Abstract
Purpose
In a recent Phase II clinical trial, low dose (100 mg/kg) gemcitabine showed promise as a radiosensitiser in bladder cancer but underlying mechanisms lack elucidation. Here we investigated the mechanism of radiosensitisation by low-dose gemcitabine in bladder cancer cell lines.
Experimental Design
Four bladder cancer cell lines were screened for radiosensitisation by low-dose gemcitabine using clonogenic assay, and gemcitabine-resistant RT112gem and CALgem cells created by exposure to increasing gemcitabine doses. Four key gemcitabine-regulatory genes were knocked down by transient siRNA. Nude mice carrying CALgem subcutaneous xenografts were exposed to 100 mg/kg gemcitabine+/−ionizing radiation (IR) and response assessed by tumour growth delay.
Results
Gemcitabine was cytotoxic in the low nanomolar range (10 to 40 nM) in four bladder cancer cell lines and radiosensitised all four lines. Sensitiser enhancement ratios (SER) at 10% survival were: RT112 1.42, CAL29 1.55, T24 1.63 and VMCUB1 1.47. Transient siRNA knockdown of deoxycytidine kinase (dCK) significantly reduced radiosensitisation by gemcitabine (P= 0.02). RT112gem and CALgem cells displayed robust decreases of dCK mRNA and protein levels; re-expression of dCK restored gemcitabine sensitivity. However, CALgem xenografts responded better to combination gemcitabine/IR than either treatment alone (P <0.001) with dCK strongly expressed in the tumour vasculature and stroma.
Conclusions
Gemcitabine resistance in bladder cancer cell lines was associated with decreased dCK expression, but gemcitabine-resistant xenografts were responsive to combination low-dose gemcitabine/IR. We propose that dCK activity in tumour vasculature renders it gemcitabine-sensitive, which is sufficient to invoke a tumour response and permit tumour cell kill in gemcitabine-resistant tumours.
Keywords: gemcitabine, bladder cancer, deoxycytidine kinase, gemcitabine resistance, tumour vasculature
Introduction
Bladder cancer is the 9th most common cancer worldwide and muscle-invasive disease can be treated by either radical cystectomy or radiotherapy, with similar outcomes (1). Addition of neoadjuvant chemotherapy or concurrent chemoradiotherapy modestly improves survival rates, but there is an urgent need to identify predictive biomarkers of response, to identify those patients best suited for a particular therapy.
The pyrimidine antimetabolite gemcitabine, a fluorinated analogue of cytidine (2′,2′-difluorodeoxycytidine, dFdC), has been used successfully as a single agent chemotherapy to treat many tumour types, including breast (2), pancreatic (3), ovarian (4) and bladder cancer (5) and is an effective radiosensitiser clinically (6-8). The primary mode of entry for gemcitabine in cancer cells is through the human equilibrative nucleoside transporter 1 (hENT1) (9), and after entering the cell requires phosphorylation to become the active antimetabolite (10). Gemcitabine can be deaminated and subsequently excreted as dFdU by the enzyme cytidine deaminase (CDA) (11); dFdU has also been reported as having radiosensitising effects on cells (12).
The rate-limiting enzyme responsible for conversion of gemcitabine to active antimetabolite is deoxycytidine kinase (dCK). This initial phosphorylation event leads to the rapid production of gemcitabine triphosphate, which competes with endogenous deoxycytidine triphosphate for incorporation into newly synthesised DNA (13). Gemcitabine triphosphate incorporation allows the addition of one further nucleotide before the blocked DNA polymerase is unable to add more nucleotides to the DNA strand (10, 14). This stalled replication is termed masked chain termination as the gemcitabine nucleotide is-not recognised as misincorporation and can lead to replication fork collapse and subsequent DNA double strand break formation.
Gemcitabine has also been reported to inhibit ribonucleotide reductase (RNR) (15), which converts nucleotide 5′-diphosphates to deoxynucleotide diphosphates, generating the nucleotides required for DNA synthesis and repair. Gemcitabine diphosphate (dFdCDP) binds irreversibly to the active site of RNR, thus inhibiting production of nucleotide di- and triphosphates, leading to a decrease in intracellular pools of endogenous nucleotides (14). This depletion feeds back to dCK and stimulates further phosphorylation of gemcitabine thus potentiating the effects of the drug.
In a recent bladder cancer Phase II clinical trial, weekly intravenous low-dose gemcitabine (100 mg/m2) was given as a radiosensitiser with promising results (16). Forty-six out of 50 patients recruited tolerated the treatment well, with a three-year cause specific survival of 82% and overall survival of 75%. However, the underlying mechanisms of radiosensitisation by low-dose gemcitabine have yet to be elucidated, so here we investigated the mechanisms both in vitro and in vivo.
Methods
Cell lines
CAL29, T24 and VMCUB1 cell lines were purchased from DSMZ-German collection of Microorganisms and Cell Cultures (ACC-515, ACC-400 and ACC-376). DSMZ authenticates lines by microsatellite short tandem repeat DNA typing (http://www.dsmz.de/catalogues/catalogue-human-and-animal-cell-lines/quality-assurance/identity-control/authentication-of-cell-lines.html). RT112 cells were a gift from Margaret Knowles, University of Leeds and were authenticated by extensive genomic analysis (microsatellite typing, conventional karyotypic analysis, M-FISH and array based copy number analysis). HUVECs were purchased from Lonza and grown in EGM-2 medium. 3T3 cells were a kind gift from Eric O’Neill and were verified by short tandem repeat profiling (DNA Diagnostics Centre, UK). RT112 and T24 cells were grown in RPMI medium (Sigma) supplemented with 10% foetal bovine serum (Invitrogen). CAL29, VMCUB1 and 3T3 were grown in Dulbecco’s modified Eagle medium (DMEM, Invitrogen), supplemented with 10% foetal bovine serum. All cells were maintained at 37°C in 5% CO2 in exponential growth phase. Resistant cell lines were generated over three months by gradually increasing doses of drug added to the medium, until cells reached resistance to 10 μM gemcitabine. These cell lines were named RT112gem and CALgem.
Western blots
Cells were processed for western blotting as outlined in Supplementary Methods. Antibodies used were rabbit polyclonal RRM1 10526-1-AP (ProteinTech Group), mouse monoclonal RRM2 MCA3434Z (AbD Serotec), rabbit polyclonal dCK ab96599 (Abcam), rabbit polyclonal CDA ab82347 (Abcam), rabbit polyclonal hENT1 ab48607 (Abcam), γH2AX 05-636 (Millipore), H2AX 07-627 (Millipore) and mouse monoclonal β actin ab8226 (Abcam).
