Abstract
P74, an envelope protein of the occlusion-derived virus (ODV) of Autographa californica M nucleopolyhedrovirus (AcMNPV), is critical for oral infection of Trichoplusia ni larvae. The role of P74 during primary infection, however, is unknown. Here we provide evidence that P74 facilitates binding of AcMNPV ODV to a specific receptor within the larval midgut epithelia of another host species, Heliothis virescens. We adapted a fluorescence dequenching assay to compare binding, fusion, and competition of wild-type AcMNPV ODV in vivo with itself and with the ODV of a p74-deficient AcMNPV mutant. We found that relative to wild-type ODV, binding and fusion of ODV deficient in P74 were both qualitatively and quantitatively different. Unlike wild-type ODV, an excess of P74-deficient ODV failed to compete effectively with wild-type ODV binding, and the overall binding level of the mutant ODV was one-third that of the wild type. These results implicated P74 as an ODV attachment protein that binds to a specific receptor on primary target cells within the midgut.
Autographa californica M nucleopolyhedrovirus (AcMNPV) (family Baculoviridae, genus Nucleopolyhedrovirus) produces two viral phenotypes during productive infection: occlusion-derived virus (ODV) and budded virus (BV). Each viral phenotype has a specific biological function: ODV establishes the first round of infection within its larval lepidopteran host, and BV, produced by the ODV-infected midgut cells, transmits infection to juxtaposed tracheal cells servicing them (4, 19, 20, 21, 22, 23). Subsequent generations of BV transmit infection throughout the host until ultimately the host dies and liquefies releasing millions, if not a billion, viral occlusions (5a).
Each viral phenotype performs its function under extremely different environmental conditions and targets different cell types. BV is a generalist; it infects a broad spectrum of cell types and circulates within the hemolymph, a protein-rich medium typically between pH 6.4 and 6.8 (3). BV is the more infectious of the two phenotypes in cell culture, and for AcMNPV, this difference is ≈1,700-fold (on a PFU per picogram viral protein basis) (18). Accordingly, most of what we understand about baculovirus infection pertains to BV in cell culture. In contrast to BV, ODV is a specialist; it targets highly differentiated columnar epithelial cells within the larval midgut. ODV accesses these cells apically after having been released from occlusions, polyhedral crystals of pH-sensitive virally encoded protein that both occlude and protect the virions in the environment. These occlusions, upon being ingested by feeding lepidopteran larvae, encounter highly alkaline midgut fluids (pH 9.2 to 11) that induce their rapid dissolution. The ODV thus liberated must survive the harsh alkaline digestive fluids of the host, penetrate the protective peritrophic membrane bordering the midgut epithelium, and make contact with the apical microvilli of columnar cells in order to establish infection (5a).
The only described mode of nucleocapsid entry into midgut cells is fusion of the ODV envelope with the microvillar membrane. These ODV-microvillar fusion events, as well as the presence of unenveloped nucleocapsids within microvilli, have been clearly documented by electron microscopy (1, 6, 11, 15, 16). Existing data, therefore, suggest that primary infection begins with fusion of the ODV envelope and an apical microvillar membrane of a columnar cell, but functional infection by this mode of entry has not been confirmed. Very little is known about the factors and events that mediate primary infection in the caterpillar host, even though primary infection is clearly a critical determinant of host range.
We do know that ODV infection of midgut columnar epithelial cells is a process that requires special cross talk between ODV and its target cell. This cross talk is mediated, in part, by the products of a few highly conserved per os infectivity factor genes (PIFs) that are essential for ODV infectivity but completely dispensable for BV infectivity (13). In the absence of any one of the pif gene products, infection is blocked prior to viral gene expression in midgut cells (13; J. Washburn, unpublished results) indicating that the PIFs are structural proteins involved in early infection events (13). AcMNPV p74 was the first of the pif genes to be reported (5, 11). More recently, two additional pif genes, Spodoptera littoralis NPV orf 7 (homologous to AcMNPV orf 119) (9a) and Spodoptera exigua orf 35 (homologous to AcMNPV orf 022) (14), have been described. These two genes have been named pif and pif-2, respectively (9a, 14).
In order to investigate whether these critical ODV gene products are involved in viral entry, we adapted an octadecyl rhodamine B chloride (R-18) dequenching assay (7) to measure ODV binding and fusion in vivo. Using newly molted fourth instar Heliothis virescens larvae and AcMNPV ODV, we established the validity of the assay by showing that R-18-labeled ODV binding could reach saturation, labeled ODV could be displaced, progressively, by coadministration of increasing dosages of excess unlabeled ODV, and a portion of the ODV that bound also fused. We next used the assay to compare control and P74-deficient AcMNPV ODVs in binding, fusion, and competition experiments. We found that the mutant ODV bound to midgut cells at approximately one-third the level of wild-type ODV but that, after binding, there was no difference in the fraction of bound virus that fused. These results indicated that binding and fusion, per se, could not account for the vast difference in oral infection capacity between the two ODVs. Competition experiments, however, revealed a qualitative difference in binding that could impact functional entry. Unlike excess wild-type ODV, competition assays revealed that excess ODV lacking P74 could not displace R-18-labeled control ODV binding, suggesting that P74's essential role in infection may be to bind a specific cellular receptor.
