Abstract
Rats emit 50 kHz ultrasonic vocalizations (USVs) in situations of increased motivation, such as during the anticipation of palatable food or drugs of abuse. Whether the same holds true for the anticipation of alcohol intake remains unknown. Alcohol drinking in a nondependent state is thought to be mediated by its rewarding effects (positive reinforcement), whereas drinking in the dependent state is motivated by alcohol’s stress-relieving effects (negative reinforcement). Here, we measured context-elicited 50 kHz USVs in alcohol-dependent (alcohol vapor-exposed) and nondependent rats immediately before operant alcohol self-administration sessions. Dependent rats showed escalated levels of alcohol intake compared with nondependent rats. Overall, dependent and nondependent rats showed similar levels of anticipatory 50 kHz USVs. However, the number of anticipatory USVs was positively correlated with alcohol intake in dependent rats but not nondependent rats. Additionally, dependent rats with higher alcohol intake displayed increased anticipatory 50 kHz USVs compared with rats that had lower alcohol intake, whereas no difference was observed between rats with high and low alcohol intake in the nondependent group. Increased 50 kHz USVs were specific for the anticipation of alcohol self-administration and did not generalize to a novel environment. These findings suggest that anticipatory 50 kHz USVs may be an indicator of context-elicited negative reinforcement learning.
Keywords: Ultrasonic vocalization, Reward, Motivation, Ethanol, Alcoholism, Addiction
1. Introduction
Fifty kilohertz (kHz) ultrasonic vocalizations (USVs) have been linked with positive affective states in rats [1–5]. However, these calls are also elicited by aversive stimuli [6,7], and the affective state(s) that they represent are not well understood. Previous reports have shown that 50 kHz USVs are elicited by cues that predict rewarding stimuli, such as food [8,9], copulation [10], cocaine [11], and rewarding brain stimulation [8]. Thus, 50 kHz USVs may be an index of motivation during reward anticipation [1,11,12]. Most studies that have investigated the relationship between 50 kHz USVs and drugs of abuse have focused on psychostimulants; few studies have examined 50 kHz USVs in relation to alcohol self-administration.
Rats that are exposed to chronic, intermittent alcohol vapor display several physical and motivational symptoms of alcohol dependence compared with air-exposed nondependent rats (for review, see [13]). In this model, during alcohol abstinence, rats exhibit tail stiffness, abnormal gait/posture, tremor [14], increased anxiety-like behavior [15], hyperalgesia [16,17], increased 22 kHz USVs [18–20], and dysphoria, reflected by elevated brain reward thresholds [21]. Dependent rats also display escalated alcohol self-administration, increased motivation for alcohol, and persistent alcohol drinking despite punishment [22,23]. It has been hypothesized that moderate (recreational) alcohol drinking is mediated by its rewarding effects (positive reinforcement). In the transition to dependence, brain stress systems are sensitized, and drinking mainly occurs to alleviate negative affective states during with-drawal (negative reinforcement [24]).
Therefore, 50 kHz USVs are observed during several situations of increased emotional and motivational valence, and alcohol dependence affects brain reward and stress systems. The hypothesis in the present study was that rats exposed to alcohol vapor to the point of dependence would present a different pattern or amount of 50 kHz USVs compared with nondependent rats. The objective of the present study was to compare 50 kHz USVs emitted during the anticipation of alcohol self-administration in dependent and nondependent rats.
2. Materials and methods
2.1. Animals
A total of 107 adult male Wistar rats, weighing 250–400 g at the beginning of the experiment, were used. The rats were housed in groups of two to three in plastic cages in a temperature-controlled (21°C) vivarium on a 12-h light:12-h dark cycle (lights on at 8:00 p.m.). Food and water were provided ad libitum in the home cages except during behavioral testing. All of the behavioral tests were conducted during the dark cycle between 1:00 and 6:00 p.m. All of the procedures adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute.
2.2. Operant self-administration
Self-administration sessions were conducted in standard operant conditioning chambers (Med Associates, St. Albans, VT) as previously reported [23]. First, the rats were given free-choice access to alcohol (10%, w/v) and water for 1 day in their home cages to habituate them to the taste of alcohol. Second, the rats were subjected to an overnight session in the operant chambers with access to one lever (right lever) that delivered water. Food was available ad libitum in the operant chambers during this training. Third, after 1 day off, the rats were subjected to one 2 h session and one 1 h session the next day, with one lever that delivered alcohol (right lever).All of the subsequent baseline and test sessions lasted 30 min, and two levers were available (left lever: water; right lever: alcohol). All of the operant sessions were conducted under a fixed-ratio 1 schedule of reinforcement, in which every lever press resulted in fluid delivery (0.1 ml). Once stable levels of baseline intake were reached, the rats were split into two groups: vapor-exposed (dependent) and air-exposed (nondependent).
2.3. Alcohol vapor chambers
The rats were made dependent by chronic, intermittent exposure to alcohol vapor as previously described [23]. They underwent cycles of 14 h on (blood alcohol levels during vapor exposure ranged between 150 and 250 mg%) and 10 h off, during which behavioral testing for acute withdrawal occurred (i.e., 6–8 h after vapor was turned off, when brain and blood alcohol levels were negligible; [25]). Nondependent rats were not exposed to alcohol vapor but were tested for alcohol self-administration together with the dependent rats.
