phase conditions and blue is Bvg− phase conditions. **, P = 0.005 by Student’s Unpaired T-test.
Additionally, we asked if the composition of LCVs (i.e., the ratio of Bvg− to Bvg+ phase bacteria) changed over time. We hypothesized that this ratio would change due to bacterial division as well as the rate of conversion of Bvg− phase-trapped bacteria to Bvg+ phase bacteria. Because we expected a unidirectional conversion of phenotypes (Bvg− to Bvg+ phase only) under Bvg+ phase conditions, we predicted that the ratio of Bvg− to Bvg+ phase bacteria would decrease as the bacterial population increased. To determine if the compositions changed after an additional day of growth, we compared GFP+ LCVs plated after our standard incubation time (48h) (Figure 7A III) to GFP+ LCVs plated after 72 h (Figure 7A IV). When GFP+ LCVs were plated after 48h of incubation, we obtained an average of 12 ± 1.2% GFP+ cfu under Bvg− phase conditions, whereas after 72h, we obtained an average of 5.75 ± 1% GFP+ cfu under the same conditions (P=0.005) (Figure 7C). These data indicate that the frequency of GFP+ (and therefore Bvg− phase-trapped) bacteria in an LCV decreases over time.
These data also strongly suggest (as discussed in a previous result) that GFP− LCVs are a result of the background activation of cyaA in RBX9PcyaAflp, as the background cyaA-flp activation under conditions of inactivity (Bvg− phase conditions) was the same as the frequency of GFP− LCVs (to total LCVs) under Bvg+ phase conditions (Figure 7A II).
Modulation of RBX9 in vivo occurs at a very low frequency
All data published thus far strongly suggest that wild-type Bordetella do not modulate to the Bvgi or Bvg− phase in vivo and that the Bvg+ phase is necessary and sufficient for infection (Cotter and Miller, 1994; Akerley et al., 1995; Tejada et al., 1998; Merkel et al., 1998; Vergara-Irigaray et al., 2005; Nicholson et al., 2012). The recovery of LCVs from mouse lung homogenates and the fact that LCVs were recovered in vitro only following modulation, however, supports the hypothesis that RBX9 modulates during infection. To test this hypothesis, we constructed strain RBX9flaAFLP, a strain containing the pGFLIP cassette in which the flaA promoter drives expression of flp (Byrd et al., 2013). Previously, using the same PflaA-flp containing cassette in wild-type RB50, we showed that flaA was not activated to a detectable level in RB50 during murine infection (Byrd et al., 2013). RB50flaAFLP and RBX9flaAFLP bacteria were grown under Bvg+ phase conditions with Km selection to minimize background resolution prior to inoculation. Mice were inoculated intranasally with 7.5×104 – 1×105 cfu and lungs were harvested at 3, 24, 30 and 72 hours post-inoculation. We conducted this experiment several times. In all experiments, a low proportion (≤1%) of GFP− bacteria was recovered from the lungs of both RB50flaAFLP- and RBX9flaAFLP-inoculated animals (data not shown). This low proportion was not significantly different, however, from the proportion of GFP− bacteria present in the samples used for inoculation (plated after inoculating the animals). Data from previous work with RB50flaAFLP (Byrd et al., 2013) and our experiments with RBX9flaAFLP indicate that resolution of the PflaA-flp cassette is BvgAS-dependent, and GFP− bacteria were not recovered from strains containing the bvgS-C3 mutation, which locks the bacteria in the Bvg+ phase (data not shown). For the RBX9flaAFLP-inoculated animals, most of the GFP− colonies recovered from the mouse lungs were LCVs and no GFP+ LCVs were recovered, indicating that formation of LCVs in vivo correlates with, and is most likely caused by, BvgAS modulation. Together, these data suggest that a very small proportion of RBX9 modulates to the Bvg− phase during infection. However, our data neither support nor refute the possibility that a small number of RB50 bacteria modulates as well.
To determine if bacteria modulate to the Bvgi phase during infection, we attempted to construct strains with Bvgi phase promoters, including the bipA promoter, driving flp. However, we were unable to construct these strains, presumably because the level of expression of these genes in Bvg+ phase conditions was above the threshold of flp activation required for recombination and loss of GFP and KmR.
If BvgAS modulation occurs in vivo, it does not alter the outcome of infection
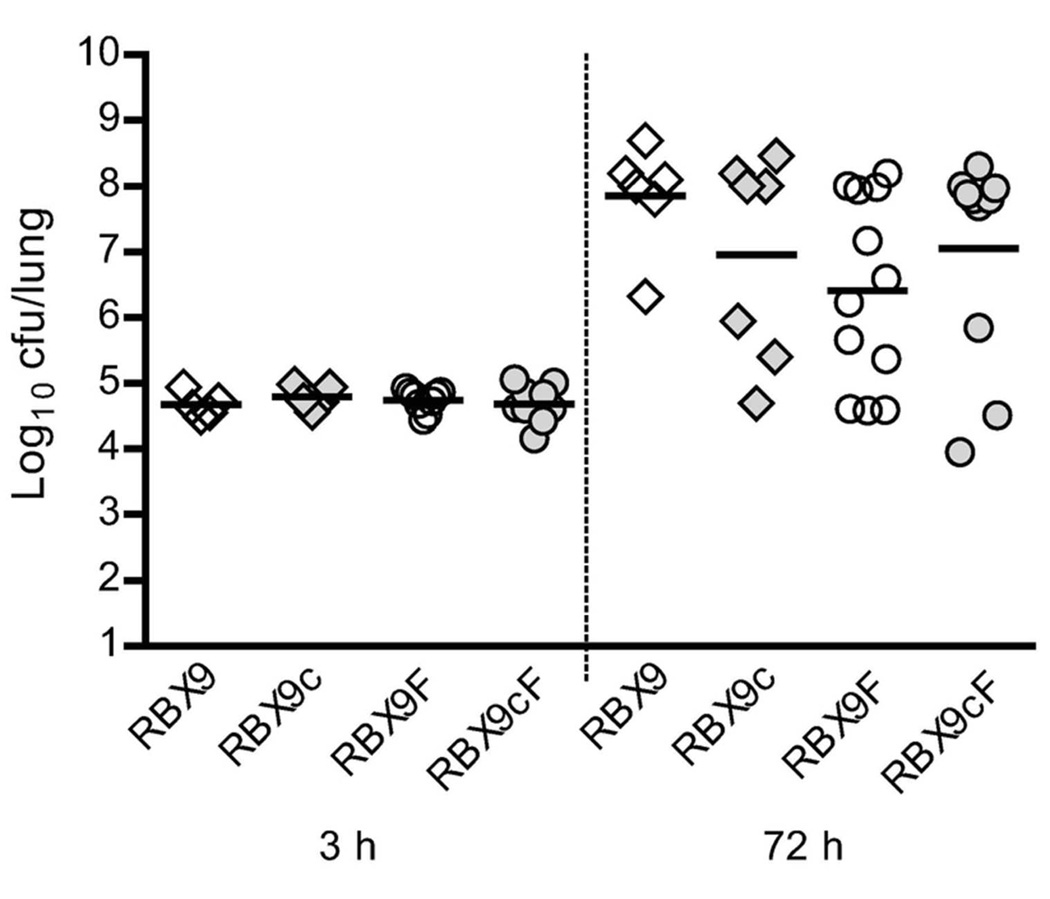
