Abstract
Contrary to the long held belief that chemotherapy is immunosuppressive, emerging evidence indicates that the anticancer activity of cisplatin is not limited to its ability to inhibit mitosis, but that cisplatin also has important immunomodulatory effects. We therefore methodically examined the relevant preclinical literature and identified four main mechanisms of cisplatin-induced antitumor immunomodulation: (1) MHC class I expression upregulation; (2) recruitment and proliferation of effector cells; (3) upregulation of the lytic activity of cytotoxic effectors; and (4) downregulation of the immunosuppressive microenvironment. Cisplatin-based combination chemotherapy’s antitumor immunomodulatory effects are also beginning to be harnessed in the clinic; we therefore additionally reviewed the applicable clinical literature and discussed how monitoring various components of the immune system (and their responses to cisplatin) can add new levels of sophistication to disease monitoring and prognostication. In summation, this growing body of literature on cisplatin-induced antitumor immunomodulation ultimately highlights the therapeutic potential of synergistic strategies that combine traditional chemotherapy with immunotherapy.
Introduction
The history of cisplatin (CDDP) dates back more than 160 years. Cisplatin was first synthesized by Michel Peyrone in 1845; some 50 years later, it played a pivotal role in the establishment of Alfred Werner’s theory of coordination chemistry, for which he won a Nobel Prize in 1913 (1). Then, in the mid-1960s, University of Michigan biophysicist Barnett Rosenberg unexpectedly discovered that CDDP could inhibit cellular division in Escherichia coli (2–4). Subsequently, Rosenberg became interested in testing CDDP for anticancer activity; he would go on to demonstrate the molecule’s potent antitumor activity in a murine model of sarcoma (5, 6). Rosenberg’s promising results in mice paved the way for trials in humans and ultimately led to CDDP becoming one of the most widely-used chemotherapeutic agents in clinical practice today.
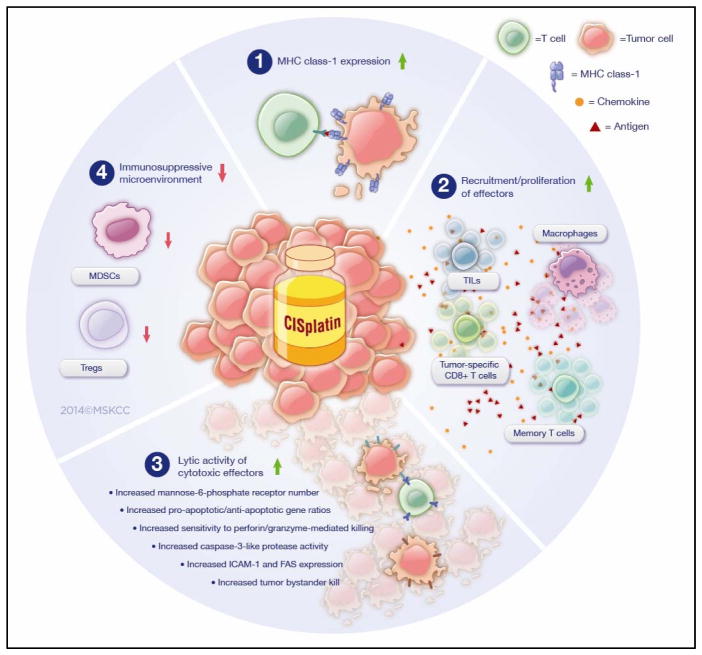
The anticancer activity of CDDP is not merely limited to its ability to cross-link DNA and inhibit mitosis (these events, in turn, lead to apoptosis). Rather, we are now learning that CDDP also has immunomodulatory effects. These effects may be quite important for combating tumors and stand in stark contrast to the more well-known immuno-(killing of circulating lymphocytes) and myelosuppressive (bone marrow impairment) actions of most chemotherapies (including CDDP). What is more, our improved understanding of this CDDP-induced antitumor immunomodulation comes at a time when emerging antineoplastic strategies are increasingly engaging the immune system directly (e.g., check-point blockade and adoptive T-cell therapies). Interestingly, recent developments in oncology involve synergizing such novel immunotherapies with conventional chemotherapeutics such as doxorubicin and CDDP-based treatments. These combinations may have initially sought to exploit CDDP’s direct cytotoxic effects; but, as we are now learning, such combinations also serendipitously harness CDDP’s ability to favorably modulate the immune system. However, fully exploiting the potential benefits of these new CDDP-combined modalities first requires a thorough understanding of how CDDP itself interacts with the immune system. We therefore methodically examined both the current preclinical and clinical evidence addressing CDDP-mediated antitumor immunomodulation and herein summarize the relevant contemporary literature. In doing so, we identified four main mechanisms by which CDDP could modulate the immune system to further promote the drug’s antitumor efficacy: 1) CDDP upregulates MHC class I expression; 2) CDDP promotes the recruitment and proliferation of effector cells; 3) CDDP upregulates the lytic activity of cytotoxic effectors; and 4) CDDP downregulates the immunosuppressive microenvironment (Fig. 1).
