Abstract
Purpose
Imiquimod is a toll-like receptor 7 agonist utilized topically to manage genital warts and basal cell carcinoma. We examine the combination of topical imiquimod with intramuscular administration of CRT/E7, a therapeutic HPV vaccination that comprises a naked DNA vector expressing calreticulin fused to HPV16 E7.
Experimental Design
Using an orthotopic HPV16 E6/E7+ syngeneic tumor, TC-1, as a model of high-grade cervical/vaginal/vulvar intraepithelial neoplasia, we show that combining CRT/E7 vaccination with cervicovaginal deposition of imiquimod results in synergistic immune-mediated tumor clearance.
Results
Imiquimod induces cervicovaginal accumulation of activated E7-specific CD8+ T cells elicited by CRT/E7 vaccination. Recruitment was not dependent upon the specificity of the activated CD8+ T cells, but was significantly reduced in mice lacking the IFNγ receptor. Intravaginal imiquimod deposition induced upregulation of CXCL9 and CXCL10 mRNA expression in the genital tract. These chemokines are expressed upon IFNγ receptor activation and attract cells expressing their receptor, CXCR3. In this study, T cells attracted by imiquimod to the cervicovaginal tract expressed CXCR3 as well as the tissue resident memory T cell (Trm) marker CD49a, a mucosal homing integrin. Our results indicate that intramuscular CRT/E7 vaccination in conjunction with intravaginal imiquimod deposition recruits antigen-specific CXCR3+CD8+ T cells to the genital tract.
Conclusions
Our study has potential clinical relevance because imiquimod is FDA approved for condyloma accuminata and basal cell carcinoma and intramuscular vaccination with pNGVL4a-CRT/E7(detox) is currently undergoing clinical testing, suggesting potential for their synergistic action to induce strong antigen-specific Trm-mediated immune responses and antitumor effects in genital mucosa.
Keywords: immunotherapy, tissue resident memory T cell, HPV vaccine, imiquimod
Introduction
Human papillomaviruses (HPVs) are the primary etiologic agents for cervical cancer and subsets of vaginal, vulvar and anal cancers and head and neck cancers (1). Approximately 90% of vaginal, vulvar and anal cancers associated with HPV are attributable to HPV16 (1). Moreover, while surgical treatment is quite effective and well tolerated for precancer and early cancer lesions of the cervix, a subset of treated patients are at risk for preterm delivery and/or cervical incompetence, and premature rupture of membranes (2), which can be devastating to young women. Furthermore, surgical treatment of a subset of vaginal, vulvar and anal intraepithelial lesions, particularly multicentric and recurrent lesions, is associated with significant morbidity and high recurrence rates (3, 4). As such, it is important to develop effective alternative therapeutic methodologies to treat HPV-associated precancer lesions and cancers, particularly for the non-cervical sites that are predominantly associated with HPV16.
Immunotherapy can potentially be used to treat HPV-associated disease. HPV oncoproteins E6 and E7 are required for the induction and maintenance of cellular transformation, lack many of the concerns for immune tolerance of self-antigens, and are consistently co-expressed in all HPV-infected, but not normal cells. Therapeutic vaccines, through activation of the adaptive immune system, can elicit a specific immune response to target precancer and cancer cells while sparing normal cells (for review see (5)). A long peptide-based therapeutic vaccine targeting HPV16 E6 and/or E7 shows partial efficacy against HPV16+ high grade VIN (6). These findings suggest that the viral oncoproteins have evolved to be weak antigens and therefore need approaches to render them more immunogenic and thus more potently activate HPV-specific cytotoxic T cell responses.
DNA vaccines targeting E6/E7 are promising because the antigen is produced intracellularly and can therefore enter the MHC class I pathway that is critical for the induction of cytotoxic T cell responses. To circumvent the poor immunogenicity of the HPV oncoproteins, we have developed an approach to enhance their immune presentation upon expression from a naked DNA vector. In this therapeutic HPV DNA vaccine, HPV16 E7 is linked to calreticulin (CRT/E7), a heat shock-related chaperone protein that enhances antigen processing and directs binding to, and activation of antigen-presenting cells (7). Vaccination by intramuscular administration of CRT/E7 DNA generated potent systemic cell-mediated immune responses against HPV16 E7 and subcutaneous TC-1 tumor, a syngeneic model of advanced cervical cancer that expresses HPV16 E6 and E7 (8–17). Furthermore, the clinical grade version of this vaccine, pNGVL4a-CRT/E7 (detox), is being tested in patients with HPV16+ high grade cervical intraepithelial neoplasia (CIN2/3) using different routes of administration (NCT00988559) (18).
Studies of the naturally occurring systemic immune responses of patients with high grade CIN indicate that HPV16-specific T cell responses in peripheral blood did not predict lesion regression (19). Furthermore, in a Phase I trial of a therapeutic HPV vaccine, systemic HPV-specific T cell responses were again not correlated with lesion clearance (20). Rather, local responses at the lesion site have been associated with disease clearance, suggesting the importance of both eliciting an HPV immune response and appropriately targeting it to the lesion site, possibly by altering the local immune microenvironment (21). Therapeutic HPV vaccination may potentially be combined with local innate inflammatory activation, such as with imiquimod, to attract antigen-specific immune responses to the site of application, and also promote a favorable immune microenvironment within the lesion to effect viral clearance (22).
Recently, tissue-resident memory T cells (Trm) have been shown to play significant roles in local immune responses involved in protection from infection by vaccination (23, 24). As such, vaccination that is able to elicit robust HPV-specific Trm has potential for the treatment of mucosal tumors (25). For example, intranasal vaccination generated a significantly enhanced therapeutic effect against an orthotopic head and neck tumor model and induced mucosal-specific CD8+ T cells as compared to intramuscular administration (26). This study suggests that it is important to consider a unique strategy to generate Trm using our therapeutic HPV vaccine to control HPV-associated diseases occurring in mucosal tissue.
Application of an adjuvant locally can further enhance site-specific immunity. For example, Luci et al linked the nontoxic B subunit of cholera toxin (CTB) to model antigen OVA and found that its administration intravaginally increased OVA-specific CD8+ T cells in the draining lymph nodes and genital mucosa (27). Additionally, Wille-Reece et al found that vaccination of non-human primates using toll-like receptor (TLR) agonists as adjuvants generated stable antigen-specific CD8+ T cells (28).
