Abstract
Background
Several studies have shown a favorable effect of supervised exercise training on obstructive sleep apnea (OSA). This meta-analysis was conducted to analyze the data from these studies on the severity of OSA (primary outcome) in adults. Secondary outcomes of interest included body mass index (BMI), sleep efficiency, daytime sleepiness and cardiorespiratory fitness.
Methods
Two independent reviewers searched PubMed and Embase (from inception to March 6, 2013) to identify studies on the effects of supervised exercise training in adults with OSA. Pre- and postexercise training data on our primary and secondary outcomes were extracted.
Results
A total of 5 studies with 6 cohorts that enrolled a total of 129 study participants met the inclusion criteria. The pooled estimate of mean pre- to postintervention (exercise) reduction in AHI was −6.27 events/h (95 % confidence interval [CI] −8.54 to −3.99; p < 0.001). The pooled estimates of mean changes in BMI, sleep efficiency, Epworth sleepiness scale and VO2 peak were −1.37 (95 % CI −2.81 to 0.07; p = 0.06), 5.75 % (95 % CI 2.47–9.03; p = 0.001), −3.3 (95 % CI −5.57 to −1.02; p = 0.004), and 3.93 mL/kg/min (95 % CI 2.44–5.42; p < 0.001), respectively.
Conclusions
This meta-analysis shows a statistically significant effect of exercise in reducing the severity of sleep apnea in patients with OSA with minimal changes in body weight. Additionally, the significant effects of exercise on cardiorespiratory fitness, daytime sleepiness, and sleep efficiency indicate the potential value of exercise in the management of OSA.
Keywords: Exercise, Obstructive sleep apnea, Cardiorespiratory fitness, Daytime sleepiness
Introduction
Obstructive sleep apnea (OSA) is a common medical condition characterized by repetitive upper airway obstruction during sleep [1, 2]. OSA is linked to a wide range of adverse health consequences, including daytime sleepiness, cognitive impairment, and several cardiovascular and metabolic disorders [3, 4].
The American Academy of Sleep Medicine recommends the use of continuous positive airway pressure (CPAP) [5] or oral appliances for treating mild to moderate OSA, whereas CPAP is recommended as the first-line and oral appliances as second-line treatments for severe OSA patients [6]. Although highly efficacious when used, the utility of CPAP is limited by poor patient adherence to CPAP. Other treatment options for OSA include weight loss [7–9] and upper airway surgery [10, 11]. However, the effects of weight loss on the severity of OSA could be delayed [12]. Surgical modifications of the upper airway for the treatment of OSA continue to evolve, and the data are not as robust due to the relative paucity of randomized, controlled trials (RCTs) [13].
Exercise training in patients with sleep apnea has received accelerated attention. Not only has exercise been shown to be effective in improving OSA, but it also has been found to decrease the severity of central sleep apnea in chronic heart failure patients [14, 15]. Moreover, exercise could be uniquely helpful to ameliorate numerous sequalae of OSA, including cardiovascular disease, impaired glucose tolerance, and fatigue. Additionally, analyses from a large community-based cohort showed that vigorous physical activity was associated with a decrease in the prevalence of OSA [16]. The mechanisms by which exercise training leads to improvement in OSA are not well-understood. Of course, exercise could reduce OSA indirectly by facilitating decreases in body weight and fat. However, epidemiologic [17, 18] and experimental studies [19–21] have shown that the effect of exercise on sleep apnea is independent of body weight reduction. Besides the beneficial effects on OSA severity, regular physical activity also has been found to be associated with subjective well being in patients with OSA irrespective of the severity [22].
The primary purpose of this study was to evaluate the efficacy of exercise training on OSA severity reduction in adults with OSA. Secondary objectives were to evaluate the effects of exercise training on body mass index (BMI), sleep efficiency, daytime sleepiness, and cardiorespiratory fitness. In much of the literature on the effects of exercise on OSA, the degree to which participants indeed exercised or were encouraged to do so has often been unclear. Therefore, the present meta-analysis was restricted to studies that documented exercise training via improvements in cardiorespiratory fitness.
Methods
This meta-analysis was performed according to the guidelines reported in meta-analysis of observational studies in epidemiology [23] and preferred reporting items for systematic reviews and meta-analyses statements [24].
Search Strategy and Selection Criteria
We searched PubMed and Embase databases from their inception to March 6, 2013. We used combinations of the following keywords: “exercise,” “obstructive sleep apnea,” and “sleep-disordered breathing.” The search from PubMed yielded all the studies included in this meta-analysis. To ensure a thorough search of the literature, we hand-searched the reference lists of the included studies and previously published meta-analyses [25, 26] on lifestyle interventions and OSA severity. For inclusion in our meta-analysis, we considered only those studies that reported exercise as a primary component of an intervention and also reported changes in cardiorespiratory fitness. Inclusion criteria for RCTs included a diagnosis of OSA and randomization to exercise training or to control condition. The control condition could include individuals who received either stretching exercises or were left untrained. We excluded studies that reported additional intervention with CPAP. We also excluded studies that reported data in median and interquartile range. If the required data from articles were ambiguous or missing, we contacted the study authors; after two unanswered attempts, we excluded these studies from the analysis.