Clonogenic assays
Cells (1 × 106) were seeded in duplicate onto 90 mm tissue culture dishes and treated with appropriate concentrations of gemcitabine 24 h later. After a further 24 h, cells were trypsinised, counted and replated onto 90 mm dishes immediately prior to IR treatment. Cells were irradiated with Cs-137 at a dose of 1.7 Gy min−1 in a GSR D1 irradiator (Gamma-Service Medical GmbH, Leipzig, Germany). After two weeks, cells were washed and fixed in 93% methanol, 7% acetic acid and stained with Coomassie blue (Sigma). Colonies containing >50 cells were counted using a Colcount automated cell counter (Oxford Optronix).
siRNA transfection
Cells (5 × 105) were plated 24 h prior to treatment with 50 nM siRNA (Invitrogen). BLOCK-iT Fluorescent Oligo (Invitrogen) was used to assess transfection efficiency (Supplementary Figure S1). siRNA was combined with Oligofectamine (Invitrogen) and added to serum-free medium for 4 h prior to replenishment with medium containing 10% v/v foetal bovine serum. Cells were then either harvested for western blot 48 h later, or trypsinised and replated for clonogenic assay. BLOCK-iT Fluorescent Oligo treated samples were fixed with 4% formaldehyde 6 h after transfection and transfection efficiency determined by fluorescence microscopy.
dCK overexpression
Full length dCK was cloned into pcDNA3.1 (Invitrogen) from cDNA generated from RT112 cells using the following primers: dCK F-5′-AAAGTCAAACCCCGACACCC-3′; dCK-R 5′-TTGGCTGCCTGTAGTCTTCAG-3′. Ten micrograms of DNA was transfected into subconfluent cells with 30 μl Fugene HD (Roche) and expression verified by western blot 24-48 h post transfection.
Flow cytometry
Cells were scraped in PBS 24 h after treatment with gemcitabine or dFdU and spun at 1000 rpm for 5 min. Cell pellets were resuspended in PBS containing 40 μg/ml propidium iodide (Sigma) and 100μg/ml RNase A, incubated at 37°C for 1 h and then analysed on a FACScan flow cytometer (Beckton Dickinson). Cell cycle phases were modelled using ModFit LT (Verity Software House).
Realtime qPCR
Relative quantitation of dCK in cell lines was measured using the ΔΔCt method with dCK TaqMan probe Hs00176127_m1 (catalogue number 785259, Applied Biosystems) and normalised to ACTB, using TaqMan probe Hs99999803_m1 (catalogue number 763630, Applied Biosystems). Samples were run on an Applied Biosystems 7500 Fast Real-Time PCR system.
Determination of deoxynucleotides by HPLC
Cells (2 × 107- 1 × 108) were treated with 20 nM gemcitabine for 24 h prior to trypsinisation, counting and extraction of deoxynucleotides with 100 μ1 cold 6% trichloroacetic acid. Samples were then processed for high performance liquid chromatography (HPLC) as outlined in Supplementary Methods.
Xenograft model
Cells (2 × 106 cells/ml) were prepared in phenol red free matrigel (BD Biosciences) and 50 μl injected into the flank of six week old female athymic nude mice (Harlan Laboratories). Tumours were measured daily with callipers, using the formula a × b × c × π/6 and were allowed to reach 100 mm3 before a single intraperitoneal injection of 100 mg/kg gemcitabine, followed by daily doses of 2 Gy ionising radiation (IR) for 5 consecutive days, as appropriate. All work was done in accordance with UK Home Office Guidelines and under project licence PPL 30/2922 at the University of Oxford.
Immunohistochemistry
A formalin-fixed paraffin-embedded human bladder tumour sample was purchased from ProteoGenex, Inc. Untreated CAL29 and CALgem xenografts (n=2 each) were also processed; the CAL29 xenografts were less than 100 mm3. Immunohistochemical staining was performed on 4 μm-thick formalin-fixed paraffin-embedded tissue sections using the Novolink Polymer Detection System (Novocastra Laboratories) and analysed as outlined in the Supplementary Methods. Rabbit polyclonal anti-dCK (Abcam, ab96599) was used at 1:100 dilution, rabbit polyclonal anti-CD31 (Abcam, ab28364) at 1:100 dilution, all incubated for 1 h at room temperature.
Statistical analysis
All statistical analyses were two-sided and the statistical analysis was performed using GraphPad Prism (version 4.0b; GraphPad Software Inc). Statistical significance was defined as P <0.05. Student’s t-test was used to compare the mean values between two groups and two-way analysis of variance used to compare drug and radiation interactions. Data are presented as mean values and standard error of the mean (SEM). Gemcitabine was determined to have a supra additive effect on all cell lines at doses of ionizing radiation of 4 Gy and above, using the isobologram technique previously described (17).
Results
Gemcitabine cytotoxicity and levels of key proteins in its metabolism differ between bladder cancer lines
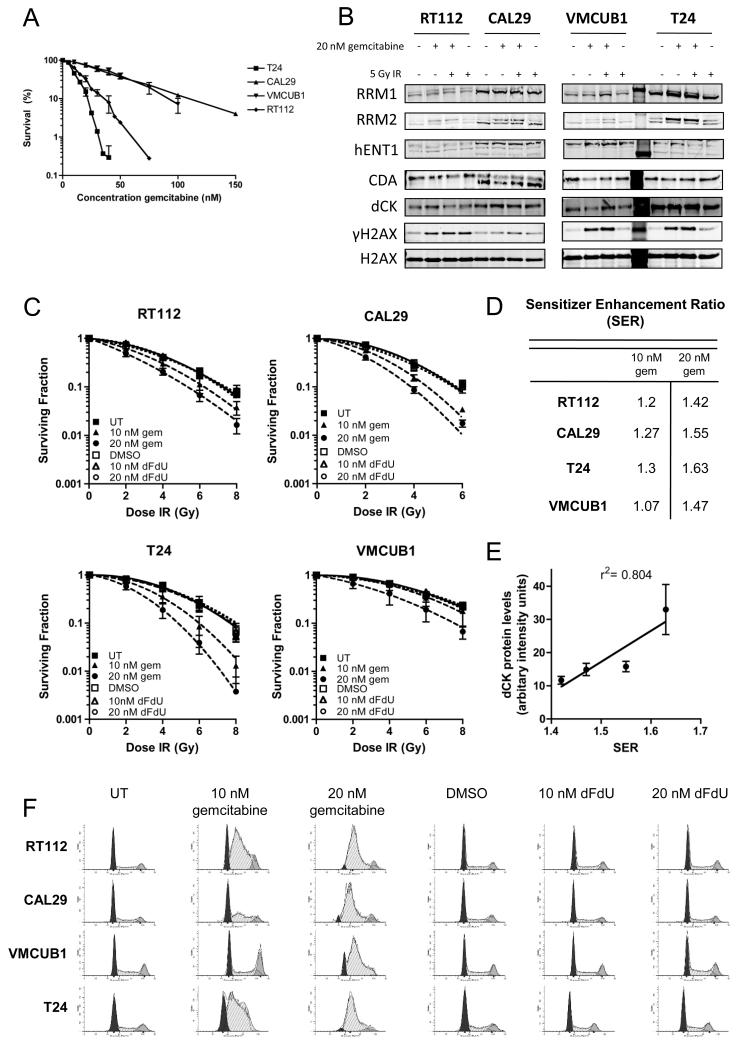
All four bladder cancer cell lines were sensitive to gemcitabine in the nanomolar range, with IC50’s of 14 nM for RT112 (95% confidence interval (CI) 12.6-14.5 nM), 10 nM for T24, (9.5-11.1 nM), 38 nM for CAL29 (34-41 nM) and 40 nM for VMCUB1 (35-46 nM), respectively, as determined by clonogenic assay (Figure 1A). There was no consistent pattern of changes in protein levels in five proteins described as key regulators of gemcitabine cellular uptake and metabolism across the cell lines in response to gemcitabine, IR and combination gemcitabine/IR by western blot (Figure 1B and Supplementary Figure S2).