2.4. Ultrasonic vocalization recording and analysis
Condenser microphones (CM16/CMPA, 10–200 kHz frequency range, Avisoft Bioacoustics, Berlin, Germany) were coupled to an Ultra Sound Gate 816H data acquisition device (250 kHz sampling rate, 16-bit resolution, Avisoft Bioacoustics). Ultrasonic vocalizations between 10 and 100 kHz were recorded and analyzed using Avisoft SASLab Pro (version 5.1, Avisoft Bioacoustics). Spectrograms were generated with a fast Fourier transform length of 512 points and overlap of 50% (FlatTop window, 100% frame size), providing a frequency resolution of 419 Hz and time resolution of 1.19 ms. Vocalizations were categorized as trill, other FM, or flat using criteria described in Buck et al. [9]. Here, trill calls included the “trill FM”and “step-trill FM” categories. Other FM calls included “other FM” and “step FM.” Flat calls included the “flat” category from Buck et al.[9]. Microphones were placed inside the sound-attenuating cubicle and outside a grate in the wall of the operant chamber. Upon placing the rats in the operant chambers with the levers retracted, anticipatory 50 kHz USVs were recorded for 2 or 3 min. The operant alcohol self-administration session then began, and USV recording stopped. Each rat was always tested in the same operant chamber.
2.5. Inclusion criteria
Five different cohorts of rats were used, but no cohort effects were detected. Rats that emitted fewer than 15 USVs in every session were excluded (two dependent rats and three nondependent rats). One rat with high vocalizations at baseline (>300 calls in 2 min) was excluded. Additionally, rats that had an average of <10 alcohol reinforcers in sessions 4–6 were excluded (three dependent rats and four nondependent rats). Following these criteria, a total of 13 rats were excluded, and the total number was 94.
2.6. Novelty-induced USVs
A separate group of rats (n = 15) was trained to self-administer alcohol (see Section 2.2 above) and made dependent on alcohol (see Section 2.3 above). Alcohol anticipatory USVs were recorded as described above (Section 2.4). To test novelty-induced USVs, the rats were individually placed in clean housing cages with fresh bed-ding and wire lids. The microphones were placed 10 cm above the lids, and USVs were recorded for 3 min. The test was performed in a dark room.
2.7. Statistical analyses
The data are expressed as the mean and standard error of the mean (SEM). Prior to the analysis, the dependent variables were tested for normality using Shapiro-Wilk’s W-test. Ultra-sonic vocalization counts were square-root transformed to achieve normality. Ultrasonic vocalization counts were analyzed using two-way repeated-measures analysis of variance (ANOVA), followed by the Fisher Least Significant Difference post hoc test when appropriate. Student’s t-test was used for group comparisons. Pearson’s test was used for correlation analysis. For USV call subtypes, the proportions of total calls were analyzed using two-way repeated-measures ANOVA. The statistical analyses were performed using Statistica 10 (StatSoft) and Prism 5 (GraphPad) software. For all of the tests, two-tailed values of p < 0.05 were considered statistically significant.
3. Results
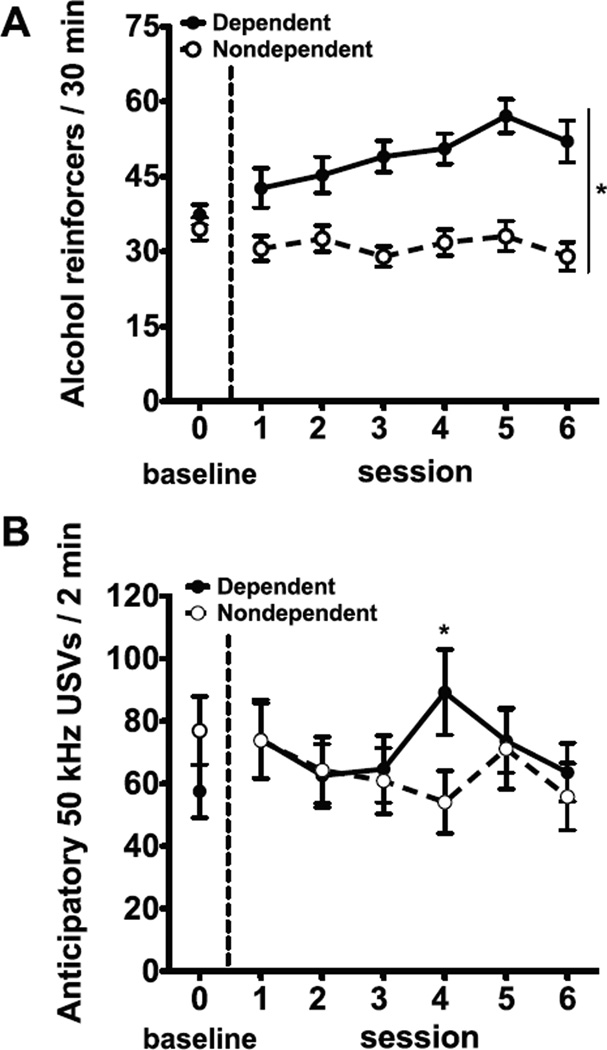
Fig. 1 shows the results of alcohol self-administration and anticipatory 50 kHz USVs in alcohol-dependent and -nondependent rats. At baseline (i.e., before the induction of alcohol dependence), the two groups of rats displayed similar levels of alcohol intake (t77= 0.92, p = 0.36). During alcohol vapor exposure, dependent rats increased their alcohol intake compared with non-dependent rats (group effect: F1,77 = 4.16, p < 0.001; Fig. 1A). For alcohol intake in g/kg/30 min (sessions 4–6), dependent rats had higher alcohol intake (1.2 ± 0.1 g/kg/30 min) compared with non-dependent rats (0.6 ± 0.04 g/kg/30 min; group effect: F1,77 = 53.42, p < 0.001; post hoc comparisons: p < 0.01). Dependent and non-dependent rats’ baseline anticipatory 50 kHz USVs were not significantly different (t77 = 1.15, p = 0.22). The group × session repeated-measures ANOVA revealed a significant group × session interaction (F5,385 = 2.37, p < 0.05). Post hoc comparisons indicated that dependent rats displayed increased anticipatory USVs compared with nondependent rats in session 4 only (Fig. 1B).