Imiquimod activates the innate immune response through TLR7-MyD88-dependent signaling, by interacting with TLR7 expressed by myeloid dendritic cells (DCs), plasmacytoid DCs, monocytes and macrophages (29). TLR7 activation induces secretion of various proinflammatory cytokines and also enhances DC maturation and antigen presentation (29). Furthermore, TLR7 ligands, including imiquimod, have been shown to lead to plasmacytoid DC activation and generate a curative effect against a breast tumor model and a melanoma model (30, 31). In particular, imiquimod stimulates IFN-γ production (32). IFN-γ in turn induces the production of CXCL9 and CXCL10, chemokines that attract CXCR3+ T cells (for review see (33)). Non-specific immunotherapy by topical application of imiquimod to HPV16+ high-grade vulvar intraepithelial neoplasia (VIN) shows significant therapeutic activity, but is less effective against large lesions (34). Additionally, imiquimod has been shown to increase the number of CD4+ and CD8+ in VIN lesions in combination with a therapeutic HPV fusion protein vaccine (22). These studies suggest that the use of local adjuvant, such as imiquimod, may enhance vaccines to induce antigen-specific tissue-resident T cells. In the current study, we examined whether deposition of imiquimod cream in the cervicovaginal tract following intramuscular CRT/E7 DNA vaccination could attract the systemic E7-specific cellular immune response to the genital tract and therein facilitate the cure of mice with TC-1 tumors, a syngeneic model of HPV16+ anogenital cancer, growing in the vaginal wall.
Materials and Methods
Experimental mice and tumor cells
6- to 8-week-old female C57BL/6 mice were purchased from the National Cancer Institute (Frederick, MD). IFN-γ receptor 1-deficient (ifngr1tm1Agt) mice (IFNγR−/−) and CXCR3-deficient (B6.129P2-Cxcr3tm1Dgen/J) mice (CXCR3−/−) have been previously described (Jackson Laboratories) (35). Mice were housed in the oncology animal facility of the Johns Hopkins Hospital (Baltimore, MD). All animal procedures were performed according to approved protocols and in accordance with recommendations for the proper use and care of laboratory animals.
The tumor cell line used in our study was TC-1. TC-1 cells were subjected to RapidMAP (Taconic Farms, Rensselaer, NY) testing, a panel of PCR tests for rodent viruses, most recently in May 2011 with negative results. In order to trace the tumor growth in vivo, TC-1 transfected with Luciferase (TC-1-Luc) was generated by lentivirus stable transfection as previously described (36). Cells were cultured in RPMI1640 medium containing 10% FBS, 2mM L-glutamine, 10% sodium pyruvate, 10% non-essential amino acids, and 100pg/ml streptomycin in a humidified atmosphere of 5% CO2/95% air at 37°C.
Luciferase-expressing E7-specific CD8+ T cell and OT-1 T cell preparation and adoptive transfer in mice
E7-specific T cells were generated from splenocytes of E7 vaccinated mice and were stimulated with irradiated TC-1 cells and 10 IU interleukin-2 (IL-2) weekly. In order to trace the E7-specific CD8 T cells in vivo, we have previously generated E7-specific cytotoxic T cells expressing luciferase (E7-Luc T cell) (36). OT-1 T cells were generated from OT-1 Rag−/− TCR transgenic mice from our lab as previously mentioned (37). T cell adoptive transfer was performed by injecting 5×105 T cells in 200μL PBS through the tail veins of mice.
DNA vaccine and drug treatments
The pNGVL4a-CRT-E7 (detox) plasmid was prepared by the NIH Rapid Access to Interventional Development (RAID) program. Imiquimod (Aldara) 5% cream was purchased from Taro Pharmaceutical Industries, Ltd (Haifa Bay, Israel). Mice were injected in the tibialis muscle of the shaved hind leg with a 28G syringe containing 40μg of DNA plasmid diluted in a total volume of 40μL of PBS. Each leg was injected with 20μg DNA vaccine. After the mice were under anesthesia with ketamine (75mg/kg)/xylazine (20mg/kg), 0.4 mg of imiquimod was applied in the vagina using a micropipette tip.
In vivo luciferase-based Bioluminescence Imaging
Luciferin (Sigma) was used to test for firefly luciferase activity in vivo (38). Mice were injected with the substrate luciferin 40mg/kg intraperitoneally and sedated by inhaling isoflurane USP (Baxter International, Inc). Bioluminescence of the mice was detected via the IVIS Imaging System 200 Series. The region of interest from displayed images was designated and quantified as total photon counts using Living Image 2.50 software (Xenogen).
Orthotopic cervical cancer model and treatment
Mice (5 per group) were challenged intravaginally with 2×104 TC-1-Luc cells/mice using methods previously described (39). Briefly, four days before the tumor implantation, female mice were diestrus synchronized by injection of medroxyroprogesterone (Greenstone LLC, NJ) s.c. (3mg/mouse), and all vaginal procedures performed upon isoflurane anesthesia. Mice were administered 4% nonoxynol-9 (N9, Igepal, Sigma) intravaginally one day before tumor challenge. On day one, the genital tracts were washed with PBS before injecting 2×104 TC-1-Luc cells into the vaginal tract. Tumor growth was confirmed by IVIS 2000 system on day 7 before immunization began. Mice were vaccinated with 40μg of CRT-E7 DNA plasmid via intramuscular injection 3 times at 3 day intervals. 0.4 mg of imiquimod 5% cream was deposited in the vagina on day 13 after the last boost vaccination and was subsequently applied every seven days. Mice were euthanized when they demonstrated stress or when they lost more than 20% of their body weight, based on animal care regulations.
Analysis of the immune cells in vaginal and tumor tissues
The genital tracts and tumor tissue of mice were dissected. Tissue samples were cut into small pieces and digested with 0.05 mg/ml collagenase I, 0.05 mg/ml collagenase IV, 0.025 mg/ml hyaluronidase IV, 0.25 mg/ml DNase I, 100 U/ml penicillin, and 100 μg/ml streptomycin and incubated at 37 °C for 60 minutes as previously described (17, 40). The tissue digest was then filtered through a 70μm nylon filter mesh to remove undigested tissue fragments.
Flow cytometry analysis and intracellular cytokine staining to detect IFN-γ secretion by E7-specific CD8+ T cells in the tumor microenvironment
H2-2Db tetramers labeled with phycoerythrin (PE) and complexed to the HPV-16 E7 (RAHYNIVTF) peptide were gifts from the NIH (41, 42). Single-cell suspended splenocytes, genital lymph nodes, and cervicovaginal cells from immunized and control mice were incubated in FACS buffer (0.5% BSA, 2 mM EDTA, 0.1% NaN3 in PBS). FITC-labeled anti-CD8a antibodies (BD 53-6.7) and PE-labeled tetramers were added and incubated at 4°C for 30 min. Samples were also stained with 7-AAD to exclude dead cells (BD). For analysis of the expression of integrin and chemokine receptors, CD8+ T cells were co-stained with anti-mouse CD103 APC-mAb (eBioscience 2E7), anti-mouse CD49a Alexa Fluor 647 (BD Ha3118) mAb, and CXCR3/CD183 APC-mAB (BD CXCR3-173). Cells were incubated with Fc blocker anti-CD16/CD32 (eBioscience) at 4°C for 5 min.
