Abstract
Background
Sorafenib is the standard systemic therapy for un-resectable or recurrent hepatocellular carcinoma (HCC) with minimal increase in survival. Therefore, there is a great need to develop novel therapies for advanced or recurrent HCC. One emerging field of cancer treatment involves oncolytic viruses that specifically infect, replicate within, and kill cancer cells. In this study we look at the ability of GLV-1h68, a recombinant vaccinia virus derived from the vaccine strain that was used to eradicate smallpox, to kill sorafenib-resistant HCC.
Methods
Four sorafenib-resistant HCC cell lines were generated by repeated passage in the presence of sorafenib. Median inhibitory concentration was determined for all cell lines. The infectivity, viral replication and cytotoxicity of GLV-1h68 were assayed for both parental and sorafenib-resistant HCC cells.
Results
Infectivity increased in a time and concentration dependent manner in all cell lines. All cell lines supported efficient replication of virus. No significant difference between the rates of cell death between the parental and sorafenib-resistant cell lines was observed.
Conclusions
Our results demonstrate that oncolytic vaccinia virus GLV-1h68 efficiently kills both parental and sorafenib-resistant HCC cell lines. This study indicates that patients who have failed treatment with sorafenib remain viable candidates for oncolytic therapy.
Keywords: Oncolytic Virus, Immunotherapy, Vaccinia, Sorafenib-resistance, Hepatocellular Carcinoma
Introduction
Hepatocellular carcinoma (HCC) is the second leading cause of cancer death in men worldwide. It is the most common primary malignancy of the liver accounting for 70–85% of the total liver cancer burden.(1) The only curative treatments available for HCC are surgery and ablative therapies for limited disease. Unfortunately, most patients are not surgical candidates due to advanced disease at presentation or prohibitive comorbidities. Even with a multidisciplinary approach using systemic chemotherapy and other adjuvant therapies, overall survival of HCC remains dismal with a cause-specific survival of less than 50% at 1 year.(2) Molecular targeted drugs have been emerging as promising agents to prolong overall survival in patients with un-resectable or recurrent HCC. Currently, the only approved treatment option for advanced stage HCC is sorafenib.(3)
Sorafenib is a multi-kinase inhibitor that has been shown to have both anti-angiogenic and anti-proliferative effects in HCC.(4) As a therapy sorafenib has been shown to create an overall improvement in survival and a delay in disease progression. Unfortunately, patients treated with sorafenib have minimal improvement showing only a modest increase of 2–3 months in overall survival when compared to placebo. More concerning is that sorafenib was shown to result in limited increase in median time to disease progression and to have a low partial response rate due to drug resistance.(5) While exact rates of sorafenib resistance are not known; patients who become resistant to sorafenib have no further approved therapies available to them. The mechanism that accounts for resistance to sorafenib is multi-factorial and currently remains unclear. Genetic heterogenicity of HCC means that many tumors are resistant to sorafenib even prior to first treatment. Those tumors that are initially sensitive to treatment and later become resistant can do so through numerous different pathways. The multifactorial nature of sorafenib-resistance makes it difficult to find therapies that can synergize with sorafenib. Thus, it is indispensable to develop novel treatment options that can be given to patients with HCC, irrespective of sorafenib-resistance.(6)
Oncolytic viral therapy has shown great promise in preclinical and clinical testing as a novel cancer therapy. Oncolytic viral therapy relies on the ability of oncolytic viruses to selectively infect, replicate within and destroy tumor tissue. Tumor destruction is mediated not only by cell lysis but also by tumor vaccination and can be augmented by the insertion of targeted genes into the viral genome that modulate the tumor microenvironment or cancer cells directly.(7) Vaccinia virus (VACV) has shown great promise as an oncolytic virus. Having been used in the WHO campaign to eradicate smallpox, it has a well documented history of safe use in humans.(8) In addition it replicates within the cytoplasm, thereby decreasing the risk of recombinant events with the host genome. Finally, VACV has a large, 192-kb genome that can easily accept insertions of foreign DNA without substantially compromising viral replication, thus allowing clinicians to deliver targeted gene therapy to tumor cells and the tumor microenvironment.
GLV-1h68 is a replication-competent oncolytic VACV that has been genetically modified by creating interruptions in the thymidine kinase, F14.5L and hemagglutinin genes. These interruptions confer tumor selectivity to the virus and decrease it’s virulence in normal tissue. Of note, infection with vaccinia has been shown to temporarily inhibit of apoptosis to allow sufficient time for successful viral replication.(9) VACV mediates this apoptotic delay via upregulation of molecular pathways that have also been associated with sorafenib resistance. This correlation would suggest that HCC cells that have become resistant to sorafenib may be more susceptible to viral therapy.
GLV-1h168 has been shown in preclinical trials to efficiently kill and lyse HCC tumor tissue. Currently, four stage I clinical trials looking at the safety profile of GLV-1h68 for assorted advanced stage solid tumors, head and neck tumors, peritoneal carcinomatosis and malignant pleural effusions are underway. Each of these studies exploits a different route of administration (intravenous, intrapleural and intraperitoneal) in addition to dose escalation adjustments. When completed, the results of these clinical trials will help to establish the safety of oncolytic vaccinia virus treatment and provide a more complete picture of the therapeutic possibilities of GLV-1h168. As GLV-1h68 completes phase I clinical trials and enters stage II clinical trials, we believe its greatest impact will be in diseases with limited treatment options such as advanced HCC. In this study we report that GLV-1h68 effectively kills HCC and that it’s tumor cell-killing ability is unaffected by the development of resistance to sorafenib.