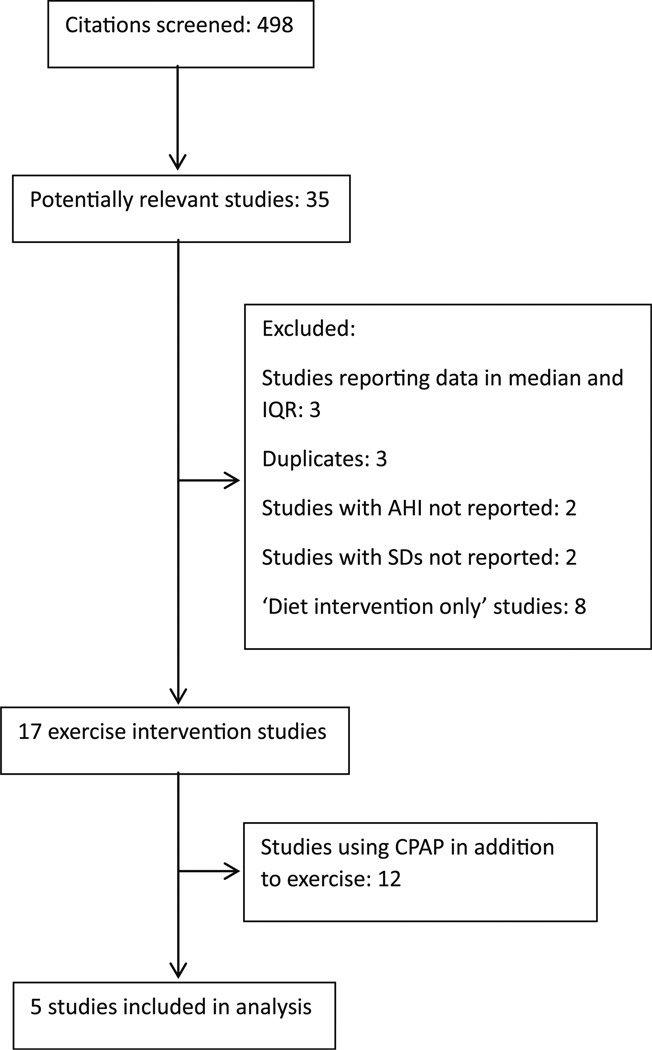
Two investigators (IHI and CEK) independently searched the studies and performed the final screening. In those instances when there was a disagreement between the investigators, a third investigator (SDY) reviewed the article and disagreements were resolved through discussion. Figure 1 summarizes the results of the selection and exclusion process.
Fig. 1.
Flow diagram of articles identified and evaluated during the study selection process
Study Outcomes
Our primary study outcome was OSA severity, as measured by the apnea hypopnea index (AHI; i.e., number of apnea or hypopnea events per hours of sleep). Secondary outcomes included changes in BMI, sleep efficiency, daytime sleepiness, and cardiorespiratory fitness. As a measure of body mass, BMI was calculated as weight in kilograms divided by height in meters squared. As an indicator of overall sleep quality, sleep efficiency was measured by overnight polysomnography and expressed as a percentage of time in bed that was spent asleep. Subjective daytime sleepiness was measured by the Epworth Sleepiness Scale [27]. Cardiorespiratory fitness was indicated by peak oxygen consumption (VO2 peak, measured in mL/kg/min) obtained during a graded cardiopulmonary exercise test.
Data Abstraction
Data were extracted on a prespecified worksheet. This included first author’s name, year of publication, number of participants, mean of pre- and postexercise training AHI, BMI, sleep efficiency, Epworth Sleepiness Scale, and VO2 peak with standard deviations. One study reported separate data for the two exercise intervention arms (aerobic exercise and combined aerobic/resistance exercise) [28]; as a result, these two groups were separately analyzed in our analyses. Some data were specifically requested from the corresponding authors of two studies included in our meta-analysis [20, 28]. Kline et al. [20] confirmed that Epworth Sleepiness Scale and cardiorespiratory fitness data were published separately from the included study [29, 30]. The authors of two studies provided additional BMI data for their study populations [19, 26]. One of the studies reported cardiorespiratory fitness data as metabolic equivalents (METs) [31]. Therefore, we transformed the data to peak relative oxygen consumption using the following equation: 1 MET = 3.5 mL O2/kg/min. Five of the study participants in one study [31] used CPAP during the intervention. Because individual pre- and postexercise training AHI data were provided, for our meta-analysis on the effects of exercise training on AHI, we excluded the data of those who concurrently used CPAP.
Quantitative Data Synthesis
The mean changes in the outcomes from exercise training along with their 95 % confidence intervals (CIs) were estimated by pooling available data using comprehensive meta analysis software version 2.2.064. Results are displayed in the form of forest plots (Figs. 2, 3, 4, 5, 6). Similarly, we also conducted separate analyses of the difference in each outcome between control and exercise groups in RCTs. We conducted both random-effects methods and fixed-effects meta-analyses to account for variance between and within the studies, respectively [32]. However, we chose to include the results from the random-effects method to account for any heterogeneity within and between the studies. Statistical heterogeneity was assessed with the I2 statistic [33]. An I2 > 60 % indicated significant heterogeneity. We also performed sensitivity analyses to assess the influence of each study on estimates of the overall effect. This was done separately for the pooled estimates of change in AHI, BMI, sleep efficiency, and VO2 peak. To check for publication bias, we constructed funnel plots of effect size and standard error [32, 34] and also analyzed results by using the Begg and Mazumdar rank correlation test [35].
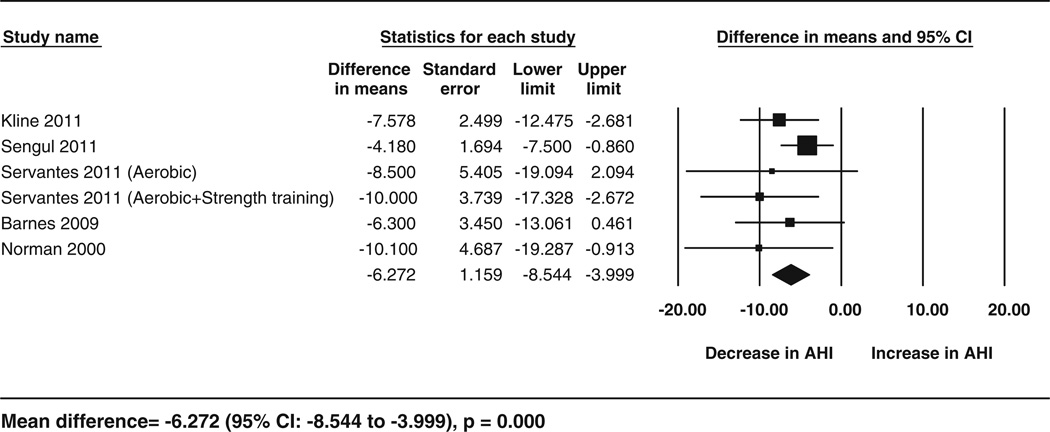
Fig. 2.
