Abstract
Lymphopenia is induced by lymphoablative therapies and chronic viral infections. We assessed the impact of lymphopenia on cardiac allograft survival in recipients conditioned with peri-transplant costimulatory blockade (CB) to promote long-term graft acceptance. After vascularized MHC-mismatched heterotopic heart grafts were stably accepted through CB, lymphopenia was induced on day 60 post-transplant by 6.5 Gy irradiation or by administration of anti-CD4 plus anti-CD8 mAb. Long-term surviving allografts were gradually rejected after lymphodepletion (MST=74 ± 5 days post-irradiation). Histological analyses indicated signs of severe rejection in allografts following lymphodepletion, including mononuclear cell infiltration and obliterative vasculopathy. Lymphodepletion of CB conditioned recipients induced increases in CD44high effector/memory T cells in lymphatic organs and strong recovery of donor-reactive T cell responses, indicating lymphopenia-induced-proliferation and donor alloimmune responses occurring in the host. T regulatory (CD4+Foxp3+) cell and B cell numbers as well as donor-specific antibody titers also increased during allograft rejection in CB conditioned recipients given lymphodepletion. These observations suggest that allograft rejection following partial lymphocyte depletion is mediated by lymphopenia-induced proliferation of donor-reactive memory T cells. As lymphopenia may cause unexpected rejection of stable allografts, adequate strategies must be developed to control T cell proliferation and differentiation during lymphopenia.
Keywords: Lymphopenia-induced proliferation, Costimulatory blockade, Memory T cell
Introduction
Although new immunosuppressive drugs have been shown to effectively prevent acute rejection of allogeneic grafts, the risk of rejection remains a serious problem (1, 2). Immune effector mechanisms dependent on CD4 helper T cells, CD8 cytotoxic T cells, and alloreactive antibody production have been implicated in both acute and chronic allograft rejection (3–6). To prevent transplant rejection by cell-mediated and humoral mechanisms, studies demonstrating the importance of lymphocyte depletion during induction of tolerance have led to clinical use of peri-transplant T cell-depleting therapies (7, 8).
Lymphopenia is observed in some pathological and physiological processes, including chronic viral infection, emigration of newly selected T cells from the thymus into the periphery prior to complete development of the repertoire, and the sequential administration of calcineurin inhibitors (9, 10). Several studies in humans and rodents have indicated that lymphopenia may be associated with autoimmune manifestations, such as insulin-dependent diabetes mellitus, rheumatoid arthritis, Sjögren’s syndrome, and lupus (9) (11). Signs of autoimmunity are also frequently observed in HIV-infected patients with consequent lymphopenia (9).
In lymphopenic hosts, naïve T cells undergo spontaneous slow proliferation and lymphopenia-induced proliferation (LIP) and differentiate into memory-like cells (12, 13). It is currently unknown whether transient lymphopenia and the resultant LIP of residual T cells affects established transplant tolerance (14, 15). Although partial T cell depletion inhibits the generation of alloreactive effector T cells, lymphopenia and the subsequent LIP of residual T cells increases resistance to tolerance and accelerates graft rejection (12, 16). Using a cynomolgus monkey model to produce sustained bone marrow chimerism by a non-myeloablative strategy, we have demonstrated that LIP of residual T cells is an inevitable barrier to the induction of tolerance by lymphocyte depletion and a CB treatment regimen (17). These findings have important implications for transplantation protocols incorporating partial T cell depletion. To date, however, the effects of transient lymphopenia on established transplant tolerance and allograft rejection have not been investigated.
The present study examined whether transient lymphopenia can induce rejection of accepted cardiac allografts. We demonstrate that induced lymphopenia and the resultant LIP of residual T cells initiated the gradual rejection of allogeneic cardiac grafts that had been accepted by peri-transplant treat of the recipient with co-stimulation blockade (CB) treatment. This rejection was accompanied by diffuse infiltration of mononuclear cells and obliterative vasculopathy. Our results indicate that a transient lymphopenia is sufficient for the induction of allograft rejection in tolerant recipients.
Materials and Methods
Mice
Female BALB/c (H-2d) and C57BL/6 (H-2b) mice were obtained from Japan SLC Co. Ltd. (Hamamatsu City, Japan). Mice were 7–12 weeks old and housed in a specific pathogen-free facility. All experiments were performed in accordance with protocols approved by the Animal Care and Use Committee of Tokyo University of Science.
Flow cytometry
Cell suspensions were prepared in FACS medium and aliquots incubated first with unlabeled anti-FcγR (2.4G2) to block nonspecific binding, and then stained with each Ab. We used a FACS Calibur with CellQuest software (BD Biosciences, San Jose, CA) for four-color flow cytometric analyses. Absolute numbers of CD4+ T cells, CD8+ T cells, regulatory T cells, and B cells in each tissue were determined using a modification of the methodology published by Afanasyeva et al. (18, 19). In BrdU incorporation assays, mice were fed water containing 0.8mg/ml BrdU for 6 days before assay. BrdU incorporation was detected by FITC-anti BrdU mAb (eBioscience, San Diego, CA).
Cervical heart transplantation
Vascularized heterotopic cervical heart transplantation from BALB/c donors to recipient B6 mice was performed using a non-suture cuff technique (20). Rejection was defined as loss of palpable cardiac contractions. Recipient mice were treated with 500 μg of anti-CD40L and/or 500 μg of hCTLA4Ig (costimulatory blockade; CB) on the day of transplantation, and every other day for 6 days (21).
Induction of lymphopenia and lymphopenia-induced proliferation
To induce lymphopenia, mice were subjected to sublethal (6.5 Gy), whole body irradiation (TBI) using a Cs137 source 60 days after transplantation. T cell depletion was also induced by treating accepted allograft recipients with anti-CD4 Ab GK1.5 plus anti-CD8 Ab 2.43 (Bio X Cell, West Lebanon, NH) via i.p. injection of 200 ug of each on days 58, 59 and 60 posttransplant. This protocol has previously been shown to cause ~85% CD4, CD8 T cells depletion (16, 15).
Mixed lymphocyte reaction assay
T cell-depleted stimulator cells were purified from spleen cell suspensions by treatment with mouse anti-mouse Thy1.2 IgM (HO13.4) and rabbit complement HLA-ABC (Invitrogen, Carlsbad, CA). To obtain T cell-enriched responder cells, total splenocytes and cervical lymphocytes were added to plates (Iwaki, Tokyo, Japan) coated with rabbit antibodies specific for mouse IgM (Cappel, Costa Mesa, CA) and after 40 min at 37°C in a CO2 incubator, the non-adherent cells were recovered and used as responders. To compare alloreactive CD4+ and CD8+ T cell priming, each cell population was enriched from recipient cell suspensions using negative selection columns (R&D Systems, Minneapolis, MN, USA) to remove CD4+ or CD8+ T cells and the enriched CD4+ or CD8+ responder T cells (1–4×105) were co-cultured with 7×105 allogeneic stimulator cells for 96 h in 200 μL of complete medium (RPMI 1640 supplemented with 10% fetal calf serum, 100 U/mL penicillin, 100 μg/mL streptomycin, 10 mM HEPES, pH 7.55, and 50 μM 2-mercaptoethanol) in 96-well flat-bottomed plates (Becton Dickinson Labware, Franklin Lakes, NJ). Cultures were pulsed with 0.5 μCi of 3H-thymidine for the final 8 h.
T regulatory cell activity assay
Treg cell activity was determined by a proliferation-suppression assay (22, 23). CD4+CD25+ T cells (Treg cells) from spleens and lymph nodes from tolerant mice and LIP mice were fractionated using magnetic beads (Stem Cell Technologies, Vancouver, BC). T cells from LIP mice were then flow sorted to isolate CD62Llow, CD44high T cell populations (> 98% purity) using a FACSAria (BD Biosciences, San Jose, CA) for use as effector/memory T cells, responders. Cultures of responder cells and Treg cells at a ratio of 1:2, 1:1, and 1:0.5 were stimulated with 1 μg/ml anti-CD3 (145–2C11) in the presence of T cell-depleted spleen cells that served as APCs. Proliferation of the CFSE-labeled cells was assessed after 3–4 days by flow cytometry. All cultures were performed and analyzed in triplicate, and levels of proliferation in the absence of Tregs present was set at 100%.
Quantitation of cytokine-producing T cells
Priming of graft donor-reactive T cells to IFN-γ and IL-2 producing cells was quantified by ELISPOT assays as previously described (18). Briefly, lymph node responder cells and mitomycin C-treated self, donor and third-party stimulator cell populations were cocultured for 24 h at 37°C in serum-free HL-1 media in 96 well Multiscreen-IP (Millipore, Billerica, MA) plates coated with anti-IFN-γ or anti-IL-2 capture mAb. To compare alloreactive CD4+ and CD8+ T cell priming, each cell population was enriched from recipient cell suspensions using negative selection columns (R&D Systems, Minneapolis, MN) to remove CD4+ or CD8+ T cells from the suspension and the enriched CD4+ or CD8+ responder T cells were stimulated with T cell depleted splenocytes from graft donor, third-party allogeneic or recipient strain mice. After 24 h, all cells were washed from the plate and biotinylated anti-IFN-γ or anti-IL-2 detecting mAb (BD Biosciences, San Jose, CA) was added, followed by alkaline phosphatase-conjugated anti-biotin antibody (Vector Laboratories, Burlingame, CA). After development with nitroblue tetrazolium/5-bromo-4-chloro-30-indolyl substrate (Bio-Rad Laboratories, Hercules, CA), the total number of spots per well was quantified using an ImmunoSpot Series 3 Analyzer (Cellular Technology Ltd., Shaker Heights, OH).