Materials and Methods
Reagents
Sorafenib was purchased from BIOTANG Inc. (Cat No. RS045; Lexington, MA). Sorafenib was dissolved in dimethyl sulfoxide (DMSO) for a stock concentration of 100mg/mL and stored at −20 °C.
Virus
GLV-1h68 was obtained from (Genelux Corp; San Diego, CA) and was derived from the VACV LIVP strain, as described previously.(7)
Cell Culture and Cell Lines
Hepatocellular Carcinoma (HCC) cell lines: Huh-7, Hep 3B and SNU-449 were purchased from the American Type Culture Collection (ATCC; Rockville, MD, USA). SNU-739 cell line was obtained through Korean Cell Line Bank (KCLB; Chongno-gu, Seoul, Korea). All cell lines were exposed to increasing concentrations of sorafenib over a three-month period to achieve a panel of sorafenib-resistant (SR) HCC cell lines. The Huh-7 cell line was cultured in Roswell Park Memorial Institute medium, Hep 3B, SNU-449 and SNU-739 cell lines were cultured in Dulbecco modified Eagle medium. African green monkey kidney fibroblast cells (CV-1) were obtained from ATCC and grown in minimum essential medium. All media was supplemented with 10% fetal calf serum, 100 units/mL penicillin, and 100 µg/mL streptomycin. All cell lines were incubated at 37 °C in a humidified 5 % CO2 atmosphere.
In-Vitro Growth Inhibition Assay
Parental and sorafenib-resistant HCC cell lines were seeded at a density of 2.5 × 103 cells/well in a 96-well plate and incubated for 24 hours. Cells were then exposed to 0.1 – 50 µM sorafenib and assessed for growth inhibition after a 72-hour exposure, using the Dojindo cell counting kit-8 (CCK-8; Rockville, MD). CCK-8 dye was added to phenol red free medium at a ratio of 1:10. The medium in each well was replaced with 110 µL of the dye solution and incubated for 4 hours. The plate was then read on a spectrophotometer (EL321e; Bio-Tek Instruments, Winooski, VT) at 450 nm wavelength. All samples were analyzed in triplicates.
Green Fluorescent Protein Expression
HCC parental and sorafenib-resistant cells were seeded as previously mentioned. After 24 hours of incubation, cells were infected with oncolytic vaccinia virus GLV-1h68 at an MOI of 0.001, 0.01, 0.1 and 1. To trace viral infection, 24 hours after infection, cells were examined using an inverted fluorescence microscope (Nikon Eclipse TE300; Nikon, Tokyo, Japan) for GFP expression and imaging was performed daily for 5 days.
Viral Plaque Assay
After infection of cells with virus and prior to daily cytotoxicity assay, supernatants from each infected well was collected daily for five days and immediately frozen at −80°C for storage. After thawing, serial dilutions of each supernatant samples were made to perform standard viral plaque assays on confluent CV-1 culture plates. All samples were measured in triplicate and the results averaged.
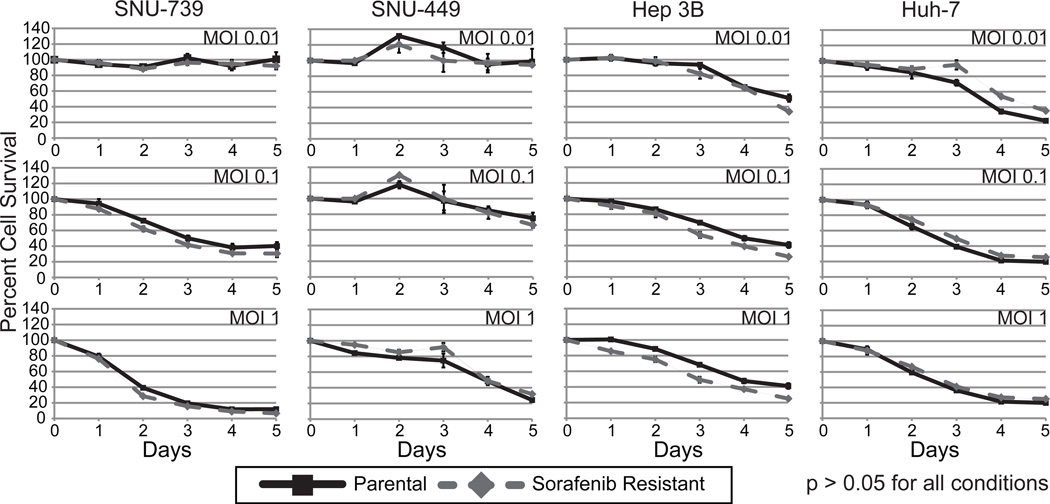
Cytotoxicity Assay
HCC parental and sorafenib-resistant cells were seeded as previously mentioned. After 24 hours of incubation, cells were infected with oncolytic vaccinia virus GLV-1h68 at an MOI of 0 (control), 0.001, 0.01, 0.1 or 1. Viral cytotoxicity was measured at days 0 through 5 for all 8 cell lines using the CCK-8 kit mentioned above. Survival curves were created showing the percent of viable cells as compared to uninfected control (Figure 4). All samples were analyzed in triplicate.
Figure 4.