Our data suggest that a small proportion RBX9 (and possibly RB50) bacteria may modulate to the Bvg− phase during infection. Although several previous experiments have shown that wild-type and Bvg+ phase-locked B. bronchiseptica strains are indistinguishable in animal models (Cotter and Miller, 1994; Tejada et al., 1998; Merkel et al., 1998; Vergara-Irigaray et al., 2005; Nicholson et al., 2012), we considered the possibility that the proportion of RBX9 bacteria that modulate in vivo could actually be greater than that of RB50, but not apparent from the PflaA-flp data because modulated RBX9 bacteria are killed in the host (i.e., that modulated bacteria, and perhaps specifically modulated RBX9 bacteria, are more susceptible to host-mediated clearance than modulated RB50 bacteria). To test this hypothesis, we compared RBX9, RBX9c (the Bvg+ phase-locked derivative of RBX9), RBX9F (the ΔPfimA derivative of RBX9 which is not defective for bvgAS autoregulation), and RBX9cF (a Bvg+ phase-locked derivative of RBX9F) in mice. The results of three independent experiments are shown in Figure 8. In no case did a statistically significant difference in bacterial burden occur amongst the various strains. These data negate our hypothesis and provide strong evidence that the low level of BvgAS modulation that occurs in vivo (based on the recovery of LCVs) does not impact the outcome of infection.
Figure 8.
Comparison of RBX9, RBX9c, RBX9F, and RBX9cF burdens in the mouse lung after 3h and 72h p.i. RBX9c and RBX9cF are Bvg+ phase-locked derivatives of RBX9 and RBX9F, respectively; four-to eight-week-old BALB/C mice were intranasally infected with 1×105 cfu in 50µl and lungs were harvested at each time point; each diamond or circle indicates the number of cfu recovered from a single animal and each horizontal line indicates the geometric mean for each group; these data represent three independent experiments with at least two mice per strain per time point.
Discussion
The discovery and characterization of LCVs in B. bronchiseptica yielded several interesting findings, the most significant being evidence that transcriptional interference can result from activity at a promoter located several hundred nucleotides 5′ to the affected promoter. Given this relatively large distance between promoters, the mechanism of interference likely does not involve direct blocking of transcription; therefore, we suggest the name “passive transcriptional interference” for this phenomenon. Our data indicate that the bvgAS P2 promoter is sensitive to passive transcriptional interference and that it results in the emergence of a bistable phenotype, apparent as LCVs, when bacteria are switched from Bvg− phase conditions to Bvg+ phase conditions. The fact that LCVs, which contain Bvg− phase-trapped bacteria, were recovered from the lungs of infected mice, provided evidence that BvgAS modulation occurs in vivo. Our experiments indicate, however, that although a small proportion of bacteria apparently do modulate during infection, this level of modulation does not alter the outcome of infection.
Bacterial populations often exhibit phenotypic heterogeneity. A common mechanism by which bacteria can generate this heterogeneity is phase variation, a reversible and heritable change in phenotype (due to either genetic or epigenetic modifications) often manifested as different colony morphologies (Hallet, 2001; Woude and Bäumler, 2004). Phase variation frequently alters the production of surface-exposed epitopes such as pili, capsule, flagella, lipopolysaccharide (LPS), and adhesins (Woude and Bäumler, 2004). Coincidently, phase variation is often associated with virulence and is an important strategy used by pathogens to avoid immune selection. Some well-characterized examples of phase variation include the opa operon encoding adhesin proteins in Neisseria species, the pap operon encoding fimbrae in E. coli, and the flagella subunits encoded by fljBA and fliC in Salmonella enterica serotype Typhimurium (Simon et al., 1980; Stern and Meyer, 1987; Hernday et al., 2002). In Bordetella, phase variation in both fim3 and bvgAS has been described (Stibitz et al., 1989; Willems et al., 1990).
More recently, a phenomenon that generates bistable populations at the single-cell level has been discovered, called feedback-based multistability (FBM) (Smits et al., 2006). FBM is distinct from phase variation in that it is not based on genetic mutations but is instead based on feedback loops of regulatory networks (Smits et al., 2006). In isogenic populations, these feedback networks can result in bistability, which occurs when individuals in a population exhibit either one of two alternative stable steady-states (but not intermediate states) (Ferrell, 2002). A well-characterized example of FBM is in Bacillius subtilis, in the regulation of competence orchestrated by the transcription factor ComK (Sinderen et al., 1995). Competence is a cellular state induced by nutrient depletion, but only occurs in a fraction of the B. subtilis population due to oscillating levels of ComK at the single-cell level (Veening et al., 2008; Espinar et al., 2013). In one study, Smits et al. removed the external regulation of comK, leaving only positive autoregulation, and showed that ComK levels continued to exhibit bistability. Therefore, the authors argue that ComK bistability can be reduced to a positive autoregulatory loop in concert with random transcriptional and translational fluctuations or “noise” (Smits et al., 2005). This claim is supported by other examples, in which feedback regulation and a non-linear input are the only required components for a bistable system (Ferrell, 2002; Maamar and Dubnau, 2005).
