Abstract
The importance of studying angiogenesis, the formation of new blood vessels from pre-existing vessels, is underscored by its involvement in both normal physiology, such as embryonic growth and wound healing, and pathologies, such as diabetes and cancer. Treatments targeting the molecular drive of angiogenesis have been developed, but many of the molecular mechanisms that mediate vascularization, as well as how these mechanisms can be targeted in therapy, remain poorly understood. The limited capacity to quantify angiogenesis properly curtails our molecular understanding and development of new drugs and therapies. Although there are a number of assays for angiogenesis, many of them strip away its important components and/or limit control of the variables that direct this highly cooperative and complex process. Here we review assays commonly used in endothelial cell biology and describe the progress toward development of a physiologically realistic platform that will enable a better understanding of the molecular and physical mechanisms that govern angiogenesis.
Keywords: endothelial cells, tubulogenesis, sprouting, growth factors, vessels, disease
Introduction
Angiogenesis is a complex and highly regulated process that plays a role in both physiological and pathological conditions. During angiogenesis, vascular sprouts from a preexisting blood supply subsequently mature into stabile vasculature that supplies local oxygen and nutrient demands. In the course of angiogenesis, endothelial cells need to escape quiescence, proliferate, migrate, and undergo tubulogenesis in order to form functional vessels. While all of the molecular events of angiogenesis have not been conclusively defined, angiogenic sprouting, vessel formation, adaptation to tissue needs, and stabilization can be considered its four major sequential events. Non-endothelial cells that participate in angiogenesis include fibroblasts, pericytes, and smooth muscle cells that, together with vascular endothelial cells, respond to a concert of growth factors, cytokines, extracellular matrix components, matrix receptors, and lipids orchestrated in response to a local need for increased blood supply (1). The environment that supports angiogenesis varies throughout the body and life of the organism. As a result, angiogenic response and the mechanisms that drive it may vary spatially and temporally, displaying distinct characteristics at different sites along the vascular tree and within different organs, at different ages, and in different physiological/pathological states. Aberrant functions of just a few of the cellular and non-cellular components that control angiogenesis can compromise the entire process, contributing to several diseases, including cancer, ischemia, hypertension, and inflammatory disorders (2–5). Consequently, understanding the cellular, biochemical, molecular, and mechanical mechanisms that control angiogenesis is crucial to devise better tools to support physiological angiogenesis and prevent or treat pathological angiogenesis.
Great strides have been made in elucidating the details of angiogenesis, and both in vitro and in vivo assays have been developed to explain individual factors that control this complex process. Despite these developments (6–8), however, there is no “gold standard” assay, and therefore angiogenesis studies rely heavily on the appropriate selection of multiple assays. In determining how well suited any single assay is for a particular study, factors such as the nature of the scientific question and the molecular mechanism under investigation, and the ultimate goal (clinical or scientific) will need to be carefully evaluated. Investigations of the molecular mechanisms of angiogenesis require assays that resolve individual aspects of the angiogenic process with precision, accuracy, and reproducibility. In vitro angiogenesis assays (i.e., tubulogenesis, proliferation) only recapitulate a few steps of the angiogenic process and, though very reproducible, are not necessarily an accurate reflection of blood vessel formation. In contrast, in vivo models (i.e., sponge assays, chorioallantoic membrane assays, cornea angiogenesis assays) evaluate an entire process that is biologically accurate, but they possess little access to and limited control of individual aspects, thereby reducing their reproducibility. Investigating the action of a particular pro- or anti-angiogenic factor requires an assay whose overall angiogenic behavior best mimics the angiogenic steps as observed under physiological and/or pathological conditions. In general, in vitro assays offer superior precision and control of components of the angiogenic process because they are isolated from confounding variables resident in the whole organism. In contrast, the comprehensive nature of in vivo assays provides biological and clinical relevance that enable translation of molecular understanding to real-life implementation. Most successful angiogenesis studies pair in vitro and in vivo assays to harness the power and overcome the limitations of both. In addition, ex vivo assays (i.e., vascular explants) that combine qualities of in vitro and in vivo assays have been developed to provide precise control over a biological system that recapitulates almost all of the mechanisms and steps of physiological angiogenesis. In this review we will describe the major steps involved in angiogenesis and the strengths and limitations of the currently available in vitro, in vivo, and ex vivo assays to study the angiogenic process.
The steps of angiogenesis
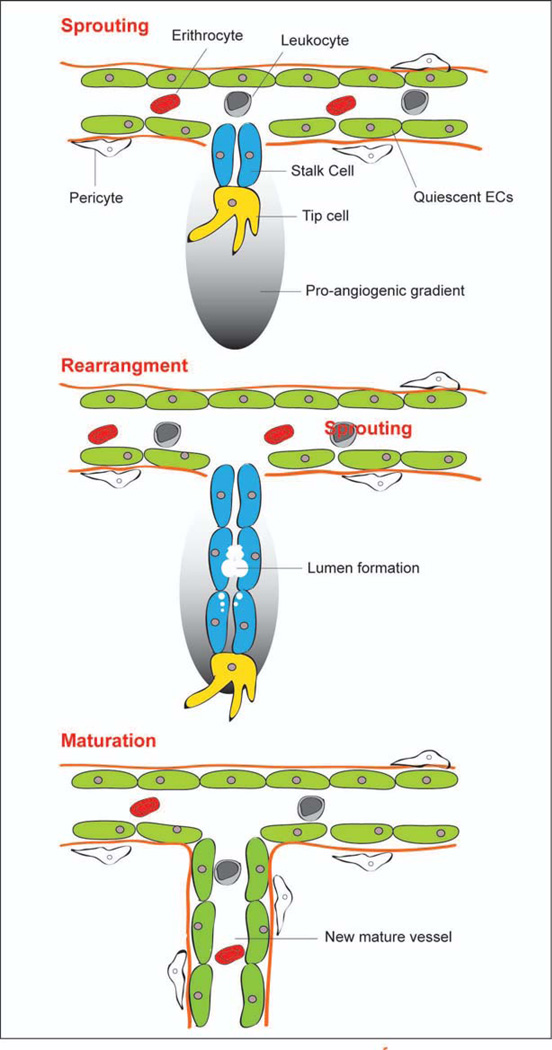
An angiogenic stimulus will cause regions of the vasculature endothelium to undergo four remodeling “steps”: 1) vascular sprouting, 2) tubule morphogenesis, 3) adaptation to tissue needs, and 4) vessel stabilization. These four major steps elicit the involvement of diverse cell types, extracellular matrix components, growth factors, and cytokines (9–14). The signals that drive angiogenesis vary temporally. In fact, several inhibitors of the early steps of vessel sprouting promote later steps of vessel maturation (15). This poses interesting challenges and opportunities to researchers who investigate ways to interrupt disease processes by targeting angiogenesis. Figure 1 describes the major events implicated in angiogenesis.
Figure 1.
Schematic representation of the major steps involved in angiogenesis.
Sprouting
Angiogenesis in adults begins with endothelial cell proliferation. In the absence of pro-angiogenic stimuli, endothelial cells will exist for years in a quiescent (i.e., non-proliferating) state. Sprouting initiates when endothelial cells receive pro-angiogenic paracrine signals released by their microenvironment in response to an increasing demand within the tissue for oxygen and nutrients or to pro-angiogenic stimuli released by cells involved in an injury or pathology such as cancer. Potent initiators of sprouting include vascular endothelial growth factor, fibroblast growth factors, angiopoietins, and hypoxia inducible factors (16). The bud of the sprouting vessels consists of two different cell types: the tip cells, which have migratory ability, and the stalk cells that contribute to the elongation of the sprouting by proliferating (17). Upon angiogenic activation of a vessel, pericytes (the cells surrounding endothelial cells) detach, proliferate, and migrate into the interstitium. Pericytes burrow through the basement membranes on which endothelial cells rest by expressing matrix metalloproteases. Fibroblasts also migrate outward, laying a provisional extracellular matrix (composed of collagen, fibronectin, and heparan sulfate proteoglycans) for the growing angiogenic sprout. Various in vitro co-culturing and vascular explant assays have provided insights about involvement of pericytes and fibroblasts in angiogenic sprouting. Endothelial proliferation and migration occur in the presence of orchestrated and spatially regulated pro-angiogenic cues so that endothelial cells do not migrate en masse toward the angiogenic stimuli (9;18–20). Molecules responsible for initiating angiogenic sprouting include VEGF-A, VEGF receptors 2 and 3, and the Notch signaling receptors (21–24). Several of the existing angiogenesis assays described in detail below incorporate a means for investigating the mechanisms of endothelial cell proliferation and migration.