GLV-1h68 demonstrates similar cytotoxic effect on both sorafenib-resistant and parental HCC cell lines. Cytotoxicity assays of 4 HCC cell lines and sorafenib-resistant cell lines developed from each of those cell lines infected with GLV-1h68 at multiplicities of infection (MOIs) of 0.01, 0.1, and 1.0 demonstrate near-complete cell kill by day 5 in all cell lines. No significant difference in cytotoxicity is demonstrated with GLV-1h68 between sorafenib-resistant and parental cell lines at all MOIs (p>0.05).
Statistics
Statistical analyses were performed using IBM Statistical Package for Social Sciences (SPSS®) software version 20 (Chicago, IL). For continuous variables Mann-Whitney U test were performed. A p-value <0.05 was considered significant.
Results
Development of sorafenib-resistant HCC cell lines
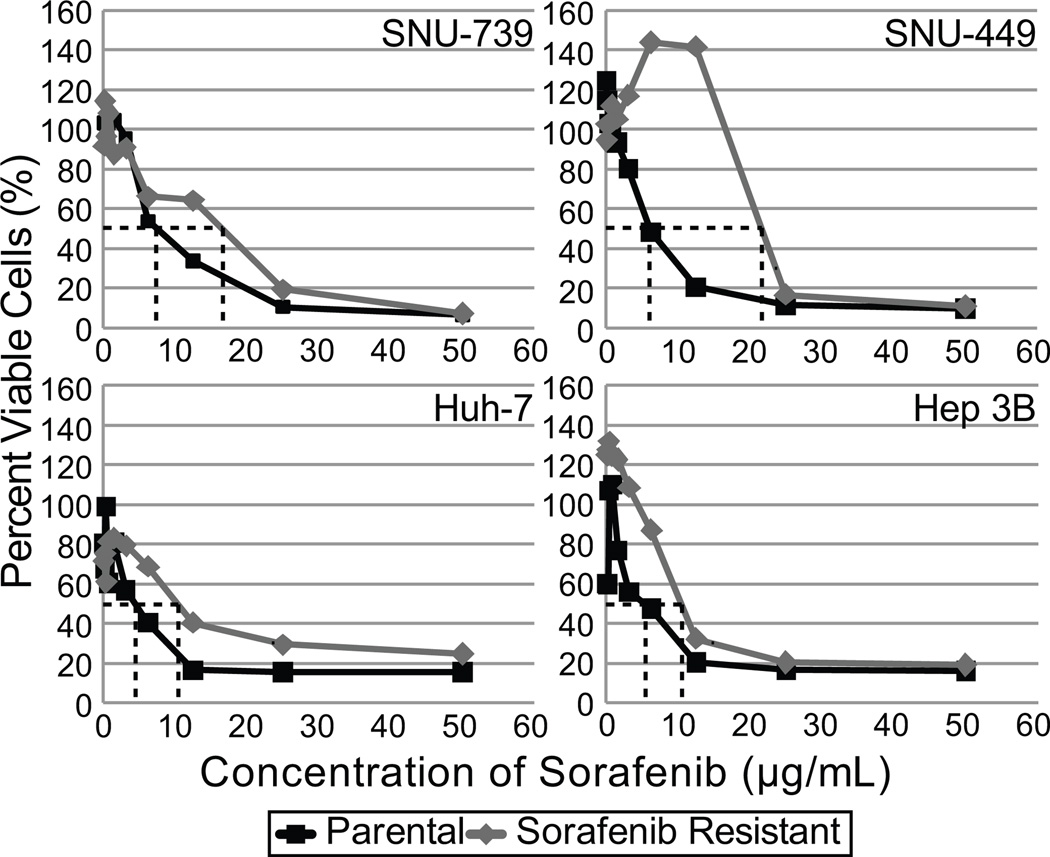
Using the cell lines SNU-739, SNU-449, Huh-7 and Hep 3B, we developed four sorafenib-resistant cell lines. Sorafenib resistance was induced by increasing the concentration of sorafenib added to cell media over repeated cell passages for a period of 3 months. All resistant cell lines showed an increase in the median inhibitory concentration of sorafenib when compared to their parental line (Figure 1).
Figure 1.
Dose response curves and the median inhibitory concentration (IC50) determination for sorafenib in SNU-739, SNU739SR, SNU-449, SNU-449SR, Huh-7, Huh-7SR, Hep 3B and Hep 3BSR cells. All cell lines, both the parental and sorafenib-resistant, were exposed to sorafenib at increasing concentrations for 72 hours. Cell viability was measured using CCK-8 assay. A. Dose response curves were plotted and the dashed lines indicate the IC50 of sorafenib for each corresponding cell line. In all cell lines the sorafenib-resistant cell line shows an increase from the IC50 of the parental cell line.