Fig. 1.
Escalation of alcohol self-administration but not anticipatory 50 kHz ultra-sonic vocalizations (USVs) in dependent rats compared with nondependent rats.(A) Mean ± SEM number of lever presses for alcohol in dependent and nondependent rats and (B) 50 kHz USVs emitted by rats during 2 min prior to alcohol self-administration. *p < 0.05, difference between dependent and nondependent rats. n = 39–40 per group.
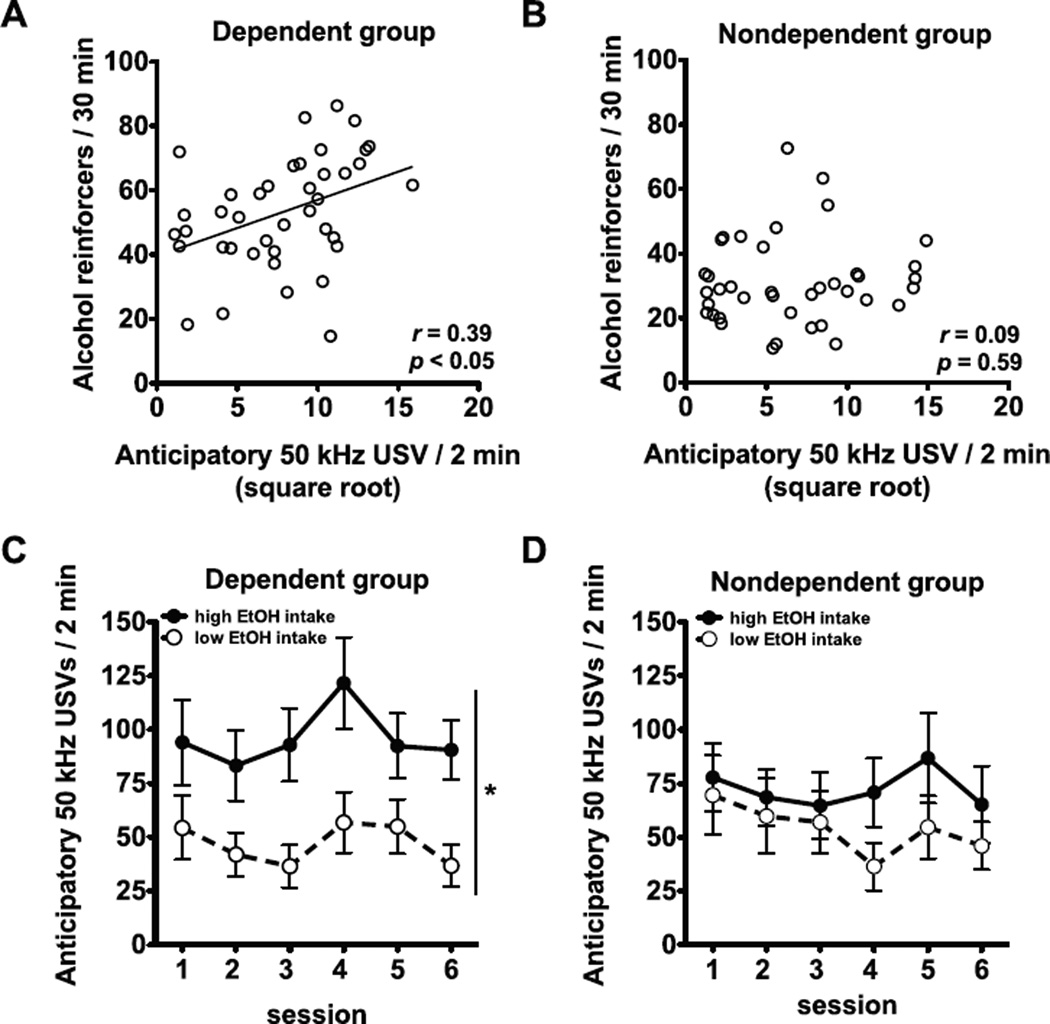
Fig. 2A and B shows the relationship between anticipatory USVs (averaged over sessions 4–6) and alcohol intake (averaged over sessions 4–6) in dependent and nondependent rats. Anticipatory USVs were positively correlated with alcohol intake independent rats (r = 0.39, p < 0.05; Fig. 2A) but not in nondependent rats (r = 0.09, p = 0.59; Fig. 2B). To further explore these results, the dependent and nondependent groups were split into high and low alcohol intake subgroups based on the median values of alcohol lever presses (averaged over sessions 4–6). Anticipatory USVs for the high and low alcohol intake subgroups are shown in Fig. 2C (dependent rats) and Fig. 2D (nondependent rats). In dependent rats, the repeated-measures ANOVA indicated that rats with high alcohol intake emitted significantly more 50 kHz USVs than rats with low alcohol intake (overall subgroup effect: F1,38 = 6.70, p < 0.05; Fig. 2C). For alcohol intake in g/kg/30 min in dependent rats, the high-intake subgroup had higher intake (1.5 ± 0.07 g/kg/30 min) than the low-intake sub-group (0.9 ± 0.06 g/kg/30 min; group effect: F1,38 = 52.39, p < 0.001;post hoc comparisons: p < 0.01). In nondependent rats, the ANOVA revealed no significant differences in anticipatory USVs between the high- and low-intake subgroups (Fig. 2D). For alcohol intake in g/kg/30 min in nondependent rats, the high-intake subgroup had higher intake (0.8 ± 0.04 g/kg/30 min) than the low-intake sub-group (0.4 ± 0.03 g/kg/30 min; group effect: F1,38 = 50.32 p < 0.001;post hoc comparisons: p < 0.01).
Fig. 2.