Cell surface marker staining for anti-mouse PE CD8 (BD RPA-T8) and intracellular cytokine staining for anti-mouse FITC IFN-γ (BD XMG1.2) as well as FACScan analysis were performed in the same conditions as those previously described (43). Lymphocytes extracted from tumor fragments were collected and incubated with 1 μg/ml of E7 peptide (RAHYNIVTF) as previously described (44) in 24-well plates for 8 hours. The number of IFN-γ-secreting CD8+ T cells was analyzed by FACScan cytometry. All analyses were performed with Flowjo 10.1.
Quantitative real-time PCR
mRNA from vaginal tissue was extracted using Trizol after homogenization as previously described (45). First strand cDNA was synthesized by reverse transcription, according to the manufacturer’s protocol (BioRad). Then, the first strain cDNA was used for quantitative real-time PCR using IQ SYBR Green (Invitrogen) on the MyiQ real-time detection system (BioRad) following the manufacturer’s protocol. The forward and reverse primers for β-actin sense-ACTGGGACGACATGGAGAAG, antisense-GGGGTGTTGAAGGTCTCAAA, TLR-7 sense-CCACAGGCTCACCCATACTTC, antisense-GGGATGTCCTAGCTGGTGACA, CXCR-9 sense-CAAATCCCTCAAAGACCTCAAAC, antisense-GATCTCCGTTCTTCAGTGTAGC, CXCR-10 sense-TCATCCCTGCGAGCCTAT, antisense-CTTGATGGTCTTAGATTCCGGAT, IFN-γ sense-ACAATGAACGCTACACACTGCAT, antisense-TGGCAGTAACAGCCAGAAACA, and β-Actin sense-ACTGGGACGACATGGAGAAG, antisense-GGGGTGTTGAAGGTCTCAAA(23). Gene expression levels were normalized to β-Actin housekeeping gene, and data were represented as fold differences by the 2−ΔΔCt method, where ΔCt = Cttarget gene − Ctβ-Actin and ΔΔCt = ΔCtinduced − ΔCtreference, as previous mentioned (46).
Statistical analysis
All data are expressed as mean ± S.E. where indicated. Comparisons between individual data points for intracellular cytokine staining with flow cytometric analysis and tumor treatment were made using Student’s t-test. In the tumor treatment experiments, the principal outcome of interest was duration until mice were sacrificed for ethical reasons (in stress, body weight lost is greater than 20%). The event time distributions for different mice were compared using the Kaplan–Meier method and the log-rank statistic by Prism 6 software. All p-values <0.05 were considered significant.
Results
Imiquimod deposition in the vagina increased the population of E7-specific CD8+ T cells locally
First, to examine the impact of inflammation in the genital tract, we examined the systemic and local effects of imiquimod treatment on the cervicovaginal tract after vaccination. Mice were vaccinated with 40μg pNGVL4a-CRT-E7 (detox) plasmid vaccine via intramuscular injection in the hind legs on days 1, 4 and 8. Imiquimod, or as a control PBS, was then applied to the vaginal tract of mice on day 15. Peripheral blood and vaginal tissue were collected on day 21. Mice treated with either imiquimod or PBS generated the similar levels of E7-specific CD8+ T cells in the circulation (Figures 1A and B). However, we observed that the genital tract of mice treated intravaginally with imiquimod displayed a 10-fold increase in total CD8+ T cells compared with mice treated with PBS (Figure 1C). Furthermore, the number of E7-specific CD8+ T cells in the vaginal tissue was also dramatically increased (8.2 fold) in mice treated with imiquimod compared to those treated with PBS, but suggests that the enrichment of activated T cells is not antigen-specific (Figure 1D and E). This suggests that local imiquimod application enhances local replication of the CD8+ T cells and/or recruits them to the site of treatment.
Figure 1. Deposition of imiquimod in the vagina attracts CD8+ T cells to the genital tract.
Groups of mice were vaccinated twice with a DNA plasmid encoding pNGVL4a-CRT-E7 (40μg) by intramuscular injection. Mice were treated with or without imiquimod. Blood was drawn one week after vaccination and cells were stained with anti-CD8 antibody, H2-Kb E7 tetramer, and 7-AAD (to exclude dead cells). (A) Representative flow cytometry analysis showing E7-specific CD8+ T cells in peripheral blood. (B) Bar graph depicting the percentage of E7-specific CD8+ T cells in circulation. (C) PBS or imiquimod 5% cream was applied in the vaginal tract one week after systemic vaccination with pNGVL4a-CRT-E7. Genital tract tissue was excised and digested. Bar graph shows the absolute number of CD8+ T cells in the genital tracts of treated mice. (D) Representative flow cytometry. (E) Bar graph depicting the absolute number of E7-specific CD8+ T cells in the genital tracts of mice treated with or without imiquimod (*p<0.05, **p<0.01).
Imiquimod application attracts luciferase-expressing E7-specific CD8+ T cells to the cervicovaginal tract
In order to determine if application of imiquimod results in the accumulation of luciferase-expressing E7-specific CD8+ T cells in the cervicovaginal tracts of treated mice, we applied imiquimod or PBS to the cervicovaginal tracts of mice and then adoptively transferred the E7-Luc CD8+ T cells (5×105/mouse) via tail vein. After 48 hours, the entire genital tract was excised in order to measure bioluminescence intensity and thereby detect presence of the luciferase-expressing E7-specific CD8+ T cells (Supplemental Figure 1A, B). Supplemental Figure 1B shows that a significantly higher number of E7-Luc T cells were present in the cervicovaginal tracts of mice treated with imiquimod compared to those treated with PBS. This data indicates that the application of imiquimod results in the accumulation of antigen-specific CD8+ T cells in the genital tract.
Systemic CRT-E7 DNA vaccination combined with local imiquimod application generated potent antitumor effects in the orthotropic cervical cancer model
Next, we wanted to test if the combination strategy of systemic vaccination with local imiquimod application could improve the treatment of an orthotropic genital mucosal tumor model, luciferase-expressing TC-1 tumor cells growing in the vaginal wall. Mice bearing vaginal TC-1-luc tumors one week after inoculation were divided into four groups, naïve, vaccination only (VAC), imiquimod only (IMQ), and vaccination plus imiquimod (VAC+IMQ) and their treatment schedule is illustrated in Figure 2A.
Figure 2. Systemic vaccination combined with local imiquimod application generated potent antitumor effects in the vaginal tract.
(A) Tumor challenge and vaccination/imiquimod application schedule. Mice were treated with pNGVL4a-CRT-E7 vaccine alone (VA), imiquimod alone (IM), or both (VA+IM). (B) Bioluminescence imaging showing the TC-1 tumors following challenge with TC-1-Luc cells (5×104/mouse) administered in the vagina. (C) Bar graph depicting bioluminescence intensity of the various treatment groups over time (**p<0.01).