Figure 1.
Preclinical evidence demonstrates CDDP-induced antitumor immunomodulation occurs via four mechanisms. MDSC, myeloid-derived suppressor cells; TIL, tumor-infiltrating lymphocytes.
While a recently published article in this journal examined the molecular pathways underlying the immunogenic effects of platinum-based chemotherapeutics, our review focuses solely on CDDP and its interactions with the solid tumor microenvironment and the influence on outcomes (7).
Preclinical Evidence
CDDP upregulates MHC class I expression
The fundamental mechanism by which the immune system attempts to keep tumor cells at bay is through recognition of cancer cell’s major histocompatibility complex (MHC) class I:peptide complex by a cytotoxic T-cell (CTL). When a CTL recognizes this complex via its T-cell receptor it becomes activated and is able to perform its cytotoxic function against the tumor cell. It is now well-established that many cancers attempt to avoid such immune recognition by downregulating their expression of MHC class I molecules (Fig. 1). A number of groups, however, have recently demonstrated that CDDP may improve the ability of CTLs to recognize cancer cells by upregulating the tumor cells’ MHC class I expression. Gameiro et al. showed that MHC class I expression increased more than 50% in the H1703 and A549 lung cancer cell lines after the cells were treated with a single, sublethal dose of CDDP/vinorelbine (8). A similar increase in MHC class I expression was observed with human head and neck squamous cell cancer (HNSCC) cell lines exposed to clinically-translatable doses of CDDP plus 5-fluorouracil (9).
It should be pointed out that the aforementioned studies used CDDP in translational settings and in combination with other standard agents (as do several other works cited in this review). While the observed results cannot be fully attributed to CDDP’s actions alone, they nevertheless highlight the important immunological impact such real-world, CDDP-based regimens may have. Still, a single, relatively low dose of CDDP has been demonstrated to elevate the expression of total cellular MHC class I in breast cancer ZR-75-1 cells (10); MHC class I expression was likewise increased with exposure of HPV-16 E7-expressing TC-1 murine tumor cells to CDDP in vitro (11).
Comparable results have been shown in vivo translating into improved antitumor efficacy. Using a murine model of plasmacytoma, Nio et al. subcutaneously inoculated cancer cells that were pretreated with either CDDP or an ethanol control (12). The potential to reject tumor was lower in mice injected with control tumor cells and flow cytometry demonstrated that the tumor cell expression of the MHC class I antigens, H-2Dd and H-2Kd, was enhanced with CDDP treatment (12). MHC class I expression in response to systemic CDDP treatment was demonstrated in a murine colorectal cancer (CRC) model (13). Cisplatin administered at IC50 doses increased the expression of MHC class I molecules in two human CRC cell lines (COLO 201 and colon 26) in vitro, analogous MHC class I upregulation was observed on cancer cells harvested from colon-26-tumor-bearing mice treated with CDDP (13).
Interestingly, CDDP’s ability to upregulate MHC class I expression may not be limited to just tumor cells but, as Jackaman et al. illustrated, CDDP may also increase MHC class I expression on antigen presenting cells (APCs) (14). In a murine mesothelioma model, the authors treated tumor-bearing mice with either two doses of CDDP or a PBS control and demonstrated that tumor-associated CD11c+ dendritic cells from mice treated with CDDP had greater MHC class I expression than did the dendritic cells from control mice (14).
Thus, accumulating evidence intriguingly suggests that even small, single doses of CDDP can be effective in upregulating MHC class I expression, both on tumor targets and on host APCs. Since the kinetics of this modulation are only on the order of several days, one can envision treatment scenarios in which a small “MHC-priming” dose of CDDP could be given prior to a complementary immunotherapy.
CDDP promotes recruitment and proliferation of effector cells
Although MHC class I expression is vital for CTL activation, the ability of the immune system to combat tumor is also dependent on the capacity of immune effector cells (including CTLs) to home to and accumulate within the tumor microenvironment. Mounting preclinical evidence now suggests that CDDP may promote the recruitment and subsequent proliferation of these effector cells (Fig. 1).
Chang et al. studied the immunomodulatory effects of dose-dense combination CDDP/paclitaxel (i.e., condensing the intervals between treatments) in a murine model of CDDP-resistant ovarian cancer (15). The investigators found that this dosing regimen yielded greater intratumoral F4/80+ macrophage and CD8+ T lymphocyte recruitment than did a standard dosing regimen; they concluded that this improved effector recruitment was responsible for enhanced antitumor efficacy (15). Nevertheless, traditional dosing regimens still appear to yield analogous results, even if to a lesser extent. For example, HPV-16 E7-expressing TC-1-tumor-bearing mice treated with two 10μg/kg CDDP doses had greater numbers of spleen-infiltrating E7-specific CD8+ T-cells than did untreated animals (11).