GLV-1h68 demonstrated similar time and dose dependent infectivity in cell culture in both the parental and their corresponding sorafenib-resistant cell lines
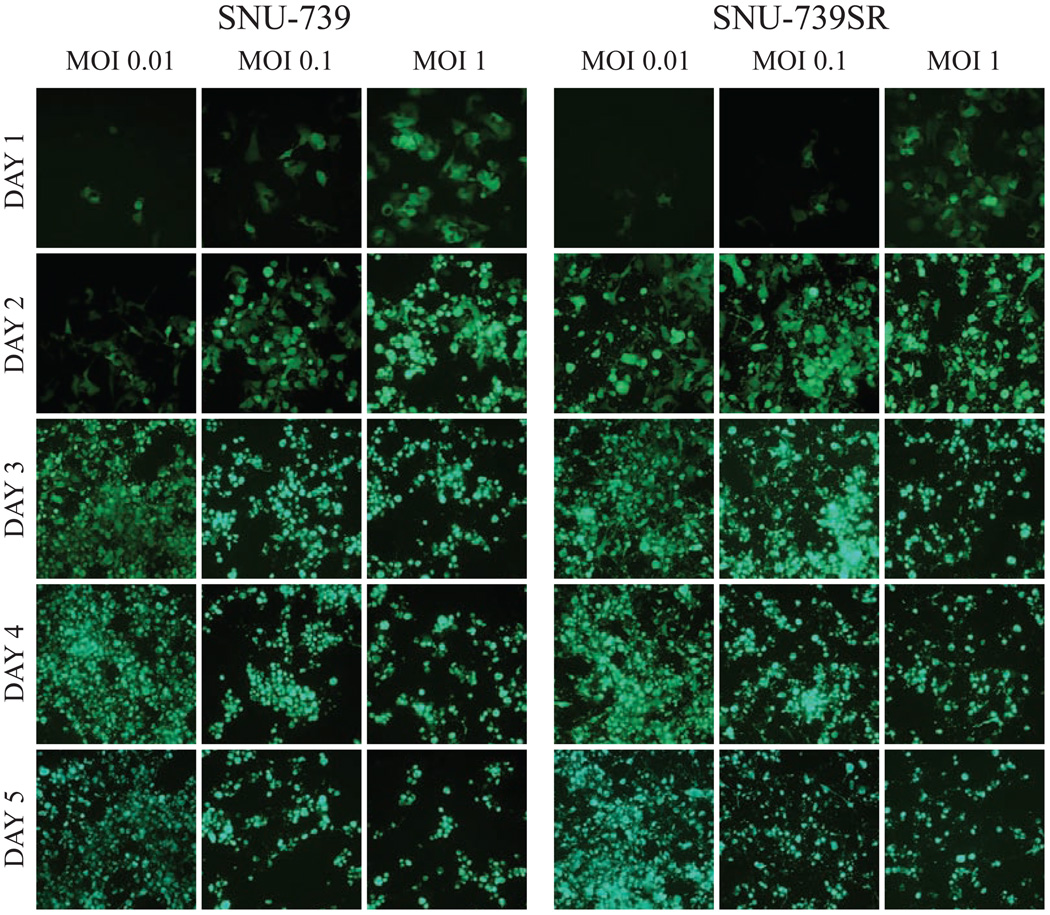
Viral infectivity via GFP expression was assessed by fluorescence microscopy in both parental and sorafenib-resistant cell lines. GFP expression confirmed viral infection by 24 hours in all cell lines and was proportional to viral concentration. GFP expression increased with time in all cell lines. Viral infection increased over the first 24 to 72 hours followed by decline in GFP expression due to viral cytotoxicity and subsequent cell death. Similar patterns of viral-mediated GFP expression were seen when comparing parental cell lines with their corresponding sorafenib-resistant cell line (Figure 2).
Figure 2.
GLV-1h68 infection of parental HCC and sorafenib-resistant HCC cell lines shows similar time and concentration dependent infection in-vitro. Above figure shows fluorescent microscopy of both the parental and sorafenib-resistant SNU-739 cell lines infected at an MOI of 0.01, 0.1 or 1.0. Both parental and sorafenib-resistant cell lines demonstrated a similar pattern of viral-mediated green fluorescent protein (GFP) expression. Both cell lines demonstrate increased GFP expression as time progresses and in a concentration dependent manner. Viral-mediated tumor cell killing at greater time points resulted in a subsequent decrease in GFP expression. While not shown above data was collected in all eight cell lines and was consistent with the above figure.
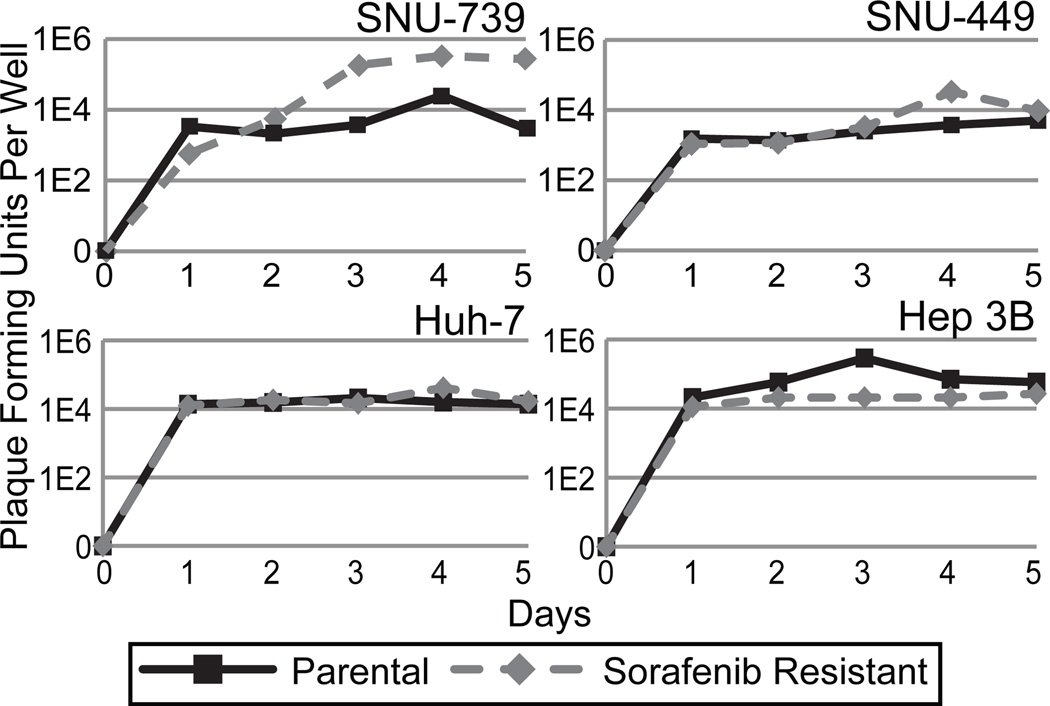
GLV-1h68 replicates efficiently in both parental and sorafenib-resistant HCC cell lines
Viral proliferation of GLV-1h68 in both parental and sorafenib-resistant SNU-739, SNU-449, Hep 3B and Huh-7 cell lines was assessed by standard viral plaque assay of supernatants collected over a 5 day period after infection (Figure 3). All cell lines supported an excess of a 4-logarithmic increase in viral titer at peak replication. The sorafenib-resistant cells lines supported GLV-1h68 replication at a level comparable to their parental sorafenib sensitive clones.