Figure 1. Low dose gemcitabine is a radiosensitiser in bladder cancer cells.
A) Clonogenic assay to determine gemcitabine cytotoxicity in a panel of bladder cancer cell lines. Subconfluent cells were treated with 20 nM gemcitabine prior to plating 600-2000 cells per dish, depending on cell line, and colony formation measured 2 weeks later.
B) Western blots for proteins involved in the uptake and metabolism of gemcitabine (n=3). RT112, CAL29, T24 and VMCUB1 bladder cancer cell lines were treated with 20 nM gemcitabine for 24 h and with 5 Gy IR where appropriate, collected 2 h post treatment and immunoblotted for RRM1 and RRM2, hENT1, CDA, and dCK. Equal loading was confirmed by blotting for total H2AX and DNA damage was confirmed by blotting for γH2AX.
C) Cells were treated as in A) and subsequently treated with IR immediately after plating. Colonies were counted 2 weeks after plating.
D) Sensitiser enhancement ratios at 10% survival. P values for comparisons were calculated using two-way ANOVA and were as follows: RT112: UT vs 10 nM gem P= 0.008, UT vs 20 nM gem P <0.0001; CAL29: UT vs 10 nM gem P= 0.004, UT vs 20 nM P <0.0001; T24: UT vs 10 nM gem P= 0.002, UT vs 20 nM gem P< 0.0001; VMCUB1: UT vs 10 nM gem P= 0.315, UT vs 20 nM gem P= 0.0013.
E) Correlation between dCK protein levels and SER. dCK protein levels were determined by densitometry of western blots from four independent experiments. These values were plotted against SERs obtained from clonogenic assays performed in triplicate.
F) Cell cycle analysis of gemcitabine and dFdU treated bladder cancer cell lines. Cells in exponential growth phase were incubated with gemcitabine or dFdU for 24 h prior to fixing and staining with propidium iodide.
Radiosensitisation of each cell line was tested after 24 h treatment with 10 nM and 20 nM gemcitabine or equivalent concentrations of its metabolite dFdU, Figure 1C. dFdU caused no radiosensitisation (all P=NS) but gemcitabine radiosensitised all four cell lines effectively, with sensitiser enhancement ratios (SERs) ranging from 1.07 to 1.3 for 10 nM gem and 1.42 to 1.63 for 20 nM gem, Figure 1D (all P<0.01 except VMCUB1 untreated (UT) vs 10 nM gem P=0.315, see Figure 1D legend for exact P-values). Interestingly, dCK protein levels correlated with SER by linear regression (r2= 0.804) (Fig 1E). Gemcitabine treatment was associated with a large accumulation of cells in S phase, while dFdU had no impact on cell cycle (Figure 1F). The extent of S phase accumulation in the four cell lines was inversely correlated with gemcitabine IC50 (Supplementary Figure S3). These data suggest that gemcitabine radiosensitises bladder cancer cells through induction of stalled replication forks. In support of this, γH2AX induction was detectable by western blot following treatment of cells with gemcitabine and gemcitabine/IR, more so than for IR alone (Figure 1B).
siRNA knockdown of dCK reduces sensitivity to gemcitabine
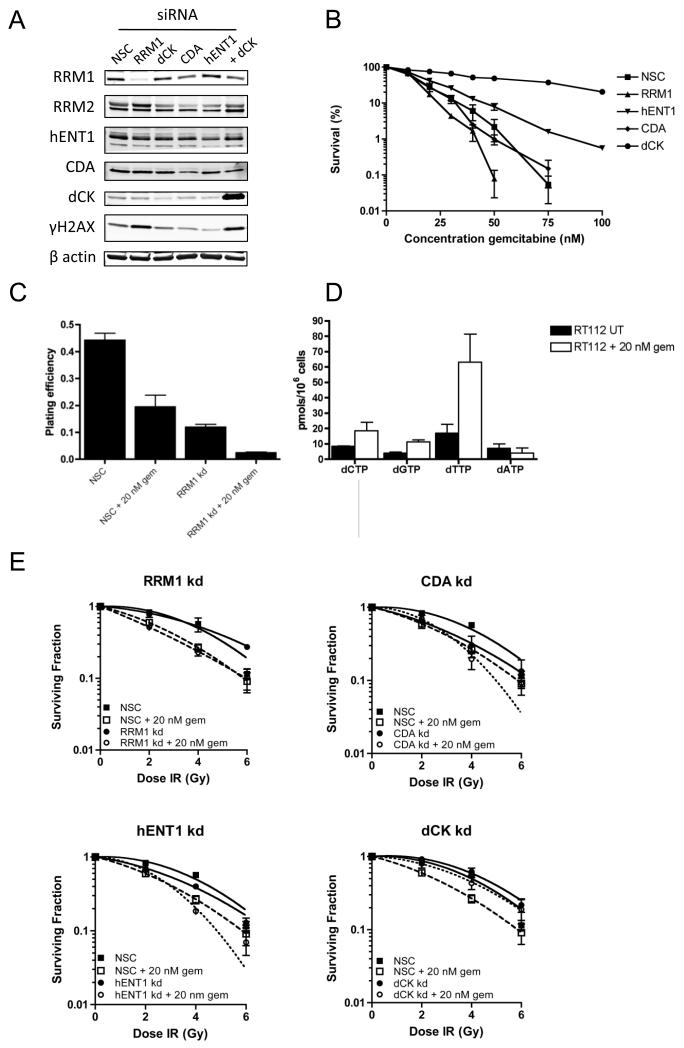
We hypothesised that altering expression levels of proteins involved in gemcitabine uptake and metabolism by siRNA knockdown would elucidate their relative importance in gemcitabine cytotoxicity and radiosensitisation. In the RT112 cell line, which had intermediate radiosensitisation by gemcitabine, we transiently knocked down RRM1, hENT1, CDA and dCK and determined effects on clonogenic cell survival (Figure 2A and B and Supplementary Figure S2). We assessed siRNA transfection efficiency by microscopy 6 h after transfection of a control, fluorescent siRNA and found that all cells analysed had taken up siRNA (Supplementary Figure S1).
Figure 2. Radiosensitisation effects of gemcitabine in RT112 cells treated with siRNAs to RRM1, hENT1, dCK and CDA.
A) Western blot analysis showing transient knockdown of RRM1, hENT1, CDA and dCK in the RT112 cell line (n=4). Knockdown was achieved using 50 nM siRNA and lysates prepared 24 h after transfection. B) Gemcitabine cytotoxicity clonogenic assay. Subconfluent cells previously treated with each siRNA were treated with increasing doses of gemcitabine for 24 h prior to reseeding at 1000 cells per 10 cm2 dish.