Notably, such CDDP-mediated recruitment has also been observed when CDDP is combined with adoptive immunotherapy. For example, Chen et al. examined the use of CDDP as a preconditioning agent for murine B16 melanoma xenografts treated with intravenously-administered cytokine-induced killer (CIK) cells (16). CIK cells are CD3+CD56+, non-MHC-restricted, natural killer-like T lymphocytes (17). Cisplatin pretreatment augmented the homing ability of CIK cells into tumors, tumor-draining lymph nodes (TDLNs), and spleen tissues (16). Moreover, endogenous effector cells – CD3+ T lymphocytes – also had increased intratumoral accumulation after CDDP pretreatment (16). These researchers also demonstrated enhanced intratumoral CD3+ T-cell accumulation with CDDP treatment and with combined CDDP/adoptive CIK therapy (as compared to controls) in a murine model of CRC (18). A combination of platinum doublet therapy (CDDP plus vinorelbine) with a recombinant yeast-CEA vaccine in a murine lung tumor model transgenic for CEA showed that both CD4+ and CD8+ CEA-specific T-cell proliferation was enhanced with combination vaccine/doublet therapy as compared to vaccine alone (19).
Though pretreatment with “priming” doses of CDDP can exert positive effects on the recruitment and proliferation of effector cells, similar CDDP-induced immunomodulation can be achieved by giving the drug adjuvantly, after immunotherapy. Fridlender et al. investigated the immune effects of CDDP plus gemcitabine when given as “boost” therapy to mice with orthotopic malignant pleural mesothelioma (MPM) or lung adenocarcinoma that were initially treated with adenoviral-based immunogene therapy (20). Adjuvant CDDP/gemcitabine therapy markedly increased numbers of antigen-specific, activated CD8+ T-cells both systemically and intratumorally (20). This treatment regimen also significantly increased the ratio of “antitumorigenic” M1 to “protumorigenic” M2 tumor-associated macrophages within the experimental mice (20).
Cisplatin-mediated, T-cell infiltration has also been studied in the setting of check-point blockade. For instance, Wu et al. investigated the antitumor and immunomodulatory effects of CDDP and combined CDDP/CTLA-4 blockade in a murine mesothelioma model (21). When quantifying relative numbers of tumor-infiltrating CD3+ T-cells, the authors found that mice treated with CDDP had significantly increased intratumoral T-cell infiltration than did untreated controls (21). Moreover, they noted that this effect became even greater when CDDP was combined with CTLA-4 blockade – an example of the synergistic potential of CDDP (21). CDDP has also been combined with PD-1 checkpoint blockade. Specifically, CDDP used in conjunction with PD-1 checkpoint blockade and CD137 (a T-cell costimulatory molecule) agonist antibody resulted in enhanced antitumor efficacy in a murine model of ovarian cancer (22). Interestingly, these beneficial effects were not seen when PD-1 blockade was excluded from the regimen (22). Qin et al. showed that CDDP leads to the upregulation of PDL-1 (the corresponding ligand to PD-1) in tumor cells; an effect also noted with the use of other chemotherapeutic agents (23, 24). The role of CDDP on PDL-1 and its combined use with PD-1 blockade both necessitate further investigation.
Cisplatin can therefore act either alone or, perhaps more powerfully, in concert with various novel immunotherapies to induce the upregulation of numerous endogenous immune effectors as well as to improve the recruitment of other adoptively-transferred cells. In fact, our own data demonstrate concordant beneficial effects: CDDP also aids in recruiting adoptively-transferred chimeric antigen receptor (CAR)-transduced T-cells. Interestingly, there is some evidence that suggests that CDDP may exert some of these recruiting/proliferative effects via more circuitous paths. Using an in vitro model, Hu et al. examined the functional consequence of CDDP treatment on CD14+ monocytes (rather than the effect of the drug on tumor or effector cells directly) and the subsequent capacity of these cells to activate CD4+ T lymphocytes (25). Cisplatin treatment led to an increased monocyte-induced CD4+ proliferation and the effect was largely mediated by the increased production of IFN-β (25). As a corollary, other studies showed that CDDP-treated mice bearing TC-1 tumors had considerably higher percentages of intratumoral CD11c+ dendritic cells (which, like monocytes, also serve as APCs) than did untreated tumor-bearing mice (26).