Figure 3.
GLV-1h68 demonstrates the ability to replicate in both parental and sorafenib-resistant HCC cell lines in-vitro. Viral replication is supported by all HCC cell lines in-vitro. Standard viral plaque assay of cells infected with an MOI of 1.0 demonstrate increase in viral titers over 5 days in all 8 cell lines tested.
GLV-1h68 demonstrated efficient cytotoxicity in both parental and sorafenib-resistant HCC cell lines
Both parental and sorafenib-resistant HCC cell lines were sensitive to the cytotoxic effects of GLV-1h68. GLV-1h68 effectively killed both the parental and sorafenib-resistant cell lines in a dose dependent manner. At day 5 with an MOI of 1.0, there was greater than 80% cell death in all cell lines. Overall, no significant difference in the cytotoxicity of GLV-1h68 was observed between the parental or corresponding sorafenib-resistant cell lines (p>0.05) (Figure 4).
Discussion
Hepatocellular carcinoma is a devastating disease with most patients presenting at an advanced stage of disease where curative therapies such as surgery, local ablation or regional therapy are no longer viable treatment options. Adjuvant therapies such as chemotherapy and radiation have little impact on survival in these patients. This has driven research into the development of molecular targeted therapies to treat HCC. Sorafenib is one such molecular targeted therapy and is currently the only systemic therapy approved for the treatment of advanced stage HCC. Unfortunately, it provides limited increase in overall survival of patients and has a low partial response rate likely due to the high levels of resistance.(5)
Sorafenib resistance in HCC, whether primary or acquired, is complicated and remains unclear even after extensive study. It is believed that primary resistance is caused by the genetic heterogenicity of HCC.(10, 11) While several promising biomarkers (phospho extracellular signaling-regulated kinase, phospho-c-Jun, c-Jun N-terminal kinase, Ang2, VEGF, c-KIT, hepatocyte growth factor) have been identified that correlate with patient survival on sorafenib therapy, studies which have attempted to conclusively link these biomarkers to sorafenib responsiveness have been thus far inconclusive.(6, 12) The lack of concrete biomarkers for sorafenib responsiveness has resulted in unnecessary treatment of primary resistant patients, thus exposing these patients to the morbidities and adverse events associated with sorafenib treatment.
Secondary resistance to sorafenib is acquired in HCC by exposure to sorafenib over time.(13) As a multi-kinase inhibitor, sorafenib functions by targeting kinases along various cellular pathways to inhibit angiogenesis and cell proliferation. Three major pathways (JAK/STAT, RAF/MAPK and PI3K/AKT) have been implicated in the acquisition of sorafenib resistance in HCC. These pathways use the mechanisms of compensatory pathways and addiction switching to overwhelm the inhibition of sorafenib on specific kinases in these pathways.(14, 15) In addition, the upregulation of numerous cell receptors (EGFR, PDGFR, VEGFR) leads to increased activation of these three pathways.(16) Mutations along these pathways have been linked to epithelialmesenchymal transformation (EMT) which is implicated in resistance of HCC to sorafenib therapy as well as poor prognosis.(17) What is becoming increasingly clear is that while this multi-targeted aspect of sorafenib is an advantage and allows it to be an effective treatment in the complex tumor environment of HCC. The disadvantage, however, is that it also allows for resistance to develop through many different mechanisms and pathways, which in turn results in the development of genetically distinct tumors.
Anticancer therapies that can be used in conjunction with sorafenib to overcome resistance and increase therapeutic efficacy are currently being investigated. Coupled with sorafenib, both chemotherapeutic agents (Doxorubicin, 5-FU and Uracil) and molecularly targeted molecules (Tegafur and Erlotinib) are being investigated in clinical trials with varied results.(18, 19) Preliminary data and early phase trials are promising and have demonstrated increased overall survival ranging from 7.4 months (Tegafur/uracil) to 13.7 months (5-fluorouracil).(20) While this increase in overall survival is a vast improvement when compared to the 2.9 month overall survival found with sorafenib alone, the fact remains that all patients with advanced HCC will inevitably succumb to this disease even with the addition of these combinational therapies. Thus it is of paramount importance that second line therapies be developed for patients after sorafenib and combinational therapy failure.