C) Clonogenic plating efficiency of cells treated with non-silencing control (NSC) siRNA or RRM1 siRNA in the presence and absence of 20 nM gemcitabine was calculated as the number of colonies containing ≥50 cells divided by the number of cell seeded. The graph displays mean plating efficiency from three independent experiments and error bars correspond to standard error of the mean (SEM).
D) HPLC analysis of deoxynucleotide triphosphate levels in RT112 untreated versus 24 h incubation with 20 nM gemcitabine. Values were calculated as mean intracellular concentration (pmoles) of dNTPs per 106 cells from two independent experiments, and error bars correspond to standard error of the mean (SEM).
E) Clonogenic assays performed as per Figure 1C. Calculated SERs are 1.3 for nonsilencing control (NSC) siRNA, 1.39 for RRM1 siRNA, 1.25 for CDA siRNA, 1.3 for hENT1 siRNA and 1.07 for dCK siRNA knockdown cells.
RRM1 of the RNR complex encodes the regulatory subunit, required by both RRM2 and RRM2B (a p53 inducible subunit) (18). Seventy percent knockdown of RRM1 had a large impact on cell viability (Figure 2C) but did not result in intrinsic radiosensitisation (P= 0.937) (Figure 2E). Gemcitabine was still able to induce radiosensitisation in RRM1 siRNA knockdown cells (P= 0.0003), indicating that the mechanism for radiosensitisation does not require RNR inhibition. Further evidence supporting lack of RNR inhibition in radiosensitisation of bladder cells was that deoxynucleotide levels in RT112 cells treated with 20 nM gemcitabine for 24 h did not show a significant decrease in deoxynucleotide pools (Figure 2D). In contrast, the most striking change in deoxynucleotide pools was a 3.6 fold increase in dTTP levels.
Knockdown of CDA to 30% of control levels had no effect on gemcitabine cytotoxicity (P=0.72) (Figure 2B), but reduction in CDA radiosensitised RT112 cells, even in the absence of gemcitabine (CDA siRNA vs NSC, P= 0.038) (Figure 2E). Reduction in hENT1 protein by 50% resulted in a slight increase in resistance to gemcitabine, with IC50 increasing from 14 nM to 18 nM, (P= 0.024, 95% CI 16.5-18.5 nM) (Figure 2B) but there was no significant change in radiosensitisation compared to non-silencing control (NSC), with 20 nM gemcitabine radiosensitising both cell lines (P <0.0001 for both hENT1 siRNA and NSC) (Figure 2E). However, a 70% reduction in dCK protein induced resistance to gemcitabine, with an increase in IC50 from 14 nM gemcitabine to 47.5 nM (P <0.0001, 95% CI 31 nM-56 nM) (Fig 2B). Knockdown of dCK also significantly reduced the radiosensitising effect of gemcitabine (P= 0.023) (Figure 2E). Complete knockdown of dCK was not achieved (Figure 2A) and this is likely to account for some of the radiosensitisation seen with 20 nM gemcitabine.
Resistance to gemcitabine is achieved by reduction of dCK
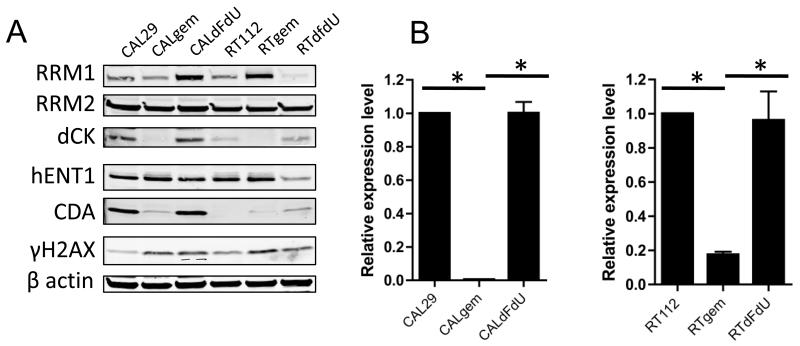
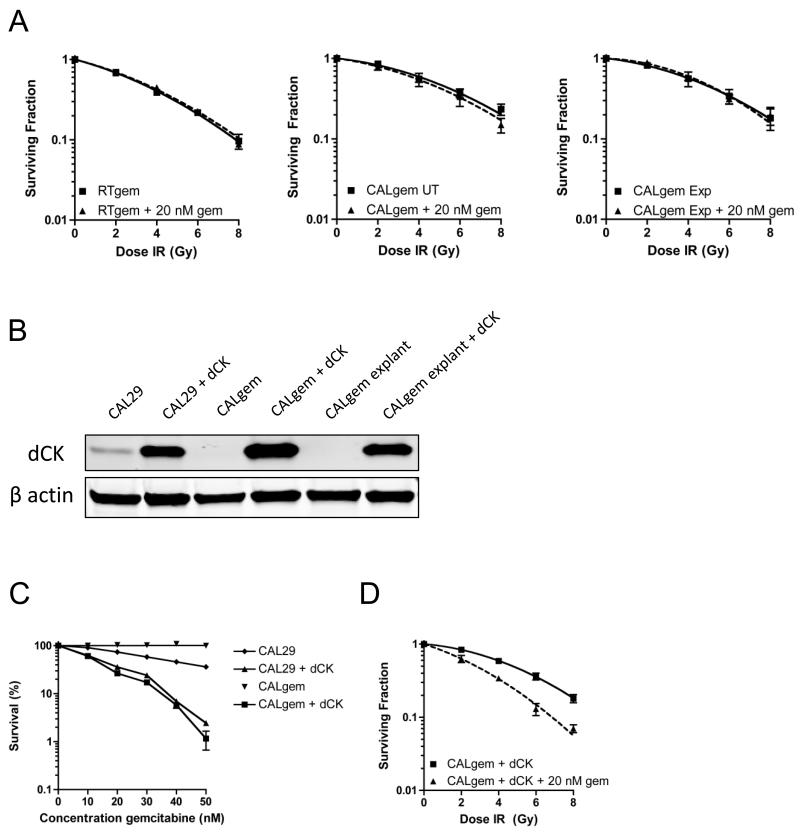
We generated RT112 and CAL29 cell lines resistant to gemcitabine and to dFdU by gradually increasing the dose of each nucleoside over several passages. Having found no radiosensitisation by dFdU, we reasoned that changes in expression of proteins seen in the gemcitabine resistant cells that did not occur in the dFdU resistant cells would be of greatest interest. In both gemcitabine resistant cell lines (RTgem and CALgem), a marked reduction in dCK was observed, consistent with this enzyme being the rate-limiting factor for gemcitabine metabolism to active antimetabolite (Figure 3A). Reduction in dCK protein level was commensurate with a significant reduction at the mRNA level, as measured by quantitative real time PCR (RT112 vs RTgem, P <0.0001, CAL29 vs CALgem P<0.0001)(Figure 3B). The reduction in dCK levels in both gemcitabine resistant cell lines rendered them refractive to the radiosensitising effects of 20 nM gemcitabine (RTgem vs RTgem + 20 nM gem P= 0.45, CALgem vs CALgem + 20 nM gem, P=0.31) (Figure 4A). Re-expression of dCK in the CALgem cell line (Figure 4B) was sufficient to re-sensitise these cells to gemcitabine, and was also sufficient to re-establish gemcitabine radiosensitivity, highlighting the importance of this enzyme in the response to gemcitabine (Figure 4C and D).