As shown in Figure 2B, the bioluminescence signals, indicating vaginal tumor load, in all groups of mice were not significantly different on day 7 and increased to a similar level by day 14. Subsequently, the signal dramatically decreased in the VAC+IMQ group by day 21. In contrast, the signals in the mice of the remaining groups continued to steadily increase, although the VAC only and IMQ only signals were about 10-fold lower than the control mice. Figure 2C shows that as early as day 21, the tumors of mice treated with DNA vaccine and imiquimod were significantly smaller than those of the other treatment groups. These results correlated with the survival rates of the groups of mice. Although mice in the VAC group and IMQ group showed improved survival compared to the control mice, mice in the VAC+IMQ group had significantly prolonged survival compared to both the VAC group (p<0.01) and the IMQ group (p<0.01) (Supplemental Figure 2A). All the mice in VAC+IMQ group survived until day 70 without any sign of tumor recurrence. Furthermore, mice treated with DNA vaccination and imiquimod had significantly higher numbers of total CD8+ T cells and E7-specific CD8+ T cells in the tumor loci compared to those in the other treatment groups (Supplemental Figure 2B and C). Here, the fold increase in E7-specific CD8+ T cells was greater than total CD8+ T cell accumulation, suggesting preferential accumulation of the activated CD8+ T cells in the tumor because of the presence of cognate antigen. Furthermore, we found that mice that cleared TC-1 tumors in Supplemental Figure 2A and were subsequently rechallenged with TC-1 tumor cells remained tumor-free for at least 60 days (data not shown). Taken together, the data shown in Figure 2 and Supplemental Figures 1 and 2 suggest that intravaginal application of imiquimod leads to the accumulation in the cervicovaginal tumor of systemic E7-specific CD8+ T cells generated by CRT-E7 DNA plasmid vaccination, resulting in the most potent antitumor effect against E7-expressing orthotopic tumors in the cervicovaginal tract.
Activation of antigen-specific CD8+ T cells in the cervicovaginal tract leads to accumulation of other antigen-specific CD8+ T cells in the cervicovaginal tract
We further explored whether the activation of antigen-specific CD8+ T cells in the cervicovaginal tract would create a suitable environment to attract other antigen-specific CD8+ T cells. Naïve C57BL/6 mice and tumor-free mice previously challenged with TC-1-Luc cells and effectively treated with CRT/E7 DNA vaccine and imiquimod (antigen-experienced mice) were used to further characterize the attraction of other antigen-specific CD8+ T cells to the cervicovaginal tract. As outlined in Figure 3A, mice were administered with E7 peptide (RAHYNIVTF) in the cervicovaginal tract one day before adoptive transfer of OVA-specific CD8+ T cells (OT-1). 5×105 OT-1 T cells were labeled with CFSE before injection into mice via the tail vein. Two days after adoptive transfer, splenocytes and vaginal tissue were collected and CFSE-labeled OT-1 T cells were quantified by flow cytometry analysis. We observed similar numbers of OT-1 T cells in the circulation after adoptive transfer in all treatment groups (Figure 3B and C). Importantly, there was a significantly greater number of OT-1 T cells that accumulated in the vaginal tracts in antigen-experienced mice that received E7 peptide administration but not in antigen-experienced mice that did not receive E7 peptide administration (Figure 3B and D). These results suggest that local inflammation induced by the activation of antigen-specific CD8+ T cells can recruit activated CD8+ T cells regardless of antigen specificity to the vaginal tissue.
Figure 3. Antigen-experienced Trm cell accumulation in the vaginal area after antigen stimulation.
(A) Illustration of assay. Briefly, E7 (RAHYNIVTF) peptide or PBS was administered locally followed by adoptive transfer of CFSE-labeled OT-1 CD8+ T cells. Two days after adoptive transfer, PBMCs and vaginal tissue were examined for CFSE labeled OT-1 CD8+ T cells. (B) Representative flow cytometry. (C) Bar graph depicting the number of CFSE+ cells in the spleens of treated mice. (D) Bar graph depicting the percentage of CFSE+ cells in the genital tracts of treated mice (*p<0.05; **p<0.01).
Imiquimod induced local expression of CXCL9 and CXCL10 leading to CXCR3+ cytotoxic T cell accumulation in the cervicovaginal tract
Previous studies have shown that imiquimod application on the cervix elevated the expression of the chemokines CXCL9 and CXCL10 in the local tissue in murine and non-human primate models (34–36). In order to characterize the expression of CXCL9 and CXCL10 mRNA, we performed qRT-PCR using mRNA derived from the cervicovaginal tracts following local imiquimod treatment. Treatment with PBS was used as a control for comparison. As shown in Figure 4A, we found that the CXCL9 and CXCL10 mRNA expression was significantly higher in imiquimod treated vaginal tissue compared to PBS treated tissue. Because CXCR3, the receptor for both CXCL9 and CXCL10, is expressed on T cells and functions to induce T cell migration to tissues displaying danger signals (33), we also examined the expression of CXCR3 on CD8+ T cells isolated from the cervicovaginal tract. Treatment with imiquimod induced a significantly increased number of CXCR3-expressing CD8+ T cells recovered from the genital tract as compared to treatment with PBS (Figure 4B). Additionally, we compared the expression of surface markers associated with T cell migration to lesions, CD103, α4β7, and CD49a, on genital tract T cells of control mice versus those treated with imiquimod (26, 47). Figure 4B shows that in imiquimod treated mice there was a significantly higher proportion of CD49a+ T cells in the cervicovaginal tract compared to mice treated with PBS.
Figure 4. Expression of CXCL9 and CXCL10 following imiquimod treatment and characterization of the CD8+ T cell accumulation in the cervicovaginal tract.
(A) Expression levels of TLR7, IFNγ, CXCL9, and CXCL10 were evaluated from mRNA extracted from vaginal tissue treated with PBS or imiquimod by qRT-PCR. Gene expression levels were normalized to β-Actin housekeeping gene, and data were represented as fold differences. (B) Surface integrin markers of cervicovaginal CD8+ T cells were screened (*p<0.05; **p<0.01).