MATERIALS AND METHODS
Inoculation and maintenance of test larvae.
Heliothis virescens and Trichoplusia ni eggs were provided by the American Cyanamid Corporation (Princeton, N.J.) or purchased from Benzon Research (Carlisle, Pa.). Larvae were reared in groups on a semisynthetic diet (Stoneville) at 28°C under constant illumination until the beginning of the penultimate instar (i.e., fourth). All larvae were injected with a 32-gauge needle fitted onto a plastic tuberculin syringe (1 ml) or a Hamilton glass syringe (250 μl) mounted onto a microapplicator (Burkhard). Newly molted fourth-instar larvae were inoculated orally with ODV or occlusions by carefully inserting a blunt needle through the mouth and into the lumen of the anterior midgut, where the virus suspension was discharged. Feeding fourth-instar larvae were injected intrahemocoelically with BV by inserting a sharp needle through the planta of one of the prolegs and delivering a 1.0-μl suspension of the virus directly into the hemocoel.
To determine mortality levels, bioassays were performed in which 24 to 32 larvae were inoculated with each viral preparation. Following inoculation, test larvae were placed into individual 25-ml plastic cups, provided with Stoneville diet, and incubated at 28°C until death or pupation. When larvae did not completely liquefy, tissues were isolated and examined by light microscopy for the presence of occlusions to confirm that the larvae had died of polyhedrosis disease. For viral binding and fusion experiments, no diet was provided to larvae after inoculation.
Viruses and virus preparation.
Two AcMNPV recombinant viruses, AcMNPV-hsp70/lacZ (4) and AcLP4 (5), and the AcMNPV wild-type AcHR3 (5) were used in this study. AcMNPV-hsp70/lacZ contains all of the genes found in the wild-type virus plus the β-galactosidase reporter gene driven by the Drosophila hsp70 promoter and is as infective as the wild-type virus when inoculated into larvae (4). AcLP4 is a mutant lacking a functional p74 gene that was generated from the wild-type AcHR3 by excising 703 nucleotides from the middle of the p74 gene and inserting the β-galactosidase gene in frame with p74. Both AcHR3 and AcLP4 were kindly provided by Peter Faulkner.
Viral occlusions were isolated from virus-killed cadavers of T. ni, partially purified by sucrose density gradient centrifugation, and stored at 4°C in a neutrally buoyant solution of glycerin and water (3:2, vol/vol) until use. ODV was liberated by exposing the occlusions to dilute alkaline saline (100 mM NaCO3, 100 mM NaCl) for 10 min at room temperature. The ODV was then neutralized by adding one-tenth volume of 1 M Tris buffer (pH 7.6) to the virus suspension. Empty calyxes and undissolved occlusions were pelleted at 2,000 × g for 10 min; subsequently, ODV in the supernatant was banded by density equilibrium centrifugation on continuous 25 to 59% sucrose gradients for 1 h at 90,000 × g. The blue-gray ODV bands were collected and pooled. To eliminate the sucrose, the ODV was diluted 1:3 in phosphate-buffered saline (PBS: 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1 mM KH2PO4, pH 7.4) and pelleted at 90,000 × g for 30 min. ODV pellets were resuspended in minimum volumes of PBS, total volumes were measured, and samples were removed to determine their protein concentrations with the BCA protein assay (Pierce). Subsequently, fetal bovine serum was added to the ODV preparations (1% final), and each ODV suspension was aliquoted into microcentrifuge tubes for storage at −80°C until use. AcMNPV BV was harvested from the medium of infected Sf-9 cells at 3 days postinfection, and the titers were determined by immunoplaque assay with Sf-9 cells (17).
R-18 labeling.
For binding, fusion, and competition assays, ODV was labeled with the self-quenching fluorescent probe octadecyl rhodamine B chloride (R-18; Molecular Probes) as described by Nussbaum and Loyter (12), with the following modification. Unbound R-18 was eliminated by dialysis with a dialysis cassette (10,000-molecular-weight cutoff; Pierce) in 1.5 liters of PBS for 12 to 16 h at 4°C in the dark. Under these conditions, R-18 was inserted into the viral envelope at self-quenching levels. For each R-18-labeled ODV preparation, a sample was removed for determination of fluorescence emission and protein concentration. Subsequently, the labeled ODV was diluted to ≈1.5 mg/ml in PBS with 1% fetal bovine serum and stored at 4°C until use. Mock-labeled ODV was prepared similarly but in the absence of R-18 probe. To confirm that all residual unbound R-18 had been eliminated from the preparations, samples of labeled ODV were centrifuged at 90,000 × g for 1 h. Triton X-100 (1% final concentration) was then added to both the supernatant and pellet, and the fluorescence was measured with a Fluorolog-3 fluorescence spectrophotometer (Instruments S.A.) fitted with a temperature-controlled cuvette holder set at 4°C at 560 and 583 nm (excitation and emission, respectively). R-18 fluorescence was associated only with the pellet, showing that all R-18 was bound to the ODV particles (data not shown).