Currently in clinical trials there are numerous anticancer drugs being tested as secondline therapies in advanced HCC. Like sorafenib, molecularly targeted drugs such as mTOR inhibitors (everolimus and temsirolimus), VEGFR inhibitors (axitinib, ramucirumab), and the EGFR inhibitor (erlotinib) in combination with VEGFR inhibitor (bevacizumab). In addition, GC33, a recombinant humanized antibody against glypican-3, is also under investigation. A retrospective study of sunitinib, a multikinase inhibitor, showed modest antitumor activity in 11 sorafenib-resistant patients with median time to progression of 3.2 months.(21) Brivanib, a selective dual inhibitor of FGFR and VEGFR, was shown to have antitumor activity against advanced HCC in a phase II clinical trial.(22) Unfortunately, phase III clinical trials failed to meet the primary endpoint of improving overall survival versus placebo. Tivatinib, a selective inhibitor of MET, showed that median time to progression was slightly improved when compared to placebo (2.7 months vs. 1.4 months) in MET-high tumors.(23) Additionally, in an 18 patient clinical trial, a drug combination of gemcitabine plus oxaliplatin has shown 4.7 month overall survival with moderate adverse events.(24) It is clear that molecularly targeted drugs have the ability to increase overall survival and prolong disease free progression in patients for a short period of time with advanced HCC. However, like sorafenib, they function by inhibiting kinase pathways in cancer cells, and thus will likely develop acquired resistance via mutations along various pathways. This reality makes it clear that finding second line therapies that target alternate anticancer mechanisms is required if we are going to substantially change the outcome paradigm of advanced HCC.
Through genetic engineering, oncolytic vaccinia virus has the ability to selectively infect, replicate within, and eradicate HCC cells. As discussed earlier, VACV has a large genome that allows for the insertion of foreign DNA that can provide targeted therapy in cancer cells. This can help to improve the development of antitumoral immunity and increase apoptosis in cancerous cells. It has previously been shown that GLV-1h68, a modified oncolytic virus, can effectively infect and destroy HCC tumors both in-vitro and in-vivo.(7) Additionally, poxvirus JX-594, which expresses granulocyte-macrophage colony stimulating factor, was able to demonstrate sensitization of tumors to sorafenib when given prior to sorafenib therapy. These results suggest that oncolytic poxviruses are good candidates for use as first line therapy. Heo et al. looked at giving poxvirus after sorafenib treatment, but in contrast to our study, the tumors treated were not sorafenib-resistant at the time of viral therapy.(25)
We believe that oncolytic vaccinia virus GLV-1h68 is also a promising second line therapy for those patients with sorafenib-resistant HCC. Infection with vaccinia virus results in inhibition of apoptosis to allow sufficient time for optimal viral replication. Wild-type VACV produces this effect through activation of EGFR via Vaccinia Growth Factor (VGF) resulting in activation of the RAF/MEK/ERK pathway(9). In GLV-1h68, a recombinant VACV, VGF has been removed via an insertional deletion to attenuate virulence. A major target of sorafenib is inhibition of Raf-1 and B-Raf kinases, resulting in increased apoptosis via inhibition of the RAF/MEK/ERK pathway. Upregulation of the upstream and/or downstream targets of Raf is one of the factors linked to development of sorafenib resistance. In this sense, sorafenib resistance could selectively increase the virulence of GLV-1h68 in tumor cells by mimicking the effect of the knocked out VGF via activation of the RAF/MEK/ERK pathways. This suggests that HCC tumors that have become resistant to sorafenib could be optimal environments for recombinant VACV replication.
As demonstrated in previous research we were able to show that GLV-1h68, a modified oncolytic VACV, efficiently infects, replicates within, and kills HCC cell lines. As part of this paper we developed four distinct sorafenib-resistant HCC cell lines (Figure 1). Vaccinia infection was demonstrated to occur in a time-dependent fashion as evidenced by a progressive increase in GFP expression in all cell lines tested over the course of 5 days. Of note, sorafenib-resistant cell lines showed similar rates of infection as their corresponding parental cell line (Figure 2). The time course of infection correlated with the cytotoxic effect of GLV-1h68 as well as the efficient viral replication demonstrated on viral plaque assay. While we hypothesized that viral replication would be increased in the sorafenib-resistant HCC cell lines as compared to parental lines, this was not observed. One sorafenib-resistant cell line, SNU-739, did demonstrate superior viral replication compared to its parental counterpart, however this effect was not significant and was not observed in the remaining cell lines tested. All cell lines showed similar cytotoxic effects when parental cell lines were compared to sorafenib-resistant cell lines at all MOIs.
In summary, hepatocellular carcinoma is a highly fatal disease with limited treatment options. Currently, surgical resection is the only curative therapy available. Unfortunately, the vast majority of patients are diagnosed at an advanced stage when surgery is no longer feasible. Sorafenib is the only first line approved systemic therapy available for patients who present at an advanced stage of disease. As a therapy for HCC, sorafenib has many downfalls. Even at the high cost of over $10,000 dollars per month, a large number of patients have poor or no response to the drug. Additionally, sorafenib only minimally extends overall survival by approximately 3 months when compared to placebo. Most importantly, all patients will develop genetically distinct tumors that are resistant to sorafenib and will eventually fail therapy. Thus, it is clear that novel therapeutic solutions must be employed to change the prognosis of this devastating disease. In this study, we observed that GLV-1h68, a modified oncolytic VACV, efficiently infects, replicates within, and kills both parental and sorafenib-resistant HCC cells. It has been shown previously that GLV-1h68 is a viable primary therapeutic option for HCC.(7) In this study, we demonstrate that after cells develop sorafenib resistance, GLV-1h68 maintained it’s ability to efficiently kill HCC cancer cells. These results strongly indicate that GLV-1h68 is a prime candidate as a first line therapy for all patients with HCC and as viable second line therapeutic option for patients with sorafenib-resistant HCC. As a therapy, GLV-1h68 merits strong consideration for future clinical trials in patients with advanced HCC.