IFNγ signaling is important for both CXCL9/10 expression and accumulation of antigen-specific CD8+ T cells in the cervicovaginal tract following imiquimod treatment
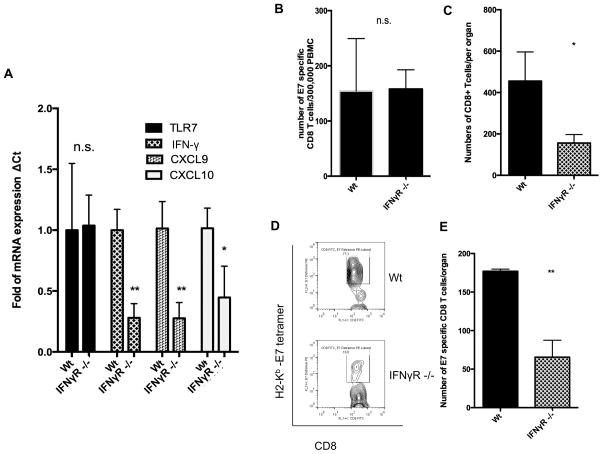
IFNγ signaling induces CXCL9/10 expression (35). In our study, we found that IFNγ expression was upregulated after local imiquimod treatment (Figure 4A). This implies that IFNγ may be a key cytokine governing CXCL9/10 upregulation and the attraction of the activated CD8+ T cell immune response after imiquimod application. In order to determine if the IFNγ signal pathway is important for the upregulated expression of CXCL9/10 in the cervicovaginal tract, we compared the genital tracts of IFNγ receptor knockout mice (IFNγR −/−) with wild type mice (Wt) following imiquimod treatment for their levels of TLR7, IFNγ, CXCL9, and CXCL10 mRNA. We found that the expression levels of IFNγ, CXCL9, and CXCL10 were significantly reduced in IFNγR −/− mice compared to Wt mice (Figure 5A). In comparison, the expression level of TLR7 was not significantly different between IFNγR −/− mice and Wt mice.
Figure 5. Recruitment of T cells from circulation to treatment location in IFNγR−/− mice.
(A) mRNA was extracted from the imiquimod-treated vaginal tissue of Wt mice and IFNγR −/− mice. The expression levels of TLR7, IFNγ, CXCL9, and CXCL10 were detected by qRTPCR and are depicted in the bar graph. (B) Bar graph showing the number of E7-specfic CD8+ T cells per 3×105 PBMCs. (C) Total number of CD8+ T cells in the genital tracts of mice. (D) Representative flow cytometry and (E) bar graph of E7-specific CD8+ T cells in vaginal tissue (*p<0.05; **p<0.01).
In order to demonstrate that IFNγ is important for the accumulation of antigen-specific CD8+ T cells in the cervicovaginal tract following imiquimod treatment, we compared the number of E7-specific CD8+ T cells in the cervicovaginal tracts of Wt and IFNγR−/− mice following DNA vaccination and local imiquimod application. As shown in Figure 5B, while the numbers of E7-specific CD8+ T cells in PBMCs were similar between Wt and IFNγR −/− mice, there were significantly fewer CD8+ T cells (Figure 5C) and E7-specific CD8+ T cells in the cervicovaginal areas of IFNγR−/− mice compared to Wt mice (Figure 5D and E). Taken together, these data suggest that the IFNγ signal pathway plays an important role in triggering CXCL9/10 chemokine expression and the accumulation of E7-specific CD8+ T cells in the cervicovaginal tract following imiquimod treatment.
CXCR3 is crucial for antigen-specific CD8+ T cell accumulation in imiquimod-treated cervicovaginal tract
In Figure 4B, we found that CXCR3+ CD8+ T cells accumulated in imiquimod-treated cervicovaginal tissue. To test the importance of CXCR3 expression on the CD8+ T cells for accumulation in the genital tract upon imiquimod treatment, we compared the number of CD8+ T cells in Wt mice and CXCR3−/− mice. All mice were vaccinated systemically with CRT-E7 DNA followed by treatment with imiquimod in the cervicovaginal tract. As shown in Figure 6, we found that in the cervicovaginal tract, the total number of CD8+ T cells and E7-specific CD8+ T cells were significantly reduced in CXCR3−/− mice compared to Wt mice. Taken together, these data suggest that the expression of CXCR3 on CD8+ T cells is crucial for the presence of antigen-experienced cytotoxic T cells in CXCL9/10-expressing tissue following local imiquimod application.
Figure 6. Characterization of CD8+ T cell migration in CXCR3 knockout mice following vaginal deposition of imiquimod.
Wild type and CXCR3 knockout mice were vaccinated with pNGVL4a-CRT-E7 and treated with imiquimod in the cervicovaginal tract. (A) Bar graph showing the absolute number of CD8+ T cells number in the cervicovaginal tracts of Wt and CXCR3−/− mice. (B) Representative flow cytometry analysis of E7-specific CD8+ T cells in the cervicovaginal tracts of Wt and CXCR3−/− mice.
Discussion
The current study examined the effects of imiquimod application following CRT/E7 DNA vaccine administration on antigen-specific CD8+ T cell-mediated immune responses and antitumor effects. We observed that following imiquimod treatment, the vaginal tissue of mice exhibited a significant increase in local E7-specific CD8+ T cells. Furthermore, upon systemic adoptive transfer of E7-Luc T cells, we found that imiquimod treatment potently attracted them to the cervicovaginal tracts of mice. We also showed that mice treated with CRT/E7 DNA vaccine followed by intravaginal imiquimod deposition, compared to either treatment alone, generated synergistic antitumor effects and dramatically improved survival. Next, we demonstrated that the local inflammation induced by the activation of antigen-specific CD8+ T cells could recruit CD8+ T cells with specificity for other antigen to the vaginal tissue. We showed that imiquimod application induces local CXCL9/10 expression and that the antigen-specific T cells that accumulate in the cervicovaginal tract following imiquimod application express CXCR3 as well as the Trm marker CD49a. Finally, we show that the IFNγ signal pathway is important for the expression of CXCL9/10 as well as the accumulation of antigen-specific CD8+ T cells in the cervicovaginal tract following imiquimod treatment.
Here, we observed that cervicovaginal application of imiquimod following CRT/E7 DNA vaccination resulted in the accumulation of antigen-specific CD8+ T cells in the cervicovaginal tract. Several mechanisms may account for our observations. For example, imiquimod may facilitate the migration of antigen-specific CD8+ T cells from the circulation to the point of imiquimod application in the cervicovaginal tract. Our data are consistent with this concept. In our study, we observed that IFNγ is essential for the accumulation of antigen-specific CD8+ T cells in the cervicovaginal tract (see Figure 5). In addition, we have observed that CXCL9/10 are upregulated following imiquimod application (Figure 4). It has been shown that CXCL9/10 can be induced by IFNγ and play an important role for attracting CXCR3+ T cells to the tissue (for review, see (33)). Thus, the local application of imiquimod can activate the IFNγ and IFNγ receptor pathway and induce CXCL9/10 expression, resulting in the migration of CXCR3+ CD8+ T cells to the cervicovaginal tract. Alternatively, expansion of the antigen-specific CD8+ T cells at the tissue location or extravasation of activated T cells from the imiquimod-treated area may also contribute to the significantly increased number of antigen-specific CD8+ T cells following imiquimod application.