Tubule morphogenesis
Endothelial cells acquire a lumen once they have migrated outward from their parent vessel. In this process, endothelial cells and stalk cells form vacuoles via pinocytosis. These vacuoles coalesce into larger, contiguous lumens, which eventually span the multiple endothelial cells. Fibroblasts induce tube formation in the angiogenic sprout by secreting tubulogenesis-stimulating molecules and by depositing extracellular matrix molecules that signal to stalk cells (25;26).
Adaptation to tissue needs
Once angiogenesis has produced a network of endothelial tubes, the angiogenic outgrowth undertakes vascular regression (which prunes parts of the angiogenic outgrowth) and vessel stabilization/maturation (which equips the nascent vessels to function long-term). Nascent angiogenic sprouts must decide whether to regress or stabilize. An abrupt loss of pro-angiogenic factors coupled with a lack of blood flow prompts endothelial tubes to regress and undergo apoptosis (27). Signals that prompt vessel stabilization include angiogenic signaling molecules, VEGF, PDGF, Ang-1, Ang-2 and blood flow. The effect of certain factors to control vessel stability depends on the overall composition of growth factors in the souring milieu. In this context, when present at the same time, VEGF and Ang-2 promote vessel formation, but in the presence of Ang-2 only, vessel regression occurs (28). Vessel regression can be studied in vivo and in angiogenesis assays that generate endothelial tubes/vascular sprouts in vitro (see below for details).
Stabilization/maturation
At this stage in angiogenesis are several processes that are the opposite of those that carry out the early steps. During vessel stabilization, the endothelium abandons its proliferative and invasive phenotype in order to revert to a non-proliferative state. Anastomoses between the vascular sprouts establish blood flow between juxtaposed capillaries. Tight junctions between adjacent cells are re-established, along with firm adhesion to the underlying extracellular matrix – a characteristic of quiescent vasculature. Pericytes and other mural cells are recruited to the vessel, matrix degradation is inhibited, and new matrix materials are deposited to generate a basement membrane for the vessel. Attached pericytes and extracellular matrix proteins inhibit further endothelial migration and proliferation, and provide pro-survival signals to the endothelial cells. Hemodynamic forces engendered in blood flow are thought to further stabilize the capillaries. Four signaling molecules, PDGF, shingosine 1, Ang-1, and TGF-β, have been identified as playing a significant role in vessel stability (9;29–31). The extracellular matrix also plays a dynamic role in stabilizing a vascular sprout by harboring angiogenic signaling molecules that become liberated as regions of the extracellular matrix get degraded by proteases.
Another factor that contributes to vessel stabilization is blood flow. Shear stress, for example, is mechanically transduced into activation of intracellular pathways that mimic signaling by VEGF and Ang-1. Hemodynamic forces engendered in blood flow (shear stress, pressurization, and vessel wall tension) have an impact on angiogenesis. In this context, increased shear stress induced by administration of adrenoreceptor agonists results in high capillary content and increased VEGF (32;33). High and low shear stress conditions also generate capillaries with differing morphologies (34). Hemodynamic signals in angiogenesis have attracted research interest, and it is suspected that mechanotransduction of blood flow-generated forces plays a more prominent role in physiological angiogenesis, while chemotransduction directs more of the pathological than the physiological angiogenic processes (35).
Angiogenesis assays
In vitro assays
In vitro assays of angiogenesis typically study the behavior of endothelial cells within a controlled environment. Fundamentally, these in vitro studies are based on purified endothelial cell cultures or carefully controlled co-culture with another cell type (i.e., fibroblasts, immune cells, pericytes, tumor cells). These assays allow researchers to study particular mechanisms or drug intervention in defined elements of angiogenesis while controlling nearly all other influencing variables. Such studies have been helpful in the identification of selective target molecules and/or key pathways controlling endothelial cell functions. In vitro endothelial cell assays typically assess proliferation, migration, and tube formation (6–8). The quantitative capacity of these in vitro studies is particularly important, as it provides a confidence that is not readily acquired with more complicated in vivo experiments. These experiments include (but are not limited to) proliferation, survival, differentiation/morphogenesis, and migration (6–8). Table 1 summarizes the major and commonly used in vitro angiogenesis assays.
Table 1.
In vitro angiogenesis assays
| Assay | Description | Uses | Cited |
|---|---|---|---|
| Cell Proliferation | Measure proliferation at baseline or in the presence of angiogenic factors. | Effect of test substance(s) on endothelial cell proliferation. | Staton et al. (8) |
| Baseline growth of endothelial cells isolated from different organs and/or transgenic animals. | |||
| Molecular mechanisms of endothelial cell proliferation. | |||
| Migration Assay | Scratch assay: endothelial cells migrate onto a denuded area. | Effect of test substance(s) on endothelial cell migration. | Staton et al. (8), Ashby et al. (38), Liang et al. (85) |
| Boyden chamber assay: endothelial cells migrate across a filter/matrix within a gradient of angiogenic factor. | Baseline migration of endothelial cells isolated from different organs and/or transgenic animals. | ||
| Stencil assay: patterned endothelial cells migrate across a protein-coated substrate. | Molecular mechanisms of endothelial cell migration. | ||
| Tube Formation Assay | Endothelial cells plated on 2D or in 3D matrices and quantification of a representative measure of tubule formation. | Effect of test substance(s) on endothelial cell tubulogenesis. | Arnaoutova and Kleinman (86) |
| Baseline tubulogenic activity of endothelial cells isolated from different organs and/or transgenic animals. | |||
| Molecular mechanisms of endothelial cell tubulogenesis. |
Proliferation
Assays that monitor endothelial cell proliferation in culture have the benefit of being rapid, reproducible, precise, and quantifiable. These assays can be used to analyze and compare the basal growth of endothelial cells isolated from a variety of sources, including primary human cell cultures (from aorta, dermal vasculature, or adipose tissue), cells obtained from syngeneic or transgenic mice, such as the immortomice (36), and established cell cultures (37). The rate of growth determines the ability of endothelial cells to respond to external stimuli (i.e., matrices, forces, growth factors). Measures of endothelial cell proliferation include traditional proliferation assays that can be achieved with manual count or automated cell counters, MTT assays that measure metabolic reduction in cells of MTT [3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide] from a yellow tetrazole to a purple formazan, and tritiated-thymidine or BrdU (Bromo-deoxyuridine) incorporation into duplicating DNA. In addition to cell proliferation, cell cycle progression is often used as a measure of endothelial cell growth. A combination of BrdU and propidium iodide can be used for this assay based on the assumption that endothelial cells in G1 phase incorporate propidium iodide only, while cells that have progressed through the S phase incorporate both propidium iodide and BrdU.
Migration
Migration assays are commonly used to determine the ability of an intrinsic molecular mechanism or an externally provided regulator, including pro- and/or anti-angiogenic factors, matrices (natural or bioengineered materials), and/or cell types (i.e., fibroblasts and/or immune cells) to promote or diminish endothelial cell migration. Boyden chambers position endothelial cells on one side of a filter (with pores of different-sized cut-offs) and facilitate migration of the cells toward a chemoattractant on the other side of the filter, or toward an extracellular matrix coated on the other side of the filter. Quantitation of endothelial cell migration is accomplished by analyzing the number of cells that completely traverse the filter in a finite time. The possibility to “create” chemotactic gradients provides the advantage of allowing detailed mechanistic analyses of endothelial cell migration. Although frequently used, drawbacks of the Boyden chamber migration assays include the difficult and time-consuming nature of quantitation, loss of the chemotactic gradient over time, and the inability to incorporate mechanical stimuli.