Figure 3. dCK expression in gemcitabine resistant bladder cancer cell lines.
A) Western blot of protein expression in CAL29 and RT112 cells treated with increasing doses of either gemcitabine or dFdU over several passages until resistant to 10 μM drug (n=2).
B) Quantitative PCR of dCK expression in gemcitabine resistant cells at the mRNA level (* denotes significance of P < 0.001).
Figure 4. dCK is required for gemcitabine sensitivity in RTgem and CALgem bladder cancer cell lines.
A) Radiation clonogenic survival curves. RTgem (left panel), CALgem (middle panel), (resistant to 10 μM gemcitabine), and CALgem explant (right panel) cell lines were treated with 20 nM gemcitabine for 24 h prior to re-plating and irradiation.
B) Western blot for expression of dCK in CAL29, CALgem and CALgem explant cell lines and β tubulin serves as a marker for equal loading (n=4).
C) Gemcitabine cytotoxicity clonogenic assay.
D) Cells treated as for Figure 1C. Colonies were counted 2 weeks after plating.
Gemcitabine resistant xenografts still respond to gemcitabine with IR
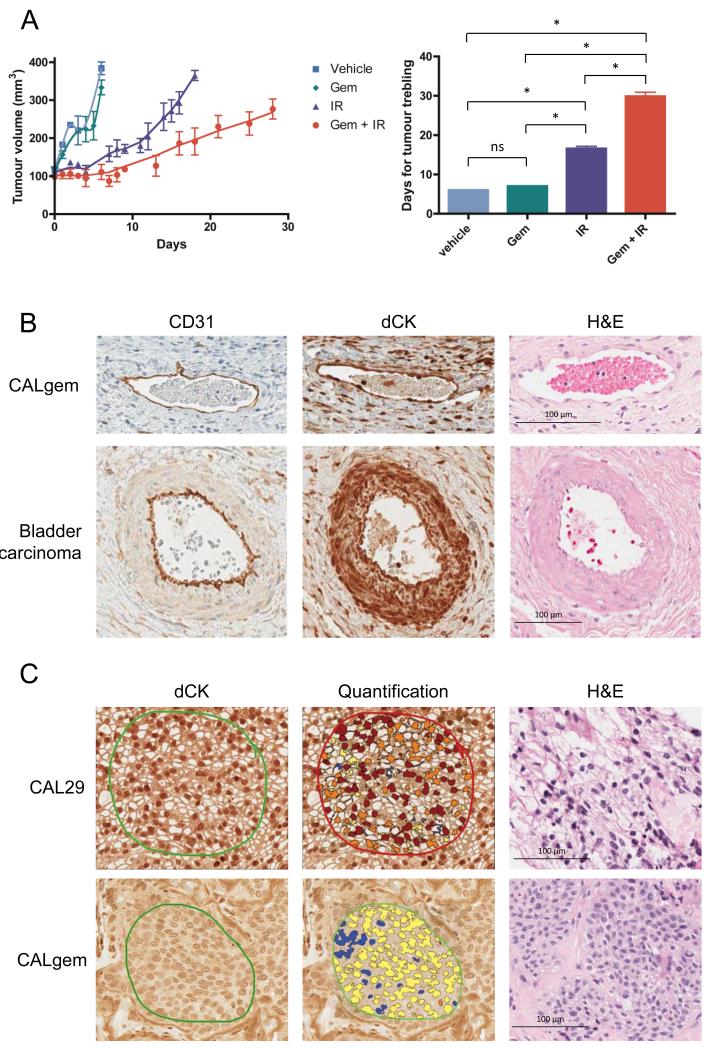
CAL29 and CALgem cells were implanted into the flanks of mice. In line with a previously published study (19), we found CAL29 xenografts to be poorly tumourigenic and an insufficient number of xenografts reached the size required for treatment. CALgem xenografts were allowed to grow to a size of 100 mm3 before being treated with daily IR, gemcitabine single dose or IR/gemcitabine at a gemcitabine dose of 100 mg/kg. Unexpectedly, xenografts treated with combination IR/gemcitabine had a significant growth delay, compared to the xenograft treated with IR alone (29.9 days to triple volume for combination IR/gemcitabine versus 16.6 days for IR alone (95% CIs 27.3-32.4 days versus 15.2-18 days, P=0.002), Figure 5A). After mouse sacrifice, tumour cells were grown out in vitro as explants in 10 μM gemcitabine, and clonogenic survival assay demonstrated no radiosensitisation with 20 nM gemcitabine (Figure 4A). Western blot confirmed that protein levels of dCK remained undetectable in these explants (Figure 4B). This intriguing result led us to study dCK expression in the tumour vasculature, in parallel with CD31, an endothelial marker used to assess tissue angiogenesis. We found strong positive nuclear staining for dCK in tumour stroma and the vasculature in both CALgem mouse xenografts and a human muscle-invasive bladder tumour (Figure 5B); this coincided with CD31 positivity in the vessel linings. Quantitative analysis of three independently stained slides from one CAL29 xenograft were strongly positive for dCK in 70% of nuclei within the tumour and in 66% of nuclei in the stroma and vasculature, whereas in three replicates of a CALgem xenograft only 18% of tumour cell nuclei were dCK positive with 61% positive nuclei in the stroma and vasculature (Figure 5C and Supplementary Table S1).
Figure 5. Tumour growth delay after gemcitabine and ionising radiation.
A) Mice bearing subcutaneous gemcitabine resistant CALgem xenografts were randomly assigned to vehicle or treatment (gemcitabine, 5 consecutive days of 2 Gy ionising radiation (IR), or gemcitabine and 5 × 2 Gy IR) groups when tumour volumes were approximately 100 mm3 and tumours were measured every 1-3 days. Mice were sacrificed when their tumour volume reached 400 mm3. (* denotes significance <0.001)
B) Immunohistochemical analysis of xenograft tumour vasculature. Thin walled vessel stained using CD31 (left panels), dCK (middle panels) and haematoxylin and eosin staining (right panels) in CALgem xenograft (top panels) and a larger vessel in human stage T2 muscle invasive bladder tumour (bottom panels).
C) Immunohistochemical analysis for dCK (left and middle panels) and haematoxylin and eosin (right panels) of xenografts from CAL29 cells (top panels) and CALgem (bottom panels). Middle panels represent dCK immunohistochemical scoring of nuclei using the Aperio nuclear v9 algorithm, performed for three independently stained replicates of the same tumours.