We also observed that the antigen-specific T cells accumulating in the cervicovaginal tract expressed the T cell homing marker CD49a after intravaginal imiquimod application (Figure 4B). CD49a is a mucosal integrin whose expression on E7-specific CD8+ T cells has been shown to be induced following intranasal mucosal vaccination (26). Furthermore, CD49a was found to be crucial to for intratumoral infiltration of the antigen-specific CD8+ T cells and the efficacy of cancer vaccines against mucosal tumors (26). Taken together, these data suggest that vaccination with CRT/E7 DNA and topical imiquimod application may together promote the generation of mucosal-homing E7-specific CD8+ T cells that are capable of infiltrating mucosal tumors and eliciting potent antitumor effects.
In order to determine whether the results of the current study can be extended to human VIN, additional considerations will need to be taken into account. For example, elimination of the VIN lesions and prevention of recurrence will likely require effector cells to concentrate at or pass through the basement membrane to target neoplastic cells that express the virus-derived antigen target. Furthermore, it will be important to determine whether our regimen also attracts other cells, in addition to CD8+ T cells, to the treated area including antagonistic cell populations.
Before this treatment strategy is ready for translation to the clinic, some additional studies will be needed. It will be important to determine the optimal regimen of local imiquimod application in relation to the therapeutic HPV vaccination regimen. Furthermore, we have created numerous therapeutic HPV vaccines and are testing these vaccines using various regimens (e.g. DNA prime-vaccinia boost (48)) and administration methods (e.g. electroporation (49)) in clinical trials (18). Specifically, administration of the DNA vaccine followed by electroporation may produce a more potent immune response. Thus, it will be important to determine when to give imiquimod relative to vaccination. Once we have identified the most potent vaccines and regimens for the generation of systemic HPV antigen-specific immune responses, they potentially can be used in conjunction with imiquimod to enhance recruitment of Trm and therapeutic anti-lesion effects. Additionally, clinicians should be aware that no controlled studies have been done to determine the safety or long term efficacy of imiquimod to date.
Our successful example of using imiquimod to generate Trm-mediated antitumor effects presents the opportunity to use other TLR ligands that may be capable of generating even stronger Trm recruitment. Currently, two TLR agonists are FDA approved for use in cancer patients in addition to imiquimod, the TLR4 agonist monophosphoryl lipid A (MPL), and the TLR2/4 agonist bacillus Calmette–Guérin (BCG). Also of interest is the TLR7/8 agonist, resiquimod, which is an imidazoquinoline like imiquimod, and has been shown to have antitumor effects (for review see (50)). TLR9 agonists are being developed for clinical application in oncology and viral infections by Pfizer, Dynavax Technologies and GlaxoSmithKline among others (for review see (51)). Future studies to compare these TLR ligands will lead to the identification of the most potent TLR ligand, which will be most suitable for further clinical translation.
In summary, we found that following therapeutic CRT/E7 DNA vaccination, local imiquimod application resulted in the accumulation of antigen-specific CXCR3+ CD8+ T cells in the cervicovaginal tracts of mice and that this phenomenon was mediated by the IFNγ pathway and local CXCL9/10 expression. This study serves as an important foundation for the future clinical application of local TLR agonists in combination with therapeutic HPV vaccines for the generation of potent cell-mediated immune responses and antitumor effects against HPV-associated lesions.
Supplementary Material
Translational Relevance.
Therapeutic HPV vaccination may potentially be combined with local innate inflammatory activation to attract antigen-specific immune responses to the site of application, and may also promote a favorable immune microenvironment within the lesion to promote viral clearance. In order to facilitate this, we employed a local adjuvant, imiquimod, to enhance the recruitment of antigen-specific CD8+ T cells generated by a naked DNA vaccine to the cervicovaginal tract using an orthotopic HPV16 E6/E7+ syngeneic tumor model, TC-1. We found that imiquimod induced cervicovaginal accumulation of activated E7-specific CD8+ T cells elicited by DNA vaccination with a naked vector expressing calreticulin fused to HPV16 E7 (CRT/E7). Furthermore, intravaginal imiquimod deposition induced upregulation of the expression of the chemokine receptor CXCR3, and the tissue resident memory T cell (Trm) marker CD49a on the T cells attracted by imiquimod to the cervicovaginal tract. Our study has high translational relevance because imiquimod is currently FDA approved for condyloma accuminata and basal cell carcinoma and intramuscular vaccination with pNGVL4a-CRT/E7(detox) is currently undergoing clinical testing, suggesting potential for their synergistic action to control HPV-associated cancers.
Acknowledgments
Financial Support: This work was supported by the National Institutes of Health/National Cancer Institute Cervical Cancer SPORE P50CA098252, Head and Neck Cancer SPORE P50 CA-DE019032, R43CA174436 and 2R01CA114425-06 grants.
Footnotes
Competing Interests: Richard Roden and T.-C. Wu hold equity in Papivax LLC and serve as scientific advisors of Papivax Biotech Inc. The terms of these arrangements are managed by Johns Hopkins University in accordance with its conflict of interest policies.