Samples of labeled ODV that were used to determine specific fluorescence were serially diluted in PBS and solubilized with Triton X-100 (1% final concentration). Protein concentrations were determined, and fluorescence measurements were made as described above. These two values were used to calculate fluorescence units per microgram of labeled ODV protein. Among the preparations, this value ranged from 1.6 × 106 to 3.8 × 106 fluorescence units/μg of labeled ODV protein, but for all experiments with paired viruses (e.g., AcHR3 versus AcLP4), the specific fluorescence values were equivalent (data not shown).
In vivo fusion assay. (i) Dissection of labeled-ODV-inoculated larvae.
Fourth-instar H. virescens larvae were inoculated orally with 2 μl of labeled ODV or PBS (negative control). At specific time points after inoculation, each larva was dissected in ice-cold separation buffer (100 mM NaCO3, 100 mM KCl, 100 mM EGTA, pH 9.5) to remove the intact digestive tract. To isolate the midgut epithelium, incisions were made along the length of the midgut and at the fore- and hindgut junctions within the midgut. The peritrophic membrane (present in all experimental larvae, newly molted and older) was removed and discarded, and the excised midgut was incubated for 10 min in ice-cold separation buffer. This incubation facilitated the subsequent physical separation of the intact midgut epithelium from the adherent basal lamina (and the associated muscles and tracheal elements) with forceps. The detached epithelium was then rinsed twice in ice-cold separation buffer in the dark for a total of 30 min. Each midgut epithelium was then transferred in 40 μl of separation buffer to a chilled methacrylate cuvette with four clear sides (Fisher) and frozen in liquid nitrogen. Cuvettes containing frozen midgut epithelia were stored in the dark at −80°C until fluorescence analysis was performed.
To identify the primary cellular targets of labeled ODV, fourth-instar H. virescens larvae were inoculated orally with labeled AcHR3 ODV (5 or 0.5 μg) or PBS. After incubation for 1 h at 28°C, larvae were dissected, and their isolated midgut epithelia were mounted onto slides for examination by light and epifluorescence compound microscopy.
(ii) Quantification of labeled ODV binding and fusion.
To quantify labeled ODV fusion levels, ice-cold separation buffer was added to each cuvette (2 ml final volume). Immediately after the midgut had thawed, it was disrupted with two to four strokes of a P1000 pipette to generate a suspension of midgut epithelial cells and cell fragments. Fluorescence associated with labeled ODV fused to each midgut preparation was immediately measured in a Fluorolog-3 fluorescence spectrophotometer (Instruments S.A.) fitted with a temperature-controlled cuvette holder set at 4°C at 560 and 583 nm (excitation and emission, respectively). To quantify the amount of labeled ODV bound to each midgut epithelium, Triton X-100 (1% final concentration) was added, and each sample was allowed to solubilize completely (100%) by incubation in the dark for 8 to 10 h at room temperature. The cuvettes were subsequently chilled to 4°C for 1 h, and then fluorescence measurements were made again as described above. Fluorescence values were corrected for the increased volume that resulted from the addition of detergent and for the background fluorescence associated with the midgut epithelial tissue of PBS-inoculated larvae in the absence of fluorescent probe.
In time course experiments, fourth-instar H. virescens larvae were inoculated orally with 3 μg of AcMNPV-hsp70/lacZ-labeled ODV and incubated in the dark at 28°C. At specific times following inoculation, larvae were dissected (14 to 16 larvae for each time point), and the midguts were processed to quantify the amount of labeled ODV that had bound and fused.
In saturation experiments, fourth-instar H. virescens larvae were inoculated orally with increasing concentrations of AcMNPV-hsp70/lacZ-labeled ODV (10 to 16 larvae for each viral dosage). Larvae were incubated at 28°C for 1 h in the dark. Subsequently, larvae were dissected, and the midguts were processed for labeled ODV binding and fusion as described above. To generate labeled ODV doses greater than 8 μg/μl, labeled ODV was concentrated by centrifugation at 90,000 × g for 30 min in a step 25 to 59% sucrose gradient. Labeled ODV was collected at the interface, and the sucrose was eliminated from the concentrated preparation by dialysis as described above.
In competition experiments with AcMNPV-hsp70/lacZ, fourth-instar H. virescens larvae were inoculated orally with 2 μg of labeled ODV with or without an excess of unlabeled AcMNPV-hsp70/lacZ ODV (12 to 15 larvae for each treatment). In competition experiments with AcHR3 and AcLP4, fourth-instar H. virescens larvae were inoculated orally with 0.5 μg of AcHR3-labeled ODV in the presence or absence of unlabeled AcLP4 ODV or AcHR3 ODV competitor (24 to 27 larvae for each treatment). Larvae were incubated at 28°C for 1 h in the dark prior to sampling. Viral binding and fusion were quantified as described above. All reported P values are from comparisons of means with a Student t test (Intercooled STATA 6 software package).