Gemcitabine radiosensitises mouse fibroblasts and human vascular endothelial cells
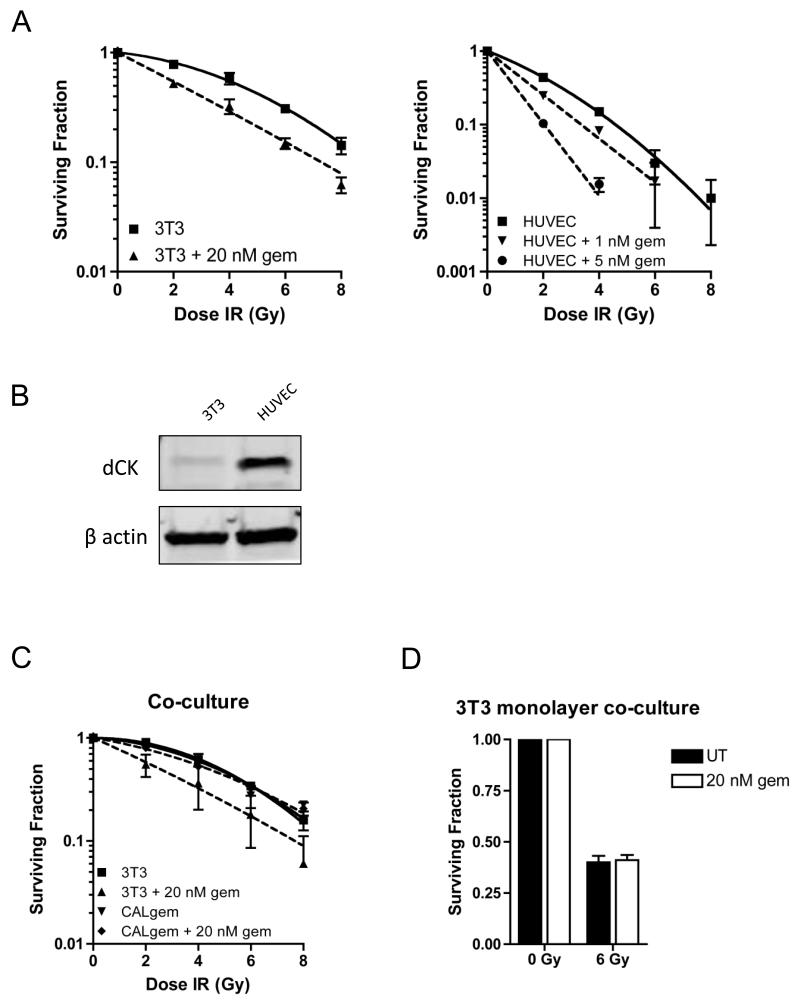
Having established that the CALgem explants had remained dCK negative and were refractive to gemcitabine, we tested the ability of gemcitabine to radiosensitise 3T3 mouse fibroblasts and HUVECs, as in vitro models for xenograft stroma and vasculature respectively. Gemcitabine radiosensitised 3T3 cells, with an SER of 1.29 for 20 nM gemcitabine. We found 20 nM gemcitabine to be almost completely inhibitory for colony formation in HUVECs (Supplementary Figure S4), but found that a dose as low as 1 nM gemcitabine was sufficient to radiosensitise these cells with an SER of 1.39 and SER increased to 1.98 with 5 nM gemcitabine (Figure 6A). Western blot demonstrated stronger expression of dCK in HUVECs than in 3T3 cells, consistent with our finding that dCK levels correlate with SER. We then sought to establish if stromal response to gemcitabine could influence the sensitivity of CALgem cells by performing a co-culture assay. We found that in this assay, 3T3 cells were still radiosensitised by gemcitabine, but that colony formation of CALgem cells was completely unaffected (Figure 6C). Following this, we plated a monolayer of 3T3 cells which were treated with and without 20 nM gemcitabine, upon which we seeded CALgem. Similarly, there was no significant difference in colony formation of CALgem cells using this method (Figure 6D).
Figure 6. Gemcitabine radiosensitises mouse fibroblasts and human vascular endothelial cells.
A) Radiation clonogenic survival curves. 3T3 cells (left panel) were treated with 20 nM gemcitabine for 24 h prior to re-plating and irradiation. HUVECs (right panel) were treated with 1 nM and 5 nM gemcitabine for 24 h prior to re-plating and irradiation.
B) Western blot for expression of dCK in 3T3 and HUVEC cell lines and β actin serves as a marker for equal loading (n=2).
C) Co-culture radiation clonogenic survival curves. 3T3 and CALgem cells were treated with 20 nM gemcitabine separately for 24 h, before being replated together and irradiated.
D) Co-culture clonogenic survival. 3T3 cells were grown as a monolayer, prior to treatment with 20 nM gemcitabine for 24 h. CALgem cells were subsequently seeded on top of 3T3 cells and irradiated.
Discussion
Gemcitabine is an effective radiosensitiser in muscle-invasive bladder cancer but also carries side effects including diarrhoea which can be dose-limiting (16). An understanding of the mechanisms of action of gemcitabine as a radiosensitiser and the enzymes involved in its metabolism might help drive the search for appropriate biomarkers of patient response, allowing optimum selection of patients for this combined treatment. We have shown that gemcitabine radiosensitises bladder cancer cells at low nanomolar concentrations and the primary mode of radiosensitisation appears to be via the activity of deoxycytidine kinase (dCK).
hENT1 knockdown to 50% of control levels only resulted in a small increase in gemcitabine resistance and did not significantly alter its radiosensitising effects. However, in vitro expression of hENT1 does increase sensitivity to gemcitabine (9, 20) and a recent study has found that high hENT1 expression in bladder tumour cells is a prognostic biomarker for prolonged survival after gemcitabine treatment (21). In pancreatic cancer, high hENT1 has been identified as a predictive marker for response to gemcitabine (22). Although siRNA knockdown of hENT1 had a limited effect on gemcitabine cytotoxicity or radiosensitisation, it should be noted that knockdown in our study was not particularly effective (approximately 50%); this may be sufficient to allow entry of low concentration gemcitabine.
RNR inhibition had a significant impact on cell viability (Figure 2C) but 70% reduction of RRM1 by siRNA did not significantly alter the radiosensitising effects of gemcitabine. The decreased plating efficiency of RRM1 knockdown cells might argue that gemcitabine does not inhibit RNR at low concentration and that, whilst RNR is important for cell viability, it is not a major contributor to the radiosensitisation caused by gemcitabine. Moreover, RRM1 knockdown itself does not lead to radiosensitisation of these cells, while gemcitabine still radiosensitises, so RRM1 inhibition does not appear to be contributing to this radiosensitisation.
RRM2 inhibition with the HDM-2 inhibitor or knockdown with siRNA synergistically enhances gemcitabine cytotoxicity, which also suggests that gemcitabine effects on RNR are not major (23, 24). Whilst we did not observe dramatic reduction in deoxynucleotide pools, we did observe a 3.6 fold increase in dTTP levels. This may be due to upregulation of thymidine kinase 1 (TK1), which has been shown to be upregulated during S phase (25) and after genotoxic stress (26). Interestingly, it has been observed that nucleotide pool perturbations cause genomic instability (27, 28) and in particular elevated dTTP levels are sufficient to cause instability (29).