Anticipatory 50 kHz ultrasonic vocalizations (USVs) are positively associated with alcohol intake in dependent rats but not nondependent rats. The number of anticipatory 50 kHz USVs (averaged over sessions 4–6) was positively correlated with the number of lever presses for alcohol (averaged over sessions 4–6) in dependent rats(A) but not in nondependent rats (B). n = 39–40 per group. (C and D) Mean ± SEM number of 50 kHz USVs emitted during 2 min immediately before the self-administration sessions in the high and low alcohol self-administration subgroups (based on a median split of average alcohol intake during sessions 4–6) in dependent rats (C) and nondependent rats (D). *p < 0.05, compared with low alcohol intake rats. n = 19–20 per subgroup.
Table 1 shows the mean proportions of trill, other FM, and flat calls during baseline and session 6. The repeated-measures ANOVA did not reveal significant differences in trill and other FM call types between dependent and nondependent rats or between baseline and session 6. Flat calls accounted for an average of less than 5% of calls; therefore, this call subtype was not statistically analyzed.
Table 1.
Proportions (mean ± SEM) of anticipatory 50 kHz call types displayed by dependent and nondependent rats during baseline and session 6.
| Group | Session | 50 kHz call types | ||
|---|---|---|---|---|
| Trill | Other FM | Flat | ||
| Dependent | Baseline | 0.22 ± 0.03 | 0.75 ± 0.03 | 0.03 ± 0.01 |
| Session 6 | 0.22 ± 0.03 | 0.74 ± 0.03 | 0.03 ± 0.01 | |
| Nondependent | Baseline | 0.29 ± 0.03 | 0.70 ± 0.03 | 0.01 ± 0.00 |
| Session 6 | 0.22 ± 0.03 | 0.75 ± 0.03 | 0.03 ± 0.01 | |
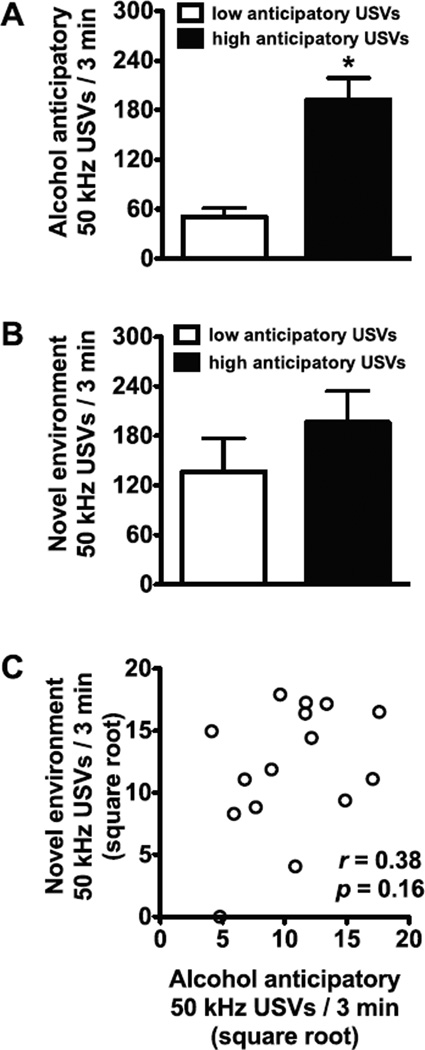
A separate group of dependent rats was split into subgroups of high (n = 8) and low (n = 7) anticipatory 50 kHz USVs based on the median value of anticipatory 50 kHz USVs for alcohol self administration. The subgroup with high anticipatory USVs had significantly more anticipatory USVs than the low subgroup (t13 = 5.60, p < 0.001;Fig. 3A), but these subgroups did not significantly differ in the number of 50 kHz USVs emitted in a novel environment (t13 = 1.06, p = 0.31; Fig. 3B). Furthermore, in this group of dependent rats, no significant correlation was found between alcohol anticipatory USVs and novel environment-induced USVs (r = 0.38, p = 0.16; Fig. 3C).