Acknowledgments
Supported in part by training grant T 32 CA09501 (J.C.), grants RO1 CA 76416 and RO1 CA/DK80982 (Y.F.) from the National Institutes of Health grant BC024118 from the US Army (Y.F.), grant IMG0402501 from the Susan G. Komen Foundation (Y.F.), and grant 032047 from the Flight Attendant Medical Research Institute (Y.F.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the 9th Annual Academic Surgical Congress in San Diego, CA, Feb. 4–6, 2014
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA: a cancer journal for clinicians. 2011 Mar-Apr;61(2):69–90. doi: 10.3322/caac.20107. PubMed PMID: 21296855. Epub 2011/02/08. eng. [DOI] [PubMed] [Google Scholar]
- 2.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009 Mar 20;27(9):1485–1491. doi: 10.1200/JCO.2008.20.7753. PubMed PMID: 19224838. Pubmed Central PMCID: Pmc2668555. Epub 2009/02/20. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology (Baltimore, Md) 2011 Mar;53(3):1020–1022. doi: 10.1002/hep.24199. PubMed PMID: 21374666. Pubmed Central PMCID: Pmc3084991. Epub 2011/03/05. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer research. 2004 Oct 1;64(19):7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. PubMed PMID: 15466206. Epub 2004/10/07. eng. [DOI] [PubMed] [Google Scholar]
- 5.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. The New England journal of medicine. 2008 Jul 24;359(4):378–390. doi: 10.1056/NEJMoa0708857. PubMed PMID: 18650514. Epub 2008/07/25. eng. [DOI] [PubMed] [Google Scholar]
- 6.Villanueva A, Llovet JM. Targeted therapies for hepatocellular carcinoma. Gastroenterology. 2011 May;140(5):1410–1426. doi: 10.1053/j.gastro.2011.03.006. PubMed PMID: 21406195. Pubmed Central PMCID: Pmc3682501. Epub 2011/03/17. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gentschev I, Muller M, Adelfinger M, Weibel S, Grummt F, Zimmermann M, et al. Efficient colonization and therapy of human hepatocellular carcinoma (HCC) using the oncolytic vaccinia virus strain GLV-1h68. PloS one. 2011;6(7):e22069. doi: 10.1371/journal.pone.0022069. PubMed PMID: 21779374. Pubmed Central PMCID: Pmc3133637. Epub 2011/07/23. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fenner F. Nature, nurture and my experience with smallpox eradication. The Medical journal of Australia. 1999 Dec 6–20;171(11–12):638–641. doi: 10.5694/j.1326-5377.1999.tb123833.x. PubMed PMID: 10721355. Epub 2000/03/18. eng. [DOI] [PubMed] [Google Scholar]
- 9.Postigo A, Martin MC, Dodding MP, Way M. Vaccinia-induced epidermal growth factor receptor-MEK signalling and the anti-apoptotic protein F1L synergize to suppress cell death during infection. Cellular microbiology. 2009 Aug;11(8):1208–1218. doi: 10.1111/j.1462-5822.2009.01327.x. PubMed PMID: 19388902. Pubmed Central PMCID: PMC2730480. Epub 2009/04/25. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu L, Cao Y, Chen C, Zhang X, McNabola A, Wilkie D, et al. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer research. 2006 Dec 15;66(24):11851–11858. doi: 10.1158/0008-5472.CAN-06-1377. PubMed PMID: 17178882. Epub 2006/12/21. eng. [DOI] [PubMed] [Google Scholar]
- 11.Blivet-Van Eggelpoel MJ, Chettouh H, Fartoux L, Aoudjehane L, Barbu V, Rey C, et al. Epidermal growth factor receptor and HER-3 restrict cell response to sorafenib in hepatocellular carcinoma cells. Journal of hepatology. 2012 Jul;57(1):108–115. doi: 10.1016/j.jhep.2012.02.019. PubMed PMID: 22414764. Epub 2012/03/15. eng. [DOI] [PubMed] [Google Scholar]
- 12.Llovet JM, Pena CE, Lathia CD, Shan M, Meinhardt G, Bruix J. Plasma biomarkers as predictors of outcome in patients with advanced hepatocellular carcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012 Apr 15;18(8):2290–2300. doi: 10.1158/1078-0432.CCR-11-2175. PubMed PMID: 22374331. Epub 2012/03/01. eng. [DOI] [PubMed] [Google Scholar]
- 13.van Malenstein H, Dekervel J, Verslype C, Van Cutsem E, Windmolders P, Nevens F, et al. Long-term exposure to sorafenib of liver cancer cells induces resistance with epithelial-to-mesenchymal transition, increased invasion and risk of rebound growth. Cancer letters. 2013 Feb 1;329(1):74–83. doi: 10.1016/j.canlet.2012.10.021. PubMed PMID: 23111106. Epub 2012/11/01. eng. [DOI] [PubMed] [Google Scholar]