Knockdown of CDA to 30% of control levels was sufficient to radiosensitise cells, even in the absence of gemcitabine, while addition of gemcitabine further radiosensitised cells. This implies that CDA is important in maintaining normal nucleotide pool balance, but given that depletion of CDA did not increase the SER of gemcitabine, we hypothesise that deamination of gemcitabine is not a major negative regulator of gemcitabine activity at low dose.
Knockdown of dCK did not fully abolish radiosensitisation by gemcitabine, but this is likely due to only partial knockdown, calculated to be a 70% reduction compared to control cells (Figure 2A and Supplementary Figure S2). However, potent reduction in dCK levels in gemcitabine-resistant cells was observed at both the level of protein and mRNA expression and these cells were not radiosensitised by gemcitabine (Figure 3A). Plasmid-based overexpression of dCK in resistant cells was sufficient to restore gemcitabine sensitivity, indicating that dCK is of key importance in the response to gemcitabine, as observed by others (30-33).
Surprisingly, in a xenograft model, gemcitabine resistant tumours responded better to combination gemcitabine and irradiation than either treatment alone. This result was unexpected, as the gemcitabine resistant cells (CALgem) had been shown not to be radiosensitised by gemcitabine in vitro (Figure 4A). This pointed towards the possibility that the vasculature/stroma formed within the tumour remained dCK positive and that perhaps radiosensitisation of the vasculature by gemcitabine is sufficient to cause tumour kill. This hypothesis is strengthened by our immunohistochemical evidence of dCK colocalisation with CD31 staining. Our cell line experiments investigating stromal and vascular sensitivity to gemcitabine also favour this model. Furthermore, our co-culture experiments suggest that radiosensitisation of gemcitabine-resistant tumours is not a direct effect caused by a diffusible stromal factor but is an indirect effect mediated by elevated stromal dCK and thus radiosensitisation of the stroma, ultimately leading to tumour cell killing.
This finding warrants further investigation, as it implies that patients may respond to gemcitabine/IR even when their tumour is gemcitabine-resistant. We are now measuring gemcitabine and metabolite levels in blood and urine from patients receiving gemcitabine/IR (http://www.clinicaltrials.gov/ct2/show/NCT01343121) to look for prognostic biomarkers, and will study dCK expression by IHC in their pretreatment tumour specimens. It will be of particular interest to study dCK expression in the tumour vasculature and/or stroma as a potential biomarker of treatment outcome.
Supplementary Material
Translational relevance.
Gemcitabine has efficacy as a radiosensitiser in bladder cancer but with some toxic side effects, so biomarkers need to be found to optimise patient selection for this combination. We found that gemcitabine-resistant bladder cancer cells expressed reduced levels of the metabolising enzyme deoxycytidine kinase (dCK), associated with abrogation of the radiosensitising effects of gemcitabine. However, in mouse, xenografts from human gemcitabine-resistant bladder cancer cells displayed significant growth delay, but with high expression of dCK retained by the tumour vasculature and stroma. We hypothesise that radiosensitisation of the vasculature is sufficient to achieve tumour cell kill. This preclinical observation implies that patients may respond to gemcitabine/radiotherapy even when their tumour is gemcitabine-resistant, and suggests that dCK expression in the tumour vasculature and/or stroma, rather than in the tumour, may be a biomarker predictive of treatment outcome.
Acknowledgments
Financial support: This work was supported by Cancer Research UK (grant numbers C5255/A15935 to AEK, C38302/A13717 to NLS and HS and the Oxford Cancer Research Centre Development Fund (grant number C38302/A12278 to NLS).
Footnotes
Conflict of interest: none to declare
References
- 1.Kotwal S, Choudhury A, Johnston C, Paul AB, Whelan P, Kiltie AE. Similar treatment outcomes for radical cystectomy and radical radiotherapy in invasive bladder cancer treated at a United Kingdom specialist treatment center. Int J Radiat Oncol Biol Phys. 2008;70(2):456–63. doi: 10.1016/j.ijrobp.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 2.Silvestris N, Cinieri S, La Torre I, Pezzella G, Numico G, Orlando L, et al. Role of gemcitabine in metastatic breast cancer patients: a short review. Breast. 2008;17(3):220–6. doi: 10.1016/j.breast.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 3.Eckel F, Schneider G, Schmid RM. Pancreatic cancer: a review of recent advances. Expert Opin Investig Drugs. 2006;15(11):1395–410. doi: 10.1517/13543784.15.11.1395. [DOI] [PubMed] [Google Scholar]
- 4.Lorusso D, Ferrandina G, Fruscella E, Marini L, Adamo V, Scambia G. Gemcitabine in epithelial ovarian cancer treatment: current role and future perspectives. Int J Gynecol Cancer. 2005;15(6):1002–13. doi: 10.1111/j.1525-1438.2005.00331.x. [DOI] [PubMed] [Google Scholar]
- 5.Shelley MD, Cleves A, Wilt TJ, Mason MD. Gemcitabine chemotherapy for the treatment of metastatic bladder carcinoma. BJU Int. 2011;108(2):168–79. doi: 10.1111/j.1464-410X.2011.10341.x. [DOI] [PubMed] [Google Scholar]
- 6.Blackstock AW, Mornex F, Partensky C, Descos L, Case LD, Melin SA, et al. Adjuvant gemcitabine and concurrent radiation for patients with resected pancreatic cancer: a phase II study. Br J Cancer. 2006;95(3):260–5. doi: 10.1038/sj.bjc.6603270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loehrer PJ, Sr., Feng Y, Cardenes H, Wagner L, Brell JM, Cella D, et al. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: an Eastern Cooperative Oncology Group trial. J Clin Oncol. 2011;29(31):4105–12. doi: 10.1200/JCO.2011.34.8904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pauwels B, Korst AE, Lardon F, Vermorken JB. Combined modality therapy of gemcitabine and radiation. Oncologist. 2005;10(1):34–51. doi: 10.1634/theoncologist.10-1-34. [DOI] [PubMed] [Google Scholar]
- 9.Mackey JR, Mani RS, Selner M, Mowles D, Young JD, Belt JA, et al. Functional nucleoside transporters are required for gemcitabine influx and manifestation of toxicity in cancer cell lines. Cancer Res. 1998;58(19):4349–57. [PubMed] [Google Scholar]