RESULTS
Oral infectivity of labeled ODV.
Ultimately, the utility of our assay was dependent upon the degree to which the labeling procedure affected ODV infectivity. We therefore compared the oral infectivities of mock-labeled AcMNPV-hsp70/lacZ ODV and labeled ODV in newly molted fourth instar H. virescens (Fig. 1). AcMNPV-hsp70/lacZ is a recombinant virus with wild-type infectivity that we routinely use in our laboratory (see Materials and Methods). The results showed there was a fivefold reduction in oral infectivity for ODV labeled with R-18 relative to mock-labeled ODV control virus. The 50% lethal dose (LD50) of labeled ODV was 16 pg, indicating that although experimental manipulation had reduced the infectivity of both our preparations somewhat, adequate amounts of infectious ODV and labeled ODV to be useful remained in our assay.
FIG. 1.
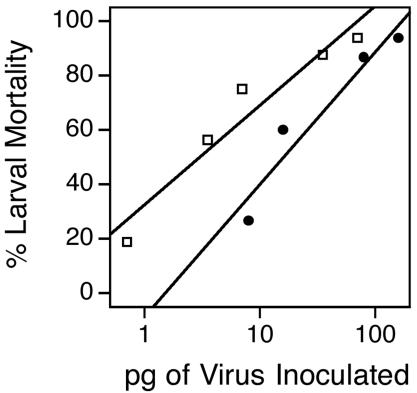
Larval mortality levels after fourth-instar H. virescens larvae were inoculated orally with AcMNPV-hsp70/lacZ mock-labeled ODV (open squares) or labeled ODV (solid circles). Viral dosages were quantified by determining protein concentrations with the BCA assay. For mock-labeled ODV, LD50 = 3 pg, y = 36.5log(x) + 32.47, and r2 = 0.93; for labeled ODV, LD50 = 16 pg, y = 48.6log(x) − 8.61, and r2 = 0.93.
Labeled ODV time course, saturation, and competition.
Time course experiments were performed to determine the time required for maximum AcMNPV-hsp70/lacZ-labeled ODV binding and fusion to the midgut epithelium of H. virescens. Maximum levels of both were achieved within the first 15 min after inoculation of 3 μg of AcMNPV-hsp70/lacZ-labeled ODV per larva, and the levels remained constant for at least 90 min after inoculation (Fig. 2). The preparation time required for each experimental larva precluded sampling at time points of less than 15 min. The amount of labeled ODV that bound ranged between 0.020 and 0.025 μg; of that, approximately 60% fused (0.012 to 0.015 μg). Because the amount of labeled ODV bound and fused remained constant between 15 and 90 min after inoculation, we used a 60-min incubation time to accommodate processing large numbers of larvae per time point in subsequent experiments.
FIG. 2.
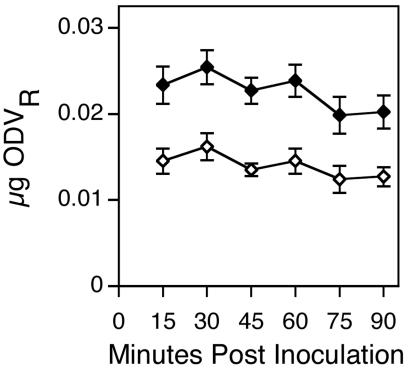
Amount (± 1 standard error) of labeled ODV bound (solid diamonds) and fused (open diamonds) to the midgut epithelia of fourth-instar H. virescens larvae inoculated orally with 3 μg of AcMNPV-hsp70/lacZ labeled ODV and incubated for increasing periods of time prior to sampling (14 to 16 larvae per time point).
Saturation experiments were conducted to determine whether the interaction between the labeled ODV and the receptors on target cells within the midgut epithelium was specific. Saturation occurred at a dosage between 8.8 and 11.7 μg of inoculated labeled ODV, demonstrating that the labeled ODV binding sites were finite on midgut epithelial cells, one indication of specificity (Fig. 3). At saturating dosages, on average, 0.155 μg of labeled ODV bound and 0.122 μg of labeled ODV fused to each larval midgut, representing an estimated 2.8 × 108 and 2.2 × 108 ODV particles, respectively (18). At saturation, approximately 78% of the labeled ODV that bound to the midgut also fused.
FIG. 3.
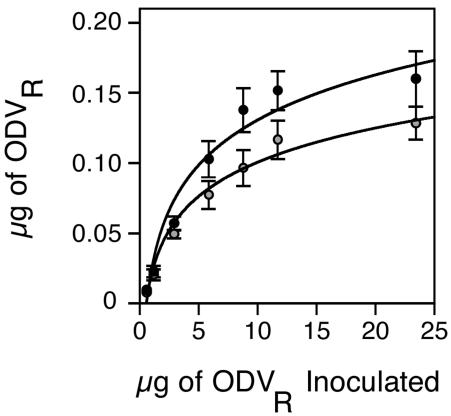
Amount (± 1 standard error) of labeled ODV (ODVR) bound (solid circles) and fused (shaded circles) to the midgut epithelia of fourth-instar H. virescens larvae inoculated orally with increasing concentrations of AcMNPV-hsp70/lacZ labeled ODV (10 to 16 larvae per dosage). For labeled ODV bound, y = 0.53log(x) + 0.061 and r2 = 0.92; for labeled ODV fused, y = 0.25log(x) + 0.039 and r2 = 0.93).