Therefore, CDDP may not only improve the recruitment and proliferation of immune effectors such as T-cells and adoptively-transferred CIKs, but it may also upregulate the accumulation of certain APCs as well – APCs that, as we learned in the previous section, have already had their MHC class I expression modulated by their exposure to CDDP. It is tempting to speculate that these now-proliferating, MHC-class-I-upregulated APCs may then go on to induce additional T-cell activation and accumulation.
CDDP upregulates the lytic activity of cytotoxic effectors
After successfully homing to the tumor microenvironment, cytotoxic effector cells then undertake their cytolytic functions. CTL-mediated killing typically occurs via the release of perforin and granzymes from the CTL and/or through the interaction between Fas (expressed on target cells) and FasL (a CTL cell-surface protein). Both the perforin/granzyme and Fas/FasL mechanisms involve activation of the caspase cascade and ultimately induce tumor-cell apoptosis. The immunomodulatory effects of CDDP have also been shown to extend into this aspect of tumor immunology (Fig. 1).
Markasz et al. undertook an expansive study of 29 frequently used chemotherapeutic agents, found giving CDDP in vitro at concentrations comparable to the maximally achieved therapeutic concentrations seen in clinical practice resulted in enhanced CTL-mediated killing of lymphoblastoid cell lines, as measured by a standard 51Cr release assay (27). Similar in vitro observations were made in CRC (28). Pretreating a primary colon adenocarcinoma cell line, SW480, with CDDP increased both ICAM-1 and Fas expression, resulting in enhanced antigen-specific CTL (i.e., ras oncogene-specific CD8+)-mediated tumor lysis (28). This improved lytic activity with CDDP was found to involve both Fas-dependent and -independent mechanisms and was accompanied by an increase in caspase-3-like protease activity (28).
In their in vitro study of platinum doublet therapy (CDDP/vinorelbine) in five human lung carcinoma cell lines (discussed earlier), Gameiro et al. found that sublethal exposure of cancer cells to CDDP/vinorelbine increased their sensitivity to perforin/granzyme-mediated CTL killing (8). This group made similar observations when CDDP/vinorelbine was used in combination with a recombinant yeast-CEA vaccine in a murine lung tumor model (19). HNSCC cells exposed to the combination sublethal CDDP/5-FU were also more susceptible to antigen-specific, MHC-restricted cytotoxic T-cell killing (9). A recent study examining the use of CDDP in combination with the chemosensitizer vitamin B6 precursor, pyridoxine, demonstrated that the synergistic cytotoxicity observed in vitro against non-small cell lung cancer (NSCLC) can translate into improved antitumor efficacy in vivo (29). When given to immunocompetent mice bearing NSCLC tumors, the CDDP/pyridoxine regimen resulted in a 45% cure rate; when given to athymic tumor-bearing mice, this combination CDDP treatment failed to avoid tumor growth and to prolong overall survival, thereby implying that the observed therapeutic effects relied on the presence of thymic-derived T lymphocytes (29).
At the mechanistic level, it appears that CDDP and pyridoxine do not only mediate a synergistic cytotoxic effect but that they also cooperate in the induction of immunogenic cell death (ICD) hallmarks. CDDP plus pyridoxine induced more endoplasmic reticulum (ER) stress protein Calreticulin exposure, HMGB1 exodus, and ATP release than did either of the two agents alone. With regard to Calreticulin exposure, both agents interacted synergistically to elicit ER stress at the level of the phosphorylation of eIF2α, which constitutes a stringent requirement for Calreticulin to be exposed. Indeed, CDDP and pyridoxine induced a higher level of eIF2α phosphorylation, if combined rather than used separately.
Considerable evidence therefore exists supporting the notion that CDDP increases a tumor cell’s sensitivity to antigen-specific CTL attack. One possible explanation for this repeatedly-observed phenomenon was explored by Ramakrishnan et al., who reported that pretreated tumor cells were sensitized to CTL-mediated lysis in particular due to the cancer cells’ increased permeability to granzyme B after CDDP exposure (30). Of note, this increased permeability allowed antigen-specific CTLs to kill not only antigen-expressing tumor cells, but also neighboring tumor cells not expressing the tumor antigen (30). In addition, the authors demonstrated that this effect was perforin-independent and mediated via the upregulation of mannose-6-phosphate receptors on the tumor cells (30).
Therefore, cisplatin exerts its antitumor immunomodulation on effector cells via several mechanisms: it can improve the lytic activity of endogenous CTLs, induce antigen-independent tumor bystander kill, and can even promote the lytic efficacy of adoptively-transferred CIKs.
CDDP downregulates the immunosuppressive microenvironment
The immunological microenvironment of a tumor is composed of elements from two competing forces – those that promote tumor eradication (“pro-immune” factors like CTLs) and those that promote tumor progression (e.g., immunosuppressive Tregs and other suppressor cells). The studies outlined thus far illustrated how CDDP can promote various pro-immune elements to tip this balance in favor of tumor eradication. However, cancer kill can also be promoted by downregulating the immunosuppressive components of the tumor microenvironment (Fig. 1).