Forest plot for the change in AHI following exercise. The diamond reflects the 95 % confidence interval of the pooled estimate of mean difference. AHI apnea hypopnea index CI confidence intervals
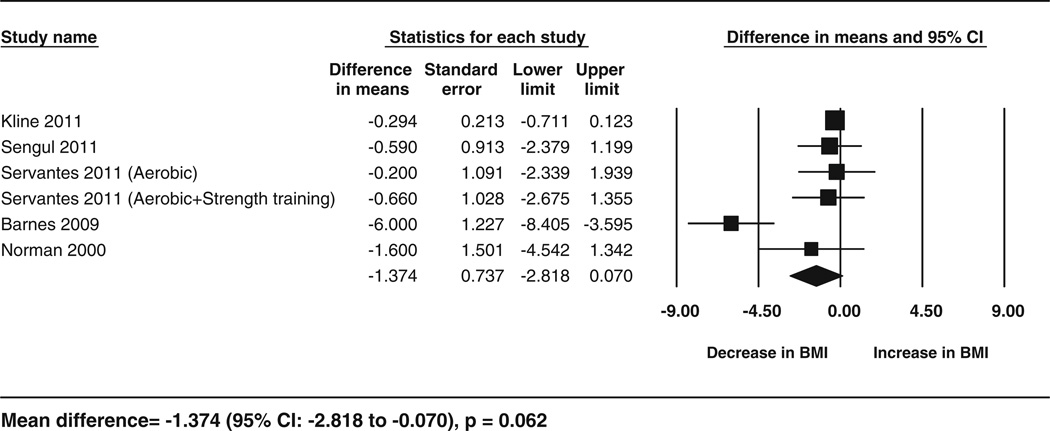
Fig. 3.
Forest plot for the change in BMI following exercise. The diamond reflects the 95 % confidence interval of the pooled estimate of mean difference. BMI body mass index CI confidence intervals
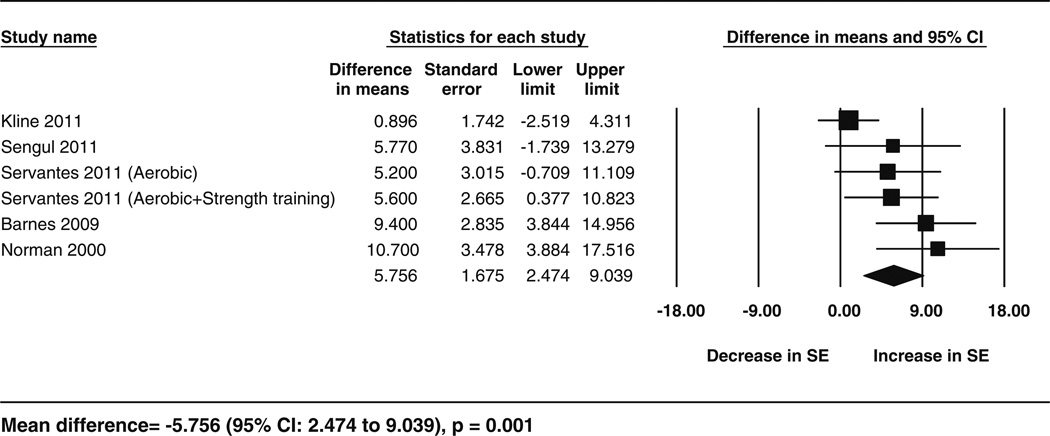
Fig. 4.
Forest plot for the change in sleep efficiency following exercise. The diamond reflects the 95 % confidence interval of the pooled estimate of mean difference. SE sleep efficiency CI confidence intervals
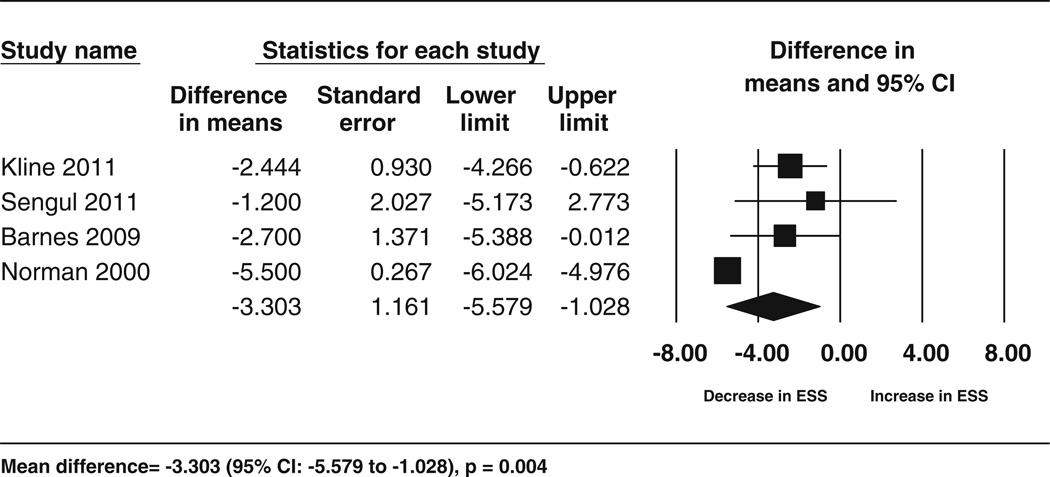
Fig. 5.