Graft histology
Transplanted hearts were harvested from recipients after irradiation at weekly intervals. Tissues were fixed in neutral buffered 20% formalin until processed, and embedded in paraffin. Sections, 5 μm, were stained with hematoxylin and eosin (H&E) and Masson’s trichrome. To evaluate allograft rejection, the number of vessels, including coronary arteries and arterioles, with obliterative vasculopathy was counted in each section, in a blinded manner by a single histologist.
Donor-specific antibody detection and quantitation by flow cytometry
Flow cytometry to detect and quantitate donor-specific antibodies in non-recipient and cardiac graft recipient serum was performed using a previously reported method (24, 23). In brief, aliquots of donor or recipient strain thymocyte suspensions were incubated with serial dilutions of recipient sera taken pretransplant and at several time points between transplantation and rejection. After 30 min, the cells were washed and suspended in staining buffer (Dulbecco’s PBS with 2% FCS/0.2% NaN3) containing FITC-conjugated goat anti-mouse IgG serum (Jackson Immuno Research, West Grove, PA). The mean channel fluorescence (MCF) of each dilution of each serum sample was determined and the dilution that returned the MCF to the level observed when Kd thymocytes were stained with a 1:4 dilution of normal wild-type serum was divided by two and reported as the titer.
Statistics
Each experiment was performed three or more times to confirm the reproducibility of the results and representative data are shown. Students’ t test was used to examine the significance of the data obtained by FACS, ELISPOT, and MLR analyses when applicable. Differences in graft survival between different experimental groups were tested for statistical significance using the log-rank sum test. For the suppression assays, one-way ANOVA with Dunnet’s post-test was performed. In all analyses, P values <0.05 were considered statistically significant differences.
Results
Lymphopenia leads to the rejection of accepted cardiac allografts
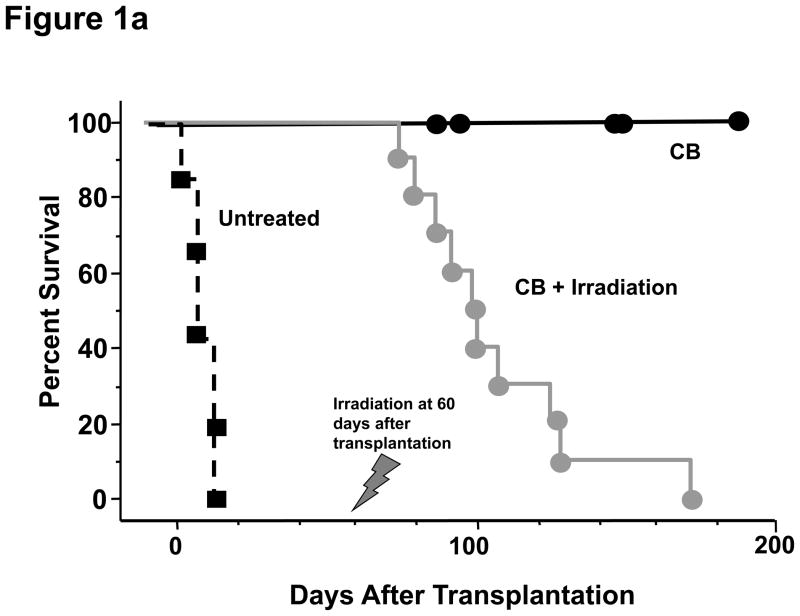
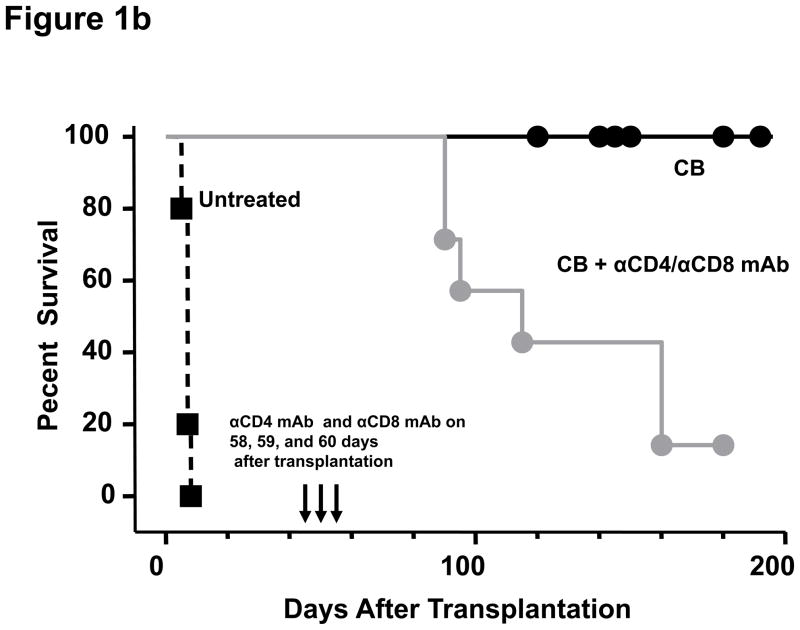
In order to test the effect of induced lymphopenia on accepted cardiac allografts, C57BL/6 mice received full MHC-mismatched BALB/c hearts and were conditioned with CTLA4-Ig and anti-CD40L mAb on days 0, 2, 4, and 6 post-transplant, and then were subjected to 6.5 Gy TBI on day 60 post-transplant. Consistent with a previous report (21), costimulation blockade prolonged survival of all allografts more than 200 days. Recipients treated with TBI on day 60 post-transplant rejected the allografts within 35–167 days after irradiation (median survival 74 ± 5 days after irradiation; Figure 1a). Syngeneic grafts in B6 recipients showed no graft loss even 300 days after irradiation, indicating that the rejection of allografts was due to alloimmune responses and not due to non-specific radiation-induced injury (Figure 2a). To the impact of LIP on accepted allografts using a different lymphodepletion strategy, recipients of accepted allografts were treated with CD4- plus CD8-depleting antibodies on days 58, 59, and 60 post-transplant (Figure 1b). This treatment also provoked rejection of the allografts within 55–115 days after antibody treatment (median survival 78 ± 4 days after depletion; Figure 1b).
Figure 1. Transient lymphopenia leads to rejection of cardiac allografts in CB conditioned recipients.
C57BL/6 mice received complete MHC-mismatched BALB/c heterotopic heart transplants with or without 500 μg of anti-CD40L mAb plus 500 μg of hCTLA4-Ig (CB conditioning) on the day of transplantation and every other day for 6 days to promote long-term allograft survival. One group of the CB conditioned recipients was (a) sub-lethally irradiated (6.5 Gy on day 60 post-transplant; and, (b) another group was treated on days 58, 59, and 60 post-transplant with CD4− and CD8− depleting antibodies. Allograft survival was monitored by palpation.
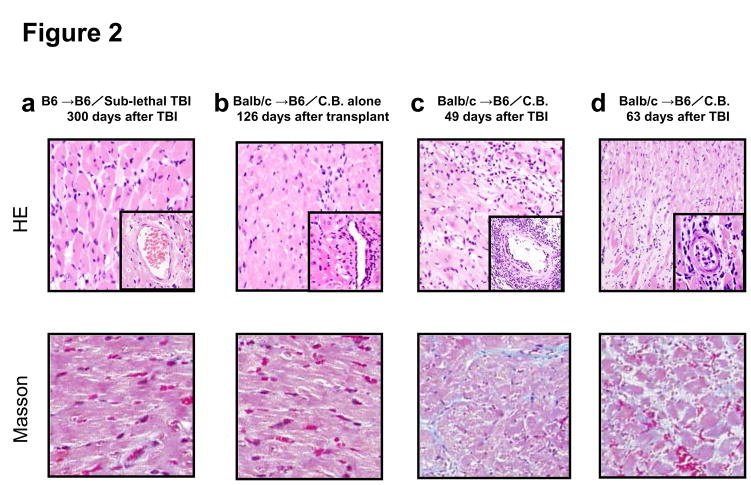
Figure 2. Histological examination of representative cardiac grafts in irradiated hosts.