Fig. 3.
Alcohol anticipatory 50 kHz ultrasonic vocalizations (USVs) are not associated novel environment-induced 50 kHz USVs in dependent rats. The mean ± SEM number of anticipatory 50 kHz USVs is shown. Dependent rats were split into high and low alcohol anticipatory USV groups based on a median split. (A) Mean + SEM number of alcohol anticipation USVs emitted during 3 min immediately before the self-administration sessions in the high and low anticipatory 50 kHz USV sub-groups (based on a median split of anticipatory USVs). n = 7–8 per subgroup. (B) Mean + SEM number of novel environment-induced USVs emitted during 3 min in the high and low anticipatory 50 kHz USV subgroups. n = 7–8 per subgroup. (C) Correlation between alcohol anticipatory USVs (square-root-transformed) and novel environment-induced USVs (square-root-transformed). *p < 0.05, compared with low anticipatory USV rats. n = 15.
4. Discussion
In the present study, we report that alcohol-dependent and-nondependent rats emitted similar levels of 50 kHz USVs in anticipation of alcohol self-administration, despite showing differences in alcohol intake. However, dependent rats displayed a positive correlation between anticipatory 50 kHz USVs and alcohol intake, whereas no relationship was observed in nondependent rats. More-over, in dependent rats but not nondependent rats, those that displayed high levels of alcohol intake also displayed an increase in the number of anticipatory 50 kHz USVs compared with rats that displayed low alcohol intake. Baseline alcohol anticipatory 50 kHz USVs (i.e., before dependence induction) did not correlate with sub-sequent alcohol intake in either dependent or nondependent rats(data not shown). Finally, dependent rats that showed high 50 kHz USVs in anticipation of alcohol showed no difference in 50 kHz USV emission in a novel environment compared with rats that showed low 50 kHz USVs.
Alcohol anticipatory 50 kHz USVs did not substantially differ between dependent and nondependent rats, suggesting that anticipatory 50 kHz USVs are not a general marker of alcohol dependence. However, anticipatory 50 kHz USVs were positively and significantly correlated with alcohol intake in dependent rats but not nondependent rats. This finding indicates that anticipatory 50 kHz USVs may be qualitatively different for these two groups. Consistent with this hypothesis, in dependent rats but not nondependent rats, we observed that a subset of rats that displayed high alcoholin take also had higher levels of anticipatory 50 kHz USVs compared with low-alcohol-intake rats. Previous studies have reported an increase in 50 kHz USVs in response to cues paired with appetitive stimuli, such as food [8,9], copulation [10,11], and cocaine [11,12]. Rats that had extinguished methamphetamine self-administration and then given a methamphetamine priming injection or exposed to drug-paired cues robustly emitted 50 kHz USVs and rein-stated drug-seeking behavior [12]. If these cue-induced USVs are thought to reflect an increased appetitive or motivational state, it may suggest that a subgroup of alcohol-dependent rats (i.e., the high drinkers) in the present study demonstrated an increase in motivational salience (i.e., an increase in alcohol-seeking behavior/craving/incentive salience), reflected by higher anticipatory50 kHz USVs.
Other studies have also observed individual differences in anticipatory 50 kHz USVs [26–28]. High- and low-50 kHz USV groups were separated based on rats’ initial USV response to amphetamine. Compared with low-USV rats, high-USV rats showed increased amphetamine-anticipatory 50 kHz USVs along with increased conditioned place preference for amphetamine-paired stimuli [26,28]. Unlike amphetamine, acute alcohol does not potently elicit 50 kHz USVs in rats [29,30]. In the current study, however, a dependence-induced increase in alcohol intake was associated with higher anticipatory USVs, indicating that USVs may be a general marker of interindividual differences in motivational states.
Paulus et al. [31] suggested that interoception (i.e., the conscious awareness of internal states) under specific conditions (e.g., with-drawal) may be a strong component associated with subsequent drug intake. Thus, the situation of anticipation for alcohol drinking may trigger interoceptive states that serve as cues for both eliciting USVs and favoring a learning process, in which dependent rats associate an environment/situation with subsequent alcohol-induced stress relief [32]. Interestingly, rats bred for high 50 kHz USVs have shown enhanced associative learning of negative con-textual conditioning [33].
Most of the anticipatory 50 kHz USVs observed herein were trill or other FM calls. These call types, especially trills, have been hypothesized to indicate a positive affective state [34]. Others have hypothesized that 50 kHz USVs indicate incentive salience, which may not necessarily be related to a positive affective state [35].Because alcohol vapor-exposed rats display several signs of negative affective-like states during withdrawal [13], we speculate that the observed 50 kHz USVs in dependent rats were indicative of negative reinforcement-related incentive salience. However, we cannot exclude the possibility of positive affective states in anticipation of alcohol.