In addition to the Boyden chamber, the “scratch assay” can be used as a two-dimensional model of endothelial cell migration. In a scratch assay, an area of an endothelial cell monolayer is denuded via scratching or other means, and the ability of endothelial cells to migrate into the denudation is measured over time. These scratch assays have the advantages of being fast and allowing continuous monitoring of angiogenesis. However, the extent of confluence and scratch size vary, and quantification methods are arbitrary and prone to bias errors. Use of stencils overcomes variations in scratch size; e.g., magnetically attachable stencils, or MATs, create a smooth, controlled denudation area (38). Stencils also permit application of an underlying gradient of surface-bound ligands, as they could advance protein printing techniques (39).
Tubulogenesis
Tube formation assays are conducted by placing endothelial cells on or within an extracellular matrix (fibrin, collagen, or Matrigel) and monitoring tube formation over time. Quantitation is accomplished by counting the lengths and/or number of the formed tubes and/or the number of branch points. Tube formation assays can be two-dimensional (plating on top of a thin layer of extracellular matrix) or three-dimensional (placing cells within an extracellular matrix). These assays are rapid, reliable, and sensitive to composition and mechanical properties of the extracellular matrix (8;40). In addition, the matrices can be layered alone and/or in combination with pro- and anti-angiogenic factors, thus allowing one to analyze how matrix and soluble factors control tubulogenesis. However, the tubes that form are quite homogeneous in length and thus not entirely representative of in vivo angiogenesis. Quantitation of tube formation assays also requires technical skill, and three-dimensional assays have the additional technical challenges of processing confocal images.
There is an increasing interest in utilizing microfabrication and/or microfluidics to study the process of tubulation, with the goal of achieving patent microvessels that can be perfused. A first step toward this was to demonstrate that tubulogenesis observed in a Transwell tri-culture environment (41) could be guided with structural patterning of the matrix (42). More recently, co-culture microfluidic devices have been developed that support the self-organization of perfusable capillary networks (43–46). The most important observation of these studies was that the endothelial cells forming the tubules would adhere to the surface of the microfluidic channel in the immediate vicinity of the three-dimensional culture region, suggesting that this approach provides an in vitro connection between a macroscopic perfusion system and both ends of a self-assembled, perfused capillary network. Initially, a controlled balance of interstitial flow and diffusion through a hydrogel matrix containing fibroblasts and microvascular endothelial cells guides the formation of patent and durable microvascular networks, which can then support the transport of oxygen, nutrients, and waste to the surrounding tissue (44;46). One advantage of microfluidic devices is that they can provide controlled, spatiotemporal gradient in fluid pressures, cell types, chemokines, oxygen, and other molecular species and enable the observation of how these gradients affect the resulting sprouting and other physiological responses (44–47). The direction of sprouting can also be affected by extracellular matrix fiber orientation (48), so that contact guidance by aligned fibrin might be used to affect network architecture.
In vivo assays
In vivo angiogenesis assays (i.e., experiments utilizing an intact organism to model angiogenic behavior) present a complete and fully functional angiogenic process that acts alongside an entire set of the processes that maintain the state of the organism. In vivo models of angiogenesis carry out all the steps of angiogenesis and vessel maturation to produce fully functional vascular networks or vessels characteristic of certain disease states. In vivo assays typically investigate drug effects on angiogenesis and validate observations about the molecular mechanisms of angiogenesis from in vitro studies. As a result, these assays have proven indispensable to the understanding of angiogenesis and the development of angiogenic therapies. Table 2 summarizes the most commonly used in vivo angiogenesis assays.
Table 2.
In vivo angiogenesis assays
| Assay | Description | Uses | Cited |
|---|---|---|---|
| Chorioallantoic Membrane Assay (CAM) | Test substances (e.g., xenograft material, cell, or tissue) are applied on or within the CAM, in order to continuously monitor local angiogenesis. | Effect of test substances on angiogenesis. | Auerbach et al. (6), Staton et al. (7), Staton et al. (8), Ribatti et al. (49) |
| Interaction between CAM vasculature and the test substance. | |||
| In vivo Matrix Invasion Assay | Test substances (e.g., xenograft material, tissue, cell, cytokine, or small molecule) are loaded into Matrigel or matrix containing polymer scaffold. The Matrigel or polymer scaffold is loaded subcutaneously. Explanted Matrigel plugs or polymer scaffolds are evaluated for invasion of angiogenic sprouts post hoc. | Effect of test substances on angiogenesis. | Auerbach et al. (6), Staton et al. (7), Staton et al. (8), Akhtar et al. (60) |
| Molecular mechanisms of angiogenic sprouting, vessel formation, regression, and stabilization. | |||
| Retinopathy of Prematurity Model | Retinopathy is induced in neonatal mammals by exposure to hyperoxia followed by normoxia. Explanted retinas are evaluated for angiogenesis post hoc. | Effect of test substances on angiogenesis in retinopathy. | Yanni and Penn (87) |
| Molecular mechanisms of angiogenic sprouting, vessel formation, regression, and stabilization in retinopathy. | |||
| Fluorescent Zebrafish Assay | Live transgenic fluorescent embryo is exposed to small molecule angiogenic inhibitors and extent of angiogenesis is measured via fluorescence confocal imaging. | Effect of test substances on angiogenesis. | Weinstein et al. (58), Serbedzija et al. (88) |
| Molecular mechanisms of angiogenic sprouting, vessel formation, regression, and stabilization. | |||
| Dorsal Air Sac Model and Chamber Assay | A chamber is implanted across dorsal skin of the mouse, or (in some chamber assays) across thin layers of tissue (e.g., the ear or mouse femur). Test substances are introduced in the chamber. Local angiogenesis is measured post hoc (dorsal air sac model) and throughout the experiment (chamber assay). | Effect of test substances on angiogenesis. | Staton et al. (8), Yonezawa et al. (61) |
| Molecular mechanisms of angiogenic sprouting, vessel formation, regression, and stabilization. | |||
| Tumor Mouse Model | Fluorescent tumor cells are implanted subcutaneously in nude mice. Other test substances are administered as well. | Molecular mechanisms of tumor angiogenic sprouting, vessel formation, regression, and stabilization. | Staton et al. (8), Yang et al. (89), Amoh et al. (90) |
| Vascularization and growth of the tumor are monitored throughout the experiment, as well as occurrence of metastases. | Tumor-host interaction. |
Chick chorioallantoic membrane (CAM) assay
The CAM assay provides the full complement of biological components comprising a complex tissue, including surface epithelium enclosing a stromal compartment that contains fibroblasts, intact vasculature, and inflammatory cell types. Nevertheless, this extraordinarily thin membrane is relatively simple in architecture and a readily overseen experimental model. Like immunodeficient mice, avian embryos lack immune response to exogenous cells and tissues, therefore allowing engraftment of exogenous materials.
The CAM assay has become the most utilized in vivo angiogenesis assay (8;49). In the chick embryo, a change in vascular density in and around a test site on the CAM results from the topical or intravenous addition of test substances to the CAM. This change in vascularization implies an effect on angiogenesis. Test substances include soluble angiogenic growth factors, angiogenic inhibitors, tumor cells, and tissues. Encapsulation or immobilization of the test substance in polymer pellets, gelatin sponges, and air-dried filters accomplishes slowed or controlled release of the test substance. The chick CAM assay can be conducted in ovo, with the test substance added to the CAM through a small hole cut into the shell of the chick’s egg, or ex ovo, where the entire embryo and CAM are cultured outside of the shell (6;8;50–52). In ovo experiments require less maintenance, and angiogenesis can be tracked through the later stages of embryo development. Ex ovo models improve access to the CAM and permit repeated administration of the test substances and repeated time-course imaging, as well as multiple test sites per embryo. Angiogenesis is measured visually by counting vessels, or semi-quantitatively by scoring vascular density. Dyes and fluorescent micro- and nanoparticles injected into the vasculature better resolve the sprouts and identify patent vessels. The chick CAM is simple, scalable, and allows repeated/time-course imaging (53–57).