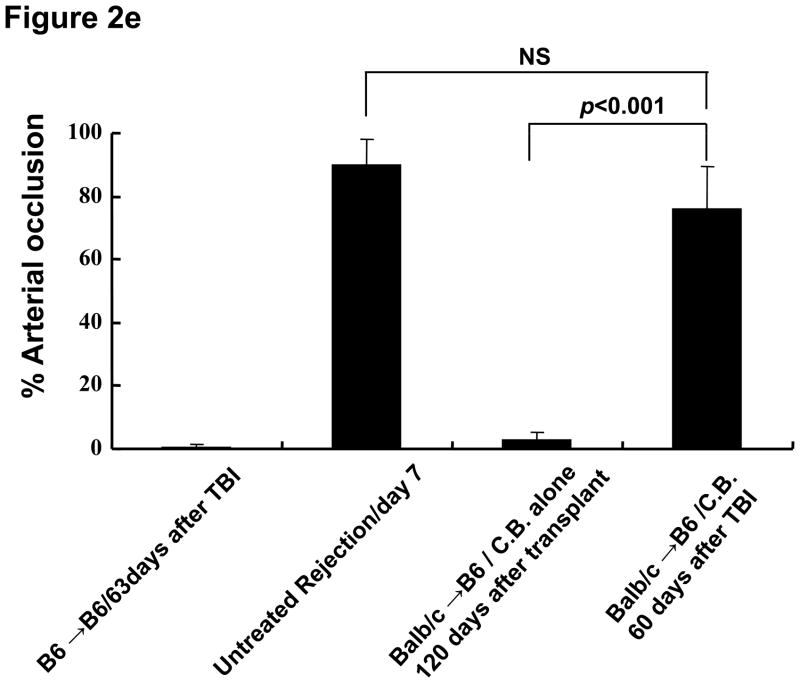
a–d) Cardiac grafts were harvested from B6 recipients at the indicated time points post-transplant and prepared sections stained with H&E (upper row) or Masson’s trichrome (lower row). Original magnification ×200; inserts ×400. e) Bars represent the combined mean ± SEM of the occlusion area of heart grafts observed in 10–20 microscopic frames of view taken from no fewer than six different cardiac grafts per group.
To investigate mechanisms underlying the loss of accepted cardiac allografts in recipients subjected to lymphopenia, we examined graft histology at days 49 and 63 after TBI (i.e. days 109 and 126 post-transplant). The histology of cardiac allografts in recipients conditioned with CB without TBI maintained normal appearance at 126 days after transplantation (Figure 2b). In contrast, allografts in CB conditioned recipients receiving TBI began to show changes characteristic of severe rejection, including diffuse infiltration of mononuclear cells and obliterative vasculopathy with diffuse intimal thickening in coronary arteries 30 days after TBI (Figure 2c and 2d).
Despite continued graft survival as assessed by palpation, histologic analysis of allografts after recipient TBI exhibited evidence of fibrosis (Figure 2c and 2d). The number of coronary arteries and arterioles affected with obliterative vasculopathy in this group ranged from 64–87% of vessels observed at 63 days after TBI (Figure 2e). These results suggest that TBI breaks tolerance to cardiac allografts that were accepted by CB treatment and that this leads to rejection of the allograft.
Memory phenotype T cells accumulate in the spleen and lymph nodes after induction of lymphopenia
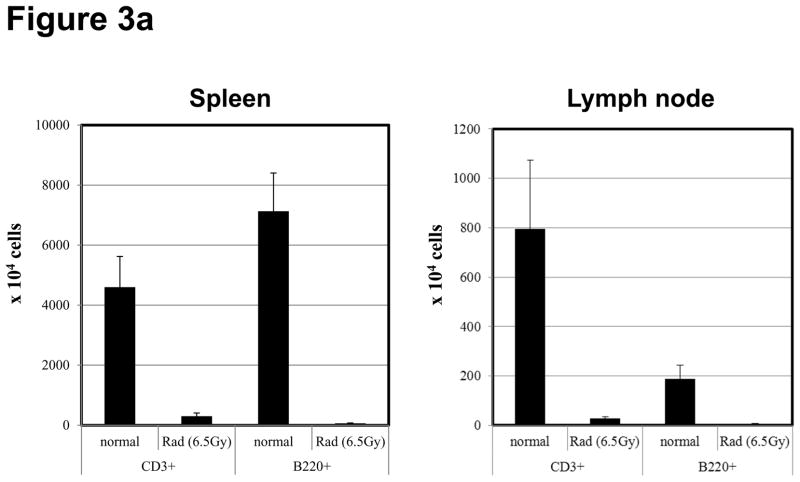
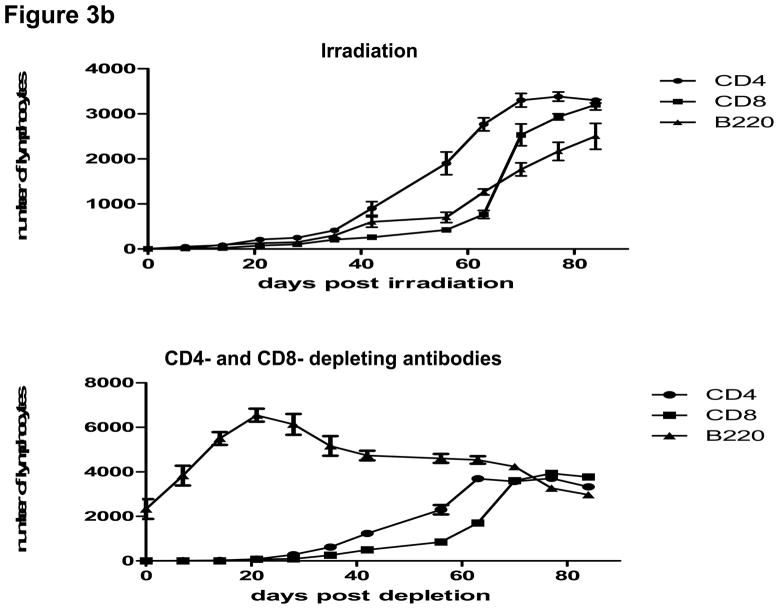
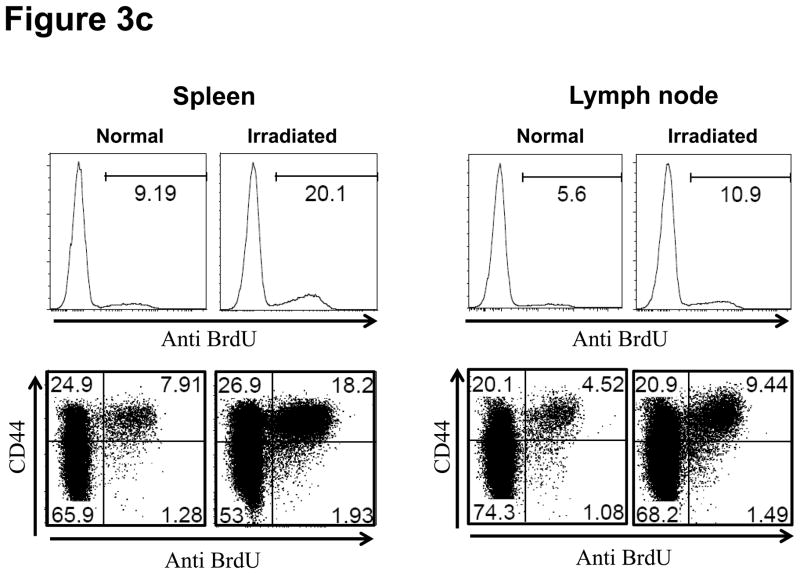
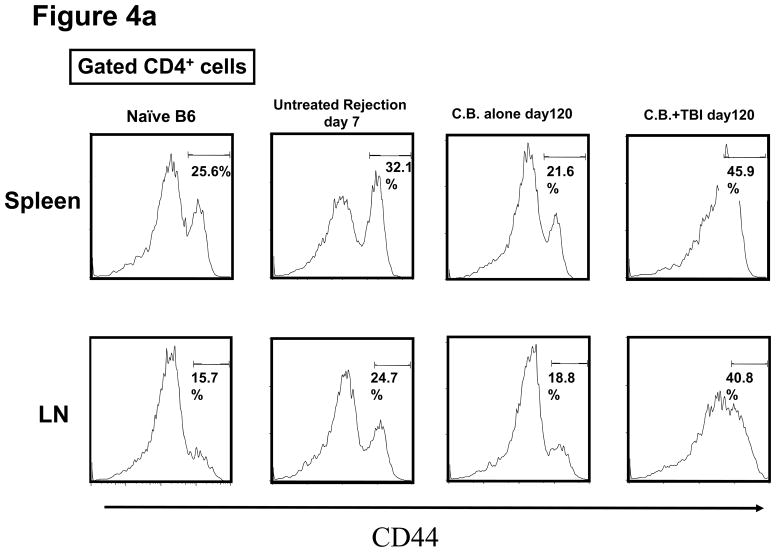
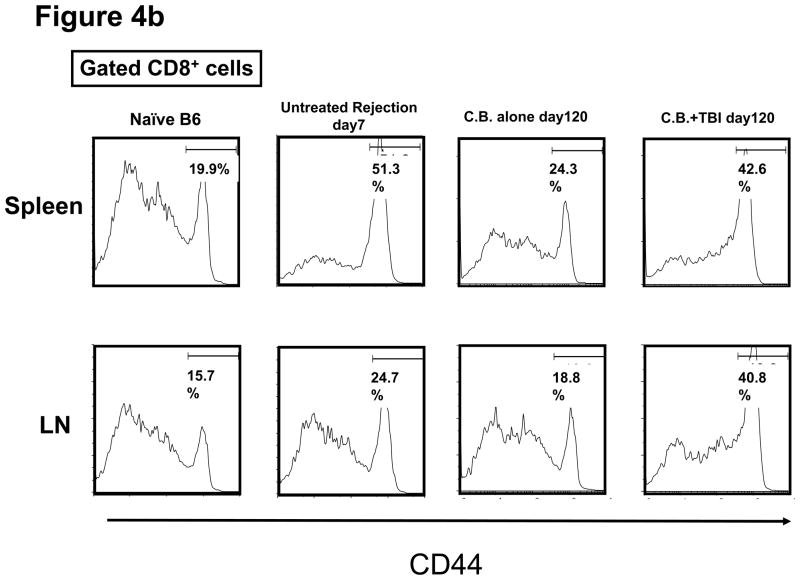
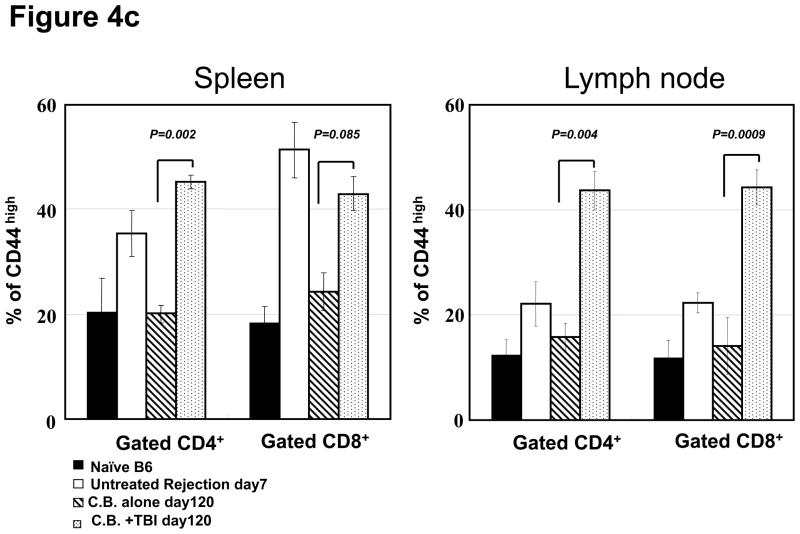
T and B cell numbers in the spleen and lymph nodes (LN) of naïve mice were significantly reduced 5 days after TBI (Figure 3a). Lymphopenia lasted approximately 28 days following irradiation and then rapid recovery of T and B cells was observed in the peripheral blood (Figure 3b). In CB-conditioned heart allograft recipients, lymphopenia lasted approximately 40 days following irradiation and then rapid recovery of T and B cells was observed in the peripheral blood, with recovery of the CD4 T cells occurring more rapidly (Figure 3b). When CB-conditioned allograft recipients were treated with CD4- plus CD8-depleting antibodies, a similar recovery from lymphopenia began 40 days after antibody treatment. In both treatment strategies, severe T cell lymphopenia was followed by the expansion of T cells in the periphery, suggesting lymphopenia-induced proliferation (LIP). To address this, the proliferation of the remaining endogenous T cells in naïve mice subjected to TBI was examined through BrdU incorporation and vigorous proliferation of T cells in the spleen and LN was observed 30 days after TBI (Figure 3c). Following LIP, T cells increase expression of effector/memory marker molecules such as CD44 and differentiate directly into functional memory T cells (13). In order to determine whether the rejection provoked by LIP was associated with increased effector/memory T cells, we analyzed the phenotypes of CD4+ and CD8+ T cells in the spleen and LNs of CB conditioned allograft recipients given TBI on day 60 post-transplant. CD4+ and CD8+ T cells from CB-conditioned recipients not rejecting their allografts expressed similar levels of CD44 as T cells in naive B6 mice (Figure 4a and b). In CB conditioned allograft recipients given TBI to induce lymphopenia, there were substantial increases in CD44high populations of both CD4+ and CD8+ T cells n the spleen and LN within 60 days after irradiation (Figure 4c). Overall, the results suggest that CD44high T cells arising during LIP may play an important role for rejection of the accepted cardiac grafts.
Figure 3. Lymphopenia and subsequent lymphopenia-induced proliferation of residual T cells following lymphocytes depletion.