- 10.Plunkett W, Huang P, Xu YZ, Heinemann V, Grunewald R, Gandhi V. Gemcitabine: metabolism, mechanisms of action, and self-potentiation. Semin Oncol. 1995;22(4 Suppl 11):3–10. [PubMed] [Google Scholar]
- 11.Heinemann V, Xu YZ, Chubb S, Sen A, Hertel LW, Grindey GB, et al. Cellular elimination of 2′,2′-difluorodeoxycytidine 5′-triphosphate: a mechanism of self-potentiation. Cancer Res. 1992;52(3):533–9. [PubMed] [Google Scholar]
- 12.Pauwels B, Korst AE, Lambrechts HA, Pattyn GG, de Pooter CM, Lardon F, et al. The radiosensitising effect of difluorodeoxyuridine, a metabolite of gemcitabine, in vitro. Cancer Chemother Pharmacol. 2006;58(2):219–28. doi: 10.1007/s00280-005-0158-5. [DOI] [PubMed] [Google Scholar]
- 13.Huang P, Chubb S, Hertel LW, Grindey GB, Plunkett W. Action of 2′,2′-difluorodeoxycytidine on DNA synthesis. Cancer Res. 1991;51(22):6110–7. [PubMed] [Google Scholar]
- 14.Plunkett W, Huang P, Gandhi V. Preclinical characteristics of gemcitabine. Anticancer Drugs. 1995;6(Suppl 6):7–13. doi: 10.1097/00001813-199512006-00002. [DOI] [PubMed] [Google Scholar]
- 15.Heinemann V, Xu YZ, Chubb S, Sen A, Hertel LW, Grindey GB, et al. Inhibition of ribonucleotide reduction in CCRF-CEM cells by 2′,2′-difluorodeoxycytidine. Mol Pharmacol. 1990;38(4):567–72. [PubMed] [Google Scholar]
- 16.Choudhury A, Swindell R, Logue JP, Elliott PA, Livsey JE, Wise M, et al. Phase II study of conformal hypofractionated radiotherapy with concurrent gemcitabine in muscle-invasive bladder cancer. J Clin Oncol. 2011;29(6):733–8. doi: 10.1200/JCO.2010.31.5721. [DOI] [PubMed] [Google Scholar]
- 17.Steel GG, Peckham MJ. Exploitable mechanisms in combined radiotherapy-chemotherapy: the concept of additivity. Int J Radiat Oncol Biol Phys. 1979;5(1):85–91. doi: 10.1016/0360-3016(79)90044-0. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka H, Arakawa H, Yamaguchi T, Shiraishi K, Fukuda S, Matsui K, et al. A ribonucleotide reductase gene involved in a p53-dependent cell-cycle checkpoint for DNA damage. Nature. 2000;404(6773):42–9. doi: 10.1038/35003506. [DOI] [PubMed] [Google Scholar]
- 19.Cattan N, Rochet N, Mazeau C, Zanghellini E, Mari B, Chauzy C, et al. Establishment of two new human bladder carcinoma cell lines, CAL 29 and CAL 185. Comparative study of cell scattering and epithelial to mesenchyme transition induced by growth factors. Br J Cancer. 2001;85(9):1412–7. doi: 10.1054/bjoc.2001.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mackey JR, Yao SY, Smith KM, Karpinski E, Baldwin SA, Cass CE, et al. Gemcitabine transport in xenopus oocytes expressing recombinant plasma membrane mammalian nucleoside transporters. J Natl Cancer Inst. 1999;91(21):1876–81. doi: 10.1093/jnci/91.21.1876. [DOI] [PubMed] [Google Scholar]
- 21.Matsumura N, Hara I. [The prognostic significance of human equilibrative nucleoside transporter1 (hENT1) expression in metastatic bladder cancer patients treated with gemcitabine-cisplatin based combination chemotherapy] Hinyokika Kiyo. 2011;57(3):157–61. [PubMed] [Google Scholar]
- 22.Greenhalf W, Ghaneh P, Neoptolemos JP, Palmer DH, Cox TF, Lamb RF, et al. Pancreatic Cancer hENT1 Expression and Survival From Gemcitabine in Patients From the ESPAC-3 Trial. J Natl Cancer Inst. 2014;106(1):djt347. doi: 10.1093/jnci/djt347. [DOI] [PubMed] [Google Scholar]
- 23.Duxbury MS, Ito H, Zinner MJ, Ashley SW, Whang EE. RNA interference targeting the M2 subunit of ribonucleotide reductase enhances pancreatic adenocarcinoma chemosensitivity to gemcitabine. Oncogene. 2004;23(8):1539–48. doi: 10.1038/sj.onc.1207272. [DOI] [PubMed] [Google Scholar]
- 24.Jones RJ, Baladandayuthapani V, Neelapu S, Fayad LE, Romaguera JE, Wang M, et al. HDM-2 inhibition suppresses expression of ribonucleotide reductase subunit M2, and synergistically enhances gemcitabine-induced cytotoxicity in mantle cell lymphoma. Blood. 2011;118(15):4140–9. doi: 10.1182/blood-2011-03-340323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bello LJ. Regulation of thymidine kinase synthesis in human cells. Exp Cell Res. 1974;89(2):263–74. doi: 10.1016/0014-4827(74)90790-3. [DOI] [PubMed] [Google Scholar]
- 26.Chen YL, Eriksson S, Chang ZF. Regulation and functional contribution of thymidine kinase 1 in repair of DNA damage. J Biol Chem. 2010;285(35):27327–35. doi: 10.1074/jbc.M110.137042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.D’Angiolella V, Donato V, Forrester FM, Jeong YT, Pellacani C, Kudo Y, et al. Cyclin F-mediated degradation of ribonucleotide reductase M2 controls genome integrity and DNA repair. Cell. 2012;149(5):1023–34. doi: 10.1016/j.cell.2012.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bester AC, Roniger M, Oren YS, Im MM, Sarni D, Chaoat M, et al. Nucleotide deficiency promotes genomic instability in early stages of cancer development. Cell. 2011;145(3):435–46. doi: 10.1016/j.cell.2011.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ke PY, Kuo YY, Hu CM, Chang ZF. Control of dTTP pool size by anaphase promoting complex/cyclosome is essential for the maintenance of genetic stability. Genes Dev. 2005;19(16):1920–33. doi: 10.1101/gad.1322905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Bree C, Castro Kreder N, Loves WJ, Franken NA, Peters GJ, Haveman J. Sensitivity to ionizing radiation and chemotherapeutic agents in gemcitabine-resistant human tumor cell lines. Int J Radiat Oncol Biol Phys. 2002;54(1):237–44. doi: 10.1016/s0360-3016(02)02891-2. [DOI] [PubMed] [Google Scholar]
- 31.Ruiz van Haperen VW, Veerman G, Eriksson S, Boven E, Stegmann AP, Hermsen M, et al. Development and molecular characterization of a 2′,2′-difluorodeoxycytidine-resistant variant of the human ovarian carcinoma cell line A2780. Cancer Res. 1994;54(15):4138–43. [PubMed] [Google Scholar]
- 32.Galmarini CM, Clarke ML, Jordheim L, Santos CL, Cros E, Mackey JR, et al. Resistance to gemcitabine in a human follicular lymphoma cell line is due to partial deletion of the deoxycytidine kinase gene. BMC Pharmacol. 2004;4:8. doi: 10.1186/1471-2210-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakano Y, Tanno S, Koizumi K, Nishikawa T, Nakamura K, Minoguchi M, et al. Gemcitabine chemoresistance and molecular markers associated with gemcitabine transport and metabolism in human pancreatic cancer cells. Br J Cancer. 2007;96(3):457–63. doi: 10.1038/sj.bjc.6603559. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.