Zebrafish model of angiogenesis
The zebrafish model provides a powerful and inexpensive in vivo screening of angiogenesis stimulators and inhibitors. Zebrafish angiogenesis assays are conducted by injecting a biomolecular test substance into the yolk sac of a zebrafish embryo. Conveniently, lipophilic test substances added to the water can freely diffuse into the embryos. Utilization of mutagens and antisense morpholinos facilitates genetic engineering of the zebrafish model for investigating the molecular mechanism of angiogenesis. The facts that zebrafish embryos develop outside the mother and are transparent allow researchers to measure angiogenesis by visual inspection. Patent vasculature is visualized via injection of fluorescent dye, quantum dots, or microspheres, followed by confocal microscopy and image reconstruction (58). Transgenic zebrafish with GFP-labeled endothelial markers (Fli-GFP, mTie2-GFP, and Flk-GFP) grow fluorescent vasculature, which eases visualization (59).
The zebrafish angiogenesis assay is inexpensive, scalable, rapid, and quantifiable via imaging, but there are some drawbacks. The relevance of angiogenesis in the zebrafish embryo as a model of angiogenesis in human adults has been questioned. In this context, in the zebrafish assay neovascularization results from both vasculogenesis and angiogenesis, and distinguishing between the two is quite difficult. In addition, regions that participate only in angiogenesis are debated, although angiogenesis is accepted to occur in the subintestinal vein.
Cornea angiogenesis assay
Once considered the “gold standard” assay of angiogenesis, the cornea assay features angiogenesis from mammalian vasculature, which better represents angiogenesis in humans (6;8). The assay is conducted by cutting a pocket into the corneal stroma of a mouse, rat, or rabbit and implanting into it a test substance (e.g., tumor (or other) tissue or cells, conditioned media, growth factors, etc.). To overcome the difficulty of controllably delivering the test substance to the corneal pocket, various slow-release polymer pellets have been employed (6;8). Angiogenesis can also occur in response to injury to the cornea, delivered via chemical cauterization or mechanical scraping. Quantification or analysis of the angiogenic response is accomplished visually, by explanting the cornea and counting the number of vessels and measuring the length, caliber, or density of the new vessels. The corneal angiogenesis assay is reliable and quantifiable, and genetic engineering in mice allows it to be used to investigate the molecular mechanisms of angiogenesis. The initially avascular cornea permits a low background measurement of angiogenesis; however, the relevance of ectopic angiogenesis into the normally avascular cornea has been questioned (8). The cornea is a two-dimensional environment for angiogenesis, while human angiogenesis typically occurs in three dimensions. Other limitations of the assay include that it is time-consuming, expensive, and technically demanding to run (more so in smaller mammals). Angiogenesis in this assay is not amenable to repeated or time-course imaging. There also exist ethical qualms regarding the invasive use of a major sensory organ.
In vivo matrix invasion assays
These assays facilitate mammalian angiogenesis in a natural extracellular matrix biomaterial (e.g., Matrigel) (60). Such assays are performed by injecting Matrigel (which gels into a plug upon injection) or implanting a polymer scaffold subcutaneously in the mouse, rat, or rabbit, then monitoring for angiogenic ingrowth. Synthetic sponge matrices, composed of polyvinyl acid, polyethylene, or polyurethane, have been utilized in the matrix invasion assays as a scaffold. The Matrigel plug or scaffold material typically contains a test substance (e.g., growth factor, cells, tumor, and tissue explant) that should recruit and/or repel host-derived endothelial cells. Measurements of angiogenesis occur at the end of the assay (typically 7–10 days post implantation), when the matrix material plug or scaffold material is explanted, sectioned, and stained for endothelial cell markers. In certain cases, the hemoglobin content in the matrix material or scaffold can be measured, thus providing an indirect measure of angiogenesis, but it may become inflated by deposition of hemoglobin due to hemorrhaging or leaky vessels. Nevertheless, this assay is commonly used to analyze the ability of a scaffold and/or given molecule to promote and/or inhibit physiological host-mediated angiogenic responses.
Dorsal air sac model of angiogenesis
This method is constructed by lifting the dorsal skin on a mouse, injecting air, and implanting a chamber through a transverse section cut on the back (61). The chamber is loaded with a test substance, such as tumor tissue or cells or angiogenic cytokines. The angiogenesis response is assessed upon explantation of the chamber by counting the newly formed vessels. To facilitate the visualization of angiogenesis, injection of dye or 51Cr-labeled erythrocytes followed by the measurement of the volume of dye or 51Cr that accumulates in the chamber is often utilized. This assay is simple, amenable to genetic engineering, and permits facile administration of the test substance. However, the dorsal air sac assay is difficult to quantitate and does not permit repeated or time-course measurement of angiogenesis.
Efforts to overcome these limitations resulted in the use of chamber assays, which are prepared by assembling a chamber around a region of thin tissue (e.g., rabbit or mouse ear, and dorsal skinfold). The chamber is typically loaded with a test substance (e.g., tumor or other tissue or cells, growth factors, cytokines, and angiogenic inhibitors). The thinness of the tissue inside the chamber allows repeated/time-course measurements of angiogenesis to be performed visually via transillumination. Vessel density and diameter are measures of angiogenesis utilized in the chamber assay. Injection of fluorescent dyes allows non-functional and patent vasculature to be distinguished and can be used to measure vascular permeability. A significant advantage of the chamber assay is that it allows repeated measurements on a mammalian model of angiogenesis. Windows implanted into the cranial bone or across the mouse femur window allow visualization of angiogenesis in organotypic sites (the brain and femur). The chamber assay and dorsal air sac assays are reliable and amenable to genetic engineering; however, they are prone to irritation from the surgery and implant. As a result, angiogenesis is subject to influences (noise) from cytokines released by inflammatory and wound healing responses. The assay is also expensive, difficult, and invasive.
Tumor-associated angiogenesis
In vivo models of pathological angiogenesis include tumor-associated angiogenesis. In this assay, mice are injected with tumor cells (i.e., subcutaneous, intra-cardiac, orthotopic, intra-bone, intra-spleen injection) and then left untreated or treated with a drug of interest. After a given time that varies depending on the tumor type and site of injection, the mice are sacrificed, tumors are retrieved, and the amount of tumor-associated angiogenesis analyzed by traditional immunohistochemical assays. If the tumors are subcutaneously implanted, in vivo imaging on anesthetized mice can be performed in order to analyze in real time the amount of blood flow within tumors, the amount of capillary network, the amount of oxygen consumption, and the metabolic profile of the tumor. In vivo imaging, although quite expensive, allows multiple analysis of the same mouse and easy quantification of the potential pro- and/or anti-angiogenic action of the drug(s) tested.
Ex vivo assays
As an intermediate method between more physiologically relevant in vivo angiogenesis assays and more precise in vitro angiogenesis assays, the organ explant angiogenesis assay has gained wide use. Organ explant assays initiate angiogenic sprouting, outward growth, and (to an extent) stabilization of new blood vessels from explanted segments of vasculature, bone, or embryonic tissue. Often termed an “ex vivo” model, organ explant assays are considered the most complete in vitro model of in vivo angiogenesis. The “ex vivo” vascular explant assays synergistically combine qualities of in vitro and in vivo angiogenesis assays to provide precise control over a biological system that recapitulates almost all of the mechanisms and steps of physiological angiogenesis. In addition, these assays recapitulate the spatial organization of heterogeneous cell types and extracellular matrices, the multitude of paracrine and juxtacrine signaling events, and the endogenously generated spatial-temporal gradients of pro- and/or anti-angiogenic active biomolecules. Therefore, researchers utilize organ explant assays to reliably investigate angiogenic mechanisms (including vessel stabilization and regression steps not accessed in the other angiogenesis assays) and the test substances that influence angiogenesis (62;63). Table 3 lists the types of organs that are utilized as a platform for studying ex vivo angiogenesis.