References
- 1.Chaturvedi AK. Beyond cervical cancer: burden of other HPV-related cancers among men and women. The Journal of adolescent health: official publication of the Society for Adolescent Medicine. 2010;46:S20–6. doi: 10.1016/j.jadohealth.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 2.Armarnik S, Sheiner E, Piura B, Meirovitz M, Zlotnik A, Levy A. Obstetric outcome following cervical conization. Archives of gynecology and obstetrics. 2011;283:765–9. doi: 10.1007/s00404-011-1848-3. [DOI] [PubMed] [Google Scholar]
- 3.Cardosi RJ, Bomalaski JJ, Hoffman MS. Diagnosis and management of vulvar and vaginal intraepithelial neoplasia. Obstetrics and gynecology clinics of North America. 2001;28:685–702. doi: 10.1016/s0889-8545(05)70229-1. [DOI] [PubMed] [Google Scholar]
- 4.Abbasakoor F, Boulos PB. Anal intraepithelial neoplasia. The British journal of surgery. 2005;92:277–90. doi: 10.1002/bjs.4967. [DOI] [PubMed] [Google Scholar]
- 5.Su JH, Wu A, Scotney E, Ma B, Monie A, Hung CF, et al. Immunotherapy for cervical cancer: Research status and clinical potential. BioDrugs: clinical immunotherapeutics, biopharmaceuticals and gene therapy. 2010;24:109–29. doi: 10.2165/11532810-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kenter GG, Welters MJ, Valentijn AR, Lowik MJ, Berends-van der Meer DM, Vloon AP, et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. The New England journal of medicine. 2009;361:1838–47. doi: 10.1056/NEJMoa0810097. [DOI] [PubMed] [Google Scholar]
- 7.Cheng WF, Hung CF, Chai CY, Hsu KF, He L, Ling M, et al. Tumor-specific immunity and antiangiogenesis generated by a DNA vaccine encoding calreticulin linked to a tumor antigen. The Journal of clinical investigation. 2001;108:669–78. doi: 10.1172/JCI12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chuang CM, Monie A, Hung CF, Wu TC. Treatment with imiquimod enhances antitumor immunity induced by therapeutic HPV DNA vaccination. Journal of biomedical science. 2010;17:32. doi: 10.1186/1423-0127-17-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim JW, Hung CF, Juang J, He L, Kim TW, Armstrong DK, et al. Comparison of HPV DNA vaccines employing intracellular targeting strategies. Gene therapy. 2004;11:1011–8. doi: 10.1038/sj.gt.3302252. [DOI] [PubMed] [Google Scholar]
- 10.Peng S, Trimble C, He L, Tsai YC, Lin CT, Boyd DA, et al. Characterization of HLA-A2-restricted HPV-16 E7-specific CD8(+) T-cell immune responses induced by DNA vaccines in HLA-A2 transgenic mice. Gene therapy. 2006;13:67–77. doi: 10.1038/sj.gt.3302607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tseng CW, Monie A, Wu CY, Huang B, Wang MC, Hung CF, et al. Treatment with proteasome inhibitor bortezomib enhances antigen-specific CD8+ T-cell-mediated antitumor immunity induced by DNA vaccination. J Mol Med (Berl) 2008;86:899–908. doi: 10.1007/s00109-008-0370-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tseng CW, Monie A, Trimble C, Alvarez RD, Huh WK, Buchsbaum DJ, et al. Combination of treatment with death receptor 5-specific antibody with therapeutic HPV DNA vaccination generates enhanced therapeutic anti-tumor effects. Vaccine. 2008;26:4314–9. doi: 10.1016/j.vaccine.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Best SR, Peng S, Juang CM, Hung CF, Hannaman D, Saunders JR, et al. Administration of HPV DNA vaccine via electroporation elicits the strongest CD8+ T cell immune responses compared to intramuscular injection and intradermal gene gun delivery. Vaccine. 2009;27:5450–9. doi: 10.1016/j.vaccine.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tseng CW, Trimble C, Zeng Q, Monie A, Alvarez RD, Huh WK, et al. Low-dose radiation enhances therapeutic HPV DNA vaccination in tumor-bearing hosts. Cancer immunology, immunotherapy: CII. 2009;58:737–48. doi: 10.1007/s00262-008-0596-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim D, Hung CF, Wu TC, Park YM. DNA vaccine with alpha-galactosylceramide at prime phase enhances anti-tumor immunity after boosting with antigen-expressing dendritic cells. Vaccine. 2010;28:7297–305. doi: 10.1016/j.vaccine.2010.08.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peng S, Monie A, Pang X, Hung CF, Wu TC. Vascular disrupting agent DMXAA enhances the antitumor effects generated by therapeutic HPV DNA vaccines. Journal of biomedical science. 2011;18:21. doi: 10.1186/1423-0127-18-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peng S, Lyford-Pike S, Akpeng B, Wu A, Hung CF, Hannaman D, et al. Low-dose cyclophosphamide administered as daily or single dose enhances the antitumor effects of a therapeutic HPV vaccine. Cancer immunology, immunotherapy: CII. 2013;62:171–82. doi: 10.1007/s00262-012-1322-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Center SKCC. A Pilot Study of pnGVL4a-CRT/E7 (Detox) for the Treatment of Patients With HPV16+ Cervical Intraepithelial Neoplasia 2/3 (CIN2/3) Bethesda (MD): National Library of Medicine (US); 2009. ClinicalTrialsgov[Internet] [Google Scholar]
- 19.Trimble CL, Peng S, Thoburn C, Kos F, Wu TC. Naturally occurring systemic immune responses to HPV antigens do not predict regression of CIN2/3. Cancer immunology, immunotherapy: CII. 2010;59:799–803. doi: 10.1007/s00262-009-0806-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trimble CL, Peng S, Kos F, Gravitt P, Viscidi R, Sugar E, et al. A phase I trial of a human papillomavirus DNA vaccine for HPV16+ cervical intraepithelial neoplasia 2/3. Clinical cancer research: an official journal of the American Association for Cancer Research. 2009;15:361–7. doi: 10.1158/1078-0432.CCR-08-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trimble CL, Clark RA, Thoburn C, Hanson NC, Tassello J, Frosina D, et al. Human papillomavirus 16-associated cervical intraepithelial neoplasia in humans excludes CD8 T cells from dysplastic epithelium. J Immunol. 2010;185:7107–14. doi: 10.4049/jimmunol.1002756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daayana S, Elkord E, Winters U, Pawlita M, Roden R, Stern PL, et al. Phase II trial of imiquimod and HPV therapeutic vaccination in patients with vulval intraepithelial neoplasia. British journal of cancer. 2010;102:1129–36. doi: 10.1038/sj.bjc.6605611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woodland DL, Kohlmeier JE. Migration, maintenance and recall of memory T cells in peripheral tissues. Nature reviews Immunology. 2009;9:153–61. doi: 10.1038/nri2496. [DOI] [PubMed] [Google Scholar]
- 24.Cauley LS, Lefrancois L. Guarding the perimeter: protection of the mucosa by tissue-resident memory T cells. Mucosal immunology. 2013;6:14–23. doi: 10.1038/mi.2012.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nardelli-Haefliger D, Dudda JC, Romero P. Vaccination route matters for mucosal tumors. Science translational medicine. 2013;5:172fs4. doi: 10.1126/scitranslmed.3005638. [DOI] [PubMed] [Google Scholar]