We discovered LCVs of B. bronchiseptica after plating lung homogenates of mice infected with strain RBX9 and found that they yielded a heterogeneous population upon restreaking onto BG blood agar. We did not find evidence of classical phase variation in RBX9. Instead, the mechanism by which LCVs are generated appears more similar to FBM, in which the concentration of BvgA under Bvg− phase conditions varies in the population and results in some, but not all, bacteria committing to a positive feedback loop when switched to Bvg+ phase conditions. In support of this hypothesis, we were able to label bacteria within LCVs with tags unique to the Bvg+ and Bvg− phases and demonstrate the existence of two phenotypically distinct populations within LCVs (Figure 3). The dual-tagged RBX9 strain also provided the first direct evidence that Bvgi phase cultures are not simply a mixture of Bvg+ and Bvg− phase bacteria.
Use of the recombinase-based reporter system pGFLIP (Byrd et al., 2013) showed that the ΔfhaB mutation in RBX9 causes a decrease in the efficiency of bvgAS positive autoregulation and results in Bvg− phase-trapped bacteria that decline in proportion over time and can initiate the formation of new LCVs (Figures 4, 5, and 7). Based on these results and previous data, we postulate a model of LCV formation and propagation (Figure 9). According to this model, the concentration of BvgA varies in a population and also in individual cells as they grow and divide. In RB50, the average concentration of BvgA under Bvg− phase conditions is such that 100% of the bacteria are able to respond to Bvg+ phase conditions and transition to the Bvg+ phase phenotype (Figure 9A, B). In RBX9 however, the average concentration of BvgA is decreased under Bvg− phase conditions compared to wild-type bacteria (curve shifted to the left in Figure 9A), such that a subpopulation is below the threshold level required to respond to Bvg+ phase conditions (Figure 9B). These bacteria are thus Bvg− phase trapped and remain phenotypically Bvg− phase even under Bvg+ phase conditions. Under Bvg+ phase conditions, these bacteria form LCVs, which continue to harbor Bvg− phase-trapped bacteria. The Bvg− phase-trapped bacteria within LCVs occasionally escape to become Bvg+ phase descendants, possibly through unequal distribution of BvgA upon cell division or by stochastic accumulation of BvgA (Figure 9C). Therefore, in this system, a mutation that decreases the basal concentration of the positively autoregulated factor (BvgA) results in an FBM-like phenotype, whereas in other systems, FBM is the natural mechanism by which bacteria reach a bistable state.
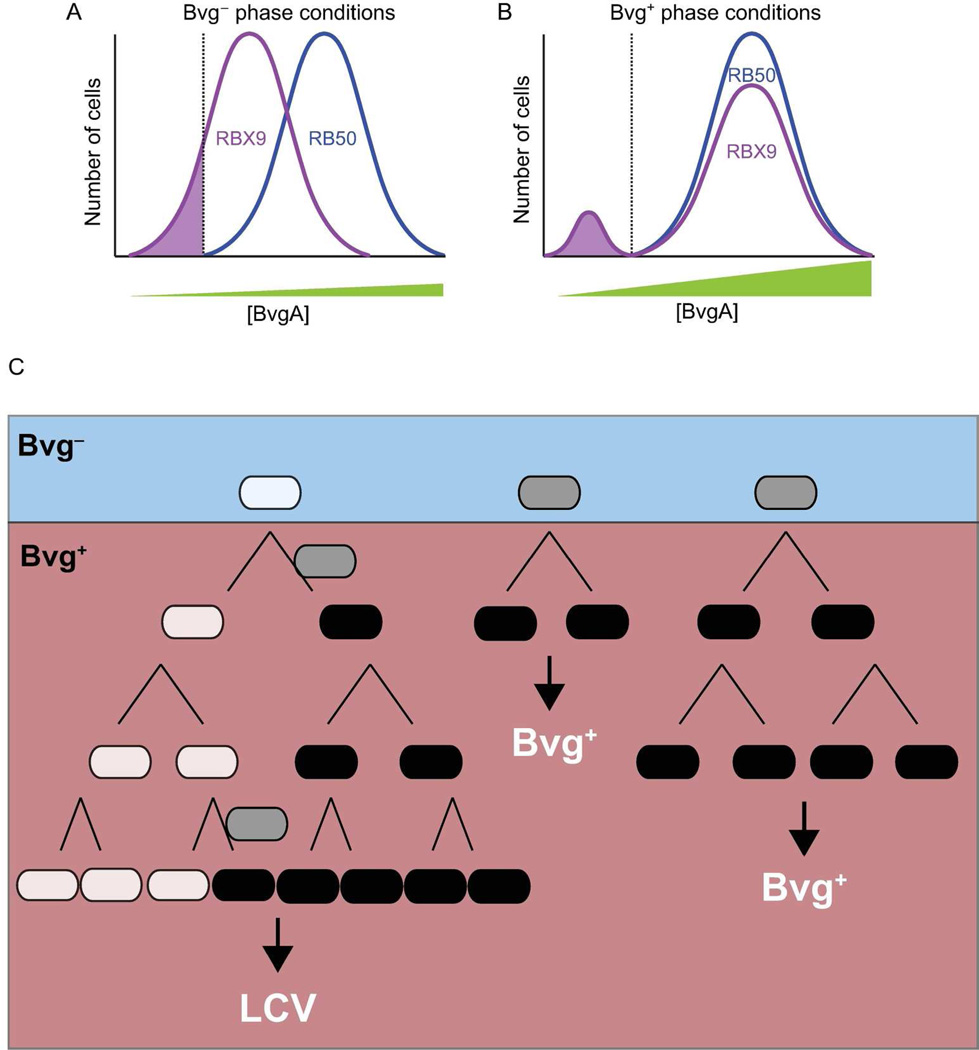
Figure 9.