Table 3.
Organs used in the vascular explant assay
| Vessel Type | Species |
|---|---|
| Aorta | Rat, Mouse, Chick, Rabbit, Cow, Dog, Human |
| Carotid Artery | Rat, Pig, Cow |
| Saphenous Vein | Human |
| Vena Cava | Rat |
| Thoracic Duct | Rat, Mouse |
| Fetal Metatarsals | Mouse |
| Placental Vein | Rat, Mouse |
Aortic ring assays
After the first observation that spontaneous angiogenic outgrowth occurs from aortas cultured in vitro (64), the aortic ring assay and other organ explant assays arose and have since developed into the most complete in vitro mimic of in vivo angiogenesis (6–8). In a vascular explant assay, the explanted vessel is cleaned of surrounding fibro/adipose tissue, cut into 1 mm slices, and imbedded in collagen, fibrin, or Matrigel (65;66), and spontaneous outgrowth is followed over time (65). This assay provides a convenient, cost-effective, reliable way to investigate the mechanisms of angiogenesis and the effects of test substances (e.g., potential therapeutic agents) on angiogenesis (6–8). Qualities that define this assay as in vitro mimics of in vivo angiogenesis include the near-physiological spatial organization of endothelial cells, paracrine and juxtacrine signals, and endogenous matrix materials (6–8;65). Vascular sprouts originating from an organ explant contain lumen, supporting pericytes, and basement membrane. Considered essentially the same as capillary sprouts arising from angiogenesis in vivo, these vessels feature nearly all the functional similarities of those in vivo except vascular maturation brought on by blood flow (65). Vascular sprouts can be counted manually or using image processing software. The count includes vessels appearing at different depths in the sample via focusing (65). The number of branch points in the outgrowth also provides a useful measure of the extent of angiogenesis (8). A more rapid quantitation of the extent of angiogenesis comes from measuring, via image processing software, the area covered by the angiogenic outgrowth (8). Vessel maturity is assessed by the number of pericytes lining the vessel and by the vessel caliber (8;65). Finally, whole mount immunostaining of the angiogenic sprouts is possible in vascular explants cultured in a thick layer of collagen (67).
Why do we need more than one assay to study angiogenesis?
As angiogenesis is a complex event that requires interactions among different cell types, cells and growth factors, and cells and matrix components, a single “gold standard” assay that recapitulates all these events is currently not available. At present the in vitro, in vivo, and ex vivo assays described above represent the best available tools to analyze endothelial cell functions outside an organism. Despite being useful, angiogenesis assays come with some pitfalls and shortcomings that need to be taken into account.
If on one hand in vitro angiogenesis assays offer high precision and control of components of the angiogenic process isolated from confounding variables resident in the whole organism, on the other they only recapitulate a few steps of the angiogenic process. In addition, in vitro assays generally create a synthetic environment that bears little similarity to the physiological environment being investigated. Angiogenesis in vitro does not always represent the in vivo situation (i.e., in several in vitro angiogenesis assays vascular maturation steps do not occur). In addition, it is conceivable that promising observations in vitro might not be recapitulated in vivo, and vice versa, thus making it difficult to determine the physiological and/or pathological relevance to the in vitro findings. Moreover, in vitro assays are usually performed with one cell type, namely endothelial cells, and they do not take into account the contribution of variables such as mural cells, extracellular matrix components, flow (shear stress, pressure, and tension), and the immune response.
A potential advantage offered by the recently developed microfluidic angiogenesis assays (43–46;46) is that they allow separate control of interstitial and vascular flow in a three-dimensional, co-culture environment well suited for high-resolution microscopy. The extent to which this new approach addresses the above-mentioned limitations has yet to be fully explored. The use of perfused hollow fibers with endothelial cells on the inside of the lumen and astrocytes on the outside (68–70) has demonstrated how the interactions between abluminal cells, luminal endothelial cells, and shear stresses can affect the realism with which the blood-brain barrier can be recreated in vitro. This work would suggest that similar effects will occur in microfluidic angiogenesis assays as their sophistication increases and more interacting cell types such as pericytes can be incorporated (71).
Ultimately, angiogenesis may prove to be critical in the development of organs on a chip, in which microfluidic bioreactors are used to culture heterogeneous human cell populations to create three-dimensional tissue constructs that present the functions of intact organs, albeit at much lower volumes and cost and greater reliability and human relevance than two-dimensional cell cultures or studies on animals or humans (72–76).
While not specifically an angiogenesis assay, preformed microfluidic channels can support functional microvascular tubes (77), and serve as surrogates for microvasculature assays that allow the observation, for example, of locations in endothelial bifurcations that exhibit preferential adhesion of leukocytes (78–81). There remain challenges in the fabrication of complex, three-dimensional microfluidic networks whose dimensions span the appropriate spatial scales (82).
As non-endothelial cell types play an indispensable role in angiogenesis, results of angiogenesis studies that lack non-endothelial cell types are less likely to translate to in vivo studies. Generally, in vitro assays are highly dependent on the endothelial cell source (6–8), which has been shown to vary greatly with respect to position along the vascular tree, organ, gender, and age of the donor. Angiogenesis models should utilize endothelial cells that best resemble the context being studied. However, the technical difficulty of isolating endothelial cells from some tissues limits the selection of available endothelial cells for in vitro study (8). Isolation disrupts the quiescent state of endothelial cells and induces proliferation (6–8). In addition, isolated endothelial cells become rapidly senescent, limiting their use (8). These problems have an impact on the characterization of endothelial cell functions, such as proliferation and migration, as well as on the analysis of pro- and anti-angiogenic compounds and pathways (6;7).
As mentioned above, in vivo assays present a complete angiogenic process capable of producing functional vasculature. However, they suffer from limitations of real-time imaging and difficulty in measuring angiogenic factors. Measurement of angiogenesis in internal tissues and/or organs often requires post hoc explanting, sectioning, and staining the organ of interest, thus making repeated/time-course imaging impossible. Moreover, the in vivo angiogenic response to an experimental condition or test substance is subject to influences (noise) from other processes also functioning to maintain the state of the organism. Finally, in vivo assays pose a challenge to controlling factors that drive angiogenesis (e.g., maintenance of gradients of growth factors). The limitations of in vivo assays are also exacerbated in assays that seek to control or study mechanical forces in angiogenesis. Measurement and actuation of mechanical influences on angiogenesis are generally restricted to single cells and two-dimensional cell monolayers, which lack several pertinent features of in vivo angiogenesis.
Vascular explants are considered the best in vitro mimic of angiogenesis in vivo. Still, they suffer limitations common to in vitro angiogenesis models. Mechanical stimuli, local blood flow, and mechanical properties of the surrounding tissues are largely absent from the organ explant assays. Another limitation is that the vascular organ explant often generates tubules not engaged in active vascularization and lack the functional hemodynamic flow of in vivo microvasculature that actively participates in angiogenesis. In addition, the ex vivo assays are quite difficult to quantify and reproduce. Variability in angiogenesis in organ explant cultures arises from variability in the matrix and serum utilized in the assay and the difference between animals utilized in the study. In mice, for example, age and genetic background strongly influence the results of the organ explant assay (83). Given the variations in endothelial phenotype along the vascular tree, discrepancies in the results between studies may arise from differences in the vessel type utilized (84). Finally, as human vessels are difficult to acquire, organ explant assays cannot fully represent angiogenesis in vivo as observed in humans.