- 26.Sandoval F, Terme M, Nizard M, Badoual C, Bureau MF, Freyburger L, et al. Mucosal imprinting of vaccine-induced CD8(+) T cells is crucial to inhibit the growth of mucosal tumors. Science translational medicine. 2013;5:172ra20. doi: 10.1126/scitranslmed.3004888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luci C, Hervouet C, Rousseau D, Holmgren J, Czerkinsky C, Anjuere F. Dendritic cell-mediated induction of mucosal cytotoxic responses following intravaginal immunization with the nontoxic B subunit of cholera toxin. J Immunol. 2006;176:2749–57. doi: 10.4049/jimmunol.176.5.2749. [DOI] [PubMed] [Google Scholar]
- 28.Wille-Reece U, Flynn BJ, Lore K, Koup RA, Miles AP, Saul A, et al. Toll-like receptor agonists influence the magnitude and quality of memory T cell responses after prime-boost immunization in nonhuman primates. The Journal of experimental medicine. 2006;203:1249–58. doi: 10.1084/jem.20052433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adams S, Kozhaya L, Martiniuk F, Meng TC, Chiriboga L, Liebes L, et al. Topical TLR7 agonist imiquimod can induce immune-mediated rejection of skin metastases in patients with breast cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2012;18:6748–57. doi: 10.1158/1078-0432.CCR-12-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Le Mercier I, Poujol D, Sanlaville A, Sisirak V, Gobert M, Durand I, et al. Tumor promotion by intratumoral plasmacytoid dendritic cells is reversed by TLR7 ligand treatment. Cancer research. 2013;73:4629–40. doi: 10.1158/0008-5472.CAN-12-3058. [DOI] [PubMed] [Google Scholar]
- 31.Kalb ML, Glaser A, Stary G, Koszik F, Stingl G. TRAIL(+) human plasmacytoid dendritic cells kill tumor cells in vitro: mechanisms of imiquimod- and IFN-alpha-mediated antitumor reactivity. J Immunol. 2012;188:1583–91. doi: 10.4049/jimmunol.1102437. [DOI] [PubMed] [Google Scholar]
- 32.Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nature immunology. 2002;3:196–200. doi: 10.1038/ni758. [DOI] [PubMed] [Google Scholar]
- 33.Groom JR, Luster AD. CXCR3 ligands: redundant, collaborative and antagonistic functions. Immunology and cell biology. 2011;89:207–15. doi: 10.1038/icb.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Seters M, van Beurden M, ten Kate FJ, Beckmann I, Ewing PC, Eijkemans MJ, et al. Treatment of vulvar intraepithelial neoplasia with topical imiquimod. The New England journal of medicine. 2008;358:1465–73. doi: 10.1056/NEJMoa072685. [DOI] [PubMed] [Google Scholar]
- 35.Pak-Wittel MA, Yang L, Sojka DK, Rivenbark JG, Yokoyama WM. Interferon-gamma mediates chemokine-dependent recruitment of natural killer cells during viral infection. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E50–9. doi: 10.1073/pnas.1220456110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim D, Hung CF, Wu TC. Monitoring the trafficking of adoptively transferred antigen- specific CD8-positive T cells in vivo, using noninvasive luminescence imaging. Human gene therapy. 2007;18:575–88. doi: 10.1089/hum.2007.038. [DOI] [PubMed] [Google Scholar]
- 37.Peng S, Monie A, Kang TH, Hung CF, Roden R, Wu TC. Efficient delivery of DNA vaccines using human papillomavirus pseudovirions. Gene therapy. 2010;17:1453–64. doi: 10.1038/gt.2010.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tannous BA, Kim DE, Fernandez JL, Weissleder R, Breakefield XO. Codon-optimized Gaussia luciferase cDNA for mammalian gene expression in culture and in vivo. Molecular therapy: the journal of the American Society of Gene Therapy. 2005;11:435–43. doi: 10.1016/j.ymthe.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 39.Zeng Q, Peng S, Monie A, Yang M, Pang X, Hung CF, et al. Control of cervicovaginal HPV-16 E7-expressing tumors by the combination of therapeutic HPV vaccination and vascular disrupting agents. Human gene therapy. 2011;22:809–19. doi: 10.1089/hum.2010.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Decrausaz L, Goncalves AR, Domingos-Pereira S, Pythoud C, Stehle JC, Schiller J, et al. A novel mucosal orthotopic murine model of human papillomavirus-associated genital cancers. International journal of cancer Journal international du cancer. 2011;128:2105–13. doi: 10.1002/ijc.25561. [DOI] [PubMed] [Google Scholar]
- 41.Lamikanra A, Pan ZK, Isaacs SN, Wu TC, Paterson Y. Regression of established human papillomavirus type 16 (HPV-16) immortalized tumors in vivo by vaccinia viruses expressing different forms of HPV-16 E7 correlates with enhanced CD8(+) T-cell responses that home to the tumor site. Journal of virology. 2001;75:9654–64. doi: 10.1128/JVI.75.20.9654-9664.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee SY, Kang TH, Knoff J, Huang Z, Soong RS, Alvarez RD, et al. Intratumoral injection of therapeutic HPV vaccinia vaccine following cisplatin enhances HPV-specific antitumor effects. Cancer immunology, immunotherapy: CII. 2013;62:1175–85. doi: 10.1007/s00262-013-1421-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soong RS, Trieu J, Lee SY, He L, Tsai YC, Wu TC, et al. Xenogeneic human p53 DNA vaccination by electroporation breaks immune tolerance to control murine tumors expressing mouse p53. PloS one. 2013;8:e56912. doi: 10.1371/journal.pone.0056912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim TW, Hung CF, Ling M, Juang J, He L, Hardwick JM, et al. Enhancing DNA vaccine potency by coadministration of DNA encoding antiapoptotic proteins. The Journal of clinical investigation. 2003;112:109–17. doi: 10.1172/JCI17293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hung CF, Tsai YC, He L, Wu TC. Control of mesothelin-expressing ovarian cancer using adoptive transfer of mesothelin peptide-specific CD8+ T cells. Gene therapy. 2007;14:921–9. doi: 10.1038/sj.gt.3302913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gunn L, Ding C, Liu M, Ma Y, Qi C, Cai Y, et al. Opposing roles for complement component C5a in tumor progression and the tumor microenvironment. J Immunol. 2012;189:2985–94. doi: 10.4049/jimmunol.1200846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Masopust D, Schenkel JM. The integration of T cell migration, differentiation and function. Nature reviews Immunology. 2013;13:309–20. doi: 10.1038/nri3442. [DOI] [PubMed] [Google Scholar]
- 48.Center SKCC. A Phase I Efficacy and Safety Study of HPV16-specific Therapeutic DNA-vaccinia Vaccination in Combination With Topical Imiquimod, in Patients With HPV16+ High Grade Cervical Dysplasia (CIN3) Bethesda (MD): National Library of Medicine (US); 2008. ClinicalTrialsgov[Internet] [Google Scholar]
- 49.Center SKCC. A Phase I Clinical Trial Assessing the Safety and Feasibility of Administration of pNGVL4a-CRT/E7(Detox) DNA Vaccine Using the Intramuscular TriGridTM Delivery System in Combination With Cyclophosphamide in HPV-16 Associated Head and Neck Cancer Patients. Bethesda (MD): National Library of Medicine (US); 2011. ClinicalTrialsgov[Internet] [Google Scholar]
- 50.Meyer T, Surber C, French LE, Stockfleth E. Resiquimod, a topical drug for viral skin lesions and skin cancer. Expert opinion on investigational drugs. 2013;22:149–59. doi: 10.1517/13543784.2013.749236. [DOI] [PubMed] [Google Scholar]
- 51.Gearing AJ. Targeting toll-like receptors for drug development: a summary of commercial approaches. Immunology and cell biology. 2007;85:490–4. doi: 10.1038/sj.icb.7100102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.