a) Naive C57BL/6 mice were subjected to 6.5Gy whole body irradiation and numbers of T and B cells in the spleen and LN were assessed 5 days later by antibody staining and flow cytometry analysis. b) The kinetics of lymphocyte population recovery in the peripheral blood of CB-conditioned heart allograft recipients following irradiation or treatment with CD4- plus CD8-depleting mAb as assessed by antibody staining and flow cytometry analysis. c) The proliferation of residual T cells in the spleen and LN 30 days of naïve mice after irradiation as assessed by BrdU incorporation.
Figure 4. Appearance of CD4 and CD8 T cells expressing high levels of CD44 following transient lymphopenia.
a and b) The presence of CD44high, memory phenotype CD4+ and CD8+ T cells in the spleen and LN of naïve C57BL/6 mice, untreated C57BL/6 recipients of BALB/c cardiac allografts on day 7 post-transplant, CB-conditioned, tolerant allograft recipients on day 120 post-transplant, and CB-conditioned allograft recipients subjected to irradiation on day 120 post-transplant. c) The frequency of CD44high T cells among total CD4+ or CD8+ T cells in the spleen and LN of the mice in analyzed in a and b. Data are expressed as mean % CD44high in gated CD4+ and CD8+ T cell populations ± SD. P values were calculated using the two-tailed paired Students’ t-test.
Alloreactivity of T cells in cervical lymph nodes is restored following irradiation of tolerant recipients
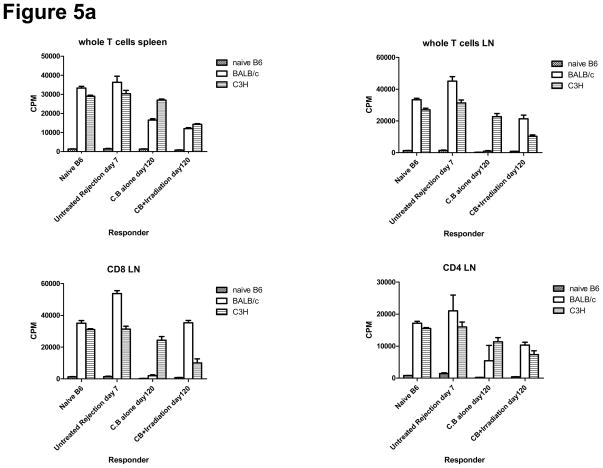
To investigate the potential restoration of anti-donor T cell reactivity in CB conditioned allograft recipients treated with TBI, we examined T cell recall responses to donor APCs by MLR and ELISPOT assays. T cells in the spleen and cervical LN from CB-conditioned recipients showed lower alloreactive proliferative responses against BALB/c donor APCs, whereas the responses to APCs from third party C3H mice were similar to that of T cells from normal B6 mice (Figure 5a). In contrast, T cells in cervical LN from allograft recipients conditioned with CB and then irradiation-induced lymphopenia had strong donor-reactive proliferative responses, though these were less than that observed in naïve B6 mice. Donor alloreactive responses of T cells in the LN of lymphopenic recipients was higher than in the spleen, suggesting that proliferating T cells encounter higher concentrations of donor alloantigens in regional/draining LN than in the spleen. Similar results were observed when CD8+and CD4+T cells were purified from recipient cervical lymph node cell suspensions and tested for proliferative responses. CD8+ T cells had higher donor-reactive proliferative responses than CD4+ T cells (Figure 5a).
Figure 5. Donor-reactive T cells responses are recovered in CB-conditioned heart allograft recipients following transient lymphopenia.
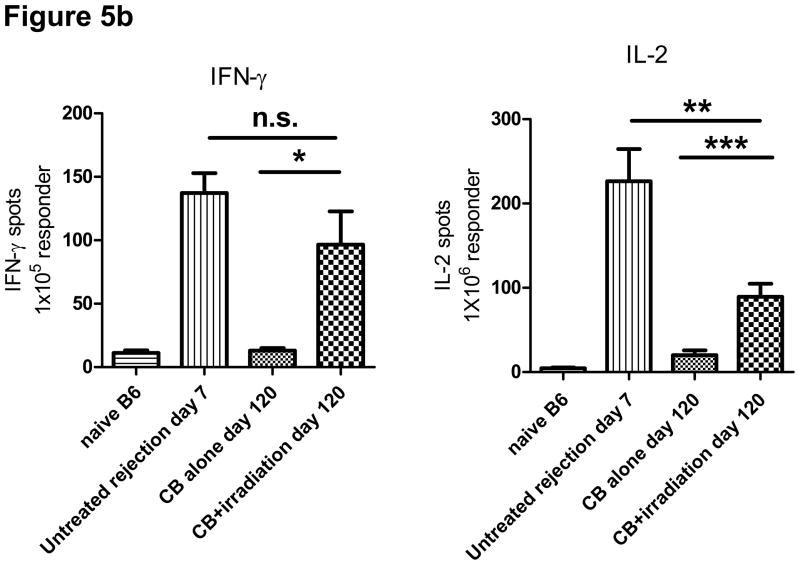
a) Spleen and LN cell suspensions were prepared from naïve C57BL/6 mice, untreated C57BL/6 recipients of BALB/c cardiac allografts on day 7 post-transplant, CB-conditioned, tolerant allograft recipients on day 120 post-transplant, and CB-conditioned allograft recipients subjected to irradiation on day 120 post-transplant. As indicated unseparated cells or enriched CD4+ or CD8+ T cell populations were cultured with syngeneic, BALB/c donor-derived, or third-party allogeneic C3H spleen cells that had been depleted of T cells for 88 hours and then pulsed with 3H-thymidinde for 8 hours before harvest and assessment of 3H-thymidine incorporation. Data represent the mean ± SEM 3H-thymidine incorporation of triplicate cultures from a single experiment that is representative of three others. b) T cells were enriched from LNs by negative selection and were cultured with BALB/c donor spleen cells that had been depleted of T cells to enumerate donor-reactive T cells producing IFN-γ and IL-2 by ELISPOT assay. The data indicate the mean number of donor-reactive T cells producing the test cytokine ± SEM. *p = 0.032 versus CB-tolerant recipients; **p = 0.014 versus CB-tolerant recipients; ***p = 0.029 versus untreated rejection recipients. Data are representative of two independent experiments.