Proposed distribution of BvgA concentration within populations of RBX9 and RB50. A, In the Bvg− phase, a proportion of RBX9 cells (shaded region) are Bvg− phase-trapped (i.e., have a concentration of BvgA below the threshold [dotted line] necessary to stimulate positive autoregulation upon transition to Bvg+ phase conditions). By contrast, all RB50 cells have a level of BvgA sufficient to initiate positive autoregulation upon transition to Bvg+ phase conditions. B, In the Bvg+ phase, the RBX9 cells that were below the threshold BvgA concentration in the Bvg− phase (shaded region as in A) maintain their low concentration of BvgA and are thus unable to switch to the Bvg+ phase. These cells are able to initiate LCV formation as described in C. Consistent with our in vitro data, all RB50 cells are able to switch to the Bvg+ phase. C, Model of LCV formation and propagation illustrated as a lineage diagram (see text for details). RBX9 bacteria exist as a heterogeneous population under Bvg− phase conditions, with some bacteria (white) being below the threshold of BvgA required to initiate positive autoregulation and others above this threshold (gray). When bacteria are switched to the Bvg+ phase, the Bvg− phase trapped bacteria form LCVs, whereas the other bacteria transition into the Bvg+ phase (black) and form Bvg+ phase colonies. Occasionally, Bvg− phase-trapped bacteria “escape” and can transition into the Bvg+ phase (indicated by gray cells between white and black cells), resulting in LCV formation after 48h.
Our pGFLIP data indicated that the bvgAS positive autoregulation defect is due to decreased activity of P2. This result explains why RBX9 has lower levels of BvgA: it has decreased transcription of bvgAS. Our data suggest that the reason for decreased transcription is the presence of a promoter located 5′ to the P2 promoter. This upstream divergent promoter exerts its negative effects on bvgAS from relatively far away (~800 bp) and this phenomenon appears to represent a previously undescribed form of transcriptional interference (the suppressive influence of one transcriptional process on another) (Shearwin et al., 2005). It is unclear whether this promoter must be highly active or divergently transcribed. However, as with other examples of transcription interference, we predict that increasing this promoter’s strength would also increase the degree of interference (Sneppen et al., 2005). Additionally, we predict that the orientation of the promoter may not be important and that reversing its orientation would not abolish interference if transcription read through was prevented. We do not understand mechanistically how this “passive” transcriptional interference occurs. One possibility is that the divergent promoter sequesters RNA polymerase away from the sensitive promoter (P2) or that transcription at this site influences DNA topology in a way that is prohibitive to P2 activation. These data suggest that the level of expression from the bvgAS P2 promoter is poised at the threshold of that required for all cells in the population to respond to Bvg+ phase conditions when they are encountered. Although the LCV phenotype appeared as an artifact of genetic manipulation, our results are important as they demonstrate a form of transcriptional interference that, to our knowledge, has not been described previously, and also because they reveal a mechanism by which in-frame deletion mutations can have unanticipated polar effects on neighboring genes. Furthermore, RBX9 and its derivatives constitute a genetically tractable system for studying additional mechanisms of transcriptional interference and details of FBM.
The LCVs also provided insight into the behavior of BvgAS during infection. The role of BvgAS-dependent modulation in the Bordetella life cycle is not completely understood and remains an important area of investigation. Several studies have attempted to determine if Bvgi or Bvg− phase bacteria exist at any point during Bordetella infection, and so far none have yielded positive results (Cotter and Miller, 1994; Tejada et al., 1998; Merkel et al., 1998; Vergara-Irigaray et al., 2005; Nicholson et al., 2012). These data, together with those demonstrating that Bvg− phase bacteria transition rapidly to the Bvg+ phase following intranasal inoculation (Veal-Carr and Stibitz, 2005; Byrd et al., 2013), have led to the conclusion that not only is the Bvg+ phase necessary and sufficient for infection, but that bacteria switch to and remain in the Bvg+ phase in vivo. The Bvgi and Bvg− phases are hypothesized to be important for transmission and survival ex vivo, however, no role for these phenotypic phases in a natural setting has been demonstrated. For B. pertussis particularly, which appears to survive outside the host only briefly during transmission to a new host, the role of BvgAS modulation remains mysterious. The isolation of LCVs from mouse lungs provides strong evidence that at least some RBX9 bacteria modulate during infection. However, the proportion of bacteria that modulated and that could be recovered from the animals was very low. Because the PflaA-flp system was unable to reliably distinguish this low proportion of modulated bacteria from background resolution, we could not determine if wild type bacteria modulate in vivo. If they do not, our data would suggest that only FHA-deficient bacteria modulate in vivo, which would suggest that FHA functions to prevent the bacteria from experiencing a Bvg− phase environment during infection. In pilot experiments, we also recovered LCVs from mice infected with ΔfhaB, ΔcyaA double mutants – in higher proportions, in some cases, than in mice infected with RBX9. These preliminary data suggest the intriguing possibility that FHA and ACT function together to prevent Bordetella from creating or entering a modulating environment in the host. Our future experiments will be aimed at testing this hypothesis.
Experimental Procedures
Strains and growth conditions
Escherichia coli were grown in lysogeny broth (LB; 10 g l−1 tryptone, 5 g l−1 yeast extract, 2.5 g l−1 NaCl) or on LB with agar (1.5%) at 37°C. Bordetella were grown in Stainer-Scholte (SS) broth (25) or on Bordet-Gengou (BG) agar (1.5%) (BD Biosciences, San Jose, CA) supplemented with 7.5% defibrinated sheep’s blood (Colorado Serum Company, Denver, CO) at 37°C (16). As required, culture media were supplemented with kanamycin (Km; 50 µg ml−1), ampicillin (Ap; 100 µg ml−1), streptomycin (Sm; 25 µg ml−1), magnesium sulfate (MgSO4; 50 mM in plates and 20 mM in liquid), and diaminopimelic acid (DAP; 400 µg ml−1) for the E. coli mobilizer strain RHO3 (Δasd ΔaphA) (19). Unless otherwise noted, all restriction enzymes and T4 DNA ligase was purchased from New England Biolabs.