To investigate further the specificity of labeled ODV-midgut interactions, competition experiments were conducted in which fourth-instar H. virescens larvae were inoculated orally with 2 μg of AcMNPV-hsp70/lacZ-labeled ODV in the presence or absence of an excess of unlabeled ODV. The amount of bound labeled ODV was inversely proportional to the amount of unlabeled ODV coinjected into larvae, with approximately one-half of the labeled ODV being displaced in the presence of a 60-fold excess of unlabeled ODV (Fig. 4). These results provided evidence that the unlabeled ODV competed with labeled ODV for specific cellular receptors within the midgut epithelia (Fig. 4).
FIG. 4.
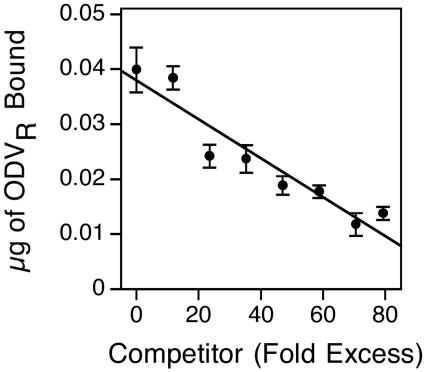
Amount (± 1 standard error) of labeled ODV bound to the midgut epithelia of fourth-instar H. virescens larvae inoculated orally with 2 μg of AcMNPV-hsp70/lacZ-labeled ODV (ODVR) with or without an excess of competitor, unlabeled AcMNPV-hsp70/lacZ ODV (12 to 15 larvae per treatment) (y = −0.0001 + 0.038; r2 = 0.90).
Comparative oral and intrahemocoelic infectivity of AcLP4 and AcHR3.
Having demonstrated that ODV binding was both saturable and competable in H. virescens, we next compared the ODVs of the p74-deficient mutant AcLP4 and its wild-type parent virus, AcHR3 (5). Because the p74-deficient phenotype had only been demonstrated in larvae of T. ni, we first had to confirm the phenotype in H. virescens. The results of our bioassays showed that the BVs of AcHR3 and AcLP4 were equally infectious when intrahemocoelically injected into H. virescens (Fig. 5A). In contrast, occlusions of the p74-deficient mutant (AcLP4) inoculated orally into fourth-instar H. virescens larvae were between five and six orders of magnitude less infectious than the parental wild-type AcHR3 (Fig. 5B). Thus, the phenotype reported in T. ni (5) was the same in H. virescens: P74 was critical for oral but not for systemic infectivity of AcMNPV in both hosts.
FIG. 5.
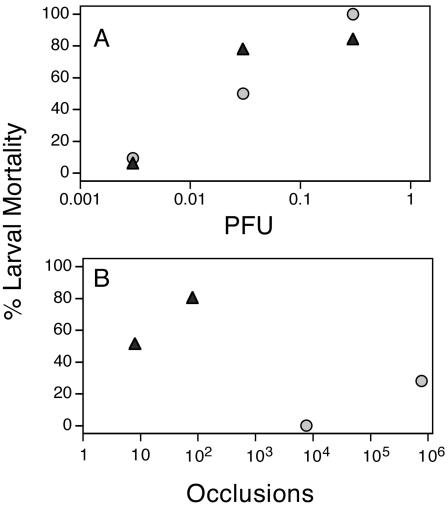
Larval mortality levels after feeding fourth-instar H. virescens larvae were injected intrahemocoelically with BV (A) or inoculated orally with occlusions (B) of wild-type AcHR3 (solid triangles) or the p74-deficent mutant AcLP4 (shaded circles); n = 25 to 30 larvae per treatment. Occlusion and BV dosages were determined from the means of 10 hemacytometer counts and eight plaque assay replicates on Sf-9 cells, respectively.
Binding and fusion of AcHR3- and AcLP4-labeled ODV.
To test the importance of P74 during ODV binding and fusion to the midgut of a host susceptible to AcMNPV, fourth-instar H. virescens larvae were inoculated orally with 3 μg of labeled ODV of either wild-type AcHR3 or AcLP4. Compared to wild-type AcHR3-labeled ODV, approximately threefold less labeled ODV of the p74 deletion mutant bound to the midgut epithelia; once bound, however, the proportions of labeled ODV that fused were similar for AcHR3 (64%)- and AcLP4 (72%)-labeled ODV (Fig. 6). Fluorescence microscopic observations of isolated midgut epithelia from labeled-ODV-treated larvae revealed that mature columnar cells displayed the greatest amount of R-18 fluorescence, confirming that they were the primary targets of infection (Fig. 7).
FIG. 6.