Forest plot for the change in Epworth Sleepiness Scale following exercise. The diamond reflects the 95 % confidence interval of the pooled estimate of mean difference. ESS Epworth Sleepiness Scale CI confidence intervals
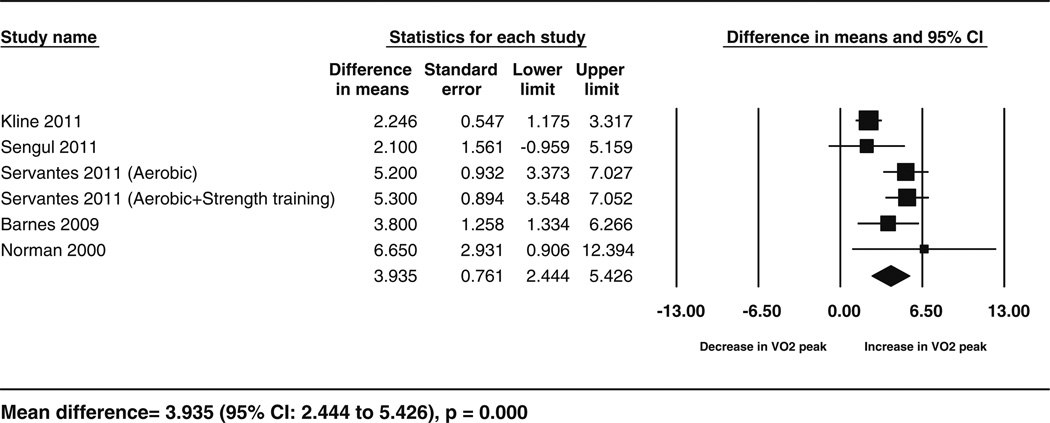
Fig. 6.
Forest plot for the change in VO2 peak following exercise. The diamond reflects the 95 % confidence interval of the pooled estimate of mean difference. VO2 peak cardiorespiratory fitness measured as peak oxygen consumption in mL/kg/min. CI confidence intervals
Results
We reviewed 498 citations and identified 5 studies [19, 20, 26, 29, 31] for inclusion in our meta-analysis (Fig. 1). Our search strategy yielded three studies that used exercise as the sole intervention [20, 21, 28]. In two other studies, exercise was the primary intervention but study participants also underwent some dietary intervention [31, 36]. Altogether, these studies enrolled a total of 129 participants. Table 1 outlines the baseline characteristics of the population in each study. Table 2 summarizes the exercise interventions used in each study. On average, study participants were ≥42 years old and had a mean BMI ≥ 26. The duration of exercise intervention lasted between 12 and 24 weeks. There were three RCTs [20, 21, 28] and two single group intervention studies [31, 36]. Two [20, 31] of the studies were conducted in the United States, one [21] in Turkey, one [28] in Brazil, and one [36] in Australia.
Table 1.
Baseline characteristics of studies
| Author | Year | Study design | Duration of exercise |
Number of participants (% of males) |
Age, mean (SD) | BMI, mean (SD) | AHI, mean (SD) |
|---|---|---|---|---|---|---|---|
| Kline et al. [20] | 2011 | RCT | 12 weeks | Exercise: 27 (56 %) Control: 16 (56 %) |
Exercise: 47.6 (6.7) Control: 45.9 (8.8) |
Exercise: 35.5.(6.2) Control: 33.6 (5.6) |
Exercise: 32.2 (29.0) Control: 24.4 (22.4) |
| Sengul et al. [21] | 2011 | RCT | 12 weeks | Exercise: 10 (100 %) Control: 10 (100 %) |
Exercise: 54.4 (6.5) Control: 48.0 (7.4) |
Exercise: 29.79 (2.6) Control: 28.42 (5.4) |
Exercise: 15.19 (5.4) Control: 17.92 (6.4) |
| Servantes et al. [28] | 2011 | RCT | 12 weeks | Aerobic exercise: 17 (47.1 %) Aerobic and resistance exercise: 17 (47.1 %) Control: 11(45.5 %) |
Aerobic exercise: 51.76 (9.8) Aerobic and resistance exercise: 50.82 (9.4) Control: 53.0 (8.1) |
Aerobic exercise: 26.87 (4.6) Aerobic and resistance exercise: 27.98 (4.4) Control: 27.73 (3.6) |
Aerobic exercise: 25.2 (24.7) Aerobic and resistance exercise: 26.4 (17.6) Control: 22.8 (17.4) |
| Barnes et al. [36] | 2009 | Single group intervention study |
16 weeks | 12 (25 %) | 42.3 (10.4) | 36.1 (4.3) | 24.6 (12.0) |
| Norman et al. [31] | 2000 | Single group intervention study |
24 weeks | 9 (88.8 %) | 48(9) | 31.2 (4.6) | 28.0 (17.9) |
BMI body mass index, SD standard deviation, AHI apnea hypopnea index, RCT randomized controlled trial
Table 2.
Characteristics of exercise intervention used in studies
| Study | Description of study intervention | Level of supervision |
|---|---|---|
| Kline et al. [20] |
|
All exercise sessions were supervised by staff trained in exercise physiology |
| Sengul et al. [21] |
|
All exercise sessions were supervised by a single physiotherapist |
| Servantes et al. [28] | Group I (aerobic exercise only):
|
First three sessions were supervised by a physiotherapist and a cardiologist; remaining 12 week of sessions were home-based |
| Barnes et al. [36] |
|
During weeks 1–8, 3 sessions/week were supervised by an exercise physiologist, with an additional 2–4 unsupervised home sessions/ week being expected; during week 9–16, at least 1 session/week was supervised, with 4–5 home sessions/week expected |
| Norman et al. [31] |
|
During months 1–4, all exercise sessions were supervised in university wellness center; during months 5–6, 1 session/week was supervised |
Effect on Primary Outcome
The effect of exercise training on our primary outcome, AHI, was assessed by using two approaches. First, the pre-to postintervention analysis of five studies (six cohorts) showed a pooled estimate of mean change in AHI of −6.27 events/h (95 % CI −8.54 to −3.99; p < 0.001) with an I2=0% (Fig. 2), which reflected a 32 % reduction in AHI from baseline in the intervention groups. Second, a separate analysis of the data from three RCTs showed an AHI reduction of 7.17 (95 % CI −1.86 to −12.48; p = 0.008) in favor of the exercise groups, with an I2 = 0 %. This effect corresponded to an improvement in AHI by 42 % following exercise compared with the control treatments (Table 3). The I2 statistic for these analyses showed no significant heterogeneity.