To assess the priming of donor-reactive T cells in CB conditioned recipients of BALB/c cardiac allografts given TBI on day 60 post-transplant, recipient LN cell suspensions were prepared on day 120 post-transplant and numbers of donor-reactive T cells producing IFN-γ and IL-2 were enumerated by ELISPOT assay. Whereas low numbers of donor-reactive T cells producing IFN-γ were observed in LN of CB-induced, allograft tolerant mice, the numbers in LIP-treated CB-conditioned recipients were markedly higher, near those observed during acute rejection of the allografts in non-treated recipients on day 7 post-transplant (Figure 5b). Numbers of donor-reactive T cells producing IL-2 in irradiated allograft recipients conditioned with CB were lower than non-treated allograft recipients rejecting their allografts but significantly higher than the numbers in LN from CB-tolerant mice. These results indicate that lymphopenia restores donor alloreactivity, mainly in regional LN, resulting in rejection of accepted cardiac allografts.
Lymphocyte depletion markedly increases the proliferation of Tregs
It has been proposed that the outcome of an immune response depends on the balance of effector and regulatory T cells. Thus, we next investigated the effect of lymphocyte depletion on the pool of Tregs in CB-conditioned allograft recipients with vs. without TBI. Specifically, we focused on CD4+FoxP3+ cells because these cells exist as a measurable minority population in naive mice and have been shown to promote long-term graft survival and tolerance in several transplantation models (25–27).
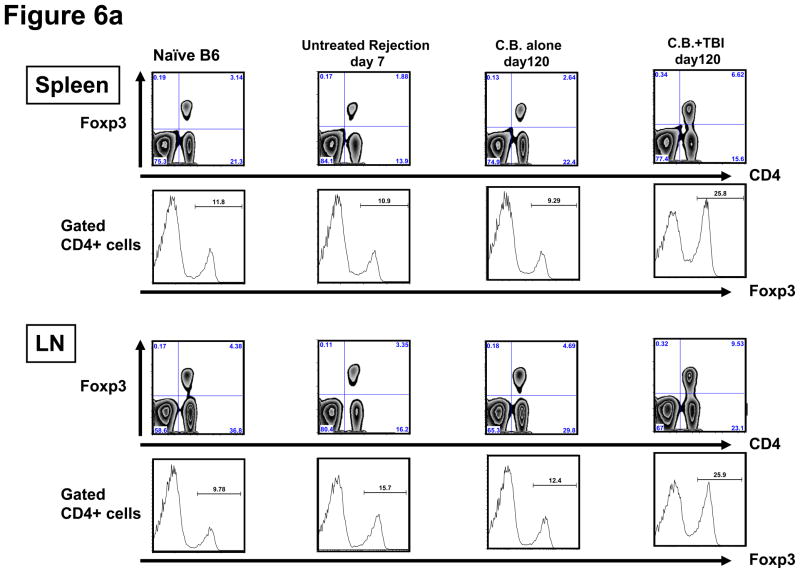
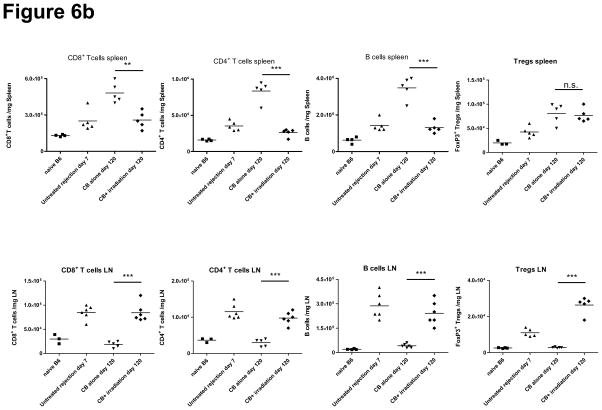
Previous reports on the expansion of Tregs during LIP have yielded contradictory results, with one study showing reduced numbers and another showing full restoration of Tregs following LIP (15, 27, 28). To determine whether Tregs go through LIP to the same extent as CD4+FoxP3− T cells, CB-conditioned allograft recipients were sacrificed 60 days after irradiation (day 120 post-transplant), and the percentage of CD4+Foxp3+ cells was assessed by intracellular staining. The percentage of Foxp3+ cells recovered from LN of recipients with LIP was significantly higher than that from LN of naïve, untreated, and CB-tolerant mice (Figure 6a). Similarly, the percentage of Foxp3+ CD4+ T cells was 1.5–2.5 fold higher in the spleen following irradiation of recipients. Approximately 25% of CD4+ cells expressed Foxp3 both in the spleen and LN. The total numbers of CD4+ T cells, CD8+ T cells, B cells, and regulatory T cells in the spleen and cervical LN are shown in Figure 6b. Interestingly, the actual numbers of each cell population in LN were significantly higher in LIP treated recipients than in CB-tolerant recipients whereas numbers of each cell population in the spleen were lower in LIP-treated recipients than in CB-tolerant recipients except for the numbers of Tregs. These results suggest that alloreactive T cells tend to accumulate in regional lymph nodes after lymphopenia.
Figure 6. Transient lymphopenia of CB-conditioned allograft recipients increases Foxp3+ CD4+ T cells in LN and spleen without suppressing the alloreactive effector/memory T cell response.
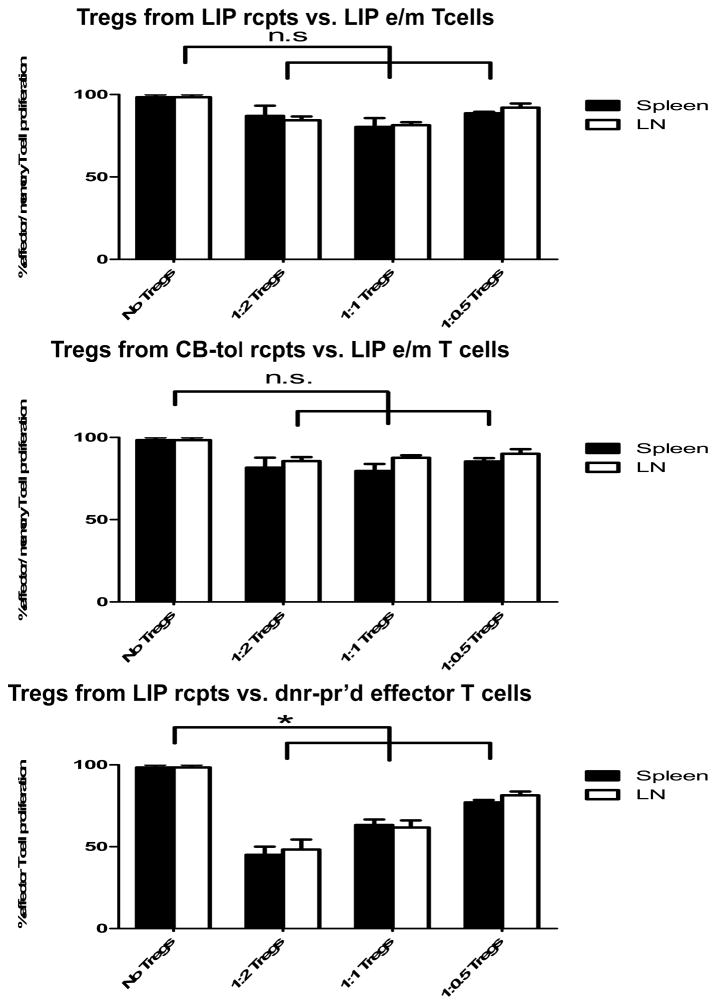
a) Spleen and LN cell suspensions were prepared from naïve C57BL/6 mice, untreated C57BL/6 recipients of BALB/c cardiac allografts on day 7 post-transplant, CB-conditioned, tolerant allograft recipients on day 120 post-transplant, and CB-conditioned allograft recipients subjected to irradiation on day 120 post-transplant and stained with anti-CD4 and anti-FoxP3 mAb to determine the percentage of double positive cells. The percentage of Foxp3+ cells in the CD4+ T cell population was higher in irradiated recipients than in the other groups (p < 0.001, for both spleen and LN). b) The total numbers of CD8 T, CD4 T, B cells, and CD4+FoxP3+ Treg cells per mg of lymphoid tissue was determined by antibody staining and flow cytometry analysis at the time of spleen and LN harvest of each group. **p < 0.01; ***p < 0.001. c) For test Treg cells, CD4+CD25+ T cells were isolated using magnetic beads (Stem Cell Technologies, Vancouver, BC) on day 120 post-transplant from the spleen and LN of either CB-conditioned, tolerant allograft recipients or CB-conditioned allograft recipients subjected to irradiation. For use as LIP effector/memory responder T cells, T cells from LIP mice on day 120 post-transplant were flow sorted to isolate CD62Llow, CD44high T cells (> 98% purity) using a FACSAria (BD Biosciences, San Jose, CA). For use as primary effector T cell responders, T cells from the spleen and lymph nodes of LIP mice were obtained on day 120 post-transplant and the CD4+CD25+ T cells removed. Responder T cell populations were labeled with CFSE and these cells and the isolated Treg cell populations added to cultures of donor-derived T cell depleted spleen cells and anti-CD3 mAb in the indicated responder T cell: regulatory T cell ratios. After 4 days of culture, the degree of responder T cell proliferation was determined by assessing CFSE dilution in flow cytometry analyses. All cultures were performed and analyzed in triplicate, and levels of proliferation in the absence of Tregs was set at 100%. n.s., p > 0.05.