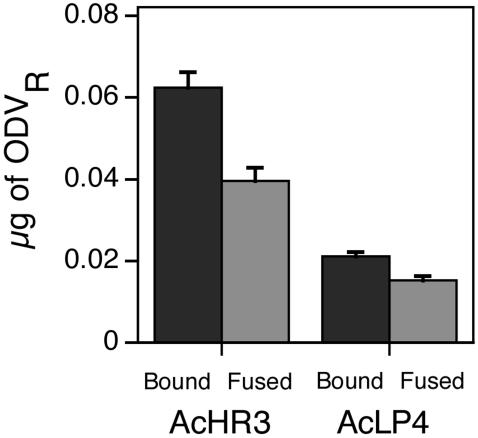
Amount (+1 standard error) of labeled ODV bound and fused to the midgut epithelium of fourth-instar H. virescens larvae inoculated orally with 3 μg of AcHR3- or AcLP4-labeled ODV (ODVR) (24 to 27 larvae per treatment).
FIG. 7.
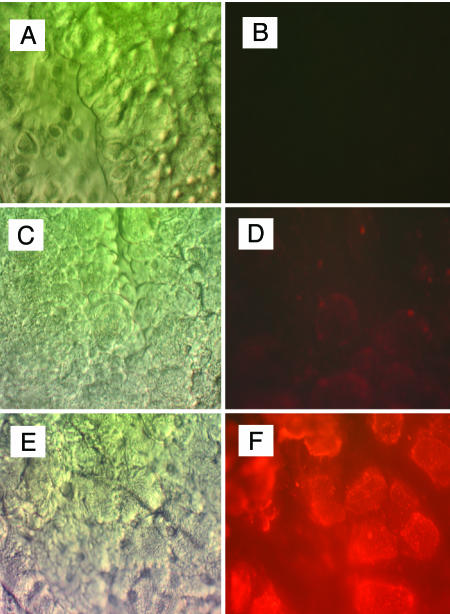
Midgut epithelia isolated from H. virescens larvae inoculated orally with 0.5 μg (C and D) or 5.0 μg (E and F) of AcHR3- labeled ODV (ODVR) or PBS (A and B) and viewed by differential interference contrast (left) and epifluorescence (right) compound microscopy. Magnification, ×400.
To determine whether the ODVs of AcHR3 and AcLP4 were bound to the same receptors, competition experiments were conducted with wild-type AcHR3-labeled ODV and unlabeled wild-type ODV or AcLP4 ODV. The results showed that increasing concentrations of unlabeled wild-type ODV progressively lowered the amount of AcHR3-labeled ODV bound to midgut tissue, with 55 and 80% reductions, respectively, at 95- and 161-fold excesses (Fig. 8). By comparison, AcLP4 ODV reduced AcHR3-labeled ODV binding by less than 25%, even at an excess of 158-fold (Fig. 8). Collectively, these results suggested that P74 was responsible for mediating specific binding of AcMNPV ODV to primary cellular targets in the midgut of H. virescens and that nonspecific binding and fusion were not sufficient to mediate productive entry of ODV into target cells.
FIG. 8.
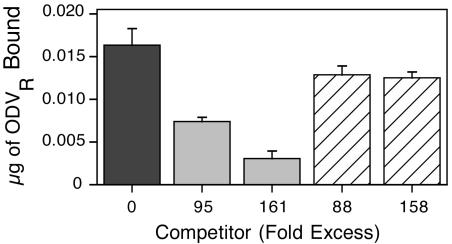
Amount (+1 standard error) of labeled ODV bound to the midgut of fourth-instar H. virescens larvae inoculated orally with 0.5 μg of AcHR3-labeled ODV (ODVR) alone (black) or in the presence of competitor, unlabeled AcHR3 ODV (shaded) or AcLP4 ODV (striped) (11 to 13 larvae per treatment).
DISCUSSION
We adapted a fluorescence dequenching assay for use in determining binding and fusion profiles of baculovirus ODV in vivo with larval lepidopteran midgut cells. ODV interaction with the microvilli of its target cells is of interest not only because of its fundamental importance in baculovirus host range determination, but also because it provides an unusual opportunity to study viral binding and fusion events in a highly alkaline environment. Fusion mechanisms of enveloped viruses have been studied thus far almost exclusively in neutral or acidic environments (3a).
Our assay is conducted in vivo, so that the cellular and tissue architecture of the midgut and conditions within the midgut lumen are preserved in their native states during the binding and fusion processes. These attributes likely contribute to a higher fidelity binding and fusion assay than those conducted with preparations of brush border membrane vesicles, especially when final-instar larvae are used to make brush border membrane vesicles (23). Relative to earlier-instar larvae, final-instar larvae are extremely resistant to baculovirus infection (2), and much of the resistance is associated with changes that occur within the midgut (10). Even so, using a Lymantria dispar brush border membrane vesicle-based fluorescence dequenching assay, Horton and Burand (8) were able to generate evidence that L. dispar MNPV ODV binds to specific receptors. Our results confirmed their observations and extended them to another baculovirus system.