We found that dependent rats that differed in the levels of anticipatory 50 kHz USVs for alcohol self-administration did not differ in 50 kHz USVs emitted in a novel environment. This indicates that the increase in USVs emitted in the self-administration chambers is not attributable to a general increase in the emission of 50 kHz USVs and is consistent with a previous study [36] that reported that rats displayed different patterns of USVs in different environmental settings.
Similar to most neuropsychiatric disorders, alcohol dependence is a heterogeneous condition determined by the interaction between environmental factors and multiple genes. Moss et al. [37] used nationally representative epidemiological data from the United States and statistically determined that alcohol dependence can be divided into five clinically distinct categories. Earlier, Cloninger [38] classified alcoholics into Type 1 and Type 2 based of drinking and personality types. Other classification schemes also exist (for review, see [39]). Despite this knowledge of the het-erogeneity of alcohol dependence, few studies have attempted to identify the neurobiological and/or behavioral bases of different subtypes in the context of alcohol dependence. In the present study, the finding that increased anticipatory 50 kHz USVs are associated with increased alcohol intake in alcohol dependence suggests that these two behaviors may share a similar neurobiological basis and that anticipatory USVs may be an potential marker for a specific subgroup of alcohol-dependent rats.
In conclusion, increases in 50 kHz USVs in anticipation of alcohol self-administration are associated with the escalation of alcoholin take during alcohol dependence. More specifically, anticipatory50 kHz USVs may be an indicator of context-elicited negative reinforcement learning. These findings provide evidence that alcohol dependence comprises a behaviorally heterogeneous group of rats and suggest that anticipatory 50 kHz USVs and high alcohol intake share some biological mechanisms. The study of 50 kHz USVs can help identify a subgroup of alcohol-dependent rats that may better model excessive alcohol drinking in the human condition and elucidate the biological mechanisms that are involved in both emotional reactivity and alcoholism subtypes.
HIGHLIGHTS.
Dependent rats self-administered more alcohol than nondependent rats.
Dependent and nondependent rats emitted 50 kHz USVs during alcohol anticipation.
Anticipatory USVs were correlated with alcohol intake for dependent rats only.
USVs may be a marker of escalated alcohol intake in alcohol dependence.
Acknowledgements
This is publication number 27013 from The Scripps Research Institute. Research was financially supported by National Institutes of Health grants AA006420, AA008459, and AA020608 from the National Institute on Alcohol Abuse and Alcoholism and by the Pearson Center for Alcoholism and Addiction Research. The authors would like to thank Larissa Lippert for help with the USV analysis and Michael Arends for proofreading the manuscript.
References
- 1.Ahrens AM, Ma ST, Maier EY, Duvauchelle CL, Schallert T. Repeated intravenous amphetamine exposure: rapid and persistent sensitization of 50-kHz ultrasonic trill calls in rats. Behav Brain Res. 2009;197:205–209. doi: 10.1016/j.bbr.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burgdorf J, Kroes RA, Moskal JR, Pfaus JG, Brudzynski SM, Panksepp J. Ultrasonic vocalizations of rats (Rattus norvegicus) during mating, play, and aggression: behavioral concomitants, relationship to reward, and self-administration of playback. J Comp Psychol. 2008;122:357–367. doi: 10.1037/a0012889. [DOI] [PubMed] [Google Scholar]
- 3.Brudzynski SM. Ethotransmission: communication of emotional states through ultrasonic vocalization in rats. Curr Opin Neurobiol. 2013;23:310–317. doi: 10.1016/j.conb.2013.01.014. http://dx.doi.org/10.1016/j.conb.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 4.Brudzynski SM, Pniak A. Social contacts and production of 50-kHz short ultra-sonic calls in adult rats. J Comp Psychol. 2002;116:73–82. doi: 10.1037/0735-7036.116.1.73. [DOI] [PubMed] [Google Scholar]
- 5.Burgdorf J, Panksepp J. Tickling induces reward in adolescent rats. Physiol Behav. 2001;72:167–173. doi: 10.1016/s0031-9384(00)00411-x. [DOI] [PubMed] [Google Scholar]
- 6.Vivian JA, Miczek KA. Morphine attenuates ultrasonic vocalization during agonistic encounters in adult male rats. Psychopharmacology (Berlin) 1993;111:367–375. doi: 10.1007/BF02244954. [DOI] [PubMed] [Google Scholar]
- 7.Wöhr M, Houx B, Schwarting RK, Spruijt B. Effects of experience and context on 50-kHz vocalizations in rats. Physiol Behav. 2008;93:766–776. doi: 10.1016/j.physbeh.2007.11.031. [DOI] [PubMed] [Google Scholar]