Construction of bacterial strains
Allelic exchange and Campbell-type integrations were done by matings using parental Bordetella and E. coli strain RHO3 harboring the appropriate suicide plasmid. The pGFLIP plasmid was delivered to the attTn7 site using tri-parental mating with the above strains and with RHO3 cells harboring pTNS3, which encodes the transposase genes tnsABCD. Integration at the attTn7 locus was confirmed via PCR. For details on specific strain constructions see the Supplemental text.
Immunofluorescence
HA epitopes on the surface of RBX9BatB-HAflaA-gfp bacteria were stained and visualized using indirect immunofluorescence. After 72h of growth at 37°C, five to twenty colonies of each morphology including Bvg+, Bvg−, Bvgi, and LCV, were scraped off of BG blood agar plates and resuspended into 1ml of 4% paraformaldehyde and were allowed to fix on ice for 30 min. Cells were pelleted and washed with 1% BSA in PBS in a microcentrifuge tube. Primary antibody (mouse monoclonal anti-HA IgG diluted 1:2000 in 1% BSA in PBS) was used to resuspend the pellet and this mixture was incubated for 1h at room temperature (RT). The pellets were washed twice in 1% BSA for 5 min. Secondary antibody (Alexa Fluor 594-conjugated goat anti-mouse IgG, diluted 1:250 in 1% BSA in PBS; Invitrogen) was used to resuspend the pellet and this mixture was then incubated for 30 min at RT in the dark. The pellets were washed twice with 1% BSA for 5 min. Four microliters of the leftover pellet and liquid was pipetted onto a slide for visualization.
Confocal Microscopy
Immunofluorescent RBX9BatB-HAflaA-gfp bacteria were visualized using a Zeiss LSM 710 confocal microscope. Secondary antibody (Alexa Fluor 594-conjugated goat anti-mouse IgG) was detected using a 594 nm laser and GFP was detected at 488 nm. We used the 63× oil objective with 3× digital zoom. Images were viewed and saved with the Zen software from Carl Zeiss Microscopy.
pGFLIP assays
For RBX9cyaAFLP, RB50PbvgAshortFLP and RB50PbvgAlongFLP, strains were grown on BG blood agar plates under promoter-inactive conditions (Bvg− phase) with Km for 48h at 37°C and were determined to be GFP positive (GFP+) using a G:BOX Chemi imaging system with an UltraBright-LED blue transilluminator and an SW06 short-pass filter (495 to 600 nm; Syngene, Frederick, MD). Single GFP+ colonies were then resuspended in PBS, diluted, and plated onto Bvg+ or Bvg− phase conditions (promoter-active and promoter-inactive conditions, respectively) in the absence of Km selection. For RBX9cyaAFLP, GFP+ LCVs were picked, diluted, and plated onto Bvg+ and Bvg− phase conditions in the absence of Km selection. Percent resolution was determined by averaging the ratio of GFP− cfu/total cfu for at least three plates.
Intranasal inoculation of mice
Four- to eight-week-old BALB/c mice (Jackson Laboratories, Bar Harbor, ME) were inoculated intranasally with 1×105 cfu of B. bronchiseptica in 50 µl of PBS. For all infections, bacteria were grown overnight in SS medium. Lungs were harvested at 1h and 72h p.i. Right lungs were homogenized in 1 ml of PBS, diluted in PBS, and plated in at least duplicate on BG agar. Figure 8 represents data from three independent experiments performed with at least two mice per strain per time point.
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. Our protocol was approved by the University of North Carolina IACUC (10–134, 12–307). All animals were properly anesthetized for inoculations, monitored regularly, euthanized when moribund, and efforts were made to minimize suffering.
Statistical analyses
Statistical analyses were performed using Prism 5.0 (GraphPad Software, Inc.). Statistical significance was determined using the unpaired Student’s t-test or analysis of variance (ANOVA) followed by Tukey’s multiple comparison test. Images were formatted using Adobe Photoshop CS5 and figures were generated using Adobe Illustrator CS5 (Adobe Systems, Inc.).
Supplementary Material
Acknowledgments
We thank members of our laboratory, especially Jeffrey Melvin, for many insightful discussions. Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award numbers R01AI43986, RO1AI094991, and U54AI065359 to P.A.C. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
The authors have no conflicts of interest to declare.