Prior to testing our in vivo system for utility in investigating ODV-midgut interactions, we determined that R-18 labeling of ODV reduced infectivity approximately fivefold in bioassays. This level of reduced infectivity is consistent with infectivity losses reported for other R-18-labeled viruses (24). We next demonstrated that H. virescens midgut cells could become saturated with R-18-labeled ODV and that labeled ODV binding could be reduced progressively by the addition of increasing amounts of excess unlabeled ODV. These results suggested that the assay would be useful for obtaining information about the specificity of binding and relative levels of fusion of ODV. With this assurance, we compared binding, fusion, and ability to compete between wild-type AcMNPV ODV from strain AcHR3 and ODV from AcLP4, a p74 mutant derived from this strain. We used viruses obtained from Peter Faulkner for our study because they were the original viruses used to show that P74 was essential for infectivity in T. ni larvae (11). In addition, Faulkner et al. (5) reported that P74 was an ODV envelope protein with properties characteristic of a viral attachment protein.
Initially, we tested AcLP4 in bioassays with H. virescens to confirm that P74 was essential for oral infectivity in this species as it is in T. ni. We found that oral administration of 1,000 AcLP4 occlusions induced no mortality, confirming the critical nature of the protein. In comparative binding and fusion studies, however, we found only a threefold difference in binding and no difference in relative fusion levels between wild-type AcHR3 and AcLP4-labeled ODV.
These results demonstrated that the lack of binding and fusion, per se, did not explain the lack of infectivity of AcLP4. The threefold difference in binding was insufficient to account for the greater than 100,000-fold difference in oral infectivity. In fact, the results clearly showed that ODV binding and fusion do not ensure that productive infection will ensue any more than binding and fusion of BV do. A number of studies conducted in vitro on baculovirus host range have shown that BV can enter a broad range of phylogenetically diverse cells but cannot infect or reach the nucleus in most of them (11a).
A result perhaps more relevant to productive infection by ODV was obtained in our competition experiments, where only 22% of AcHR3-labeled ODV could be competed by an 88-fold excess of unlabeled AcLP4 ODV (Fig. 8). The fact that binding of AcHR3-labeled ODV could not be reduced further by increased concentrations of AcLP4 ODV competitor (as it could be with AcHR3 ODV competitor) suggested that P74 was a viral attachment protein involved in specific binding of 78% of the bound AcHR3-labeled ODV. We postulate from these results that specific binding afforded by P74 is essential for establishment of primary infection. Hence, productive infection by ODV, like that by BV, requires specialized entry pathways.
Acknowledgments
Financial support was provided by USDA NRICG 2002-02549, Torrey Mesa Research Institute, Syngenta Research and Technology, San Diego, Calif., and by federal Regional Research and HATCH funds. The CNR Biological Imaging Facility supplied the equipment used for photographing and viewing fluorescence microscopic images.
REFERENCES
- 1.Adams, J. R., and J. T. McClintock. 1991. Nuclear polyhedrosis viruses of insects, p. 87-204. In J. R. Adams and J. R. Bonami (ed.), Atlas of invertebrate viruses. CRC Press, Boca Raton, Fla.
- 2.Briese, D. T. 1986. Insect resistance to baculoviruses, p. 89-108. In B. A. Federici and R. R. Granados (ed.), The biology of baculoviruses, vol. II. CRC Press, Boca Raton, Fla. [Google Scholar]
- 3.Chapman, R. F. 1998. The insects: structure and function, 4th ed., p. 116. Cambridge University Press. Cambridge, United Kingdom.
- 3a.Eckert, D. M., and P. S. Kim. 2001. Mechanisms of viral membrane fusion and its inhibition. Annu. Rev. Biochem. 70:777-810. [DOI] [PubMed] [Google Scholar]
- 4.Engelhard, E. K., L. N. Kam-Morgan, J. O. Washburn, and L. E. Volkman. 1994. The insect tracheal system: a conduit for the systemic spread of Autographa californica M nuclear polyhedrosis virus. Proc. Natl. Acad. Sci. USA 91:3224-3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faulkner, P., J. Kuzio, G. V. Williams, and J. A. Wilson. 1997. Analysis of p74, a PDV envelope protein of Autographa californica nucleopolyhedrovirus required for occlusion body infectivity in vivo. J. Gen. Virol. 78:3091-3100. [DOI] [PubMed] [Google Scholar]
- 5a.Federici, B. A. 1997. Baculovirus pathogenesis, p. 33-59. In L. K. Miller (ed.), The baculoviruses. Plenum Press, New York, N.Y.