Proliferative response of alloreactive effector/memory T cells is not inhibited by Tregs from CB-tolerant and LIP-treated recipients
The activities of Tregs in recipients with CB conditioned accepted cardiac allografts and in recipients during irradiation-induced LIP were directly tested by isolating the CD4+CD25+ T cells from the spleen and LN from each group of recipients and adding these Treg cells to co-cultures of effector/memory T cells induced by the LIP plus T cell-depleted donor APCs and anti-CD3ε mAb. Splenic and LN resident Tregs from CB-tolerant or from LIP recipients were unable to suppress the proliferation of the LIP-induced effector/memory T cells as there were no differences in this proliferation when compared to proliferation observed in the absence of the added Treg populations (Fig. 6c). However. Tregs isolated during irradiation-induced LIP significantly suppressed the proliferation of primary effector T cells induced in response to donor heart allografts. Thus, the increased CD4+FoxP3+ Tregs induced during allograft recipient LIP were unable to inhibit the activation of the effector/memory T cells induced during LIP, consistent with the allograft injury and rejection observed. The ability of the Tregs to inhibit the activation of donor-reactive primary effector T cells suggests that it is the LIP-induced effector/memory T cells that are resistant to the suppressive activity of the Tregs rather than a defect in the function of the regulatory T cells.
Anti-donor antibody formation following lymphocyte depletion
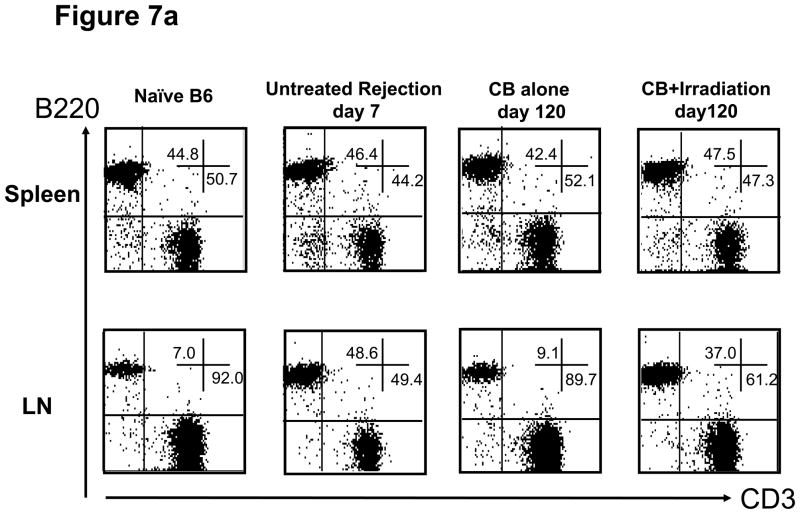
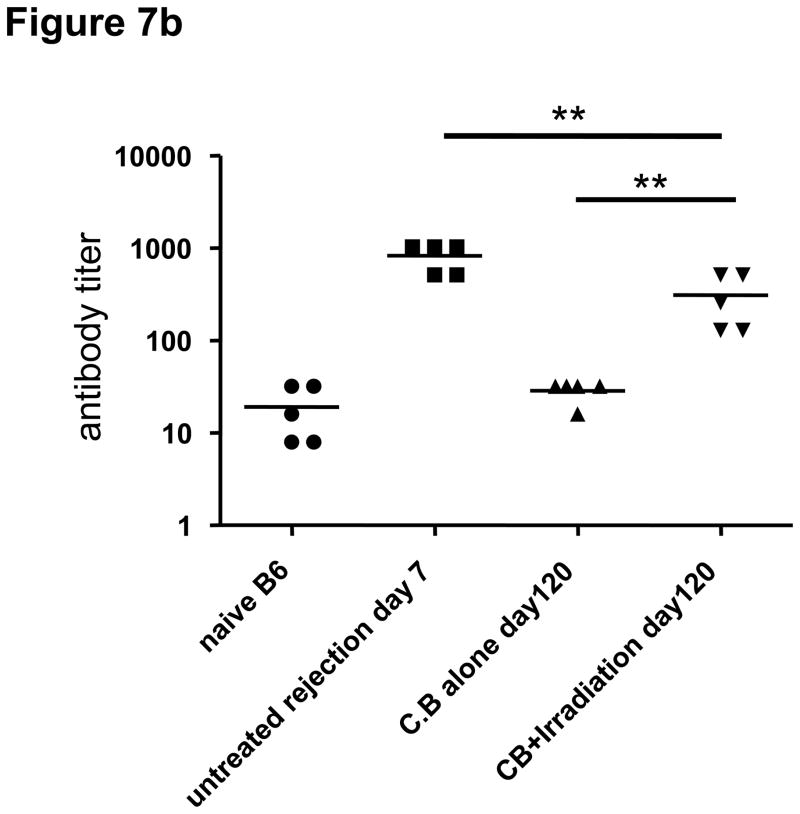
While transplant rejection in rodent models is primarily mediated by donor-reactive T cells (29–31), a role for donor-reactive antibodies in acute and chronic rejection of clinical transplants has been reported (4, 5). In our experiments, we found that the percentage and numbers of B cells in LN significantly expanded in both untreated and lymphopenia induced allograft recipients after allograft rejection when compared to B cell numbers in CB-tolerant hosts (Figures 6b and 7a). The anti-donor antibody titers in CB-tolerant mice were equivalent to those observed in naïve B6 mice whereas anti-donor antibody titers increased after lymphopenia induction and graft rejection (Figure 7b). The levels of anti-donor antibody were higher than those in CB-treated tolerized recipients, but lower than those in untreated allograft rejecting recipients. These findings demonstrate that inducible lymphopenia in CB tolerant hosts not only restores allograft donor reactive T cell responses but also restores donor-reactive antibody production that may contribute to the gradual allograft rejection.
Figure 7. Transient lymphopenia in CB conditioned allograft recipients induces the accumulation of B cells in lymph nodes and restores antibody production against allogenic antigens.
a) Flow cytometry analysis of T and B cells in the spleen and LN of naïve C57BL/6 mice, untreated C57BL/6 recipients of BALB/c cardiac allografts on day 7 post-transplant, CB-conditioned, tolerant allograft recipients on day 120 post-transplant, and CB-conditioned allograft recipients subjected to irradiation on day on day 120 post-transplant. b) Serum from naïve, untreated-rejecting allograft recipients on day 7 post-transplant, CB-conditioned, tolerant allograft recipients on day 120 post-transplant, and CB-conditioned allograft recipients subjected to irradiation on day on day 120 post-transplant was collected and tested for reactivity to BALB/c thymocytes using flow cytometry-based analyses to determine donor-reactive antibody titers. Data indicate mean titer ± SEM for n=5/group and are representative of two independent experiments. **p < 0.01
Discussion
In the current study, we demonstrate that transient lymphopenia is a factor that initiates rejection of stable cardiac allografts. During lymphopenia, qualitative histological changes such as myocardial atrophy, coronary occlusion, fibrosis and mononuclear cell infiltration were observed in the grafts. Consistent with a previous study (13), we found that both CD8+ and CD4+ T cells with a memory phenotype accumulated in the spleen and LN after induction of lymphopenia. Analysis of the proliferative capacity and the function of donor-reactive T cells revealed the reversal of T cell non-responsiveness induced by allograft recipient CB conditioning following induction of lymphopenia. These results suggest that LIP and accumulation of donor-reactive T cells are mechanistic components underlying rejection of allografts in CB-conditioned recipients. The reduction of lymphocytes by cytotoxic drugs, irradiation, or certain viruses is known to lead to lymphopenia-induced proliferation and the subsequent restoration of normal T-cell levels (32). Transplant recipients that develop a malignancy are in some instances treated with irradiation as a component of the cancer therapy, often with a reduction in immunosuppression administered for the graft. The current studies indicate that such treatment could conceivably lead to LIP with adverse effects on graft outcome.
Using an adoptive transfer of TCR transgenic T cells specific for influenza virus hemagglutinin (HA) into lymphopenic mice transgenically expressing insulin promoter driven HA (InsHA), Le Saout, et al. demonstrated that memory-like CD8+ and CD4+ T cells cooperate to break peripheral tolerance during the lymphopenic conditions, resulting in the induction of autoimmune diabetes (33). Neither adoptive transfer with only CD8+ T cells into lymphopenic hosts nor transfer with both CD4+ and CD8+ T cells into replete hosts was sufficient to induce autoimmune diabetes. Furthermore, in tumor models LIP was observed to induce IL-2 and IFN-γ production by adoptively transferred T cells resulting in suppression of tumor growth (34, 35). These reports are in accord with our observation that LIP following lymphopenia breaks transplant tolerance achieved by CB conditioning and causes allograft rejection.