Table 3.
Difference in outcomes between control and exercise groups in randomized, controlled trials
| Outcomes | Mean difference | p value |
|---|---|---|
| AHI (events/h) | −7.174 (95 % CI: −1.867 to −12.482) |
0.008 |
| BMI (kg/m2) | −0.573 (95 % CI: −1.34 to 2.486) | 0.557 |
| Sleep efficiency % | 6.188 (95 % CI: 2.021 to 10.354) | 0.004 |
| VO2 peak (mL/kg/min) |
5.695 (95 % CI: 1.698 to 9.692) | 0.005 |
AHI apnea hypopnea index, BMI body mass index, VO2 peak cardiorespiratory fitness, CI confidence interval
Effect on Secondary Outcomes
Data for the secondary outcomes of BMI, sleep efficiency, and VO2 peak were available for five studies (6 cohorts), whereas Epworth Sleepiness Scale data were available for four studies. The analyses showed significant improvements following exercise training for sleep efficiency (5.75 %; 95 % CI 2.47–9.03; p = 0.001, I2 = 53.29 %), VO2 peak (3.93 mL/kg/min; 95 % CI 2.44–5.42; p < 0.001, I2 = 65.89 %) and for Epworth Sleepiness Scale scores (−3.3; 95 % CI −5.57 to −1.02; p = 0.004,I2 = 82.52 %) but no significant change in BMI (−1.37; 95 % CI −2.81 to 0.07; p = 0.06,I2 = 76.92 %). These results are shown in the form of forest plots (Figs. 3, 4, 5, 6). The I2 statistic for these analyses showed moderate to high heterogeneity. The results corresponded to an improvement of 8 % in sleep efficiency, 24.6 % in VO2 peak, and 28 % in Epworth Sleepiness Scale scores compared with baseline following intervention. Similar results were obtained from separate analyses of the data from RCTs (Table 3). These results showed that, compared with the controls, there was a significant increase in VO2 peak and sleep efficiency in the exercise groups, but BMI did not decrease significantly. These effects corresponded to an improvement in VO2 peak and sleep efficiency by 17.65 and 5.8 % respectively, following exercise compared with the control treatments.
Sensitivity Analysis
Sensitivity analyses, done by systematically removing one study at a time, demonstrated that no single study changed the statistical significance of the overall results.
Publication Bias
The Begg and Mazumdar rank correlation tests of funnel plots for the analyses on AHI, BMI, sleep efficiency, VO2 peak, and Epworth Sleepiness Scale did not show any publication bias (see Supplementary Material, only funnel plot for data on pre- and post-AHI is provided).
Discussion
Our findings indicate that exercise training has a statistically significant effect on AHI that seems to be independent of changes in BMI. An important finding of our meta-analysis is that the reduction in OSA severity was achieved without a significant reduction in body weight. This suggests a possible role of exercise in the treatment of sleep apnea. Using a pre- to postintervention model and pooling the mean differences in AHI across the studies, we found that exercise training resulted in a mean AHI reduction of 6.27 events/h. Limiting the results to studies that used exercise as the sole intervention and using change scores to calculate the difference in AHI between the cohorts that received exercise as an intervention and the controls, we found a similar reduction in AHI. Our meta-analysis shows that BMI did not change with exercise, but there was a significant improvement in sleep efficiency and daytime sleepiness.
The AHI reduction seen in our meta-analysis seems modest compared with similar meta-analyses that evaluated the effects of dietary weight loss, surgery, oral appliances, and CPAP on OSA severity. Anandam et al. [25], in a meta-analysis of studies on dietary weight loss intervention showed a reduction in AHI by 23.1 events/h (95 % CI 8.9–37.3, p = 0.001) corresponding to a 44 % reduction compared with baseline. In another meta-analysis, oral appliances were found to reduce AHI by 12.07 events/h (95 % CI −9.7 to −14.3, p < 0.01), suggesting an improvement of 60.25 % postintervention [37]. Finally, Greenburg et al. [38] showed that bariatric surgery reduced AHI by 38.2 events/h (95 % CI 31.9–44.4), possibly suggesting a 71.11 % improvement compared to baseline.
An important finding of our meta-analysis is that the reduction in OSA severity was achieved without a significant reduction in body weight. This is an important distinction, as epidemiologic research estimates that a reduction in body weight of approximately 10 % would be necessary to achieve the 25–30 % reduction in OSA severity that we documented with exercise [7]. Furthermore, complete amelioration of OSA is not necessary to obtain significant health benefits; even modest differences in OSA severity have been associated with significantly reduced risk of adverse health outcomes (e.g., hypertension) [37]. Finally, whereas CPAP and oral appliances are dependent upon nightly use to obtain the effects, evidence indicates that exercise training elicits chronic reduction in the severity of OSA [19].
Besides changes in OSA severity, the improvements in secondary outcomes following exercise training are noteworthy. The improvement in cardiorespiratory fitness has unique health and longevity benefits [39]. Moreover, the improvement in sleep efficiency with exercise is similar to what is typically achieved with CPAP [40, 41]. Likewise, the effects on daytime sleepiness (as measured by the Epworth Sleepiness Scale) with exercise training is similar to that seen with CPAP [42, 43]. Therefore, although based upon a small number of studies, these preliminary findings suggest that the effects of exercise in adults with OSA extend past AHI reduction.
Although converging evidence suggests beneficial effects of exercise training on the severity of sleep apnea, the current body of evidence remains inconclusive on the exact mechanisms of these effects. Different theories have been proposed. An earlier study in canines found increased tone in the genioglossus muscle when the gastrocnemius muscle and sciatic nerve were stimulated [44]. Later, two studies in humans raised some interest in the possible role of strength of respiratory muscles in relation to exercise [31, 45]. However, contrary to this hypothesis, Sengul et al. [21] found no change in the strength of respiratory muscles in OSA patients who received breathing and aerobic exercise training.