Multiple groups have shown that CDDP can perform such downregulation. For example, as described above, Tseng et al. treated TC-1-tumor-bearing mice with CDDP, and showed that treatment with CDDP significantly reduced the levels of myeloid-derived suppressor cells (MDSC) and Tregs in tumor-bearing mice (11). Cisplatin also favorably modulates the immunosuppressive milieu when used in the setting of combination chemotherapy. In their study of the immunomodulatory effects of dose-dense CDDP/paclitaxel (discussed earlier), Chang et al. showed that this combined therapy significantly reduced the number of MDSCs as well as the number of Treg cells found in tumor-bearing mice (15). Gameiro et al. demonstrated that a single intraperitoneal dose of CDDP/vinorelbine induced a transient modulation of Treg function and markedly reduced the absolute number of Tregs available for immune suppression (19).
A number of researchers studying immunotherapies that are used in conjunction with CDDP have drawn similar conclusions. Chen at al used CDDP as a preconditioning agent in their study on adoptive CIK cell therapy (mentioned earlier) and observed a significant depletion of MDSCs in the TDLNs of CDDP-preconditioned mice (16). Parallel observations were made in a related study in which the ratio of intratumoral FoxP3+ Tregs to CD3+ lymphocytes was significantly reduced with CDDP treatment alone and when CDDP was combined with adoptive CIK therapy (18). Fridlender et al. saw similar reductions in Treg numbers in the spleens of mice treated with boost CDDP after initial adenoviral immunogene therapy (20).
It is intriguing that CDDP dually promotes the function and quantity of effector immune cells while simultaneously counteracting numerous immunosuppressive forces; the detailed mechanisms of these effects are still being investigated. Nevertheless, armed with the preclinical observations detailed above, we next examined the clinical literature for further evidence demonstrating the antitumor immunomodulatory effects of CDDP.
Clinical Evidence
Table 1 provides an overview of the clinical literature that supports CDDP-induced antitumor immunomodulation.
Table 1.
Summary of clinical literature with data indicating cisplatin-induced antitumor immunomodulation.
| Study | Patients (N) | Target Malignancy | Interventions | Immunological Studies | Results |
|---|---|---|---|---|---|
| Chang et al. (15) | 14 | Relapsed platinum-resistant ovarian CA | Dose-dense (DD) vs. maximum-tolerated-dose CDDP/paclitaxel regimens | Immune cell subset analysis and cytokine profiles | DD regimen: 1) Was less toxic to immune system; 2) Reduced immunosuppressive microenvironment; 3) Triggered macrophage and tumor-specific CD8 T-cell responses |
| Koufos et al. (31) | 32 | Stage III/IV, therapy-naïve NSCLC | CDDP/paclitaxel/i fosfamide | Systemic immune responses (i.e., cytokines) | 1) Treatment responders had significantly greater increases in IL-2 than did non-responders |
| Antonia et al. (32) | 29 | Extensive-stage small cell lung CA | p53 cancer vaccine combined w/ cisplatin-based chemotherapy | Phenotype & function of T-cells, DCs, and immature myeloid cells were analyzed & correlated w/ Ag-specific immune responses | 1) Ag-specific T-cell responses to vaccine seen in 57.1%; one patient w/ clinical response; 2) High rate (61.9%) of objective clinical responses to chemotherapy given immediately after vaccination |
| Roselli et al. (33) | 14 | Stage IB-IIIA NSCLC | Adjuvant CDDP/vinorelbine | Number & function of peripheral-blood Tregs | 1) Significant decrease in peripheral Tregs after successive treatment cycles; 2) Treatment significantly increased CD4 Teff:Treg ratio; 3) Marked decrease in immunosuppressive activity of circulating Tregs observed in all patients after treatment |
| Tsuchikawa et al. (34) | 18 | Therapy-naïve esophageal squamous cell carcinoma | Neoadjuvant CDDP/5-FU | HLA class I heavy chain, CD4, CD8, Treg infiltrate | Compared to non-neoadjuvant controls, neoadjuvant patients had: 1) Significantly more stromal & intratumoral CD4 T-cells; 2) significantly more stomal CD8 T-cells; 3) HLA class I expression more downregulated in controls than in neoadjuvant patients |
Chang et al., in their previously-mentioned study on the immunomodulatory effects of dose-dense CDDP/paclitaxel, also examined the use of this regimen in 14 patients with relapsed platinum-resistant ovarian cancer (15). During the course of treatment, the authors serially collected patient serum for IFN-γ and IL-2 quantification – both of which were used as surrogates for cytotoxic CD8+ T-cell activity (15). Of the four patients with disease control, the authors determined that three had serum levels of IFN-γ and IL-2 associated with cytotoxic CD8+ T-cell activity (15). Increases in serum IL-2 concentrations have similarly been observed in responders to CDDP-based therapy in stage III/IV, therapy-naïve NSCLC (31). Koufos et al. isolated the peripheral blood mononuclear cells of 32 such patients and tested the cells for the production of various immunological factors before and after treatment; the authors found that responders had significant increases in IL-2 as compared to non-responders and that patients who responded to treatment and had significant increases in IL-2 production had significantly greater median survival (31).