References
- Abramson T, Kedem H, Relman D. Proinflammatory and proapoptotic activities associated with Bordetella pertussis filamentous hemagglutinin. Infection and immunity. 2001;69:2650–2658. doi: 10.1128/IAI.69.4.2650-2658.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akerley BJ, Cotter PA, Miller JF. Ectopic expression of the flagellar regulon alters development of the Bordetella-host interaction. Cell. 1995;80 doi: 10.1016/0092-8674(95)90515-4. [DOI] [PubMed] [Google Scholar]
- Akerley BJ, Monack DM, Falkow S, Miller JF. The bvgAS locus negatively controls motility and synthesis of flagella in Bordetella bronchiseptica. Journal of bacteriology. 1992;174:980–990. doi: 10.1128/jb.174.3.980-990.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arico B, Miller JF, Roy C, Stibitz S, Monack D, Falkow S, et al. Sequences required for expression of Bordetella pertussis virulence factors share homology with prokaryotic signal transduction proteins. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:6671–6675. doi: 10.1073/pnas.86.17.6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellalou J, Sakamoto H, Ladant D, Geoffroy C, Ullmann A. Deletions affecting hemolytic and toxin Deletions Affecting Hemolytic and Toxin Activities of Bordetella pertussis Adenylate Cyclase. 1990;58 doi: 10.1128/iai.58.10.3242-3247.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher PE, Maris AE, Yang M-S, Stibitz S. The response regulator BvgA and RNA polymerase alpha subunit C-terminal domain bind simultaneously to different faces of the same segment of promoter DNA. Molecular cell. 2003;11:163–173. doi: 10.1016/s1097-2765(03)00007-8. [DOI] [PubMed] [Google Scholar]
- Boucher PE, Yang M, Schmidt DM, Stibitz S. Genetic and Biochemical Analyses of BvgA Interaction with the Secondary Binding Region of the fha Promoter of Bordetella pertussis. 2001;183:536–544. doi: 10.1128/JB.183.2.536-544.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher PE, Yang MS, Stibitz S. Mutational analysis of the high-affinity BvgA binding site in the fha promoter of Bordetella pertussis. Molecular microbiology. 2001;40:991–999. doi: 10.1046/j.1365-2958.2001.02442.x. [DOI] [PubMed] [Google Scholar]
- Boulanger A, Chen Q, Hinton DM, Stibitz S. In vivo phosphorylation dynamics of the Bordetella pertussis virulence-controlling response regulator BvgA. Molecular microbiology. 2013;88:156–172. doi: 10.1111/mmi.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd MS, Mason E, Henderson MW, Scheller EV, Cotter Pa. An improved RIVET-like reporter system reveals differential cyaA gene activation in Bordetella species. Infection and immunity. 2013;81:1295–1305. doi: 10.1128/IAI.01445-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos-Outcalt D. Pertussis: a disease re-emerges. The Journal of family practice. 2005;54:699–703. [PubMed] [Google Scholar]
- Cotter PA, Jones AM. Phosphorelay control of virulence gene expression in Bordetella. Trends in microbiology. 2003;11:1–7. doi: 10.1016/s0966-842x(03)00156-2. [DOI] [PubMed] [Google Scholar]
- Cotter PA, Miller JF. BvgAS-mediated signal transduction: analysis of phase-locked regulatory mutants of Bordetella bronchiseptica in a rabbit model. Infection and immunity. 1994;62:1–11. doi: 10.1128/iai.62.8.3381-3390.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter PA, Miller JF. A mutation in the Bordetella bronchiseptica bvgS gene results in reduced virulence and increased resistance to starvation, and identifies a new class of Bvg-regulated antigens. Molecular microbiology. 1997;24:1–15. doi: 10.1046/j.1365-2958.1997.3821741.x. [DOI] [PubMed] [Google Scholar]
- Cotter PA, Yuk MH, Mattoo S, Akerley BJ, Boschwitz J, Relman DA, Miller JF. Filamentous hemagglutinin of Bordetella bronchiseptica is required for efficient establishment of tracheal colonization. Infection and immunity. 1998;66 doi: 10.1128/iai.66.12.5921-5929.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutte L, Antoine R, Drobecq H, Locht C, Jacob-Dubuisson F. Subtilisin-like autotransporter serves as maturation protease in a bacterial secretion pathway. The EMBO journal. 2001;20:5040–5048. doi: 10.1093/emboj/20.18.5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings CA, Bootsma HJ, Relman DA, Miller JF. Species- and strain-specific control of a complex, flexible regulon by Bordetella BvgAS. Journal of bacteriology. 2006;188:1–12. doi: 10.1128/JB.188.5.1775-1785.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deora R, Bootsma HJ, Miller JF, Cotter PA. Diversity in the Bordetella virulence regulon: transcriptional control of a Bvg-intermediate phase gene. Molecular microbiology. 2001;40:1–15. doi: 10.1046/j.1365-2958.2001.02415.x. [DOI] [PubMed] [Google Scholar]
- Diavatopoulos Da, Cummings Ca, Schouls LM, Brinig MM, Relman Da, Mooi FR. Bordetella pertussis, the causative agent of whooping cough, evolved from a distinct, human-associated lineage of B. bronchiseptica. PLoS pathogens. 2005;1:e45. doi: 10.1371/journal.ppat.0010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenighini M, Relman D, Capiau C, Falkow S, Prugnola A, Scarlato, Rappuoli R. Genetic characterization of Bordetella pertussis filamentous haemagglutinin: a protein processed from an unusually large precursor. Molecular microbiology. 1990;4:1–15. doi: 10.1111/j.1365-2958.1990.tb00649.x. [DOI] [PubMed] [Google Scholar]
- Espinar L, Dies M, Cagatay T, Suel GM, Garcia-Ojalvo J. Circuit-level input integration in bacterial gene regulation. Proceedings of the National Academy of Sciences. 2013 doi: 10.1073/pnas.1216091110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrell JE. Self-perpetuating states in signal transduction: positive feedback, double negative feedback and bistability. Current Opinion in Cell Biology. 2002;14:140–148. doi: 10.1016/s0955-0674(02)00314-9. [DOI] [PubMed] [Google Scholar]
- Gerlach G, Wintzingerode F, von, Middendorf B, Gross R. Evolutionary trends in the genus Bordetella. Microbes and infection / Institut Pasteur. 2001;3:61–72. doi: 10.1016/s1286-4579(00)01353-8. [DOI] [PubMed] [Google Scholar]