In conclusion, interest in angiogenesis is growing as researchers realize its impact on several diseases. The picture of angiogenesis is incomplete, however, and although angiogenesis studies have produced useful insights for understanding and treating disease, they suffer from limitations in the current armamentarium of assays. In general, in vitro angiogenesis assays permit detailed study of a biological process that poorly represents physiological angiogenesis, while in vivo assays limit access to the variables that influence angiogenesis. Thus, in setting angiogenesis assays, it is important to consider the relevance of the model organism, the tissue type, the test site, the matrix material, and the cell type in order to closely resemble the physiological or pathological setting being investigated. We anticipate that microfluidically perfused three-dimensional bioreactors may be the next great step in angiogenesis assays.
Footnotes
This work was supported in part by the Department of Defense CDMRP Breast Cancer Research Program W81XWH-07-1-0507 (JPW); the VA Merit Review 1I01BX002025 (AP); the National Institutes of Health RO1CA143081 (AZ) and RO1CA162433 (AP); and the Vanderbilt Institute for Integrative Biosystems Research and Education.
Declarations: None
Author Contributions
MWI performed the literature research and wrote the first draft of the review in consultation with JPW and AP. AZ provided guidance with some of the in vivo assays and comments on the manuscript. AP and JPW completed the final version of the review.
References
- 1.Pozzi A, Zent R. Regulation of endothelial cell functions by basement membrane- and arachidonic acid-derived products. WIREs Syst Biol Med. 2009 Sep 1;1(2):254–272. doi: 10.1002/wsbm.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferroni P, Della-Morte D, Palmirotta R, Rundek T, Guadagni F, Roselli M. Angiogenesis and hypertension: the dual role of anti-hypertensive and anti-angiogenic therapies. Curr Vasc Pharmacol. 2012 Jul;10(4):479–493. doi: 10.2174/157016112800812836. [DOI] [PubMed] [Google Scholar]
- 3.Konisti S, Kiriakidis S, Paleolog EM. Hypoxia-a key regulator of angiogenesis and inflammation in rheumatoid arthritis. Nature Reviews Rheumatology. 2012;8(3):153–162. doi: 10.1038/nrrheum.2011.205. [DOI] [PubMed] [Google Scholar]
- 4.Mackey JR, Kerbel RS, Gelmon KA, Mcleod DM, Chia SK, Rayson D, et al. Controlling angiogenesis in breast cancer: A systematic review of anti-angiogenic trials. Cancer Treatment Reviews. 2012;38(6):673–688. doi: 10.1016/j.ctrv.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Martin A, Komada MR, Sane DC. Abnormal angiogenesis in diabetes mellitus. Medicinal Research Reviews. 2003;23(2):117–145. doi: 10.1002/med.10024. [DOI] [PubMed] [Google Scholar]
- 6.Auerbach R, Lewis R, Shinners B, Kubai L, Akhtar N. Angiogenesis assays: A critical overview. Clin Chem. 2003;49(1):32–40. doi: 10.1373/49.1.32. [DOI] [PubMed] [Google Scholar]
- 7.Staton CA, Stribbling SM, Tazzyman S, Hughes R, Brown NJ, Lewis CE. Current methods for assaying angiogenesis in vitro and in vivo. Int J Exp Pathol. 2004;85(5):233–248. doi: 10.1111/j.0959-9673.2004.00396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Staton CA, Reed MWR, Brown NJ. A critical analysis of current in vitro and in vivo angiogenesis assays. Int J Exp Pathol. 2009;90(3):195–221. doi: 10.1111/j.1365-2613.2008.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473(7347):298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bouis D, Kusumanto Y, Meijer C, Mulder NH, Hospers GAP. A review on pro- and anti-angiogenic factors as targets of clinical intervention. Pharmacol Res. 2006;53(2):89–103. doi: 10.1016/j.phrs.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 11.Gerhardt H, Betsholtz C. Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res. 2003;314(1):15–23. doi: 10.1007/s00441-003-0745-x. [DOI] [PubMed] [Google Scholar]
- 12.Moldovan NI. Role of monocytes and macrophages in adult angiogenesis: A light at the tunnel’s end. J Hematoth Stem Cell Res. 2002;11(2):179–194. doi: 10.1089/152581602753658394. [DOI] [PubMed] [Google Scholar]
- 13.Cheresh DA, Stupack DG. Regulation of angiogenesis: apoptotic cues from the ECM. Oncogene. 2008;27(48):6285–6298. doi: 10.1038/onc.2008.304. [DOI] [PubMed] [Google Scholar]
- 14.Sottile J. Regulation of angiogenesis by extracellular matrix. BBA-Rev Can. 2004;1654(1):13–22. doi: 10.1016/j.bbcan.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Shojaei F, Wu XM, Malik AK, Zhong CL, Baldwin ME, Schanz S, et al. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+ myeloid cells. Nat Biotechnol. 2007;25(8):911–920. doi: 10.1038/nbt1323. [DOI] [PubMed] [Google Scholar]
- 16.Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003;9(6):677–684. doi: 10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]
- 17.Ribatti D, Crivellato E. “Sprouting angiogenesis”, a reappraisal. Dev Biol. 2012;372(2):157–165. doi: 10.1016/j.ydbio.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 18.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438(7070):967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 19.Fidler IJ. Angiogenesis and cancer metastasis. Cancer Journal. 2000;6(Supp.2):S134–S141. [PubMed] [Google Scholar]
- 20.Secomb TW, Alberding JP, Hsu R, Dewhirst MW, Pries AR. Angiogenesis: An Adaptive Dynamic Biological Patterning Problem. Plos Comput Biol. 2013;9(3):e1002983. doi: 10.1371/journal.pcbi.1002983. Article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hellstrom M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, et al. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445(7129):776–780. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- 22.Kume T. Ligand-Dependent Notch Signaling in Vascular Formation. In: Reichrath JÃ, Reichrath S, editors. Notch Signaling in Embryology and Cancer. New York: Springer; 2012. pp. 210–222. [DOI] [PubMed] [Google Scholar]
- 23.Benedito R, Rocha SF, Woeste M, Zamykal M, Radtke F, Casanovas O, et al. Notch-dependent VEGFR3 upregulation allows angiogenesis without VEGF-VEGFR2 signalling. Nature. 2012;484(7392):110–114. doi: 10.1038/nature10908. [DOI] [PubMed] [Google Scholar]
- 24.Blanco R, Gerhardt H. VEGF and Notch in Tip and Stalk Cell Selection. Cold Spring Harbor Perspectives in Medicine. 2013 Jan 1;3(1):a006569. doi: 10.1101/cshperspect.a006569. Article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davis GE, Senger DR. Endothelial extracellular matrix - Biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ Res. 2005;97(11):1093–1107. doi: 10.1161/01.RES.0000191547.64391.e3. [DOI] [PubMed] [Google Scholar]
- 26.Berthod F, Germain L, Tremblay N, Auger FA. Extracellular matrix deposition by fibroblasts is necessary to promote capillary-like tube formation in vitro. J Cell Physiol. 2006;207(2):491–498. doi: 10.1002/jcp.20584. [DOI] [PubMed] [Google Scholar]
- 27.Dimmeler S, Zeiher AM. Endothelial cell apoptosis in angiogenesis and vessel regression. Circ Res. 2000;87(6):434–439. doi: 10.1161/01.res.87.6.434. [DOI] [PubMed] [Google Scholar]