The antitumor immunomodulatory effects of CDDP with respect to both effector and suppressor cells have also been addressed in the clinical literature. Antonia et al. studied 29 patients with extensive-stage small cell lung cancer treated with a p53 cancer vaccine in combination with CDDP-based chemotherapy (32). Here, the authors noted p53-specific T-cell responses in 57.1% of patients following vaccination; a higher (61.9%) objective clinical response rate was observed for patients then receiving chemotherapy after vaccination (32). Roselli et al. sought to study the effects of adjuvant CDDP-based chemotherapy on the number and function of Tregs in 14 patients with NSCLC (stages IB, II, and IIIA) (33). The authors found that the ratio between effector CD4+ T-cells and Tregs significantly increased in patients receiving CDDP plus vinorelbine; they further determined that the immunosuppressive activity of the Tregs was reduced in the majority of these patients (33). Similar observations were made in a study of 18 therapy-naïve squamous cell esophageal carcinoma patients (34). Specifically, patients who received neoadjuvant CDDP/5-FU had significantly higher numbers of stromal and intratumoral CD4+ T-cells than did the patients not receiving neoadjuvant treatment (34). In addition, the authors found that the number of stromal CD8+ T-cells was also significantly higher in the neoadjuvant group (34).
The aforementioned clinical findings were made in the “real world” arena of clinical trials (as opposed to a highly controllable preclinical setting) and lend credibility to the monitoring of serum cytokines and/or the quantification of infiltrating or circulating immune cells in gauging the immune responses (both positive and negative) to CDDP.
Conclusions
Increasing preclinical and clinical evidence supports the belief that the anti-cancer activity of CDDP is not simply due to its ability to cross-link DNA and cause apoptosis. In fact, CDDP can favorably modulate the immune system, both in isolation and in concert with emerging immunotherapies. We now know that (1) even small, sublethal doses of CDDP can upregulate MHC class I expression on tumor cells and APCs; (2) CDDP exposure may improve the recruitment and proliferation of immune effectors such as T-cells and adoptively-transferred CIKs, as well as certain APCs; (3) CDDP can augment the lytic activity of endogenous and adoptively-transferred effector cells and can even induce antigen-independent bystander kill; and (4) CDDP can favorably downregulate immunosuppressive players in the tumor microenvironment, both by itself and synergistically with new immunotherapies. Furthermore, we are now witnessing how CDDP’s antitumor immunomodulatory effects are being harnessed clinically, adding new levels of sophistication to disease monitoring and prognostication. The observations summarized above thus lay the foundation for further investigations that might take advantage of CDDP’s potential synergism with novel immunotherapies like checkpoint blockade and adoptive T-cell therapy. In addition to exploring the specific pathways of these phenomena in preclinical models, it will be critically important to study the kinetics of CDDP’s immunomodulatory influence in patients, as high doses of the drug may negatively impact immune cells. The above-described literature therefore reveals to us additional mechanisms by which CDDP combats tumor – mechanisms that are yet to be exploited in treating solid tumors.