Some authors have also suggested that exercise can lead to decreased leg fluid accumulation and, hence, prevent the nocturnal rostral fluid shift that may be implicated in upper airway collapse [46–50]. This mechanism would more likely be associated with a potential acute effect of exercise rather than a training effect, which could nevertheless be important if repeated frequently. However, a recent study by Jafari and Mohsenin [51] casts some doubt on this study, as they found no progressive worsening in OSA despite a demonstrable fluid shift overnight.
Slow wave sleep (SWS) has been found to be associated with decreased severity of sleep apnea [52, 53]. In the study by McSharry et al. [53] this effect was thought to be related to the increased genioglossus single motor unit activity during SWS, making the airway more stable and resistant to collapse. It also is known that exercise training is associated with increased SWS [15]. Is it possible that the effects of exercise on reducing sleep apnea severity could be related to the protective effects of SWS that it induces?. Proving such a relationship in a well designed study would indeed be more insightful. However, it is possible that no single mechanism is responsible and perhaps there is a complex interplay of factors associated with exercise training that leads to improvement in the severity of sleep apnea.
Our meta-analysis has numerous strengths. First, our analysis had enough power to detect an effect of exercise intervention in the included studies. Given a total of 87 participants that received exercise intervention, we estimate that our meta-analysis had a power of 100 % to detect a change in AHI of 12.8 assuming a standard deviation of nine. Second, there was no evidence of publication bias by funnel plots. Third, our sensitivity analyses showed no significant change in the overall statistical significance of the results. Fourth, the analysis for the change in AHI based on the pre- and postintervention effects of exercise showed no significant heterogeneity. Finally, our study showed that significant effects from exercise training occurred despite the fact that studies differed in the mode, frequency, intensity, compliance, and levels of supervision of the exercise interventions.
However, there also are limitations to our study. Most of the studies had a small sample size and the length of intervention in most studies ranged from 12 to 24 weeks. Therefore, this meta-analysis does not address the long-term efficacy of exercise training on OSA severity. With so few studies thus far, it was not possible to evaluate which exercise characteristics were associated with more favorable OSA outcomes. It also is a limitation of our study that the data on secondary outcomes had moderate to high heterogeneity.
In conclusion, our meta-analysis demonstrates that exercise training leads to a significant reduction in AHI, improvement in sleep efficiency, and daytime sleepiness, independent of the effects on BMI. Although the effect size in AHI reduction seems smaller compared with CPAP and oral appliances, exercise training may be an ideal adjunct therapy, especially considering its effects on sleep efficiency and daytime sleepiness. Given the preliminary nature of our findings, RCTs involving a larger number of participants and longer duration of intervention are needed to determine whether the beneficial effects of exercise training in patients with sleep apnea can be sustained over a longer period of time.
Supplementary Material
Acknowledgment
This work was supported in part by HL78566 (to SDY) and HL082610 (to CEK).
Footnotes
Conflict of interest The authors report no potential conflicts of interest with any companies/organizations whose products or services may be discussed in this article.
Electronic supplementary material The online version of this article (doi:10.1007/s00408-013-9511-3) contains supplementary material, which is available to authorized users.
Contributor Information
Imran H. Iftikhar, Email: Imran.Iftikhar@uscmed.sc.edu, Division of Pulmonary, Critical Care and Sleep Medicine, Department of Medicine, University of South Carolina, One Richland Medical Park, Suite 300, Columbia, SC 29203, USA.
Christopher E. Kline, Email: klinec@upmc.edu, Department of Psychiatry, University of Pittsburgh School, of Medicine, Pittsburgh, PA, USA.
Shawn D. Youngstedt, Email: syoungst@mailbox.sc.edu, Departments of Exercise Science and Psychology, University of South Carolina, Columbia, SC, USA; WJB Dorn VA Medical Center, Columbia, SC, USA.
References
- 1.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165(9):1217–1239. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 2.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 3.Parish JM, Somers VK. Obstructive sleep apnea and cardiovascular disease. Mayo Clin Proc. 2004;79(8):1036–1046. doi: 10.4065/79.8.1036. [DOI] [PubMed] [Google Scholar]
- 4.Levinson PD, McGarvey ST, Carlisle CC, Eveloff SE, Herbert PN, Millman RP. Adiposity and cardiovascular risk factors in men with obstructive sleep apnea. Chest. 1993;103(5):1336–1342. doi: 10.1378/chest.103.5.1336. [DOI] [PubMed] [Google Scholar]
- 5.Kushida CA, Littner MR, Hirshkowitz M, Morgenthaler TI, Alessi CA, Bailey D, et al. Practice parameters for the use of continuous and bilevel positive airway pressure devices to treat adult patients with sleep-related breathing disorders. Sleep. 2006;29(3):375–380. doi: 10.1093/sleep/29.3.375. [DOI] [PubMed] [Google Scholar]