Alloimmune responses involve both effector lymphocytes and regulatory T cells and it is the balance between the functions of these two populations that determines allograft outcome (36). Human and non-human primate recipients with acquired donor-specific hyporesponsiveness often have Foxp3+ lymphocyte infiltrates in allografts and their presence is associated with excellent graft function (37, 38). Moreover, recent studies showed that adoptive transfer with in vitro expanded regulatory T cells can induce long term allograft tolerance (39, 40). Therefore, the localization and significance of Foxp3+ CD4+ T cells in transplantation is of considerable clinical and immunological interest. In our study, during lymphopenia, the proportions of CD4+ FoxP3+ T cells were elevated by approximately 2.5-fold and 1.6-fold in the spleen and LN, respectively, when compared with those in untreated, allograft-rejecting recipients. Similar results were obtained with the absolute number of Tregs in spleen and LN following LIP of CB conditioned allograft recipients. Our findings regarding the modest increases in regulatory T cells in lymphopenic hosts are consistent with recent reports modeling LIP in RAG knockout mice or inducible lymphopenia by antibody-mediated depletion of T cells (12, 15). Despite higher levels of Foxp3+ cells in lymphatic tissues, CB-treated recipients that developed lymphopenia rejected their allografts, suggesting that CD62LlowCD44high effector /memory T cells that have undergone proliferation after lymphopenia are resistant to the suppressive activities of Tregs and mediate allogeneic cardiac graft rejection. This interpretation is supported by studies in other models indicating that reactivated memory T cells are resistant to regulation by Treg cells (41, 42).
Our results demonstrate that anti-donor antibody production was also restored after induction of lymphopenia, whereas anti-donor antibody titers in CB-tolerized hosts were similar to those in naïve mice (Figure 7b). The restoration of anti-donor antibody production might lead to the activation of antibody dependent cellular cytotoxicity and complement dependent cytotoxicity against donor cells; however, the precise mechanisms underlying chronic antibody mediated rejection in transplantation remain unclear (4). Several previous studies suggest that the lymphopenia-induced expansion of CD4+ T cells can induce systemic autoimmunity accompanied by hypergammaglobulinemia and autoantibody production (43, 44). In the transplantation setting, our results indicate that tolerance of T cells is broken in graft-draining lymph nodes following LIP and that expansion of B cells is also observed in lymph nodes of lymphopenic hosts, suggesting that the restoration of the alloreactive CD4+ T cell response induces the activation of B cells. Thus, transient lymphopenia is sufficient to initiate both cellular and humoral alloimune mechanisms involved in allograft rejection.
In summary, our results demonstrate an unfavorable effect of lymphopenia in CB-conditioned recipients in solid organ transplantation. Even after CB treatment has established donor-specific tolerance, its effect can be negated following induction of lymphopenia. Selective costimulatory blockade is now being used clinically to induce tolerance or prolong graft survival (45, 46). However, investigators and clinicians should be aware that induced lymphopenia may cause later allograft rejection. Thus, it is necessary to develop strategies to obtain the optimal extent of depletion or to control proliferation caused by lymphopenia to avoid the subsequent accumulation of pathogenic donor-reactive cell types that could lead to graft rejection.
Acknowledgments
Thanks are due to Sakiko Kobayashi for preparation of Abs, and the members of Science Ser vice, Inc. for care of the experimental animals. We thank Dr. William M. Baldwin, III, Department of Immunology; Lerner Research Institute, Cleveland Clinic for helpful discussions. This work was supported in part by PO1 AI087506 from the NIH (AV and RLF).
List of abbreviations
- CB
costimulatory blockade
- HIV
human immunodeficiency virus
- MLR
mixed lymphocyte reaction
- LIP
lymphopenia-induced-proliferation
- PBS
phosphate buffered saline
- RPMI
Roswell Park Memorial Institute
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- LN
lymph node
- APCs
antigen presenting cells
- TCR
T cell receptor
- HA
hemagglutinin (HA)
- InsHA
Insulin promoter driven HA hemagglutinin
- hCTLA4-Ig
Human cytotoxic T-lymphocyte antigen 4-immunoglobulin
- IFN-γ
Interferon-γ
- IFN-2
Interleukin-2
- CR
Chronic rejection
- 2.4G2
Anti-γFc Receptor
- MST
Mean survival time
- mAbs
Monoclonal antibodies
Footnotes
Disclosures
The authors have no financial conflict of interest to declare.
References
- 1.Baczkowska T, Durlik M. Calcineurin inhibitor sparing immunosuppressive regimens in kidney allograft recipients. Pol Arch Med Wewn. 2009 May;119(5):318–25. [PubMed] [Google Scholar]
- 2.Vathsala A. Preventing renal transplant failure. Ann Acad Med Singapore. 2005 Jan;34(1):36–43. [PubMed] [Google Scholar]
- 3.Boisgerault F, Liu Y, Anosova N, Ehrlich E, Dana MR, Benichou G. Role of CD4+ and CD8+ T cells in allorecognition: lessons from corneal transplantation. J Immunol. 2001 Aug 15;167(4):1891–9. doi: 10.4049/jimmunol.167.4.1891. [DOI] [PubMed] [Google Scholar]
- 4.Baldwin WM, 3rd, Valujskikh A, Fairchild RL. Antibody-mediated rejection: emergence of animal models to answer clinical questions. Am J Transplant. May;10(5):1135–42. doi: 10.1111/j.1600-6143.2010.03065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Billing H, Rieger S, Ovens J, Susal C, Melk A, Waldherr R, et al. Successful treatment of chronic antibody-mediated rejection with IVIG and rituximab in pediatric renal transplant recipients. Transplantation. 2008 Nov 15;86(9):1214–21. doi: 10.1097/TP.0b013e3181880b35. [DOI] [PubMed] [Google Scholar]
- 6.Libby P, Pober JS. Chronic rejection. Immunity. 2001 Apr;14(4):387–97. doi: 10.1016/s1074-7613(01)00119-4. [DOI] [PubMed] [Google Scholar]
- 7.Knechtle SJ, Pirsch JDH, Fechner JJ, Becker BN, Friedl A, Colvin RB, et al. Campath-1H induction plus rapamycin monotherapy for renal transplantation: results of a pilot study. Am J Transplant. 2003 Jun;3(6):722–30. doi: 10.1034/j.1600-6143.2003.00120.x. [DOI] [PubMed] [Google Scholar]
- 8.Kirk AD, Hale DA, Mannon RB, Kleiner DE, Hoffmann SC, Kampen RL, et al. Results from a human renal allograft tolerance trial evaluating the humanized CD52-specific monoclonal antibody alemtuzumab (CAMPATH-1H) Transplantation. 2003 Jul 15;76(1):120–9. doi: 10.1097/01.TP.0000071362.99021.D9. [DOI] [PubMed] [Google Scholar]
- 9.Haluszczak C, Akue AD, Hamilton SE, Johnson LD, Pujanauski L, Teodorovic L, et al. The antigen-specific CD8+ T cell repertoire in unimmunized mice includes memory phenotype cells bearing markers of homeostatic expansion. J Exp Med. 2009 Feb 16;206(2):435–48. doi: 10.1084/jem.20081829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sai P, Senecat O, Martignat L, Gouin E. Neonatal injections of cyclosporin enhance autoimmune diabetes in non-obese diabetic mice. Clin Exp Immunol. 1994 Jul;97(1):138–45. doi: 10.1111/j.1365-2249.1994.tb06592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baccala R, Theofilopoulos AN. The new paradigm of T-cell homeostatic proliferation-induced autoimmunity. Trends Immunol. 2005 Jan;26(1):5–8. doi: 10.1016/j.it.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 12.Moxham VF, Karegli J, Phillips RE, Brown KL, Tapmeier TT, Hangartner R, et al. Homeostatic proliferation of lymphocytes results in augmented memory-like function and accelerated allograft rejection. J Immunol. 2008 Mar 15;180(6):3910–8. doi: 10.4049/jimmunol.180.6.3910. [DOI] [PubMed] [Google Scholar]
- 13.Surh CD, Sprent J. Regulation of mature T cell homeostasis. Semin Immunol. 2005;17(3):183–91. doi: 10.1016/j.smim.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 14.Pearl JP, Parris J, Hale DA, Hoffmann SC, Bernstein WB, McCoy KL, et al. Immunocompetent T-cells with a memory-like phenotype are the dominant cell type following antibody-mediated T-cell depletion. Am J Transplant. 2005 Mar;5(3):465–74. doi: 10.1111/j.1600-6143.2005.00759.x. [DOI] [PubMed] [Google Scholar]
- 15.Neujahr DC, Chen C, Huang X, Markmann JF, Cobbold S, Waldmann H, et al. Accelerated memory cell homeostasis during T cell depletion and approaches to overcome it. J Immunol. 2006 Apr 15;176(8):4632–9. doi: 10.4049/jimmunol.176.8.4632. [DOI] [PubMed] [Google Scholar]