- 8.Burgdorf J, Knutson B, Panksepp J. Anticipation of rewarding electrical brain stimulation evokes ultrasonic vocalization in rats. Behav Neurosci. 2000;114:320–327. [PubMed] [Google Scholar]
- 9.Buck CL, Vendruscolo LF, Koob GF, George O. Dopamine D1 and µ-opioid receptor antagonism blocks anticipatory 50 kHz ultrasonic vocalizations induced by palatable food cues in Wistar rats. Psychopharmacology (Berlin) 2014;231:929–937. doi: 10.1007/s00213-013-3307-2. http://dx.doi.org/10.1007/s00213-013-3307-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bialy M, Rydz M, Kaczmarek L. Precontact 50-kHz vocalizations in male rats during acquisition of sexual experience. Behav Neurosci. 2000;114:983–990. doi: 10.1037//0735-7044.114.5.983. [DOI] [PubMed] [Google Scholar]
- 11.Ma ST, Maier EY, Ahrens AM, Schallert T, Duvauchelle CL. Repeated intravenous cocaine experience: development and escalation of pre-drug anticipatory 50-kHz ultrasonic vocalizations in rats. Behav Brain Res. 2010;212:109–114. doi: 10.1016/j.bbr.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahler SV, Moorman DE, Feltenstein MW, Cox BM, Ogburn KB, Bachar M, et al. A rodent self-report measure of methamphetamine craving? Rat ultrasonic vocalizations during methamphetamine self-administration, extinction, and reinstatement. Behav Brain Res. 2013;236:78–89. doi: 10.1016/j.bbr.2012.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vendruscolo LF, Roberts AJ. Operant alcohol self-administration in dependent rats: Focus on the vapor model. Alcohol. 2014;48:277–286. doi: 10.1016/j.alcohol.2013.08.006. http://dx.doi.org/10.1016/j.alcohol.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Macey DJ, Schulteis G, Heinrichs SC, Koob GF. Time-dependent quantifiable withdrawal from ethanol in the rat: effect of method of dependence induction. Alcohol. 1996;13:163–170. doi: 10.1016/0741-8329(95)02030-6. [DOI] [PubMed] [Google Scholar]
- 15.Zhao Y, Weiss F, Zorrilla EP. Remission and resurgence of anxiety-like behavior across protracted withdrawal stages in ethanol-dependent rats. Alcohol Clin Exp Res. 2007;31:1505–1515. doi: 10.1111/j.1530-0277.2007.00456.x. [DOI] [PubMed] [Google Scholar]
- 16.Egli M, Koob GF, Edwards S. Alcohol dependence as a chronic pain disorder. Neurosci Biobehav Rev. 2012;36:2179–2192. doi: 10.1016/j.neubiorev.2012.07.010. http://dx.doi.org/10.1016/j.neubiorev.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edwards S, Vendruscolo LF, Schlosburg JE, Misra KK, Wee S, Park PE, et al. Development of mechanical hypersensitivity in rats during heroin and ethanol dependence: alleviation by CRF1 receptor antagonism. Neuropharmacology. 2012;62:1142–1151. doi: 10.1016/j.neuropharm.2011.11.006. http://dx.doi.org/10.1016/j.neuropharm.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berger AL, Williams AM, McGinnis MM, Walker BM. Affective cue-induced escalation of alcohol self-administration and increased 22-kHz ultrasonic vocalizations during alcohol withdrawal: role of kappa-opioid receptors. Neuropsy-chopharmacology. 2013;38:647–654. doi: 10.1038/npp.2012.229. http://dx.doi.org/10.1038/npp.2012.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kissler JL, Sirohi S, Reis DJ, Jansen HT, Quock RM, Smith DG, et al. The one-two punch of alcoholism: role of central amygdaladynorphins/kappa-opioid receptors. Biol Psychiatry. 2014;75:774–782. doi: 10.1016/j.biopsych.2013.03.014. http://dx.doi.org/10.1016/j.biopsych.2013.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams AM, Reis DJ, Powell AS, Neira LJ, Nealey KA, Ziegler CE, et al. The effect of intermittent alcohol vapor or pulsatile heroin on somatic and negative affective indices during spontaneous withdrawal in Wistar rats. Psychopharmacology (Berlin) 2012;223:75–88. doi: 10.1007/s00213-012-2691-3. http://dx.doi.org/10.1007/s00213-012-2691-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schulteis G, Markou A, Cole M, Koob GF. Decreased brain reward produced byethanol withdrawal. Proc Natl Acad Sci USA. 1995;92:5880–5884. doi: 10.1073/pnas.92.13.5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edwards S, Baynes BB, Carmichael CY, Zamora-Martinez ER, Barrus M, Koob GF, et al. Traumatic stress reactivity promotes excessive alcohol drinking and alters the balance of prefrontal cortex-amygdala activity. Transl Psychiatry. 2013;3:e296. doi: 10.1038/tp.2013.70. http://dx.doi.org/10.1038/tp.2013.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vendruscolo LF, Barbier E, Schlosburg JE, Misra KK, Whitfield TW, Jr, Logrip ML, et al. Corticosteroid-dependent plasticity mediates compulsive alcohol drinking in rats. J Neurosci. 2012;32:7563–7571. doi: 10.1523/JNEUROSCI.0069-12.2012. http://dx.doi.org/10.1523/JNEUROSCI.0069-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koob GF, Buck CL, Cohen A, Edwards S, Park PE, Schlosburg JE, et al. Addiction as a stress surfeit disorder. Neuropharmacology. 