- 28.Lobov IB, Brooks PC, Lang RA. Angiopoietin-2 displays VEGF-dependent modulation of capillary structure and endothelial cell survival in vivo. PNAS (US) 2002;99(17):11205–11210. doi: 10.1073/pnas.172161899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9(6):685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- 30.Takuwa Y, Du W, Qi X, Okamoto Y, Takuwa N, Yoshioka K. Roles of sphingosine-1-phosphate signaling in angiogenesis. World J Biol Chem. 2010 Oct 26;1(10):298–306. doi: 10.4331/wjbc.v1.i10.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fantin A, Vieira JM, Gestri G, Denti L, Schwarz Q, Prykhozhij S, et al. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood. 2010;116(5):829–840. doi: 10.1182/blood-2009-12-257832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hudlicka O, Egginton S, Brown MD. Angiogenesis in the Heart and Skeletal Muscle - Models for Capillary Growth. In: Maragoudakis ME, editor. Angiogenesis: models, modulators, and clinical applications. New York: Plenum Press; 1998. pp. 19–33. [Google Scholar]
- 33.Egginton S, Zhou AL, Brown MD, Hudlicka O. Unorthodox angiogenesis in skeletal muscle. Cardiovasc Res. 2001;49(3):634–646. doi: 10.1016/s0008-6363(00)00282-0. [DOI] [PubMed] [Google Scholar]
- 34.Egginton S. Physiological factors influencing capillary growth. Acta Physiol. 2011;202(3):225–239. doi: 10.1111/j.1748-1716.2010.02194.x. [DOI] [PubMed] [Google Scholar]
- 35.Egginton S. In vivo shear stress response. Biochem Soc Trans. 2011;39:1633–1638. doi: 10.1042/BST20110715. [DOI] [PubMed] [Google Scholar]
- 36.Su XJ, Sorenson C, Sheibani N. Isolation and characterization of murine retinal endothelial cells. Molecular Vision. 2003;9(25–26):171–178. [PubMed] [Google Scholar]
- 37.Shao R, Guo X. Human microvascular endothelial cells immortalized with human telomerase catalytic protein: a model for the study of in vitro angiogenesis. Biochem Biophys Res Commun. 2004;321(4):788–794. doi: 10.1016/j.bbrc.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 38.Ashby W, Wikswo JP, Zijlstra A. Magnetically Attachable Stencils and the Non-Destructive analysis of the Contribution Made by the Underlying Matrix to Cell Migration. Biomaterials. 2012;33(33):8189–8203. doi: 10.1016/j.biomaterials.2012.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Georgescu W, Jourquin J, Estrada L, Anderson ARA, Quaranta V, Wikswo JP. Model-controlled hydrodynamic focusing to generate multiple overlapping gradients of surface-immobilized proteins in microfluidic devices. Lab Chip. 2008;8:238–244. doi: 10.1039/b716203k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arnaoutova I, George J, Kleinman HK, Benton G. The endothelial cell tube formation assay on basement membrane turns 20: state of the science and the art. Angiogenesis. 2009;12(3):267–274. doi: 10.1007/s10456-009-9146-4. [DOI] [PubMed] [Google Scholar]
- 41.Martins-Green M, Li QJ, Yao M. A new generation organ culture arising from cross-talk between multiple primary human cell types. FASEB J. 2005;18(15):222–224. doi: 10.1096/fj.04-1725fje. [DOI] [PubMed] [Google Scholar]
- 42.Liu Y, Markov D, Wikswo J, McCawley L. Microfabricated scaffold-guided endothelial morphogenesis in three-dimensional culture. Biomed Microdevices. 2011 Jun 28;13(5):837–846. doi: 10.1007/s10544-011-9554-2. [DOI] [PubMed] [Google Scholar]
- 43.Yeon JH, Ryu HR, Chung M, Hu QP, Jeon NL. In vitro formation and characterization of a perfusable three-dimensional tubular capillary network in microfluidic devices. Lab Chip. 2012;12(16):2815–2822. doi: 10.1039/c2lc40131b. [DOI] [PubMed] [Google Scholar]
- 44.Hsu YH, Moya ML, Abiri P, Hughes CCW, George SC, Lee AP. Full range physiological mass transport control in 3D tissue cultures. Lab Chip. 2013;13(1):81–89. doi: 10.1039/c2lc40787f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim S, Lee H, Chung M, Jeon NL. Engineering of functional, perfusable 3D microvascular networks on a chip. Lab Chip. 2013;13(8):1489–1500. doi: 10.1039/c3lc41320a. [DOI] [PubMed] [Google Scholar]
- 46.Moya ML, Hsu Y-H, Lee AP, Hughes CCW, George SC. In vitro Perfused Human Capillary Networks. Tissue Eng Pt C. 2013;19(9):730–737. doi: 10.1089/ten.tec.2012.0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baker BM, Trappmann B, Stapleton SC, Toro E, Chen CS. Microfluidics embedded within extracellular matrix to define vascular architectures and pattern diffusive gradients. Lab Chip. 2013;13(16):3246–3252. doi: 10.1039/c3lc50493j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morin KT, Tranquillo RT. Guided sprouting from endothelial spheroids in fibrin gels aligned by magnetic fields and cell-induced gel compaction. Biomaterials. 2011;32(26):6111–6118. doi: 10.1016/j.biomaterials.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 49.Ribatti D, Nico B, Vacca A, Presta M. The gelatin sponge-chorioallantoic membrane assay. Nat Protoc. 2006;1(1):85–91. doi: 10.1038/nprot.2006.13. [DOI] [PubMed] [Google Scholar]
- 50.Phelps EA, Garcia AJ. Update on therapeutic vascularization strategies. Regen Med. 2009 Jan 1;4(1):65–80. doi: 10.2217/17460751.4.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dohle DS, Pasa SD, Gustmann S, Laub M, Wissler JH, Jennissen HP, et al. Chick ex ovo culture and ex ovo CAM assay: How it really works. JoVE 2009 Nov. 30(33):e1620. doi: 10.3791/1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Palmer TD, Lewis J, Zijlstra A. Quantitative Analysis of Cancer Metastasis Using an Avian Embryo Model. JoVE. 2011 May;30(51):e2815. doi: 10.3791/2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zijlstra A, Lewis JD. Visualization and Quantification of De Novo Angiogenesis in Ex Ovo Chicken Embryos. In: Zudaire E, Cuttitta F, editors. The Textbook of Angiogenesis and Lymphangiogenesis: Methods and Applications. Dordrecht: Springer Netherlands; 2012. pp. 217–240. [Google Scholar]
- 54.Pink DBS, Schulte W, Parseghian MH, Zijlstra A, Lewis JD. Real-Time Visualization and Quantitation of Vascular Permeability In Vivo: Implications for Drug Delivery. PloS One. 2012;7(3):e33760. doi: 10.1371/journal.pone.0033760. Article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leong HS, Steinmetz NF, Ablack A, Destito G, Zijlstra A, Stuhlmann H, et al. Intravital imaging of embryonic and tumor neovasculature using viral nanoparticles. Nat Protoc. 2010;5(8):1406–1417. doi: 10.1038/nprot.2010.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zijlstra A, Mikolon D, Stupack DG. Angiogenesis assays in the chick. In: Staton C, Lewis CE, Bicknell RJ, editors. Angiogenesis assays: a critical appraisal of current techniques. Chichester, England: J. Wiley & Sons; 2006. pp. 183–201. [Google Scholar]
- 57.Lewis JD, Destito G, Zijlstra A, Gonzalez MJ, Quigley JP, Manchester M, et al. Viral nanoparticles as tools for intravital vascular imaging. Nat Med. 2006;12(3):354–360. doi: 10.1038/nm1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weinstein BM, Stemple DL, Driever W, Fishman MC. Gridlock, A Localized Heritable Vascular Patterning Defect in the Zebrafish. Nat Med. 1995;1(11):1143–1147. doi: 10.1038/nm1195-1143. [DOI] [PubMed] [Google Scholar]
- 59.Motoike T, Loughna S, Perens E, Roman BL, Liao W, Chau TC, et al. Universal GFP reporter for the study of vascular development. Genesis. 2000;28(2):75–81. doi: 10.1002/1526-968x(200010)28:2<75::aid-gene50>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 60.Akhtar N, Dickerson EB, Auerbach R. The sponge/Matrigel angiogenesis assay. Angiogenesis. 2002;5(1–2):75–80. doi: 10.1023/a:1021507031486. [DOI] [PubMed] [Google Scholar]