- Gross R, Keidel K, Schmitt K. Resemblance and divergence: the “new” members of the genus Bordetella. Medical microbiology and immunology. 2010;199:155–163. doi: 10.1007/s00430-010-0148-z. [DOI] [PubMed] [Google Scholar]
- Hallet B. Playing Dr Jekyll and Mr Hyde: combined mechanisms of phase variation in bacteria. Current opinion in microbiology. 2001;4:570–581. doi: 10.1016/s1369-5274(00)00253-8. [DOI] [PubMed] [Google Scholar]
- Henderson MW, Inatsuka CS, Sheets AJ, Williams CL, Benaron DJ, Donato GM, et al. Contribution of Bordetella filamentous hemagglutinin and adenylate cyclase toxin to suppression and evasion of IL-17-mediated inflammation. Infection and immunity. 2012;80:1–48. doi: 10.1128/IAI.00148-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernday A, Krabbe M, Braaten B, Low D. Self-perpetuating epigenetic pili switches in bacteria. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(Suppl 4):16470–16476. doi: 10.1073/pnas.182427199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inatsuka CS, Julio SM, Cotter PA. Bordetella filamentous hemagglutinin plays a critical role in immunomodulation, suggesting a mechanism for host specificity. Proceedings of the National Academy of Sciences of the United States of America. 2005;102 doi: 10.1073/pnas.0507910102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie Y, Mattoo S, Yuk MH. The Bvg Virulence Control System Regulates Biofilm Formation in Bordetella bronchiseptica. Journal of bacteriology. 2004;186 doi: 10.1128/JB.186.17.5692-5698.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AM, Boucher PE, Williams CL, Stibitz S, Cotter PA. Role of BvgA phosphorylation and DNA binding affinity in control of Bvg-mediated phenotypic phase transition in Bordetella pertussis. Molecular microbiology. 2005;58 doi: 10.1111/j.1365-2958.2005.04875.x. [DOI] [PubMed] [Google Scholar]
- Julio SM, Inatsuka CS, Mazar J, Dieterich C, Relman DA, Cotter PA. Natural-host animal models indicate functional interchangeability between the filamentous haemagglutinins of Bordetella pertussis and Bordetella bronchiseptica and reveal a role for the mature C-terminal domain, but not the RGD motif, during infection. Molecular microbiology. 2009;71:1574–1590. doi: 10.1111/j.1365-2958.2009.06623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimova G, Bellalou J, Ullmann A. Phosphorylation-dependent binding of BvgA to the upstream region of the cyaA gene of Bordetella pertussis. Molecular microbiology. 1996;20:1–8. doi: 10.1046/j.1365-2958.1996.5231057.x. [DOI] [PubMed] [Google Scholar]
- Maamar H, Dubnau D. Bistability in the Bacillus subtilis K-state (competence) system requires a positive feedback loop. Molecular microbiology. 2005;56:615–624. doi: 10.1111/j.1365-2958.2005.04592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marconi GP, Ross LA, Nager AL. An upsurge in pertussis: epidemiology and trends. Pediatric emergency care. 2012;28:215–219. doi: 10.1097/PEC.0b013e318248b0cd. [DOI] [PubMed] [Google Scholar]
- Mattoo S, Cherry JD. Molecular Pathogenesis, Epidemiology, and Clinical Manifestations of Respiratory Infections Due to Bordetella pertussis and Other Bordetella Subspecies. clinical microbiology reviews. 2005;18:326–382. doi: 10.1128/CMR.18.2.326-382.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattoo S, Miller JF, Cotter PA. Role of Bordetella bronchiseptica fimbriae in tracheal colonization and development of a humoral immune response. Infection and immunity. 2000;68 doi: 10.1128/iai.68.4.2024-2033.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazar J, Cotter PA. Topology and maturation of filamentous haemagglutinin suggest a new model for two-partner secretion. Molecular microbiology. 2006;62:1–14. doi: 10.1111/j.1365-2958.2006.05392.x. [DOI] [PubMed] [Google Scholar]
- McGuirk P, Mills KH. Direct anti-inflammatory effect of a bacterial virulence factor: IL-10-dependent suppression of IL-12 production by filamentous hemagglutinin from Bordetella pertussis. European journal of immunology. 2000;30:415–422. doi: 10.1002/1521-4141(200002)30:2<415::AID-IMMU415>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Merkel TJ, Stibitz S, Keith JM, Leef M, Shahin R. Contribution of regulation by the bvg locus to respiratory infection of mice by Bordetella pertussis. Infection and immunity. 1998;66:4367–4373. doi: 10.1128/iai.66.9.4367-4373.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson TL, Brockmeier SL, Loving CL, Register KB, Kehrli ME, Stibitz SE, Shore SM. Phenotypic modulation of the virulent Bvg phase is not required for pathogenesis and transmission of Bordetella bronchiseptica in swine. Infection and immunity. 2012;80:1–13. doi: 10.1128/IAI.06016-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez Vidakovics MLa, Lamberti Y, Pol W-L, van der Yantorno O, Rodriguez ME. Adenylate cyclase influences filamentous haemagglutinin-mediated attachment of Bordetella pertussis to epithelial alveolar cells. FEMS immunology and medical microbiology. 2006;48:140–147. doi: 10.1111/j.1574-695X.2006.00136.x. [DOI] [PubMed] [Google Scholar]
- Prugnola A, Aricò B, Manetti R, Rappuoli R, Scarlato, Scarlato V. Response of the bvg regulon of Bordetella pertussis to different temperatures and short-term temperature shifts. Microbiology (Reading, England) 1995;141(1):1–6. doi: 10.1099/13500872-141-10-2529. [DOI] [PubMed] [Google Scholar]
- Roy CR, Falkow S. Identification of Bordetella pertussis regulatory sequences required for transcriptional activation of the fhaB gene and autoregulation of the bvgAS operon. Journal of bacteriology. 1991;173:1–9. doi: 10.1128/jb.173.7.2385-2392.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y, Sato H. Development of acellular pertussis vaccines. Biologicals : journal of the International Association of Biological Standardization. 1999;27:61–69. doi: 10.1006/biol.1999.0181. [DOI] [PubMed] [Google Scholar]