2014;76(Pt B):370–382. doi: 10.1016/j.neuropharm.2013.05.024. http://dx.doi.org/10.1016/j.neuropharm.2013.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gilpin NW, Smith AD, Cole M, Weiss F, Koob GF, Richardson HN. Operant behavior and alcohol levels in blood and brain of alcohol-dependent rats. Alcohol Clin Exp Res. 2009;33:2113–2123. doi: 10.1111/j.1530-0277.2009.01051.x. http://dx.doi.org/10.1111/j. 1530-0277.2009.01051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahrens AM, Nobile CW, Page LE, Maier EY, Duvauchelle CL, Schallert T. Individual differences in the conditioned and unconditioned rat 50-kHz ultrasonic vocalizations elicited by repeated amphetamine exposure. Psychopharmacology (Berlin) 2013;229:687–700. doi: 10.1007/s00213-013-3130-9. http://dx.doi.org/10.1007/s00213-013-3130-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taracha E, Hamed A, Krząścik P, Lehner M, Skórzewska A, Płaźnik A, et al. Inter-individual diversity and intra-individual stability of amphetamine-induced sensitization of frequency-modulated 50-kHz vocalization in Sprague-Dawley rats. Psychopharmacology (Berlin) 2012;222:619–632. doi: 10.1007/s00213-012-2658-4. http://dx.doi.org/10.1007/s00213-012-2658-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taracha E, Kaniuga E, Chrapusta SJ, Maciejak P, Sliwa L, Hamed A, et al. Diverging frequency-modulated 50-kHz vocalization, locomotor activity and conditioned place preference effects in rats given repeated amphetamine treatment. Neuropharmacology. 2014;83:128–136. doi: 10.1016/j.neuropharm.2014.04.008. http://dx.doi.org/10.1016/j.neuropharm.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 29.Blanchard RJ, Yudko EB, Blanchard DC, Taukulis HK. High-frequency(35–70 kHz) ultrasonic vocalizations in rats confronted with anesthetized conspecifics: effects of gepirone, ethanol, and diazepam. Pharmacol Biochem Behav. 1993;44:313–319. doi: 10.1016/0091-3057(93)90467-8. [DOI] [PubMed] [Google Scholar]
- 30.Willey AR, Spear LP. Effects of ethanol on social approach and 50 kHz ultra-sonic vocalization production in adolescent male Sprague-Dawley rats. DevPsychobiol. 2014;56:857–863. doi: 10.1002/dev.21143. http://dx.doi.org/10.1002/dev.21143. [DOI] [PubMed] [Google Scholar]
- 31.Paulus MP, Tapert SF, Schulteis G. The role of interoception and alliesthesia in addiction. Pharmacol Biochem Behav. 2009;94:1–7. doi: 10.1016/j.pbb.2009.08.005. http://dx.doi.org/10.1016/j.pbb.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walker BM. Conceptualizing withdrawal-induced escalation of alcohol self-administration as a learned, plasticity-dependent process. Alcohol. 2012;46:339–348. doi: 10.1016/j.alcohol.2012.01.001. http://dx.doi.org/10.1016/j.alcohol.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Webber ES, Harmon KM, Beckwith TJ, Peña S, Burgdorf J, Panksepp J, et al. Selective breeding for 50 kHz ultrasonic vocalization emission produces alterations in the ontogeny and regulation of rough-and-tumble play. Behav Brain Res. 2012;229:138–144. doi: 10.1016/j.bbr.2012.01.012. http://dx.doi.org/10.1016/j.bbr.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 34.Burgdorf J, Panksepp J, Moskal JR. Frequency-modulated 50 kHz ultrasonic vocalizations: a tool for uncovering the molecular sub-strates of positive affect. Neurosci Biobehav Rev. 2011;35:1831–1836. doi: 10.1016/j.neubiorev.2010.11.011. http://dx.doi.org/10.1016/j.neubiorev.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 35.Meyer PJ, Ma ST, Robinson TE. A cocaine cue is more preferred and evokes more frequency-modulated 50-kHz ultrasonic vocalizations in rats prone to attribute incentive salience to a food cue. Psychopharmacology (Berlin) 2012;219:999–1009. doi: 10.1007/s00213-011-2429-7. http://dx.doi.org/10.1007/s00213-011-2429-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mu P, Fuchs T, Saal DB, Sorg BA, Dong Y, Panksepp J. Repeated cocaine expo-sure induces sensitization of ultrasonic vocalization in rats. Neurosci Lett. 2009;453:31–35. doi: 10.1016/j.neulet.2009.02.007. http://dx.doi.org/10.1016/j.neulet.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moss HB, Chen CM, Yi HY. Subtypes of alcohol dependence in a nationally representative sample. Drug Alcohol Depend. 2007;91:149–158. doi: 10.1016/j.drugalcdep.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cloninger CR. Neurogenetic adaptive mechanisms in alcoholism. Science. 1987;236:410–416. doi: 10.1126/science.2882604. [DOI] [PubMed] [Google Scholar]
- 39.Pombo S, Lesch OM. The alcoholic phenotypes among different multidimensional typologies: similarities and their classification procedures. Alcohol Alcohol. 2009;44:46–54. doi: 10.1093/alcalc/agn080. http://dx.doi.org/10.1093/alcalc/agn080. [DOI] [PubMed] [Google Scholar]