- 61.Yonezawa S, Asai T, Iku N. Dorsal Air Sac Model. In: Staton CA, Lewis CE, Bicknell RJ, editors. Angiogenesis Assays a Critical Appraisal of Current Techniques. Chichester, UK: John Wiley & Sons; 2007. pp. 229–238. [Google Scholar]
- 62.Seano G, Chiaverina G, Gagliardi PA, di Blasio L, Sessa R, Bussolino F, et al. Modeling human tumor angiogenesis in a three-dimensional culture system. Blood. 2013;121(21):129–137. doi: 10.1182/blood-2012-08-452292. [DOI] [PubMed] [Google Scholar]
- 63.Angiogenesis assays: a critical appraisal of current techniques. Chichester, England: J. Wiley & Sons; 2006. [Google Scholar]
- 64.Nicosia RF, Tchao R, Leighton J. Histotypic angiogenesis in vitro: Light microscopic, ultrastructural, and autoradiographic studies. In Vitro. 1982;18(6):538–549. doi: 10.1007/BF02810077. [DOI] [PubMed] [Google Scholar]
- 65.Aplin AC, Fogel E, Zorzi P, Nicosia RF. The aortic ring model of angiogenesis. Met Enzymol. 2008;443:119–136. doi: 10.1016/S0076-6879(08)02007-7. [DOI] [PubMed] [Google Scholar]
- 66.Burbridge MF, West DC. Rat Aortic Ring 3D Model of Angiogenesis In Vitro. In: Murray JC, editor. Angiogenesis protocols. Totowa, N.J.: Humana Press; 2001. pp. 185–204. [DOI] [PubMed] [Google Scholar]
- 67.Zhu WH, Nicosia RF. The thin prep rat aortic ring assay: A modified method for the characterization of angiogenesis in whole mounts. Angiogenesis. 2002;5(1–2):81–86. doi: 10.1023/a:1021509004829. [DOI] [PubMed] [Google Scholar]
- 68.Cucullo L, McAllister MS, Kight K, Krizanac-Bengez L, Marroni M, Mayberg MR, et al. A new dynamic in vitro model for the multidimensional study of astrocyte-endothelial cell interactions at the blood-brain barrier. Brain Res. 2002;951(2):243–254. doi: 10.1016/s0006-8993(02)03167-0. [DOI] [PubMed] [Google Scholar]
- 69.Cucullo L, Hossain M, Rapp E, Manders T, Marchi N, Janigro D. Development of a humanized in vitro blood-brain barrier model to screen for brain penetration of antiepileptic drugs. Epilepsia. 2007;48(3):505–516. doi: 10.1111/j.1528-1167.2006.00960.x. [DOI] [PubMed] [Google Scholar]
- 70.Davies PF, Remuzzi A, Gordon EJ, Dewey CF, Gimbrone MA. Turbulent Fluid Shear-Stress Induces Vascular Endothelial-Cell Turnover Invitro. PNAS (US) 1986;83(7):2114–2117. doi: 10.1073/pnas.83.7.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alcendor DJ, Charest AM, Zhu WQ, Vigil HE, Knobel SM. Infection and upregulation of proinflammatory cytokines in human brain vascular pericytes by human cytomegalovirus. J Neuroinflamm. 2012;9:95. doi: 10.1186/1742-2094-9-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huh D, Torisawa YS, Hamilton GA, Kim HJ, Ingber DE. Microengineered physiological biomimicry: Organs-on-Chips. Lab Chip. 2012;12(12):2156–2164. doi: 10.1039/c2lc40089h. [DOI] [PubMed] [Google Scholar]
- 73.Sung JH, Esch MB, Prot JM, Long CJ, Smith A, Hickman JJ, et al. Microfabricated mammalian organ systems and their integration into models of whole animals and humans. Lab Chip. 2013;13(7):1201–1212. doi: 10.1039/c3lc41017j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.van der Meer AD, van den Berg A. Organs-on-chips: breaking the in vitro impasse. Integr Biol. 2012;4:461–470. doi: 10.1039/c2ib00176d. [DOI] [PubMed] [Google Scholar]
- 75.Wikswo J, Curtis EL, Eagleton ZE, Evans BC, Kole A, Hofmeister LH, et al. Scaling and systems biology for integrating multiple organs-on-a-chip. Lab Chip. 2013;13:3496–3511. doi: 10.1039/c3lc50243k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wikswo JP, Block FE, III, Cliffel DE, Goodwin CR, Marasco CC, Markov DA, et al. Engineering Challenges for Instrumenting and Controlling Integrated Organ-on-a-Chip Systems. IEEE Trans Biomed Eng. 2013;60(3):682–690. doi: 10.1109/TBME.2013.2244891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chrobak KM, Potter DR, Tien J. Formation of perfused, functional microvascular tubes in vitro. Microvascular Research. 2006;71(3):185–196. doi: 10.1016/j.mvr.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 78.Prabhakarpandian B, Wang Y, Rea-Ramsey A, Sundaram S, Kiani MF, Pant K. Bifurcations: Focal Points of Particle Adhesion in Microvascular Networks. Microcirculation. 2011;18(5):380–389. doi: 10.1111/j.1549-8719.2011.00099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Prabhakarpandian B, Pant K, Scott RC, Patillo CB, Irimia D, Kiani MF, et al. Synthetic microvascular networks for quantitative analysis of particle adhesion. Biomed Microdevices. 2008;10(4):585–595. doi: 10.1007/s10544-008-9170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rosano JM, Tousi N, Scott RC, Krynska B, Rizzo V, Prabhakarpandian B, et al. A physiologically realistic in vitro model of microvascular networks. Biomed Microdevices. 2009;11(5):1051–1057. doi: 10.1007/s10544-009-9322-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tousi N, Wang B, Pant K, Kiani MF, Prabhakarpandian B. Preferential adhesion of leukocytes near bifurcations is endothelium independent. Microvascular Research. 2010;80(3):384–388. doi: 10.1016/j.mvr.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Huang JH, Kim J, Agrawal N, Sudarson AP, Maxim JE, Jayaraman A, et al. Rapid Fabrication of Bio-inspired 3D Microfluidic Vascular Networks. Adv Mater. 2009;21(35):3567–3571. [Google Scholar]
- 83.Zhu WH, Iurlaro M, MacIntyre A, Fogel E, Nicosia RF. The mouse aorta model: Influence of genetic background and aging on bFGF-and VEGF-induced angiogenic sprouting. Angiogenesis. 2003;6(3):193–199. doi: 10.1023/B:AGEN.0000021397.18713.9c. [DOI] [PubMed] [Google Scholar]
- 84.Nicosia RF, Zhu WH, Fogel E, Howson KM, Aplin AC. A new ex vivo model to study venous angiogenesis and arterio-venous anastomosis formation. J Vasc Res. 2005;42(2):111–119. doi: 10.1159/000083457. [DOI] [PubMed] [Google Scholar]
- 85.Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2(2):329–333. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- 86.Arnaoutova I, Kleinman HK. In vitro angiogenesis: endothelial cell tube formation on gelled basement membrane extract. Nat Protoc. 2010;5(4):628–635. doi: 10.1038/nprot.2010.6. [DOI] [PubMed] [Google Scholar]
- 87.Yanni SE, Penn JS. Animal Models of Retinopathy of Prematurity. In: Pang IH, Clark AF, editors. Animal models for retinal diseases. Totowa, N.J.: Humana Press; 2010. pp. 99–111. [Google Scholar]
- 88.Serbedzija G, Flynn E, Willett C. Zebrafish angiogenesis: A new model for drug screening. Angiogenesis. 1999 Dec 1;3(4):353–359. doi: 10.1023/a:1026598300052. [DOI] [PubMed] [Google Scholar]
- 89.Yang M, Li LN, Jiang P, Moossa AR, Penman S, Hoffman RM. Dual-color fluorescence imaging distinguishes tumor cells from induced host angiogenic vessels and stromal cells. PNAS (US) 2003;100(24):14259–14262. doi: 10.1073/pnas.2436101100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Amoh Y, Katsuoka K, Hoffman RM. Color-Coded Fluorescent Protein Imaging of Angiogenesis: The AngioMouse (R) Models. Curr Pharm Design. 2008;14(36):3810–3819. doi: 10.2174/138161208786898644. [DOI] [PubMed] [Google Scholar]