- Scarlato, Prugnola A, Aricó B, Rappuoli R. Positive transcriptional feedback at the bvg locus controls expression of virulence factors in Bordetella pertussis. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:1–6. doi: 10.1073/pnas.87.24.10067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarlato, Rappuoli R, Scarlato V. Differential response of the bvg virulence regulon of Bordetella pertussis to MgSO4. Journal of bacteriology. 1991;173:1–5. doi: 10.1128/jb.173.22.7401-7404.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearwin KE, Callen BP, Egan JB. Transcriptional interference--a crash course. Trends in genetics : TIG. 2005;21:339–345. doi: 10.1016/j.tig.2005.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M, Zieg J, Silverman M, Mandel G, Doolittle R. Phase variation: evolution of a controlling element. Science (New York, NY) 1980;209:1370–1374. doi: 10.1126/science.6251543. [DOI] [PubMed] [Google Scholar]
- Sinderen D, Van, Luttinger A, Kong U, Kong L, Dubnau D, Venema G, Hamoen L. comK encodes the competence transcription factor, the key regulatory protein for competence development in Bacillus subtilis. Molecular Microbiology. 1995;15:455–462. doi: 10.1111/j.1365-2958.1995.tb02259.x. [DOI] [PubMed] [Google Scholar]
- Smits WK, Eschevins CC, Susanna Ka, Bron S, Kuipers OP, Hamoen LW. Stripping Bacillus: ComK auto-stimulation is responsible for the bistable response in competence development. Molecular microbiology. 2005;56:604–614. doi: 10.1111/j.1365-2958.2005.04488.x. [DOI] [PubMed] [Google Scholar]
- Smits WK, Kuipers OP, Veening J-W. Phenotypic variation in bacteria: the role of feedback regulation. Nature reviews Microbiology. 2006;4:259–271. doi: 10.1038/nrmicro1381. [DOI] [PubMed] [Google Scholar]
- Sneppen K, Dodd IB, Shearwin KE, Palmer AC, Schubert Ra, Callen BP, Egan JB. A mathematical model for transcriptional interference by RNA polymerase traffic in Escherichia coli. Journal of molecular biology. 2005;346:399–409. doi: 10.1016/j.jmb.2004.11.075. [DOI] [PubMed] [Google Scholar]
- Steffen P, Goyard S, Ullmann A. Phosphorylated BvgA is sufficient for transcriptional activation of virulence-regulated genes in Bordetella pertussis. The EMBO Journal. 1996;15:102–109. [PMC free article] [PubMed] [Google Scholar]
- Stern A, Meyer TF. Common mechanism controlling phase and antigenic variation in pathogenic neisseriae. Molecular microbiology. 1987;1:5–12. doi: 10.1111/j.1365-2958.1987.tb00520.x. [DOI] [PubMed] [Google Scholar]
- Stibitz S, Aaronson W, Monack D, Falkow S. Phase variation in Bordetella pertussis by frameshift mutation in a gene for a novel two-component system. Nature. 1989;338:266–269. doi: 10.1038/338266a0. [DOI] [PubMed] [Google Scholar]
- Stockbauer KE, Fuchslocher B, Miller JF, Cotter PA. Identification and characterization of BipA, a Bordetella Bvg-intermediate phase protein. Molecular microbiology. 2001;39:1–14. doi: 10.1046/j.1365-2958.2001.02191.x. [DOI] [PubMed] [Google Scholar]
- Tejada GM, Cotter PA, Heininger U, Camilli A, Akerley BJ, Mekalanos JJ, Miller JF. Neither the Bvg- phase nor the vrg6 locus of Bordetella pertussis is required for respiratory infection in mice. Infection and immunity. 1998;66 doi: 10.1128/iai.66.6.2762-2768.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejada GM, Miller JF, Cotter PA. Comparative analysis of the virulence control systems of Bordetella pertussis and Bordetella bronchiseptica. Molecular. 1996;22 doi: 10.1046/j.1365-2958.1996.01538.x. [DOI] [PubMed] [Google Scholar]
- Veal-Carr WL, Stibitz S. Demonstration of differential virulence gene promoter activation in vivo in Bordetella pertussis using RIVET. Molecular microbiology. 2005;55:1–11. doi: 10.1111/j.1365-2958.2004.04418.x. [DOI] [PubMed] [Google Scholar]
- Veening J-W, Smits WK, Kuipers OP. Bistability, epigenetics, and bet-hedging in bacteria. Annual review of microbiology. 2008;62:193–210. doi: 10.1146/annurev.micro.62.081307.163002. [DOI] [PubMed] [Google Scholar]
- Vergara-Irigaray N, Chavarri-Martinez A, Rodriguez-Cuesta J, Miller JF, Cotter PA, Tejada GM. Evaluation of the role of the Bvg intermediate phase in Bordetella pertussis during experimental respiratory infection. Infection and immunity. 2005;73 doi: 10.1128/IAI.73.2.748-760.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems R, Paul A, Heide HGJ, van der Avest AR, ter, Mooi FR. Fimbrial phase variation in Bordetella pertussis: a novel mechanism for transcriptional regulation. The EMBO journal. 1990;9:2803–2809. doi: 10.1002/j.1460-2075.1990.tb07468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CL, Boucher PE, Stibitz S, Cotter PA. BvgA functions as both an activator and a repressor to control Bvg phase expression of bipA in Bordetella pertussis. Molecular microbiology. 2005;56 doi: 10.1111/j.1365-2958.2004.04526.x. [DOI] [PubMed] [Google Scholar]
- Williams CL, Cotter PA. Autoregulation is essential for precise temporal and steady-state regulation by the Bordetella BvgAS phosphorelay. Journal of bacteriology. 2007;189:1974–1982. doi: 10.1128/JB.01684-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CL, Haines R, Cotter PA. Serendipitous discovery of an immunoglobulin-binding autotransporter in Bordetella species. Infection and immunity. 2008;76:1–12. doi: 10.1128/IAI.00323-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woude MW, Van Der, Bäumler AJ. Phase and Antigenic Variation in Bacteria. clinical microbiology reviews. 2004;17 doi: 10.1128/CMR.17.3.581-611.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaretzky FR, Gray MC, Hewlett EL. Mechanism of association of adenylate cyclase toxin with the surface of Bordetella pertussis: a role for toxin-filamentous haemagglutinin interaction. Molecular microbiology. 2002;45:1589–1598. doi: 10.1046/j.1365-2958.2002.03107.x. [DOI] [PubMed] [Google Scholar]
- Zu T, Manetti R, Rappuoli R, Scarlato Differential binding of BvgA to two classes of virulence genes of Bordetella pertussis directs promoter selectivity by RNA polymerase. Molecular microbiology. 1996;21:1–9. doi: 10.1111/j.1365-2958.1996.tb02564.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.