Acknowledgments
Grant Support
The laboratory work of P. Adusumilli was supported by grants from the NIH (R21 CA164568-01A1, R21 CA164585-01A1, U54 CA137788, P30 CA008748, and P50 CA086438-13), grants from the U.S. Department of Defense (PR101053 and LC110202), the Mr. William H. Goodwin and Mrs. Alice Goodwin and the Commonwealth Foundation for Cancer Research, and the Experimental Therapeutics Center of Memorial Sloan Kettering Cancer Center.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Lebwohl D, Canetta R. Clinical development of platinum complexes in cancer therapy: an historical perspective and an update. Eur J Cancer. 1998;34:1522–34. doi: 10.1016/s0959-8049(98)00224-x. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg B, Vancamp L, Krigas T. Inhibition of Cell Division in Escherichia Coli by Electrolysis Products from a Platinum Electrode. Nature. 1965;205:698–9. doi: 10.1038/205698a0. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg B, Renshaw E, Vancamp L, Hartwick J, Drobnik J. Platinum-induced filamentous growth in Escherichia coli. J Bacteriol. 1967;93:716–21. doi: 10.1128/jb.93.2.716-721.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenberg B, Van Camp L, Grimley EB, Thomson AJ. The inhibition of growth or cell division in Escherichia coli by different ionic species of platinum(IV) complexes. J Biol Chem. 1967;242:1347–52. [PubMed] [Google Scholar]
- 5.Rosenberg B, VanCamp L, Trosko JE, Mansour VH. Platinum compounds: a new class of potent antitumour agents. Nature. 1969;222:385–6. doi: 10.1038/222385a0. [DOI] [PubMed] [Google Scholar]
- 6.Rosenberg B, VanCamp L. The successful regression of large solid sarcoma 180 tumors by platinum compounds. Cancer Res. 1970;30:1799–802. [PubMed] [Google Scholar]
- 7.Hato SV, Khong A, de Vries IJ, Lesterhuis WJ. Molecular pathways: the immunogenic effects of platinum-based chemotherapeutics. Clin Cancer Res. 2014;20:2831–7. doi: 10.1158/1078-0432.CCR-13-3141. [DOI] [PubMed] [Google Scholar]
- 8.Gameiro SR, Caballero JA, Hodge JW. Defining the molecular signature of chemotherapy-mediated lung tumor phenotype modulation and increased susceptibility to T-cell killing. Cancer Biother Radiopharm. 2012;27:23–35. doi: 10.1089/cbr.2012.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gelbard A, Garnett CT, Abrams SI, Patel V, Gutkind JS, Palena C, et al. Combination chemotherapy and radiation of human squamous cell carcinoma of the head and neck augments CTL-mediated lysis. Clin Cancer Res. 2006;12:1897–905. doi: 10.1158/1078-0432.CCR-05-1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wan S, Pestka S, Jubin RG, Lyu YL, Tsai YC, Liu LF. Chemotherapeutics and radiation stimulate MHC class I expression through elevated interferon-beta signaling in breast cancer cells. PLoS One. 2012;7:e32542. doi: 10.1371/journal.pone.0032542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tseng CW, Hung CF, Alvarez RD, Trimble C, Huh WK, Kim D, et al. Pretreatment with cisplatin enhances E7-specific CD8+ T-Cell-mediated antitumor immunity induced by DNA vaccination. Clin Cancer Res. 2008;14:3185–92. doi: 10.1158/1078-0432.CCR-08-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nio Y, Hirahara N, Minari Y, Iguchi C, Yamasawa K, Toga T, et al. Induction of tumor-specific antitumor immunity after chemotherapy with cisplatin in mice bearing MOPC-104E plasmacytoma by modulation of MHC expression on tumor surface. Anticancer Res. 2000;20:3293–9. [PubMed] [Google Scholar]
- 13.Ohtsukasa S, Okabe S, Yamashita H, Iwai T, Sugihara K. Increased expression of CEA and MHC class I in colorectal cancer cell lines exposed to chemotherapy drugs. J Cancer Res Clin Oncol. 2003;129:719–26. doi: 10.1007/s00432-003-0492-0. [DOI] [PubMed] [Google Scholar]
- 14.Jackaman C, Majewski D, Fox SA, Nowak AK, Nelson DJ. Chemotherapy broadens the range of tumor antigens seen by cytotoxic CD8(+) T cells in vivo. Cancer Immunol Immunother. 2012;61:2343–56. doi: 10.1007/s00262-012-1307-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang CL, Hsu YT, Wu CC, Lai YZ, Wang C, Yang YC, et al. Dose-dense chemotherapy improves mechanisms of antitumor immune response. Cancer Res. 2013;73:119–27. doi: 10.1158/0008-5472.CAN-12-2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen J, Huang X, Huang G, Chen Y, Chen L, Song H. Preconditioning chemotherapy with cisplatin enhances the antitumor activity of cytokine-induced killer cells in a murine melanoma model. Cancer Biother Radiopharm. 2012;27:210–20. doi: 10.1089/cbr.2011.1116. [DOI] [PubMed] [Google Scholar]