- 6.Kushida CA, Morgenthaler TI, Littner MR, Alessi CA, Bailey D, Coleman J, Jr, et al. Practice parameters for the treatment of snoring and obstructive sleep apnea with oral appliances: an update for 2005. Sleep. 2006;29(2):240–243. doi: 10.1093/sleep/29.2.240. [DOI] [PubMed] [Google Scholar]
- 7.Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284(23):3015–3021. doi: 10.1001/jama.284.23.3015. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz AR, Gold AR, Schubert N, Stryzak A, Wise RA, Per-mutt S, et al. Effect of weight loss on upper airway col-lapsibility in obstructive sleep apnea. Am Rev Respir Dis. 1991;144(3 Pt 1):494–498. doi: 10.1164/ajrccm/144.3_Pt_1.494. [DOI] [PubMed] [Google Scholar]
- 9.Smith PL, Gold AR, Meyers DA, Haponik EF, Bleecker ER. Weight loss in mildly to moderately obese patients with obstructive sleep apnea. Ann Intern Med. 1985;103(6 Pt 1):850–855. doi: 10.7326/0003-4819-103-6-850. [DOI] [PubMed] [Google Scholar]
- 10.Bridgman SA, Dunn KM. Surgery for obstructive sleep apnoea. Cochrane Database Syst Rev. 2000;2:CD001004. doi: 10.1002/14651858.CD001004. [DOI] [PubMed] [Google Scholar]
- 11.Sundaram S, Bridgman SA, Lim J, Lasserson TJ. Surgery for obstructive sleep apnoea. Cochrane Database Syst Rev. 2005;4:CD001004. doi: 10.1002/14651858.CD001004.pub2. [DOI] [PubMed] [Google Scholar]
- 12.Morgenthaler TI, Kapen S, Lee-Chiong T, Alessi C, Boehlecke B, Brown T, et al. Practice parameters for the medical therapy of obstructive sleep apnea. Sleep. 2006;29(8):1031–1035. [PubMed] [Google Scholar]
- 13.Aurora RN, Casey KR, Kristo D, Auerbach S, Bista SR, Chow-dhuri S, et al. Practice parameters for the surgical modifications of the upper airway for obstructive sleep apnea in adults. Sleep. 2010;33(10):1408–1413. doi: 10.1093/sleep/33.10.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamamoto U, Mohri M, Shimada K, Origuchi H, Miyata K, Ito K, et al. Six-month aerobic exercise training ameliorates central sleep apnea in patients with chronic heart failure. J Card Fail. 2007;13(10):825–829. doi: 10.1016/j.cardfail.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 15.Ueno LM, Drager LF, Rodrigues AC, Rondon MU, Braga AM, Mathias W, Jr, et al. Effects of exercise training in patients with chronic heart failure and sleep apnea. Sleep. 2009;32(5):637–647. doi: 10.1093/sleep/32.5.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quan SF, O’Connor GT, Quan JS, Redline S, Resnick HE, Shahar E, et al. Association of physical activity with sleep-disordered breathing. Sleep Breath. 2007;11(3):149–157. doi: 10.1007/s11325-006-0095-5. [DOI] [PubMed] [Google Scholar]
- 17.Awad KM, Malhotra A, Barnet JH, Quan SF, Peppard PE. Exercise is associated with a reduced incidence of sleep-disordered breathing. Am J Med. 2012;125(5):485–490. doi: 10.1016/j.amjmed.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peppard PE, Young T. Exercise and sleep-disordered breathing: an association independent of body habitus. Sleep. 2004;27(3):480–484. doi: 10.1093/sleep/27.3.480. [DOI] [PubMed] [Google Scholar]
- 19.Giebelhaus V, Strohl KP, Lormes W, Lehmann M, Netzer N. Physical exercise as an adjunct therapy in sleep apnea: an open trial. Sleep Breath. 2000;4(4):173–176. doi: 10.1007/s11325-000-0173-z. [DOI] [PubMed] [Google Scholar]
- 20.Kline CE, Crowley EP, Ewing GB, Burch JB, Blair SN, Durstine JL, et al. The effect of exercise training on obstructive sleep apnea and sleep quality: a randomized controlled trial. Sleep. 2011;34(12):1631–1640. doi: 10.5665/sleep.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sengul YS, Ozalevli S, Oztura I, Itil O, Baklan B. The effect of exercise on obstructive sleep apnea: a randomized and controlled trial. Sleep Breath. 2011;15(1):49–56. doi: 10.1007/s11325-009-0311-1. [DOI] [PubMed] [Google Scholar]
- 22.Hong S, Dimsdale JE. Physical activity and perception of energy and fatigue in obstructive sleep apnea. Med Sci Sports Exerc. 2003;35(7):1088–1092. doi: 10.1249/01.MSS.0000074566.94791.24. [DOI] [PubMed] [Google Scholar]
- 23.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 24.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65–W94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 25.Anandam A, Akinnusi M, Kufel T, Porhomayon J, El-Solh AA. Effects of dietary weight loss on obstructive sleep apnea: a meta-analysis. Sleep Breath. 2013;17(1):227–234. doi: 10.1007/s11325-012-0677-3. [DOI] [PubMed] [Google Scholar]
- 26.Thomasouli MA, Brady EM, Davies MJ, Hall AP, Khunti K, Morris DH, et al. The impact of diet and lifestyle management strategies for obstructive sleep apnoea in adults: a systematic review and meta-analysis of randomised controlled trials. Sleep Breath. 2013;17(3):925–935. doi: 10.1007/s11325-013-0806-7. [DOI] [PubMed] [Google Scholar]
- 27.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 28.Servantes DM, Pelcerman A, Salvetti XM, Salles AF, de Albuquerque PF, de Salles FC, et al. Effects of home-based exercise training for patients with chronic heart failure and sleep apnoea: a randomized comparison of two different programmes. Clin Rehabil. 2012;26(1):45–57. doi: 10.1177/0269215511403941. [DOI] [PubMed] [Google Scholar]
- 29.Kline CE, Crowley EP, Ewing GB, Burch JB, Blair SN, Durstine JL, et al. Blunted heart rate recovery is improved following exercise training in overweight adults with obstructive sleep apnea. Int J Cardiol. 2012;167(4):1610–1615. doi: 10.1016/j.ijcard.2012.04.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kline CE, Ewing GB, Burch JB, Blair SN, Durstine JL, Davis JM, et al. Exercise training improves selected aspects of daytime functioning in adults with obstructive sleep apnea. J Clin Sleep Med. 2012;8(4):357–365. doi: 10.5664/jcsm.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Norman JF, Von Essen SG, Fuchs RH, McElligott M. Exercise training effect on obstructive sleep apnea syndrome. Sleep Res Online. 2000;3(3):121–129. [PubMed] [Google Scholar]