- 16.Wu Z, Bensinger SJ, Zhang J, Chen C, Yuan X, Huang X, et al. Homeostatic proliferation is a barrier to transplantation tolerance. Nat Med. 2004 Jan;10(1):87–92. doi: 10.1038/nm965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Setoguchi K, Kishimoto H, Kobayashi S, Shimmura H, Ishida H, Toki D, et al. Potential role of host effector memory CD8+ T cells in marrow rejection after mixed chimerism induction in cynomolgus monkeys. Transpl Immunol. Aug;23(4):194–203. doi: 10.1016/j.trim.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 18.Shprecher DR, Flanigan KM, Smith AG, Smith SM, Schenkenberg T, Steffens J. Clinical and diagnostic features of delayed hypoxic leukoencephalopathy. J Neuropsychiatry Clin Neurosci. 2008 Fall;20(4):473–7. doi: 10.1176/jnp.2008.20.4.473. [DOI] [PubMed] [Google Scholar]
- 19.Afanasyeva M, Georgakopoulos D, Belardi DF, Ramsundar AC, Barin JG, Kass DA, et al. Quantitative analysis of myocardial inflammation by flow cytometry in murine autoimmune myocarditis: correlation with cardiac function. Am J Pathol. 2004 Mar;164(3):807–15. doi: 10.1016/S0002-9440(10)63169-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsuura A, Abe T, Yasuura K. Simplified mouse cervical heart transplantation using a cuff technique. Transplantation. 1991 Apr;51(4):896–8. doi: 10.1097/00007890-199104000-00031. [DOI] [PubMed] [Google Scholar]
- 21.Larsen CP, Elwood ET, Alexander DZ, Ritchie SC, Hendrix R, Tucker-Burden C, et al. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996 May 30;381(6581):434–8. doi: 10.1038/381434a0. [DOI] [PubMed] [Google Scholar]
- 22.Reddy J, Illes Z, Zhang X, Encinas J, Pyrdol J, Nicholson L, et al. Myelin proteolipid protein-specific CD4+CD25+ regulatory cells mediate genetic resistance to experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2004 Oct 26;101(43):15434–9. doi: 10.1073/pnas.0404444101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nozaki T, Amano H, Bickerstaff A, Orosz CG, Novick AC, Tanabe K, et al. Antibody-mediated rejection of cardiac allografts in CCR5-deficient recipients. J Immunol. 2007 Oct 15;179(8):5238–45. doi: 10.4049/jimmunol.179.8.5238. [DOI] [PubMed] [Google Scholar]
- 24.Bickerstaff A, Nozaki T, Wang JJ, Pelletier R, Hadley G, Nadasdy G, et al. Acute humoral rejection of renal allografts in CCR5(−/−) recipients. Am J Transplant. 2008 Mar;8(3):557–66. doi: 10.1111/j.1600-6143.2007.02125.x. [DOI] [PubMed] [Google Scholar]
- 25.Dai Z, Li Q, Wang Y, Gao G, Diggs LS, Tellides G, et al. CD4+CD25+ regulatory T cells suppress allograft rejection mediated by memory CD8+ T cells via a CD30-dependent mechanism. J Clin Invest. 2004 Jan;113(2):310–7. doi: 10.1172/JCI19727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee MK, Moore DJ, Jarrett BP, Lian MM, Deng S, Huang X, et al. Promotion of allograft survival by CD4+CD25+ regulatory T cells: evidence for in vivo inhibition of effector cell proliferation. J Immunol. 2004 Jun 1;172(11):6539–44. doi: 10.4049/jimmunol.172.11.6539. [DOI] [PubMed] [Google Scholar]
- 27.Kingsley CI, Karim M, Bushell AR, Wood KJ. CD25+CD4+ regulatory T cells prevent graft rejection: CTLA-4- and IL-10-dependent immunoregulation of alloresponses. J Immunol. 2002 Feb 1;168(3):1080–6. doi: 10.4049/jimmunol.168.3.1080. [DOI] [PubMed] [Google Scholar]
- 28.Komatsu N, Hori S. Full restoration of peripheral Foxp3+ regulatory T cell pool by radioresistant host cells in scurfy bone marrow chimeras. Proc Natl Acad Sci U S A. 2007 May 22;104(21):8959–64. doi: 10.1073/pnas.0702004104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oberbarnscheidt MH, Ng YH, Chalasani G. The roles of CD8 central and effector memory T-cell subsets in allograft rejection. Am J Transplant. 2008 Sep;8(9):1809–18. doi: 10.1111/j.1600-6143.2008.02335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grazia TJ, Plenter RJ, Weber SM, Lepper HM, Victorino F, Zamora MR, et al. Acute cardiac allograft rejection by directly cytotoxic CD4 T cells: parallel requirements for Fas and perforin. Transplantation. 2010 Jan 15;89(1):33–9. doi: 10.1097/TP.0b013e3181be6bc7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nozaki T, Rosenblum JM, Ishii D, Tanabe K, Fairchild RL. CD4 T cell-mediated rejection of cardiac allografts in B cell-deficient mice. J Immunol. 2008 Oct 15;181(8):5257–63. doi: 10.4049/jimmunol.181.8.5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boyman O, Letourneau S, Krieg C, Sprent J. Homeostatic proliferation and survival of naive and memory T cells. Eur J Immunol. 2009 Aug;39(8):2088–94. doi: 10.1002/eji.200939444. [DOI] [PubMed] [Google Scholar]
- 33.Le Saout C, Mennechet S, Taylor N, Hernandez J. Memory-like CD8+ and CD4+ T cells cooperate to break peripheral tolerance under lymphopenic conditions. Proc Natl Acad Sci U S A. 2008 Dec 9;105(49):19414–9. doi: 10.1073/pnas.0807743105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown IE, Blank C, Kline J, Kacha AK, Gajewski TF. Homeostatic proliferation as an isolated variable reverses CD8+ T cell anergy and promotes tumor rejection. J Immunol. 2006 Oct 1;177(7):4521–9. doi: 10.4049/jimmunol.177.7.4521. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki T, Ogawa S, Tanabe K, Tahara H, Abe R, Kishimoto H. Induction of antitumor immune response by homeostatic proliferation and CD28 signaling. J Immunol. 2008 Apr 1;180(7):4596–605. doi: 10.4049/jimmunol.180.7.4596. [DOI] [PubMed] [Google Scholar]
- 36.Zheng XX, Sanchez-Fueyo A, Sho M, Domenig C, Sayegh MH, Strom TB. Favorably tipping the balance between cytopathic and regulatory T cells to create transplantation tolerance. Immunity. 2003 Oct;19(4):503–14. doi: 10.1016/s1074-7613(03)00259-0. [DOI] [PubMed] [Google Scholar]
- 37.Veronese F, Rotman S, Smith RN, Pelle TD, Farrell ML, Kawai T, et al. Pathological and clinical correlates of FOXP3+ cells in renal allografts during acute rejection. Am J Transplant. 2007 Apr;7(4):914–22. doi: 10.1111/j.1600-6143.2006.01704.x. [DOI] [PubMed] [Google Scholar]
- 38.Oderup C, Malm H, Ekberg H, Qi Z, Veress B, Ivars F, et al. Costimulation blockade-induced cardiac allograft tolerance: inhibition of T cell expansion and accumulation of intragraft cD4(+)Foxp3(+) T cells. Transplantation. 2006 Dec 15;82(11):1493–500. doi: 10.1097/01.tp.0000244064.66136.04. [DOI] [PubMed] [Google Scholar]
- 39.Joffre O, Santolaria T, Calise D, Al Saati T, Hudrisier D, Romagnoli P, et al. Prevention of acute and chronic allograft rejection with CD4+CD25+Foxp3+ regulatory T lymphocytes. Nat Med. 2008 Jan;14(1):88–92. doi: 10.1038/nm1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nadig SN, Wieckiewicz J, Wu DC, Warnecke G, Zhang W, Luo S, et al. In vivo prevention of transplant arteriosclerosis by ex vivo-expanded human regulatory T cells. Nat Med. Jul;16(7):809–13. doi: 10.1038/nm.2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang J, Brook MO, Carvalho-Gaspar M, Zhang J, Ramon HE, Sayegh MH, et al. Allograft rejection mediated by memory T cells is resistant to regulation. Proc Natl Acad Sci U S A. 2007 Dec 11;104(50):19954–9. doi: 10.1073/pnas.0704397104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dutton RW, Bradley LM, Swain SL. T cell memory. Annu Rev Immunol. 1998;16:201–23. doi: 10.1146/annurev.immunol.16.1.201. [DOI] [PubMed] [Google Scholar]
- 43.Khiong K, Murakami M, Kitabayashi C, Ueda N, Sawa S, Sakamoto A, et al. Homeostatically proliferating CD4 T cells are involved in the pathogenesis of an Omenn syndrome murine model. J Clin Invest. 2007 May;117(5):1270–81. doi: 10.1172/JCI30513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cassani B, Poliani PL, Marrella V, Schena F, Sauer AV, Ravanini M, et al. Homeostatic expansion of autoreactive immunoglobulin-secreting cells in the Rag2 mouse model of Omenn syndrome. J Exp Med. 2010 Jul 5;207(7):1525–40. doi: 10.1084/jem.20091928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haanstra KG, Ringers J, Sick EA, Ramdien-Murli S, Kuhn EM, Boon L, et al. Prevention of kidney allograft rejection using anti-CD40 and anti-CD86 in primates. Transplantation. 2003 Mar 15;75(5):637–43. doi: 10.1097/01.TP.0000054835.58014.C2. [DOI] [PubMed] [Google Scholar]
- 46.Linsley PS, Nadler SG. The clinical utility of inhibiting CD28-mediated costimulation. Immunol Rev. 2009 May;229(1):307–21. doi: 10.1111/j.1600-065X.2009.00780.x. [DOI] [PubMed] [Google Scholar]