- 14.Tai WT, Cheng AL, Shiau CW, Liu CY, Ko CH, Lin MW, et al. Dovitinib induces apoptosis and overcomes sorafenib resistance in hepatocellular carcinoma through SHP-1-mediated inhibition of STAT3. Molecular cancer therapeutics. 2012 Feb;11(2):452–463. doi: 10.1158/1535-7163.MCT-11-0412. PubMed PMID: 22180308. Epub 2011/12/20. eng. [DOI] [PubMed] [Google Scholar]
- 15.Chen KF, Chen HL, Tai WT, Feng WC, Hsu CH, Chen PJ, et al. Activation of phosphatidylinositol 3-kinase/Akt signaling pathway mediates acquired resistance to sorafenib in hepatocellular carcinoma cells. The Journal of pharmacology and experimental therapeutics. 2011 Apr;337(1):155–161. doi: 10.1124/jpet.110.175786. PubMed PMID: 21205925. Epub 2011/01/06. eng. [DOI] [PubMed] [Google Scholar]
- 16.Ezzoukhry Z, Louandre C, Trecherel E, Godin C, Chauffert B, Dupont S, et al. EGFR activation is a potential determinant of primary resistance of hepatocellular carcinoma cells to sorafenib. International journal of cancer Journal international du cancer. 2012 Dec 15;131(12):2961–2969. doi: 10.1002/ijc.27604. PubMed PMID: 22514082. Epub 2012/04/20. eng. [DOI] [PubMed] [Google Scholar]
- 17.Maheswaran T, Rushbrook SM. Epithelial-mesenchymal transition and the liver: role in hepatocellular carcinoma and liver fibrosis. Journal of gastroenterology and hepatology. 2012 Mar;27(3):418–420. doi: 10.1111/j.1440-1746.2012.07060.x. PubMed PMID: 22353346. Epub 2012/02/23. eng. [DOI] [PubMed] [Google Scholar]
- 18.Abou-Alfa GK, Johnson P, Knox JJ, Capanu M, Davidenko I, Lacava J, et al. Doxorubicin plus sorafenib vs doxorubicin alone in patients with advanced hepatocellular carcinoma: a randomized trial. JAMA : the journal of the American Medical Association. 2010 Nov 17;304(19):2154–2160. doi: 10.1001/jama.2010.1672. PubMed PMID: 21081728. Epub 2010/11/18. eng. [DOI] [PubMed] [Google Scholar]
- 19.Finn RS. Emerging targeted strategies in advanced hepatocellular carcinoma. Seminars in liver disease. 2013 Feb;33(Suppl 1):S11–S19. doi: 10.1055/s-0033-1333632. PubMed PMID: 23457035. Epub 2013/03/15. eng. [DOI] [PubMed] [Google Scholar]
- 20.Petrini I, Lencioni M, Ricasoli M, Iannopollo M, Orlandini C, Oliveri F, et al. Phase II trial of sorafenib in combination with 5-fluorouracil infusion in advanced hepatocellular carcinoma. Cancer chemotherapy and pharmacology. 2012 Mar;69(3):773–780. doi: 10.1007/s00280-011-1753-2. PubMed PMID: 22033636. Epub 2011/10/29. eng. [DOI] [PubMed] [Google Scholar]
- 21.Worns MA, Schuchmann M, Duber C, Otto G, Galle PR, Weinmann A. Sunitinib in patients with advanced hepatocellular carcinoma after progression under sorafenib treatment. Oncology. 2010;79(1–2):85–92. doi: 10.1159/000320363. PubMed PMID: 21071995. Epub 2010/11/13. eng. [DOI] [PubMed] [Google Scholar]
- 22.Finn RS, Kang YK, Mulcahy M, Polite BN, Lim HY, Walters I, et al. Phase II, open-label study of brivanib as second-line therapy in patients with advanced hepatocellular carcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012 Apr 1;18(7):2090–2098. doi: 10.1158/1078-0432.CCR-11-1991. PubMed PMID: 22238246. Epub 2012/01/13. eng. [DOI] [PubMed] [Google Scholar]
- 23.Santoro A, Rimassa L, Borbath I, Daniele B, Salvagni S, Van Laethem JL, et al. Tivantinib for second-line treatment of advanced hepatocellular carcinoma: a randomised, placebo-controlled phase 2 study. The lancet oncology. 2013 Jan;14(1):55–63. doi: 10.1016/S1470-2045(12)70490-4. PubMed PMID: 23182627. Epub 2012/11/28. eng. [DOI] [PubMed] [Google Scholar]
- 24.Mir O, Coriat R, Boudou-Rouquette P, Ropert S, Durand JP, Cessot A, et al. Gemcitabine and oxaliplatin as second-line treatment in patients with hepatocellular carcinoma pre-treated with sorafenib. Medical oncology (Northwood, London, England) 2012 Dec;29(4):2793–2799. doi: 10.1007/s12032-012-0208-x. PubMed PMID: 22427209. Epub 2012/03/20. eng. [DOI] [PubMed] [Google Scholar]
- 25.Heo J, Breitbach CJ, Moon A, Kim CW, Patt R, Kim MK, et al. Sequential therapy with JX-594, a targeted oncolytic poxvirus, followed by sorafenib in hepatocellular carcinoma: preclinical and clinical demonstration of combination efficacy. Molecular therapy : the journal of the American Society of Gene Therapy. 2011 Jun;19(6):1170–1179. doi: 10.1038/mt.2011.39. PubMed PMID: 21427706. Pubmed Central PMCID: 3129795. [DOI] [PMC free article] [PubMed] [Google Scholar]