- 32.Sutton AAK, Jones D, Sheldon T, Song F. Methods for meta-analysis in medical research Wiley series in probability and statistics. Wiley, New York: 2000. [Google Scholar]
- 33.Huedo-Medina TB, Sanchez-Meca J, Marin-Martinez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or 12 index? Psychol Methods. 2006;11(2):193–206. doi: 10.1037/1082-989X.11.2.193. [DOI] [PubMed] [Google Scholar]
- 34.Sterne JA, Egger M, Smith GD. Systematic reviews in health care: investigating and dealing with publication and other biases in meta-analysis. BMJ. 2001;323(7304):101–105. doi: 10.1136/bmj.323.7304.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. [PubMed] [Google Scholar]
- 36.Barnes M, Goldsworthy UR, Cary BA, Hill CJ. A diet and exercise program to improve clinical outcomes in patients with obstructive sleep apnea—a feasibility study. J Clin Sleep Med. 2009;5(5):409–415. [PMC free article] [PubMed] [Google Scholar]
- 37.Iftikhar IH, Hays ER, Iverson MA, Magalang UJ, Maas AK. Effect of oral appliances on blood pressure in obstructive sleep apnea: a systematic review and meta-analysis. J Clin Sleep Med. 2013;9(2):165–174. doi: 10.5664/jcsm.2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Greenburg DL, Lettieri CJ, Eliasson AH. Effects of surgical weight loss on measures of obstructive sleep apnea: a metaanalysis. Am J Med. 2009;122(6):535–542. doi: 10.1016/j.amjmed.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 39.Sui X, LaMonte MJ, Laditka JN, Hardin JW, Chase N, Hooker SP, et al. Cardiorespiratory fitness and adiposity as mortality predictors in older adults. JAMA. 2007;298(21):2507–2516. doi: 10.1001/jama.298.21.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rodway GW, Weaver TE, Mancini C, Cater J, Maislin G, Staley B, et al. Evaluation of sham-CPAP as a placebo in CPAP intervention studies. Sleep. 2010;33(2):260–266. doi: 10.1093/sleep/33.2.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McArdle N, Douglas NJ. Effect of continuous positive airway pressure on sleep architecture in the sleep apnea-hypopnea syndrome: a randomized controlled trial. Am J Respir Crit Care Med. 2001;164(8 Pt 1):1459–1463. doi: 10.1164/ajrccm.164.8.2008146. [DOI] [PubMed] [Google Scholar]
- 42.Giles TL, Lasserson TJ, Smith BH, White J, Wright J, Cates CJ. Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database Syst Rev. 2006;3:CD001106. doi: 10.1002/14651858.CD001106.pub3. [DOI] [PubMed] [Google Scholar]
- 43.Marshall NS, Barnes M, Travier N, Campbell AJ, Pierce RJ, McEvoy RD, et al. Continuous positive airway pressure reduces daytime sleepiness in mild to moderate obstructive sleep apnoea: a meta-analysis. Thorax. 2006;61(5):430–434. doi: 10.1136/thx.2005.050583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haxhiu MA, van Lunteren E, Mitra J, Cherniack NS, Strohl KP. Comparison of the responses of the diaphragm and upper airway muscles to central stimulation of the sciatic nerve. Respir Physiol. 1984;58(1):65–76. doi: 10.1016/0034-5687(84)90045-8. [DOI] [PubMed] [Google Scholar]
- 45.O’Donnell DE, McGuire M, Samis L, Webb KA. General exercise training improves ventilatory and peripheral muscle strength and endurance in chronic airflow limitation. Am J Respir Crit Care Med. 1998;157(5 Pt 1):1489–1497. doi: 10.1164/ajrccm.157.5.9708010. [DOI] [PubMed] [Google Scholar]
- 46.Mirrakhimov AE. Physical exercise related improvement in obstructive sleep apnea. Look for the rostral fluid shift. Med Hypotheses. 2013;80(2):125–128. doi: 10.1016/j.mehy.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 47.Redolfi S, Yumino D, Ruttanaumpawan P, Yau B, Su MC, Lam J, et al. Relationship between overnight rostral fluid shift and obstructive sleep apnea in nonobese men. Am J Respir Crit Care Med. 2009;179(3):241–246. doi: 10.1164/rccm.200807-1076OC. [DOI] [PubMed] [Google Scholar]
- 48.Redolfi S, Arnulf I, Pottier M, Bradley TD, Similowski T. Effects of venous compression of the legs on overnight rostral fluid shift and obstructive sleep apnea. Respir Physiol Neurobiol. 2011;175(3):390–393. doi: 10.1016/j.resp.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 49.Redolfi S, Arnulf I, Pottier M, Lajou J, Koskas I, Bradley TD, et al. Attenuation of obstructive sleep apnea by compression stockings in subjects with venous insufficiency. Am J Respir Crit Care Med. 2011;184(9):1062–1066. doi: 10.1164/rccm.201102-0350OC. [DOI] [PubMed] [Google Scholar]
- 50.Shiota S, Ryan CM, Chiu KL, Ruttanaumpawan P, Haight J, Arzt M, et al. Alterations in upper airway cross-sectional area in response to lower body positive pressure in healthy subjects. Thorax. 2007;62(10):868–872. doi: 10.1136/thx.2006.071183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jafari B, Mohsenin V. Overnight rostral fluid shift in obstructive sleep apnea: does it affect the severity of sleep-disordered breathing? Chest. 2011;140(4):991–997. doi: 10.1378/chest.11-0044. [DOI] [PubMed] [Google Scholar]
- 52.Ratnavadivel R, Chau N, Stadler D, Yeo A, McEvoy RD, Catcheside PG. Marked reduction in obstructive sleep apnea severity in slow wave sleep. J Clin Sleep Med. 2009;5(6):519–524. [PMC free article] [PubMed] [Google Scholar]
- 53.McSharry DG, Saboisky JP, Deyoung P, Matteis P, Jordan AS, Trinder J, et al. A mechanism for upper airway stability during slow wave sleep. Sleep. 2013;36(4):555–563. doi: 10.5665/sleep.2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.