- 17.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–40. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 18.Huang X, Chen YT, Song HZ, Huang GC, Chen LB. Cisplatin pretreatment enhances anti-tumor activity of cytokine-induced killer cells. World J Gastroenterol. 2011;17:3002–11. doi: 10.3748/wjg.v17.i25.3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gameiro SR, Caballero JA, Higgins JP, Apelian D, Hodge JW. Exploitation of differential homeostatic proliferation of T-cell subsets following chemotherapy to enhance the efficacy of vaccine-mediated antitumor responses. Cancer Immunol Immunother. 2011;60:1227–42. doi: 10.1007/s00262-011-1020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fridlender ZG, Sun J, Singhal S, Kapoor V, Cheng G, Suzuki E, et al. Chemotherapy delivered after viral immunogene therapy augments antitumor efficacy via multiple immune-mediated mechanisms. Mol Ther. 2010;18:1947–59. doi: 10.1038/mt.2010.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu L, Yun Z, Tagawa T, Rey-McIntyre K, de Perrot M. CTLA-4 blockade expands infiltrating T cells and inhibits cancer cell repopulation during the intervals of chemotherapy in murine mesothelioma. Mol Cancer Ther. 2012;11:1809–19. doi: 10.1158/1535-7163.MCT-11-1014. [DOI] [PubMed] [Google Scholar]
- 22.Wei H, Zhao L, Li W, Fan K, Qian W, Hou S, et al. Combinatorial PD-1 blockade and CD137 activation has therapeutic efficacy in murine cancer models and synergizes with cisplatin. PLoS One. 2013;8:e84927. doi: 10.1371/journal.pone.0084927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qin X, Liu C, Zhou Y, Wang G. Cisplatin induces programmed death-1-ligand 1(PD-L1) over-expression in hepatoma H22 cells via Erk /MAPK signaling pathway. Cell Mol Biol (Noisy-le-grand) 2010;56(Suppl):OL1366–72. [PubMed] [Google Scholar]
- 24.Zhang P, Su DM, Liang M, Fu J. Chemopreventive agents induce programmed death-1-ligand 1 (PD-L1) surface expression in breast cancer cells and promote PD-L1-mediated T cell apoptosis. Mol Immunol. 2008;45:1470–6. doi: 10.1016/j.molimm.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 25.Hu J, Kinn J, Zirakzadeh AA, Sherif A, Norstedt G, Wikstrom AC, et al. The effects of chemotherapeutic drugs on human monocyte-derived dendritic cell differentiation and antigen presentation. Clin Exp Immunol. 2013;172:490–9. doi: 10.1111/cei.12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee SY, Kang TH, Knoff J, Huang Z, Soong RS, Alvarez RD, et al. Intratumoral injection of therapeutic HPV vaccinia vaccine following cisplatin enhances HPV-specific antitumor effects. Cancer Immunol Immunother. 2013;62:1175–85. doi: 10.1007/s00262-013-1421-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Markasz L, Skribek H, Uhlin M, Otvos R, Flaberg E, Eksborg S, et al. Effect of frequently used chemotherapeutic drugs on cytotoxic activity of human cytotoxic T-lymphocytes. J Immunother. 2008;31:283–93. doi: 10.1097/CJI.0b013e3181628b76. [DOI] [PubMed] [Google Scholar]
- 28.Bergmann-Leitner ES, Abrams SI. Treatment of human colon carcinoma cell lines with anti-neoplastic agents enhances their lytic sensitivity to antigen-specific CD8+ cytotoxic T lymphocytes. Cancer Immunol Immunother. 2001;50:445–55. doi: 10.1007/s002620100229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aranda F, Bloy N, Pesquet J, Petit B, Chaba K, Sauvat A, et al. Immune-dependent antineoplastic effects of cisplatin plus pyridoxine in non-small-cell lung cancer. Oncogene. 2014 Jul 28; doi: 10.1038/onc.2014.234. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 30.Ramakrishnan R, Assudani D, Nagaraj S, Hunter T, Cho HI, Antonia S, et al. Chemotherapy enhances tumor cell susceptibility to CTL-mediated killing during cancer immunotherapy in mice. J Clin Invest. 2010;120:1111–24. doi: 10.1172/JCI40269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koufos N, Michailidou D, Xynos ID, Tomos P, Athanasiadou K, Kosmas C, et al. Modulation of peripheral immune responses by paclitaxel-ifosfamide-cisplatin chemotherapy in advanced non-small-cell lung cancer. J Cancer Res Clin Oncol. 2013;139:1995–2003. doi: 10.1007/s00432-013-1514-1. [DOI] [PubMed] [Google Scholar]
- 32.Antonia SJ, Mirza N, Fricke I, Chiappori A, Thompson P, Williams N, et al. Combination of p53 cancer vaccine with chemotherapy in patients with extensive stage small cell lung cancer. Clin Cancer Res. 2006;12:878–87. doi: 10.1158/1078-0432.CCR-05-2013. [DOI] [PubMed] [Google Scholar]
- 33.Roselli M, Cereda V, di Bari MG, Formica V, Spila A, Jochems C, et al. Effects of conventional therapeutic interventions on the number and function of regulatory T cells. Oncoimmunology. 2013;2:e27025. doi: 10.4161/onci.27025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsuchikawa T, Md MM, Yamamura Y, Shichinohe T, Hirano S, Kondo S. The immunological impact of neoadjuvant chemotherapy on the tumor microenvironment of esophageal squamous cell carcinoma. Ann Surg Oncol. 2012;19:1713–9. doi: 10.1245/s10434-011-1906-x. [DOI] [PubMed] [Google Scholar]