- 6.Granados, R. R., and K. A. Lawler. 1981. In vivo pathway of Autographa californica baculovirus invasion and infection. Virology 108:297-308. [DOI] [PubMed] [Google Scholar]
- 7.Hoekstra, D., T. Deboer, K. Klappe, and J. H. Wilschut. 1984. Fluorescence method of measuring the kinetics of fusion between biological membranes. Biochemistry 23:5675-5681. [DOI] [PubMed] [Google Scholar]
- 8.Horton, H. M., and J. P. Burand. 1993. Saturable attachment sites for polyhedron-derived baculovirus on insect cells and evidence for entry via direct membrane fusion. J. Virol. 67:1860-1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawanish, C. Y., M. D. Summers, D. B. Stoltz, and H. J. Arnott. 1972. Entry of an insect virus in vivo by fusion of viral envelope and microvillus membrane. J. Invertebr. Pathol. 20:104-108. [DOI] [PubMed] [Google Scholar]
- 9a.Kikhno, I., S. Guitiérrez, L. Croizier, G. Croizier, and M. L. Lopéz-Ferber. 2002. Characterization of pif, a gene required for the per os infectivity of Spodoptera littoralis nucleopolyhedrovirus. J. Gen. Virol. 83:3013-3022. [DOI] [PubMed] [Google Scholar]
- 10.Kirkpatrick, B. A., J. O. Washburn, and L. E. Volkman. 1998. AcMNPV pathogenesis and developmental resistance in fifth instar Heliothis virescens. J. Invertebr. Pathol. 72:63-72. [DOI] [PubMed] [Google Scholar]
- 11.Kuzio, J., R. Jaques, and P. Faulkner. 1989. Identification of P74, a gene essential for virulence of baculovirus occlusion bodies. Virology 173:759-763. [DOI] [PubMed] [Google Scholar]
- 12.Nussbaum, O., and A. Loyter. 1987. Quantitatiave determination of virus membrane fusion events. Fusion of influenza virions with plasma membranes and membranes of endocytic vesicles in living cultured cells. FEBS Lett. 221:61-67. [DOI] [PubMed] [Google Scholar]
- 13.Ohkawa, T. 1997. Identification and characterization of genes of the baculovirus Bombyx mori nucleopolyhedrovirus (BmNPV) involved in viral pathogenesis. Ph.D. thesis. University of California, Davis.
- 14.Pijlman, G. P., A. J. P. Pruijssers, and J. M. Vlak. 2003. Identification of pif-2, a third conserved baculovirus gene required for per os infection of insects. J. Gen. Virol. 84:2041-2049. [DOI] [PubMed] [Google Scholar]
- 15.Summers, M. D. 1971. Electron microscopic observations on granulosis virus entry, uncoating and replication process during infection of the midgut cells of Trichoplusia ni. J. UltraStruct. Res. 35:606-625. [DOI] [PubMed] [Google Scholar]
- 16.Tanada, Y., R. T. Hess, and E. M. Omi. 1975. Invasion of a nuclear polyhedrosis virus in midgut of the armyworm, Pseudaletia unipuncta, and the enhancement of a synergistic enzyme. J. Invertebr. Pathol. 26:99-104. [DOI] [PubMed] [Google Scholar]
- 17.Volkman, L. E., and P. A. Goldsmith. 1982. Generalized immunoassay for Autographa californica nuclear polyhedrosis virus infectivity in vitro. Appl. Environ. Microbiol. 44:227-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Volkman, L. E., M. D. Summers, and C. H. Hsieh. 1976. Occluded and nonoccluded nuclear polyhedrosis virus grown in Trichoplusia ni: Comparative neutralization, comparative infectivity and in vitro growth studies. J. Virol. 19:820-832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Washburn, J. O., E. Y. Chan, L. E. Volkman, J. J. Aumiller, and D. L. Jarvis. 2003. Early synthesis of budded virus envelope fusion protein GP64 enhances Autographa californica multicapsid nucleopolyhedrosis virulence in orally infected Heliothis virescens. J. Virol. 77:280-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Washburn, J. O., B. A. Kirkpatrick, E. Haas-Stapleton, and L. E. Volkman. 1998. Evidence that the stilbene-derived optical brightener M2R enhances Autographa californica M nucleopolyhedrovirus infection of Trichoplusia ni and Heliothis virescens by preventing sloughing of infected midgut epithelial cells. Biol. Control 11:58-69. [Google Scholar]
- 21.Washburn, J. O., Kirkpatrick, B. A., and Volkman, L. E. 1995. Comparative pathogenesis of Autographa californica M nuclear polyhedrosis virus in larvae of Trichoplusia ni and Heliothis virescens. Virology 209:561-568. [DOI] [PubMed] [Google Scholar]
- 22.Washburn, J. O., E. H. Lyons, E. J. Haas-Stapleton, and L. E. Volkman. 1999. Multiple nucleocapsid packaging of Autographa californica nucleopolyhedrovirus accelerates the onset of systemic infection in Trichoplusia ni. J. Virol. 73:411-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolfersberger, M., P. Luethy, A. Maurer, P. Parenti, F. V. Sacchi, B. Giordana, and G. M. Hanozet. 1987. Preparation and partial characterization of amino acid transporting berush border membrane vesicles from the larval midgut of the cabbage butterfly (Pieris brassicae). Comp. Biochem. Physiol. 86:301-308. [Google Scholar]
- 24.Wunderliallenspach, H., M. Gunthert, and S. Ott. 1993. Inactivation of Pr8-influenza virus through the octadecylrhodamine-β chloride membrane marker. Biochemistry 32:900-907. [DOI] [PubMed] [Google Scholar]


